Abstract
Wolbachia pipientis bacteria are maternally inherited endosymbionts that are of interest to control the Anopheles mosquito vectors of malaria. Wolbachia does not infect Anopheles mosquitoes in nature, although cultured Anopheles cells can be infected. Here, we show that the virulent Wolbachia strain wMelPop can survive and replicate when injected into female Anopheles gambiae adults, but the somatic infections established are avirulent. These in vivo data suggest that stable Wolbachia infections of Anopheles may be possible.
Infecting up to 500 million people per year (with almost 3 million annual deaths), malaria is the most important vector-borne disease in the world (8, 30, 31, 32). The Plasmodium parasites that cause the disease are transmitted to humans by the bite of Anopheles mosquitoes, with Anopheles gambiae being the principle vector in sub-Saharan Africa (6). Malaria control is limited by the lack of a vaccine and by parasite and mosquito evolution of drug and insecticide resistance (9, 28, 31). In light of these problems, there has been a recent concerted effort to develop innovative methods for malaria control based on the genetic modification of Anopheles mosquitoes (transgenesis) or their associated symbiotic microorganisms (paratransgenesis) (5, 10, 11, 13, 15, 23, 25, 27, 36, 37).
In many mosquitoes, Wolbachia pipientis symbionts are the causative agents of cytoplasmic incompatibility, a phenomenon where matings between uninfected females and infected males have reduced egg hatch, while matings in the reciprocal cross are fertile. In a mixed population, infected females have a reproductive advantage which can allow Wolbachia to increase rapidly in frequency due to maternal inheritance. The propensity of Wolbachia to “drive” through populations has been investigated using mathematical models and has been validated by both laboratory and field investigations (20, 21, 33, 34, 35, 36).
There are three scenarios currently envisioned to use Wolbachia as part of a malaria control strategy: (i) use Wolbachia spread to “drive” refractory transgenes into Anopheles populations, converting the mosquito population into one that cannot maintain transmission of the malaria parasites (18, 21, 24, 29, 33, 36); (ii) release Wolbachia-infected males into uninfected Anopheles populations to reduce population sizes through cytoplasmic incompatibility, similar to the sterile insect technique but without exposing males to radiation or chemical sterilants that could lower their fitness (2, 4, 37); and (iii) release mosquitoes infected with pathogenic or virulent Wolbachia strains that shorten mosquito life span. Pathogens must pass through an extrinsic incubation period in the vector before they are able to be transmitted. By shortening mosquito life span, it is theoretically possible to reduce the number of mosquitoes that live through the extrinsic incubation period and become infectious (14, 15, 17, 20, 24).
All of the above strategies require the stable transfer of Wolbachia into Anopheles. Wolbachia symbionts are common in mosquitoes, but no infections have ever been identified in any species of Anopheles (12, 22, 26). The negative infection status of natural Anopheles populations offers good potential for Wolbachia-based malaria control strategies, since preexisting infections can complicate the behavior of introduced infections (33). However, the absence of natural infections in anophelines has led some to suggest that Anopheles mosquitoes may be physiologically/genetically incapable of harboring Wolbachia infections (1, 29). If this hypothesis is true, then Wolbachia-based malaria control strategies are likely doomed to fail. In vitro studies demonstrated that cultured immunocompetent Anopheles gambiae cell lines (Sua5B and Moss55) are fully competent to harbor infections of distinct Wolbachia strains (the “A” supergroup strains wRi and wMelPop from Drosophila simulans and Drosophila melanogaster and the “B” supergroup strain wAlbB from Aedes albopictus) (16, 18). Some cultured infections reached very high levels where 100% of cells were infected at extremely high levels (wAlbB in Sua5B and wMelPop in Moss55) (16, 18), while other strains were eventually eliminated from the cells (wRi in Sua5B cells) (24). The combined results of these experiments, using multiple Wolbachia strains and multiple Anopheles cell lines, indicate that there is no intrinsic genetic block to Wolbachia infection in Anopheles cells, although certain strains of Wolbachia may be more likely to colonize Anopheles than others.
In this study, we investigated the establishment of in vivo Wolbachia infections in Anopheles gambiae (Keele strain) mosquitoes by injection of the virulent Wolbachia strain wMelPop into the hemolymph of adult female mosquitoes. Approximately 200 adult mosquitoes were reared in 30-cm cube cages in a walk-in insectary held at 28°C and 80% relative humidity on a 12:12 h light/dark cycle. Mosquitoes were allowed access to a cotton wick soaked in 10% sucrose as a carbohydrate source. Adults were allowed to blood feed on an anesthetized mouse 5 days postemergence according to JHU animal use protocol MO-03H210. Two days after blood feeding, an oviposition substrate (consisting of a filter paper cone inside a 50-ml beaker half filled with water) was introduced into cages and removed the next day for egg collection. Approximately 250 eggs were placed into a 41- by 34- by 6-cm rearing tray half filled with distilled water and one pellet of dry cat food, with one additional pellet added to each tray daily after day 3. Larvae were removed, and tray water changed if polluted. Pupae were picked with an eyedropper, placed in a cup, and introduced into cages (∼200 pupae/cage) to begin the next generation.
wMelPop was cultured in Anopheles gambiae Moss55 cells (16), purified, and assessed for viability as described previously (18, 19). Purified Wolbachia cells were suspended in culture medium and adjusted to a final concentration of 108 bacteria per ml. Using a calibrated glass capillary needle, amounts of 100 to 200 nl suspended Wolbachia cells were injected into the thorax of 2-day-old, cold-immobilized adult Anopheles gambiae females. Injected mosquitoes were held at 18°C for 5 days and then transferred to the 28°C insectary. Mosquitoes were allowed to blood feed on a mouse twice per week.
Mosquito genomic DNA was extracted by salt extraction/ethanol precipitation as described previously (21), quantified using a NanoDrop spectrophotometer, and adjusted to 20 ng/μl. Wolbachia infections in individual mosquitoes were detected by PCR amplification of a fragment of the Wolbachia 16S rRNA gene (440 bp) using primers WspecF and WspecR (18). As a control, we amplified a 400-bp fragment from the Anopheles mitochondrial NADH dehydrogenase subunit 4 gene (ND4) (18). Mosquitoes were assayed for Wolbachia infection by PCR at 6, 10, 20, or 30 days postinjection (p.i.). In a second experiment, mosquitoes were assayed at 0, 3, 8, 13, 15, and 21 days p.i. Amplified fragments were separated by 1% agarose gel electrophoresis, stained with ethidium bromide, and viewed under UV light. Template DNA from infected and uninfected Moss55 cells was included as positive and negative controls.
Quantitative PCR (qPCR) was used to determine if Wolbachia could survive and replicate in Anopheles by comparing normalized Wolbachia levels in individual mosquitoes at day 6 and day 30 p.i. The relative abundance of wMelPop bacteria in each mosquito was assessed by comparing the abundance of the single-copy Wolbachia ankyrin repeat gene WD_0550 (16) to that of the single-copy Anopheles gambiae ribosomal S7 gene (forward, 5′-TCC-TGG-AGC-TGG-AGA-TGA-AC-3′, and reverse, 5′-GAC-GGG-TCT-GTA-CCT-TCT-GG-3′). For each time point, 14 mosquitoes (biological replicates) were examined. Duplicate reactions were performed for every mosquito, and the results differed by less than 3%, demonstrating consistency of the assay. qPCR was performed using an ABI Prism 7300 detection system (Applied Biosystems) with a QuantiTect SYBR green PCR kit (Qiagen). Determinations of relative abundance of wMelPop in each mosquito and relative changes in wMelPop levels between time points, confidence interval estimation, and statistical analyses were carried out as described by Yuan et al. (38).
To test for virulence of wMelPop, 2-day-old adult female mosquitoes were injected with either wMelPop purified from cell culture or filtered lysate of uninfected Moss55 cells (control) and held at 18°C for 2 days as described above. Injected mosquitoes were held an additional 3 days at 28°C to control for mortality due to the injection procedure. At day 5 p.i., mosquitoes were moved into pint-sized cup cages for life table experiments. Approximately 25 mosquitoes were placed in each cage (two replicate control cages and three replicate Wolbachia treatment cages) and the entire experiment replicated two times, for a total of four control cages and six Wolbachia treatment cages. Mosquitoes were provided a cotton pad soaked in 10% sucrose but were not given access to blood. Dead mosquitoes were removed from each cage approximately every other day. For each experiment, mortality data were used to construct treatment-specific cohort life tables (3). Because the data did not conform to parametric assumptions, they were analyzed by the Mann-Whitney U test using STATVIEW (SAS Corporation).
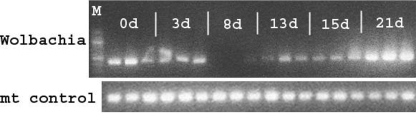
In injected mosquitoes, Wolbachia bacteria were detectable by PCR at all time points, as follows [infection frequency (95% exact binomial confidence interval)]: day 6, 0.875 (0.617 to 0.985; n = 16); day 10, 0.75 (0.401 to 0.968; n = 8); day 20, 0.722 (0.465 to 0.903; n = 18); and day 30, 1.0 (0.794 to 1.0; n = 13). In further experiments, Wolbachia bacteria were easily detectable through day 3 p.i. but were weak or not detectable by conventional PCR by day 8 p.i. After 13 days p.i., Wolbachia bacteria were easily detectable again, and the bands increased in intensity for the remainder of the time series experiment (Fig. 1). This initial decrease, followed by an increase, in the apparent infection rate is possibly due to initial clearance of some of the injected bacteria and then establishment of infection and bacterial replication. By qPCR, a highly statistically significant 42-fold increase in the normalized Wolbachia level was observed: on day 6 p.i., there were 23.7 Wolbachia genomes per host genome (95% confidence interval, 10.6 to 52.7; n = 14), and on day 30 p.i., there were 992 Wolbachia genomes per host genome (95% confidence interval, 433.6 to 2,267.4; n = 14) (Mann-Whitney U test, tied Z value = −4.319; P < 0.0001). Since Wolbachia cannot replicate in the extracellular environment (19), these results confirm that injected bacteria are able to infect cells, survive, and replicate in Anopheles gambiae in vivo.
FIG. 1.
Typical results using conventional PCR, showing changes in Wolbachia levels in injected adult Anopheles gambiae females at sequential time points postinjection. Results for mt control (host ND4 mitochondrial gene) indicate that PCR efficiency was approximately equal for all samples. M, 100-bp marker; d, days.
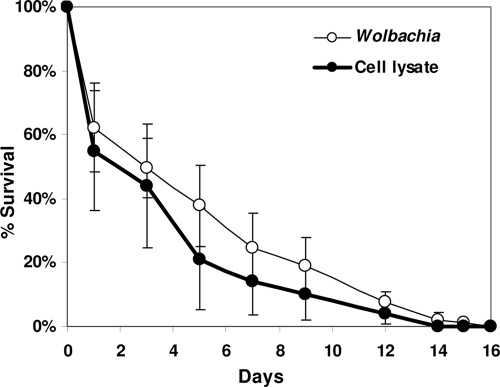
wMelPop is a virulent Wolbachia strain that reduces the life span of its host by approximately 50%. While originally found and characterized in a laboratory colony of Drosophila melanogaster, it has similar pathogenic effects when artificially transferred into Drosophila simulans (14), and recently, the yellow fever mosquito Aedes aegypti (15). However, there was no statistically significant difference in survival trajectories between Anopheles gambiae mosquitoes injected with wMelPop and mosquitoes injected with filtered uninfected cell lysate (Mann-Whitney U test, tied Z value = −1.799; P = 0.702) (Fig. 2). Although wMelPop replicates to high levels in injected Anopheles (approximately 1,000 bacterial genomes per host genome), these levels do not seem to be associated with virulence. It is possible that the virulence of wMelPop has been attenuated during its culture in Moss55 cells, although during long-term culture in an Aedes aegypti cell line, wMelPop retained its virulent phenotype when reintroduced into either Drosophila or Aedes aegypti in vivo (15, 16). The specific mechanism of wMelPop virulence is not completely understood, but it seems that increased host mortality is not simply due to overreplication and high infection levels but rather to overreplication in and damage to specific host tissues, such as the brain and central nervous system (14, 15, 17). Investigation into the tissue localization of Wolbachia in injected Anopheles mosquitoes is currently ongoing, but in light of these results, it is reasonable to hypothesize that when injected into the hemolymph, Wolbachia bacteria reach high levels in some mosquito tissues but either do not infect or do not replicate in the Anopheles central nervous system. It remains to be seen whether vertically acquired infections will show virulence in Anopheles gambiae.
FIG. 2.
Mean survival trajectories of wMelPop-injected versus cell lysate-injected Anopheles gambiae adult females. Survival trajectories do not differ significantly. Error bars show standard deviations.
Previous studies showed that cultured Anopheles gambiae cells can be infected with Wolbachia (16, 18), but no data were available to assess whether the in vitro results could be extrapolated to Anopheles mosquitoes in vivo. The experiments outlined in this paper demonstrate that Wolbachia can infect Anopheles mosquitoes in vivo. However, for a Wolbachia-based malaria control strategy to be effective, simply infecting Anopheles by injection is not sufficient—the infection must be transmitted vertically to offspring. Stable (100%) vertical transmission of Wolbachia after injection into adults has been reported for Drosophila melanogaster (7). A similar phenomenon has been reported for Aedes aegypti, but transmission was unstable (approximately 40%) (27). Experiments to determine whether Wolbachia bacteria injected into the hemolymph of adult Anopheles will be transmitted vertically to offspring are ongoing, and if efficient vertical transmission of the symbionts can be established, Wolbachia-based strategies for malaria control should be possible.
Acknowledgments
This work was supported by NIH grant R21AI070178 and funds from the Bloomberg Family Foundation to J.L.R. C.J. was partially supported by the Calvin and Helen Lang Scholarship (JHSPH).
We thank Scott O'Neill for his kind gift of the wMelPop-infected Moss55 cell line.
Footnotes
Published ahead of print on 27 March 2009.
REFERENCES
- 1.Arcà, B., F. Lombardo, J. G. Valenzuela, I. M. Francischetti, O. Marinotti, M. Coluzzi, and J. M. Ribeiro. 2005. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J. Exp. Biol. 208:3971-3986. [DOI] [PubMed] [Google Scholar]
- 2.Brelsfoard, C. L., Y. Sechan, and S. L. Dobson. 2008. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey, J. R. 1993. Applied demography for biologists with special emphasis on insects. Oxford University Press, Oxford, United Kingdom.
- 4.Dobson, S. L., C. W. Fox, and F. M. Jiggins. 2002. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. Biol. Sci. 269:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favia, G., I. Ricci, C. Damiani, N. Raddadi, E. Crotti, M. Marzorati, A. Rizzi, R. Urso, L. Brusetti, S. Borin, D. Mora, P. Scuppa, L. Pasqualini, E. Clementi, M. Genchi, S. Corona, I. Negri, G. Grandi, A. Alma, L. Kramer, F. Esposito, C. Bandi, L. Sacchi, and D. Daffonchio. 2007. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. USA 104:9047-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontenille, D., and F. Simard. 2004. Unraveling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vector populations. Comp. Immunol. Microbiol. Infect. Dis. 27:357-375. [DOI] [PubMed] [Google Scholar]
- 7.Frydman, H. M., J. M. Li, D. N. Robson, and E. Wieschaus. 2006. Somatic stem cell niche tropism in Wolbachia. Nature 441:509-512. [DOI] [PubMed] [Google Scholar]
- 8.Hay, S. I., C. A. Guerra, A. J. Tatem, A. M. Noor, and R. W. Snow. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 4:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemingway, J., and H. Ranson. 2000. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45:371-391. [DOI] [PubMed] [Google Scholar]
- 10.Ito, J., A. Ghosh, L. A. Moreira, E. A. Wimmer, and M. Jacobs-Lorena. 2002. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417:452-455. [DOI] [PubMed] [Google Scholar]
- 11.James, A. A. 2005. Gene drive systems in mosquitoes: rules of the road. Trends Parasitol. 21:64-67. [DOI] [PubMed] [Google Scholar]
- 12.Kittayapong, P., K. J. Baisley, V. Baimai, and S. L. O'Neill. 2000. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37:340-345. [DOI] [PubMed] [Google Scholar]
- 13.Marrelli, M. T., C. Li, J. L. Rasgon, and M. Jacobs-Lorena. 2007. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc. Natl. Acad. Sci. USA 104:5580-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMeniman, C. J., R. V. Lane, B. N. Cass, A. W. Fong, M. Sidhu, Y. F. Wang, and S. L. O'Neill. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141-144. [DOI] [PubMed] [Google Scholar]
- 16.McMeniman, C. J., A. M. Lane, A. W. Fong, D. A. Voronin, I. Iturbe-Ormaetxe, R. Yamada, E. A. McGraw, and S. L. O'Neill. 2008. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl. Environ. Microbiol. 74:6963-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min, K. T., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasgon, J. L., X. Ren, and M. Petridis. 2006. Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl. Environ. Microbiol. 72:7718-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasgon, J. L., C. E. Gamston, and X. Ren. 2006. Survival of Wolbachia pipientis in cell-free medium. Appl. Environ. Microbiol. 72:6934-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasgon, J. L., L. M. Styer, and T. W. Scott. 2003. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J. Med. Entomol. 40:125-132. [DOI] [PubMed] [Google Scholar]
- 21.Rasgon, J. L., and T. W. Scott. 2003. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics 165:2029-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasgon, J. L., and T. W. Scott. 2004. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J. Med. Entomol. 41:255-257. [DOI] [PubMed] [Google Scholar]
- 23.Rasgon, J. L., and F. Gould. 2005. Transposable element insertion location bias and the dynamics of gene drive in mosquito populations. Insect Mol. Biol. 14:493-500. [DOI] [PubMed] [Google Scholar]
- 24.Rasgon, J. L. 2008. Wolbachia and Anopheles mosquitoes, p. 321-327. In K. Bourtzis and T. Miller (ed.), Insect symbiosis, vol. 3. CRC Press, Boca Raton, FL. [Google Scholar]
- 25.Ren, X., E. Hoiczyk, and J. L. Rasgon. 2008. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 4:e1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci, I., G. Cancrini, S. Gabrielli, S. D'Amelio, and G. Favia. 2002. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J. Med. Entomol. 39:562-567. [DOI] [PubMed] [Google Scholar]
- 27.Ruang-Areerate, T., and P. Kittayapong. 2006. Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. USA 103:12534-12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiff, C. 2002. Integrated approach to malaria control. Clin. Microbiol. Rev. 15:278-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinkins, S. P. 2004. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 34:723-729. [DOI] [PubMed] [Google Scholar]
- 30.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talisuna, A. O., P. Bloland, and U. D'Alessandro. 2004. History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17:235-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townson, H., M. B. Nathan, M. Zaim, P. Guillet, L. Manga, R. Bos, and M. Kindhauser. 2005. Exploiting the potential of vector control for disease prevention. Bull. W. H. O. 83:942-947. [PMC free article] [PubMed] [Google Scholar]
- 33.Turelli, M., and A. A. Hoffmann. 1999. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 8:243-255. [DOI] [PubMed] [Google Scholar]
- 34.Turelli, M., and A. A. Hoffmann. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werren, J. H., L. Baldo, and M. E. Clark. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6:741-751. [DOI] [PubMed] [Google Scholar]
- 36.Xi, Z., C. C. Khoo, and S. L. Dobson. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326-328. [DOI] [PubMed] [Google Scholar]
- 37.Xi, Z., C. C. Khoo, and S. L. Dobson. 2006. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. Biol. Sci. 273:1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan, J. S., A. Reed, F. Chen, and C. N. Stewart, Jr. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]