Abstract
Formation of dental plaque is a developmental process involving initial and late colonizing species that form polymicrobial communities. Fusobacteria are the most numerous gram-negative bacteria in dental plaque, but they become prevalent after the initial commensal colonizers, such as streptococci and actinomyces, have established communities. The unusual ability of these bacteria to coaggregate with commensals, as well as pathogenic late colonizers, has been proposed to facilitate colonization by the latter organisms. We investigated the integration of Fusobacterium nucleatum into multispecies communities by employing two in vitro models with saliva as the sole nutritional source. In flow cell biofilms, numbers of cells were quantified using fluorescently conjugated antibodies against each species, and static biofilms were analyzed by quantitative real-time PCR (q-PCR) using species-specific primers. Unable to grow as single-species biofilms, F. nucleatum grew in two-species biofilms with Actinomyces naeslundii but not with Streptococcus oralis. However, enhanced growth of fusobacteria was observed in three-species biofilms, indicating that there was multispecies cooperation. Importantly, these community dynamics yielded an 18-fold increase in the F. nucleatum biomass between 4 h and 18 h in the flow cell inoculated with three species. q-PCR analysis of static biofilms revealed that maximum growth of the three species occurred at 24 h to 36 h. Lower numbers of cells were observed at 48 h, suggesting that saliva could not support higher cell densities as the sole nutrient. Integration of F. nucleatum into multispecies commensal communities was evident from the interdigitation of fusobacteria in coaggregates with A. naeslundii and S. oralis and from the improved growth of fusobacteria, which was dependent on the presence of A. naeslundii.
The human mouth contains microbiologically diverse communities. While collectively humans harbor more than 700 bacterial phylotypes, each individual is estimated to have fewer than 100 such phylotypes (1), and approximately 50% of human oral bacteria have yet to be cultivated. Although biofilm communities on tooth enamel are polymicrobial (3, 20), more than 60 to 90% of the bacteria found in initial plaque on saliva-coated tooth enamel are streptococci (6, 19). Other bacterial genera that are among the initial commensal colonizers include Actinomyces, Veillonella, and Neisseria (6, 16, 19), and these organisms contribute to the polymicrobial nature of initial plaque.
The structure of a community is dependent upon the nature of the foundation. An integral feature of an oral bacterial biofilm foundation is the ability to coaggregate, which is defined as cell-cell recognition and binding between genetically distinct bacteria. After routine oral hygiene treatment, freshly cleaned tooth enamel is quickly coated with a salivary pellicle, which provides a set of receptor molecules recognized by primary colonizing bacteria, such as streptococci and actinomyces. Besides recognizing salivary receptors, these bacteria coaggregate and provide a foundation for the subsequent attachment and growth of other bacteria, such as veillonellae, that form close metabolic relationships with streptococci (12, 15). As initial colonizers develop into biofilm communities with anaerobic microenvironments, incorporation of the obligate anaerobic fusobacteria into these communities becomes possible. Fusobacteria as a group coaggregate with all other oral bacteria and have been suggested, therefore, to be a crucial link between primary colonizing species and later colonizing pathogens (13, 14). Thus, a foundation consisting of coaggregating streptococci, actinomyces, and veillonellae populates the tooth surface, and these organisms are recognized by fusobacteria, which colonize and become the dominant gram-negative bacterial species. The new foundation is a substratum containing fusobacterial surface receptors available for recognition by late colonizing pathogens. Supporting the crucial link is clinical evidence that fusobacteria appear in dental plaque after commensal species and before the pathogenic “red” complex consisting of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia (22, 23).
Coaggregation partnerships are highly specific. A significant role for coaggregation in the formation of dental plaque biofilms and particularly in accretion of secondary colonizers to the pioneer species in plaque has been proposed (14) and has been demonstrated for the development of a spatially organized community (20). However, coaggregation may also provide some metabolic advantages (e.g., cross feeding and enzyme complementation) to neighboring cells by facilitating physical juxtaposition of partner cells, as has been shown for glucose metabolism of coaggregates of actinomyces and streptococci (7, 8). One aim of the present study was to examine the structures of two- and three-species communities composed of Actinomyces naeslundii, Streptococcus oralis, and Fusobacterium nucleatum in model biofilm systems. The first two species are initial colonizers and are considered commensals, whereas fusobacteria are secondary colonizers and are postulated to be a coaggregation bridge between initial and late colonizers (14). Our second aim was to investigate the integration and growth of fusobacteria in polymicrobial communities.
A variety of experimental methods have been developed to study the formation of biofilms. Model systems often rely on the flow of nutrients over a surface on which bacteria are able to attach and grow. In the present study we used two distinct in vitro models, a saliva-fed flow cell and a polystyrene peg immersed in static saliva. Biofilm communities form naturally and are undisturbed (3, 20, 21). The spatial organization of a multispecies community resulting from colonization and growth is preserved and can be examined noninvasively by confocal laser scanning microscopy (CLSM). In the static system, the amount of each species in multispecies biofilms formed on polystyrene pegs can be measured by real-time quantitative PCR (q-PCR). We show here with both models that fusobacteria are unable to grow as single species, but they integrate into commensal streptococcus-actinomyces communities and grow. Integration and growth are required for fusobacteria to become crucial links between commensal communities and later colonizing pathogenic communities. In the three-species community studied here, A. naeslundii is required for F. nucleatum to integrate and grow.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. oralis 34 and A. naeslundii ATCC 43146 were routinely cultured in Todd-Hewitt broth (Difco Laboratories, Detroit, MI) or on Todd-Hewitt agar. F. nucleatum ATCC 10953 was grown in brain heart infusion (Difco) broth supplemented with 0.25% l-glutamic acid. All species were grown in a Bactron anaerobic (N2-CO2-H2, 90:5:5) environmental chamber (Sheldon Manufacturing Inc., Cornelius, OR) at 37°C.
Coaggregation assay.
Coaggregation reactions were investigated by combining F. nucleatum, S. oralis, and A. naeslundii pairwise and by mixing all three species in saliva. Equal volumes (0.1 ml) of microbial suspensions (about 1 × 109 cells/ml) were combined in glass tubes (12 by 75 mm) and mixed for 10 s with a vortex mixer. After mixing, the suspensions were scored immediately for coaggregation. The scoring system of Cisar et al. (4) was used to evaluate the degree of coaggregation in the suspensions by viewing the tubes with the naked eye. The scores ranged from 0 to +4, as follows: 0, no change in turbidity and no evidence of coaggregates in the mixed suspensions; +1, turbid supernatant with finely dispersed coaggregates which did not precipitate immediately; +2, definite coaggregates easily seen, but the suspension remained turbid and there was no immediate settling of coaggregates; +3, slightly turbid supernatant and formation of large precipitating coaggregates; and +4, clear supernatant and large coaggregates that precipitated immediately. The coaggregating partners S. oralis 34, A. naeslundii ATCC 43146, and F. nucleatum ATCC 10953 were used as inocula for formation of multispecies biofilms.
Saliva preparation.
Saliva from at least six healthy individuals was collected on ice, pooled on ice, and treated with 2.5 mM dithiothreitol for 10 min with stirring to reduce salivary protein aggregation. The saliva was then centrifuged and processed as previously described (5, 21). Briefly, the supernatant was diluted with distilled water to obtain 25% saliva and then was filtered through a 0.22-μm-pore-size SFCA low-protein-binding filter (Nalge Nunc International, Rochester, NY) and stored at −20°C. Prior to use, saliva was thawed and centrifuged to remove any precipitate that resulted from freezing and thawing.
Flow cell preparation.
Two tracks (each track was 40 mm long, 3 mm wide, and 2 mm deep) were milled into a high-density polyethylene block, resulting in two chambers, each having a volume of 240 μl. A glass coverslip, which served as the attachment substratum for the growing biofilm, was secured to each of the reusable flow cells with a silicone adhesive. The flow cells were cleaned overnight with 0.1 M HCl and rinsed with 5 ml of distilled water. To disinfect the flow cells, 70% ethanol was injected into the flow cells and the flow cells were incubated for 20 min. The flow cells were then treated with 25% sterile human saliva for 15 min at 37°C in an anaerobic chamber to condition the glass surface with salivary components.
Biofilm growth conditions in flow cells.
Overnight bacterial cultures were harvested by centrifugation and washed twice with 25% sterile human saliva, and the optical density at 600 nm was adjusted to 0.1. Flow cells were inoculated with one, two, or three species. Two-species and three-species inoculants were first coaggregated (by mixing 0.1-ml portions of appropriate combinations of S. oralis 34, A. naeslundii ATCC 43146, and F. nucleatum ATCC 10953), which was followed by incubation of the flow cell in the anaerobic chamber to provide an environment favorable for F. nucleatum, a strict anaerobe. Sterile 25% saliva was supplied at a flow rate of 0.2 ml/min as the sole source of nutrients.
Biofilm staining.
Cells were stained with BacLight Live/Dead (Invitrogen, Carlsbad, CA) when strains were grown as monocultures. When multiple species were inoculated, organisms were visualized by using primary immunofluorescence with Alexa Fluor 633 (Invitrogen)-, Alexa Fluor 488 (Invitrogen)-, and Alexa Fluor 546 (Invitrogen)-conjugated immunoglobulin G of a polyclonal antiserum to S. oralis, A. naeslundii, and F. nucleatum, respectively. The immunofluorescence analysis was performed by injecting the antibody (5 μg/ml in phosphate-buffered saline) into the appropriate flow cell track and incubating the preparation for 20 min. A final wash with 1% bovine serum albumin in phosphate-buffered saline (filter sterilized with a 0.22-μm-pore-size SFCA low-protein-binding filter [Nalge Nunc International]) preceded CLSM. No cross-reactivity of staining by antibodies with nonhomologous strains was observed.
Image and statistical analysis.
A TCS-SP2 confocal microscope (Leica, Exton, PA) with a 40×, 1.25 NA oil immersion lens was used to record confocal image stacks in five random locations near the center of each flow cell, after which biofilm biovolumes were determined by volumetric analyses (IMARIS, version 5.71; Bitplane AG, Zurich, Switzerland). Fluorescence intensity thresholds were set manually for red, green, and blue pixels with cubic voxels used for the biovolume determination. Five confocal data sets were analyzed for each time point, and the mean and standard deviation were calculated. A one-way analysis of variance at the 95% confidence level with a nonparametric Tukey's pairwise comparison test was used to determine if the means were statistically different between the 4-h and 18-h time points for each species for each condition (one, two, or three species) in the flow cell studies and if the means were statistically different between the 12-h, 24-h, 36-h, and 48-h time points for each species for each condition (one, two, or three species) in the peg studies with static saliva. All images presented below are maximum projections of the entire confocal image stack produced by the Leica TCS software (Leica).
Biofilm growth conditions on polystyrene pegs.
Static biofilms were grown in 25% human saliva on polystyrene pegs (Nunc-Immuno TSP; Nunc catalog no. 445497) (2, 17) mounted in U96 MicroWell plates (Nunc catalog no. 163320) (3, 17). Microtiter plate wells were filled with 200 μl of 25% human saliva, and the pegs were then inserted for 30 min at room temperature to obtain a conditioning film. Overnight cultures (∼20 μl) of S. oralis, A. naeslundii, and F. nucleatum were added to the 200 μl of saliva in the wells to obtain an optical density at 600 nm of 0.1, which was equivalent to about 1 × 107 to 3 × 107 cells of each species in the microtiter wells; the plates were placed in a humidity chamber and incubated anaerobically at 37°C for 48 h. After 12-h intervals, the pegs were transferred to fresh, prereduced saliva in microtiter plate wells.
DNA extraction and quantification.
DNA was extracted from biofilms by using a modified alkaline lysis protocol (10). Biofilm-covered pegs were immersed in 40 μl of sterile ultrapure water plus 160 μl of 0.05 M sodium hydroxide and incubated at 60°C for 45 to 60 min, after which 18.4 μl of 1 M Tris-HCl (pH 7.0) was added to neutralize the pH. The resulting extract was used as the template DNA for the q-PCR analyses (3, 10). Bacterial genomic DNA for construction of standard curves was extracted from overnight cultures of S. oralis, A. naeslundii, and F. nucleatum with a DNA extraction kit (Qiagen, Valencia, CA) used according to the manufacturer's instructions. Genomic DNA was stored at −20°C.
Quantification of species in static biofilms by real-time q-PCR.
Species-specific primers were designed with AlleleID6 (PREMIER Biosoft International, Palo Alto, CA); no cross-reactivity of primers with nonhomologous species was observed. The primers specific for S. oralis were forward primer GATACATAGCCGACCTGAG and reverse primer TCCATTGCCGAAGATTCC, and the annealing temperature was 56°C. The primers specific for A. naeslundii were forward primer GGCTGCGATACCGTGAGG and reverse primer TCTGCGATTACTAGCGACTCC, and the annealing temperature was 56°C. The primers specific for F. nucleatum were forward primer CTTAGGAATGAGACAGAGATG and reverse primer TGATGGTAACATACGAAAGG, and the annealing temperature was 56°C. Quantification of S. oralis, A. naeslundii, and F. nucleatum in the biofilms was performed by q-PCR using the SYBR green dye to detect the 16S rRNA gene amplicons. Each reaction mixture (final volume, 25 μl) contained 3 μl of template, 3.5 μl of diethyl pyrocarbonate-treated ultrapure water, 12.5 μl of Power SYBR green PCR master mixture (Applied Biosystems, Foster City, CA), and 3 μl each of the forward and reverse primers at a concentration of 375 nM. q-PCR was performed with an MX3005P thermocycler (Stratagene, La Jolla, CA) using the thermocycle recommended for Power SYBR green PCR master mixture (95°C for 10 min, followed by 40 cycles of 30 s at 95°C and 1 min at 56°C). Dissociation curves were generated by incubating reaction products at 95°C for 1 min and at 56°C for 30 s and then incrementally increasing the temperature to 95°C. Fluorescence data were collected at the end of the 56°C primer annealing step for 40 amplification cycles and throughout the dissociation curve analysis. Analysis of the melting curves with each of the three primer sets revealed a single sharp peak. DNA concentrations (in ng/ml) were calculated based on standard curves obtained by using 10-fold serial dilutions of bacterial DNA isolated with a DNA extraction kit (Qiagen) and quantified with the PicoGreen fluorescence assay (Invitrogen). To convert nanograms of DNA to numbers of cells, the following weights and genome sizes were used: 2.41 fg/genome and 2.4 Mb for fusobacteria, 2.05 fg/genome and 2 Mb for streptococci, and 3.08 fg/genome and 3.0 Mb for actinomyces (http://www.homd.org). The data presented below are data for three independent biofilms.
RESULTS
Coaggregation of three-species community members.
Pairwise coaggregation of F. nucleatum with A. naeslundii in saliva yielded a coaggregation score of +2. A weak coaggregation score, +1, was obtained for F. nucleatum and S. oralis, and examination of the coaggregates by phase-contrast microscopy revealed numerous corncob cellular arrangements consisting of long, slender fusobacterial cells surrounded by coccal streptococci. In three-species interactions F. nucleatum participated in very strong coaggregations with a score of +4. Pairwise coaggregations of S. oralis and A. naeslundii gave a score of +3. Thus, each species is a coaggregation partner of the other two species, and the ability of the species to form multispecies biofilm communities in two model systems was investigated.
Flow cells inoculated with one species.
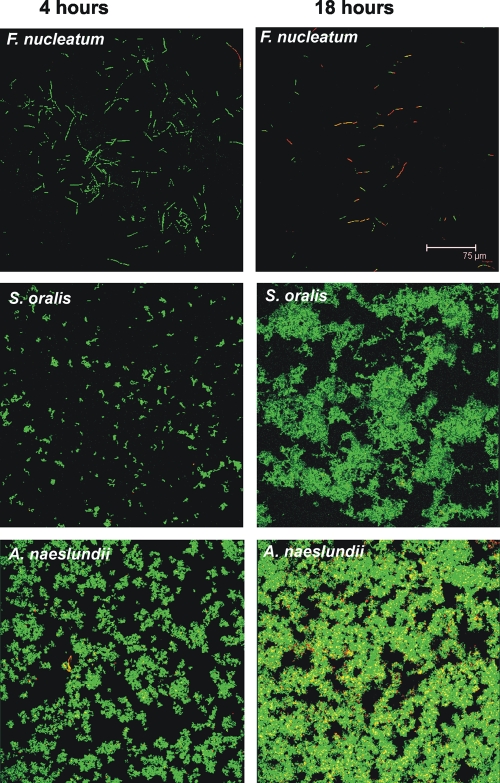
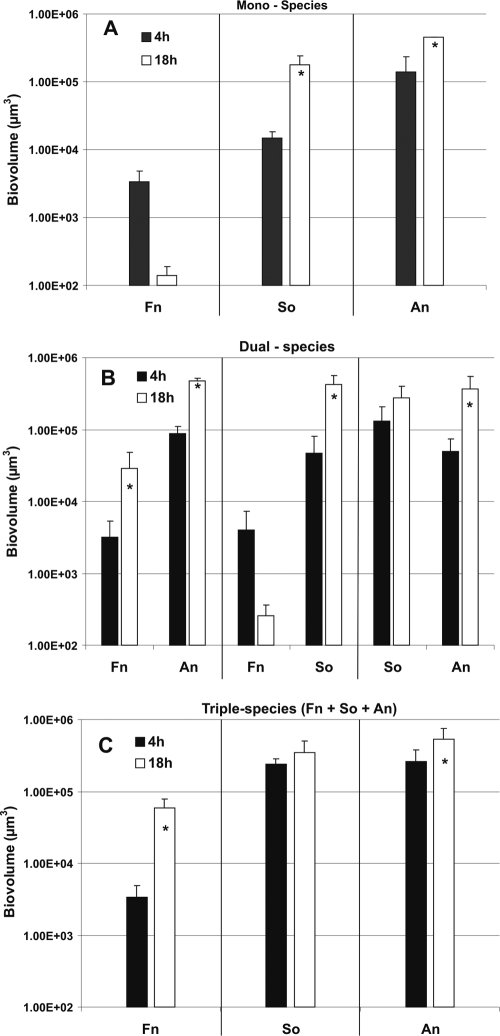
S. oralis, A. naeslundii, and F. nucleatum monocultures were inoculated into flow cells, and images of the biofilms formed were obtained after 4 h and 18 h of incubation (Fig. 1). The level of cell attachment to the substratum was quantified by determining the biovolume (Fig. 2A). Each species attached to the saliva-conditioned surface within 4 h (Fig. 1, left panels). After 18 h of incubation, the number of F. nucleatum cells decreased, indicating that there was no growth (Fig. 1, top right panel). In most experiments in this anaerobic environment, S. oralis grew well (Fig. 1, middle right panel, and Fig. 2A). Occasionally, S. oralis grew poorly, as we observed previously for aerobic environments (21). Only A. naeslundii grew consistently (Fig. 1, lower right panel, and Fig. 2A). To determine whether the cells in the biofilms were viable after 4 h and 18 h, cells were stained with the BacLight Live/Dead viability stain (Invitrogen), a fluorescent marker of membrane integrity (Fig. 1). Most cells (90%) in biofilms containing S. oralis or A. naeslundii appeared to have no membrane damage (green cells) at 4 h or 18 h. Also, at 4 h F. nucleatum appeared to have no membrane damage, which is consistent with the presence of a proper environment for the anaerobic physiology of this organism. However, after 18 h in saliva, fewer F. nucleatum cells were present, and most cells exhibited membrane damage (red cells), suggesting that saliva did not provide nutrients for growth. Thus, while F. nucleatum was unable to grow by itself, the commensals streptococcus and actinomyces formed one-species biofilms.
FIG. 1.
Confocal micrographs of biofilms formed in flow cells inoculated with one species (F. nucleatum, S. oralis, or A. naeslundii) after 4 h and 18 h of growth on 25% saliva. BacLight Live/Dead stain was used to assess the vitality of cells. Red cells have impaired membrane activity, whereas green cells have fully functional membranes.
FIG. 2.
Time-resolved changes in the biovolume (μm3 per field of view) of A. naeslundii (An), S. oralis (So), and F. nucleatum (Fn) following 4 h and 18 h of incubation in flow cells fed 25% saliva. (A) Biovolumes of single species (see Fig. 1). (B) Biovolumes of each species in two-species biofilms (see Fig. 3). (C) Biovolumes of each species in three-species biofilms (see Fig. 4). An asterisk indicates statistically significant growth.
Flow cells inoculated with two species.
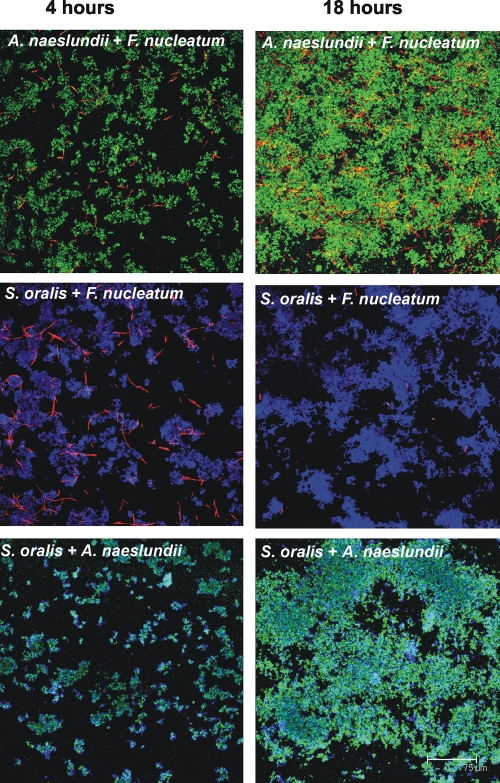
Saliva-conditioned flow cells were inoculated with pairwise combinations of coaggregating species, and the species were distinguished by labeling them with specific antibodies (Fig. 3). In the presence of A. naeslundii, F. nucleatum flourished (Fig. 3 and Fig. 2B). Although F. nucleatum attached to the substratum equally well in one-species biofilms (Fig. 1 and Fig. 2A) and in coaggregates with A. naeslundii (Fig. 2B and Fig. 3), its biovolume increased ninefold when it was in coaggregates with A. naeslundii (Fig. 2B), indicating that F. nucleatum required the actinomyces for cooperative growth. The A. naeslundii biovolume increased fivefold (Fig. 2B). In the presence of S. oralis, F. nucleatum did not grow, although the initial attachment to the substratum at 4 h was excellent (Fig. 3), whereas the S. oralis biovolume increased ninefold (Fig. 3 and Fig. 2B). As determined by Live/Dead staining, fusobacteria were green at 4 h with either coaggregation partner and at 18 h with A. naeslundii, but most of the cells were red with S. oralis after 18 h (data not shown), suggesting that the membrane integrity of F. nucleatum cells was compromised when they were paired with S. oralis. The biovolumes of S. oralis and A. naeslundii increased between 4 h and 18 h (Fig. 2B), and the increase in the A. naeslundii biovolume was statistically significant. Thus, the three species grew in saliva in some but not all pairwise combinations, leaving open the possibility of unchanged pairwise relationships or unanticipated cooperative growth of all members in three-species communities.
FIG. 3.
Representative confocal micrographs of 4-h and 18-h two-species biofilms grown in flow cells. The primary immunofluorescence indicates S. oralis (blue), A. naeslundii (green), and F. nucleatum (red) and shows that there is intimate contact among species.
Flow cell inoculated with three species.
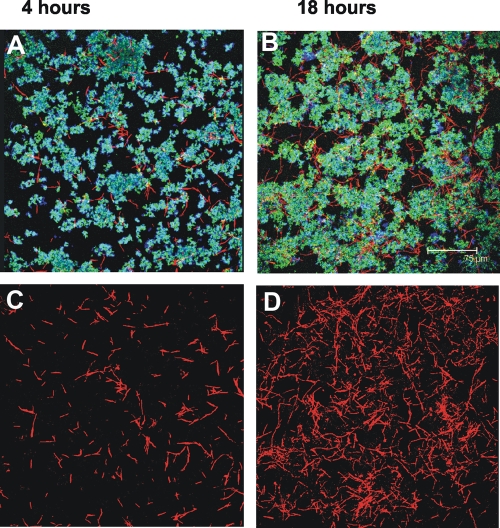
Coaggregates of the three species were inoculated into a flow cell, and all of the species attached to the saliva-conditioned substratum and grew between 4 h and 18 h during incubation (Fig. 4A and 4B). A dramatic increase in the biovolume of F. nucleatum was evident (Fig. 4C and 4D). The biovolume of F. nucleatum increased 18-fold (Fig. 2C), indicating that the cooperation between fusobacteria and actinomyces is stronger when the three species are present than when fusobacteria and actinomyces form two-species communities in the flowing saliva model system. Further, these data suggest that the outcome of interactions among three species cannot be predicted on the basis of two-species interactions.
FIG. 4.
Representative confocal micrographs of three-species biofilms grown in a flow cell. (A and B) Communities at 4 h and at 18 h, showing that there is an intimate interaction of F. nucleatum (red) with S. oralis (blue) and A. naeslundii (green) that forms the integrated multispecies communities. (C and D) Micrographs showing only the fusobacteria at 4 h and at 18 h to emphasize the dramatic increase in fusobacterial growth in the three-species communities.
Real-time q-PCR quantification of species in static biofilms on pegs immersed in saliva.
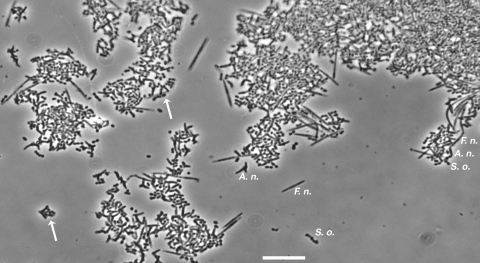
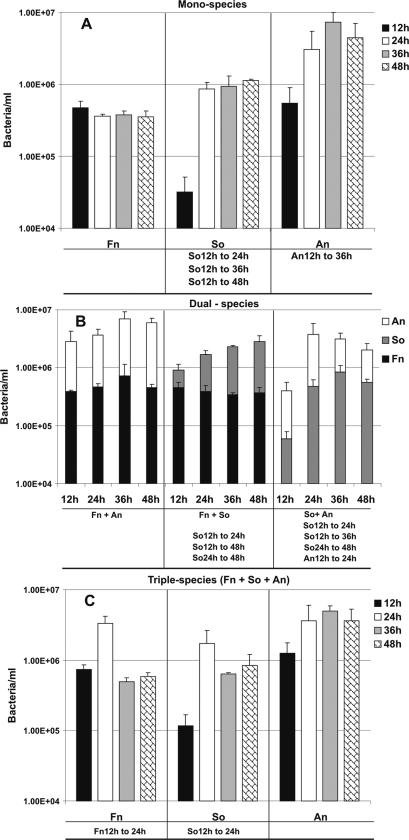
The second model system, the system with saliva-fed transferable solid-phase polystyrene pegs, was used to compare static systems and flowing systems. This system uses much less saliva and can be easily manipulated anaerobically. Coaggregates were formed easily by inoculating the species sequentially into the saliva in a well (Fig. 5). Single cells, two-species coaggregates, and large multispecies coaggregates are evident in Fig. 5. A corncob morphology consisting of a fusobacterial cell with several streptococci alongside it is also shown Fig. 5. Preliminary measurements of biomass accumulation in biofilms were obtained by crystal violet staining (data not shown) using a previously described method (3). An increased amount of accumulated stain on a peg can indicate an increase in biomass, which is acceptable for one-species biofilms and was very helpful as a screening tool for identifying potential target multispecies communities, but this technique cannot identify which bacteria are growing in two-species and three-species systems. To quantify each species in the multispecies biofilms, we used species-specific primers and conducted q-PCR with biofilms formed on pegs. When inoculated as single species, F. nucleatum did not grow, S. oralis grew rapidly between 12 h and 24 h, and the level of A. naeslundii increased gradually between 12 h and 36 h but the numbers of cells leveled off between 36 h and 48 h (Fig. 6A), suggesting that nutrient limitation had occurred. In F. nucleatum-A. naeslundii two-species biofilms, the numbers of F. nucleatum and A. naeslundii cells doubled (Fig. 6B). In contrast, in two-species biofilms with S. oralis no increase in the number of F. nucleatum cells was observed; the number of S. oralis cells increased sixfold (Fig. 6B). For the S. oralis-A. naeslundii two-species biofilm there was a 10-fold increase in the numbers of cells of both species (Fig. 6B).
FIG. 5.
Phase-contrast micrograph of a suspension of the three-species inoculum for a peg biofilm, showing mostly two-species and three-species coaggregates. The morphologies of the three species, A. naeslundii (A. n.), F. nucleatum (F. n.), and S. oralis (S. o.), are shown. The arrows indicate coaggregates consisting of A. naeslundii and S. oralis (left arrow) and consisting of F. nucleatum and S. oralis (right arrow). A chain consisting of S. oralis, A. naeslundii, and F. nucleatum attached end to end is on the right. Numerous other multispecies coaggregates are present. Bar, 10 μm.
FIG. 6.
q-PCR quantification for one-species (A), two-species (B), and three-species (C) biofilms grown for 12 h, 24 h, 36 h, or 48 h on polystyrene pegs submerged in 25% saliva. An, A. naeslundii; So, S. oralis; Fn, F. nucleatum. (A) Numbers of cells in one-species peg biofilms; (B) numbers of cells of each species in two-species peg biofilms; (C) numbers of cells of each species in three-species peg biofilms. Statistically significant increases (P < 0.05) in bacterial growth on the pegs are indicated at the bottom of each panel; for example, in the middle section of panel A, “So12h to 24h” indicates that there was significant growth of S. oralis 34 between 12 h and 24 h in a peg biofilm inoculated with one species.
Significantly, the number of F. nucleatum cells increased fivefold between 12 h and 24 h when this organism was inoculated with S. oralis and A. naeslundii (Fig. 6C). This rapid increase in the number of fusobacterial cells within 12 h was accompanied by sharp increases in the numbers of streptococcus cells (14-fold) and actinomyces cells (4-fold), suggesting that the multispecies community was particularly advantageous for all of the species. However, the rapid increases could not be sustained through 36 h and 48 h, suggesting that the maximum cell density that could be supported was approximately 1.0 × 107 cells per ml of saliva.
Collectively, the results for both model systems emphasize that it is not possible to predict multispecies community growth based solely on one-species and two-species growth patterns. Clearly, the two model systems, although distinctly different (flow versus static), yielded similar results, which may reflect a natural relationship among the three species. Of particular interest was the integration of F. nucleatum into the growing community; instead of no growth, the number of F. nucleatum cells increased 5-fold (static conditions) and 18-fold (flow cell) when A. naeslundii was part of the community.
DISCUSSION
We used two saliva-fed biofilm model systems and two quantitative methods to investigate multispecies community growth under an anaerobic atmosphere. In both model systems, the bacterial cells first had to attach to the saliva-conditioned substratum or they would be washed away by salivary flow (flow cell model) or when the substratum was lifted out of the saliva pool and inserted into a fresh saliva pool (transferable solid-phase polystyrene peg model). Although distinctly different in terms of design and operation, the two models yielded similar growth patterns for single species and multiple species, indicating that attachment to a substratum is a key element. Further, the data show that community composition and participant relationships can be consistent under very different environmental conditions. For example, flowing saliva can remove inhibitory as well as beneficial molecules; static incubation with saliva allows accumulation of these substances. Also, flowing saliva provides constant nourishment; static incubation with saliva provides a finite amount of nourishment. Given these significant differences, our data suggest that the three-species community possesses properties relevant to the development of dental plaque, such as integration of F. nucleatum into biofilms containing the initially colonizing organisms A. naeslundii and S. oralis.
In an earlier study, we failed to obtain growth of F. nucleatum in a multispecies flow cell community (9). In that study, flow cells were placed in an aerobic incubator, and it was assumed that the streptococci, actinomyces, and veillonellae used converted the biofilm environment to an environment favorable for anaerobiosis. Fusobacteria are known anaerobes, and it is likely that there was not a favorable anaerobic environment those flow cells. In the current study we incubated the saliva and the flow cells or the transferable pegs in an anaerobic chamber. Although F. nucleatum could not grow by itself on saliva as the sole source of nutrients, it grew well in two-species biofilms with A. naeslundii and exhibited significantly enhanced growth (compare Fig. 2B with Fig. 2C and Fig. 6B with Fig. 6C) in three-species biofilms with A. naeslundii and S. oralis. This dramatic shift in growth of fusobacteria in vitro is consistent with the secondary appearance of fusobacteria in dental plaque biofilms; first, streptococci, actinomyces, and veillonellae colonize enamel, and this is followed by a dramatic increase in the number of fusobacterial cells (18). Accordingly, we propose that either of the models used in this study is suitable for investigating the integration of additional species, such as the late colonizing pathogens Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, into these developing communities.
It is significant that F. nucleatum was unable to grow on saliva without A. naeslundii. Curiously, the numbers of S. oralis cells increased when S. oralis was paired with F. nucleatum (Fig. 2B and 6B), although F. nucleatum did not appear to benefit from this relationship. Such results emphasize the need to include multiple species in investigations of oral bacterial community development. Our use of q-PCR to quantify the numbers of cells of each species was essential for clarifying which species was growing in the peg biofilms. For example, only S. oralis grew after two-species inoculation with F. nucleatum, whereas both F. nucleatum and A. naeslundii grew after inoculation of these two species and all three species grew when the three species were inoculated. The transferable peg system and q-PCR are not limited in terms of the number of species, and therefore additional species, such as in vivo late colonizing pathogens, can be included.
In this study, we established that there was fusobacterial interdigitation with initial colonizing commensal species by performing CLSM with undisturbed flow cell biofilm communities. We showed that fusobacteria required A. naeslundii for biofilm growth and improved the initial colonization of streptococci in two-species biofilms. Initial colonizing streptococci produce hydrogen peroxide, which inhibits the growth of anaerobes. A. naeslundii produces catalase, which inactivates the hydrogen peroxide and could promote the growth of anaerobic fusobacteria. We reported recently that A. naeslundii protected Streptococcus gordonii from self-produced hydrogen peroxide (11); a similar protective effect of actinomyces on fusobacteria could have contributed to the enhanced growth of fusobacteria in the presence of streptococci in three-species biofilms in the current study. We can now extend these observations and design multispecies communities that mimic developing dental plaque at initial stages, secondary stages, and late stages of colonization and quantify each species in the communities.
ADDENDUM IN PROOF
After this paper was in press, we became aware of the name change for Actinomyces naeslundii genospecies 2, which is now called Actinomyces oris (U. Henssge et al., Int. J. Syst. Evol. Microbiol. 59:509-516, 2009). A. naeslundii ATCC 43146 is a genospecies 2 strain and is thus A. oris ATCC 43146.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health, and in part by a cooperative research and development agreement with the Colgate-Palmolive Co.
We thank R. J. Palmer, Jr., for his assistance with the CLSM and for helpful comments on the manuscript.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers, N. I., R. J. Palmer, Jr., J. O. Cisar, and P. E. Kolenbrander. 2008. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 190:8145-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cisar, J. O., P. E. Kolenbrander, and F. C. McIntire. 1979. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 24:742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jong, M. H., and J. S. Van der Hoeven. 1987. The growth of oral bacteria on saliva. J. Dent. Res. 66:498-505. [DOI] [PubMed] [Google Scholar]
- 6.Diaz, P. I., N. I. Chalmers, A. H. Rickard, C. Kong, C. L. Milburn, R. J. Palmer, Jr., and P. E. Kolenbrander. 2006. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 72:2837-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Distler, W., A. Kagermeier-Callaway, and A. Petschelt. 1992. Prevention of coaggregation by a dialysis membrane—influences on the glucose metabolism of mixtures of Actinomyces and streptococci. J. Dent. Res. 71:735. [Google Scholar]
- 8.Distler, W., A. Kagermeier, and A. Kroencke. 1991. Influence of coaggregation on the glucose metabolism of mixtures of Actinomyces and streptococci. J. Dent. Res. 70:764. [Google Scholar]
- 9.Foster, J. S., and P. E. Kolenbrander. 2004. Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Appl. Environ. Microbiol. 70:4340-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino, T., T. Fujiwara, and M. Kilian. 2005. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J. Clin. Microbiol. 43:6073-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubovics, N. S., S. R. Gill, M. M. Vickerman, and P. E. Kolenbrander. 2008. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol. Ecol. 66:637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson, H. F., and R. J. Lamont. 2005. Oral microbial communities in sickness and in health. Trends Microbiol. 13:589-595. [DOI] [PubMed] [Google Scholar]
- 13.Kolenbrander, P. E., R. N. Andersen, D. S. Blehert, P. G. Egland, J. S. Foster, and R. J. Palmer, Jr. 2002. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66:486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolenbrander, P. E., R. J. Palmer, Jr., A. H. Rickard, N. S. Jakubovics, N. I. Chalmers, and P. I. Diaz. 2006. Bacterial interactions and successions during plaque development. Periodontol. 2000 42:47-79. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., E. J. Helmerhorst, C. W. Leone, R. F. Troxler, T. Yaskell, A. D. Haffajee, S. S. Socransky, and F. G. Oppenheim. 2004. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 97:1311-1318. [DOI] [PubMed] [Google Scholar]
- 17.Mampel, J., T. Spirig, S. S. Weber, J. A. Haagensen, S. Molin, and H. Hilbi. 2006. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72:2885-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 19.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, R. J., Jr., S. M. Gordon, J. O. Cisar, and P. E. Kolenbrander. 2003. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 185:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer, R. J., Jr., K. Kazmerzak, M. C. Hansen, and P. E. Kolenbrander. 2001. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect. Immun. 69:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socransky, S. S., and A. D. Haffajee. 2005. Periodontal microbial ecology. Periodontol. 2000 38:135-187. [DOI] [PubMed] [Google Scholar]
- 23.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]