Abstract
Although their exact function remains enigmatic, bifidobacteria are among the first colonizers of the newborn infant gut and further develop into abundant communities, notably in response to diet. Therefore, the transcriptional responses of bifidobacteria in rapidly processed fecal samples from young infants that were fed either breast milk or a formula containing a mixture of galacto- and fructo-oligosaccharides were studied. The presence and diversity of the bifidobacterial fecal communities were determined using PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR for specific species. Changes in the total number of bifidobacteria as well as in species diversity were observed, indicating the metabolic activities of the bifidobacteria within the infant gut. In addition, total RNAs isolated from infant feces were labeled and hybridized to a bifidobacterium-specific microarray comprising approximately 6,000 clones of the major bifidobacterial species of the human gut. Approximately 270 clones that showed the most prominent hybridization with the samples were sequenced. Fewer than 10% of the hybridizing clones contained rRNA genes, whereas the vast majority of the inserts showed matches with protein-encoding genes predicted to originate from bifidobacteria. Although a wide range of functional groups was covered by the obtained sequences, the largest fraction (14%) of the transcribed genes assigned to a functional category were predicted to be involved in carbohydrate metabolism, while some were also implicated in exopolysaccharide production or folate production. A total of three of the above-described protein-encoding genes were selected for quantitative PCR and sequence analyses, which confirmed the expression of the corresponding genes and the expected nucleotide sequences. In conclusion, the results of this study show the feasibility of obtaining insight into the transcriptional responses of intestinal bifidobacteria by analyzing fecal RNA and highlight the in vivo expression of bifidobacterial genes implicated in host-related functions.
Following birth, the virtually sterile gastrointestinal tracts of neonates become rapidly colonized by microbial communities, collectively know as microbiota, which rapidly increase in complexity (13). The vast majority reside in the colon, where densities approach 1011 to 1012 cells per gram, the highest recorded for any microbial habitat (66). Here, hundreds of bacterial species form a bacterial community in which bifidobacterial species can constitute up to 60% of the total population in infants (21). It has been shown previously that various environmental factors affect the microbiota development, including the feeding regimen of the infant (21). Bifidobacteria are heterofermentative, nonmotile, non-spore-forming rods; these gram-positive bacteria have high G+C contents in their genomic DNA and belong to the Actinobacteria phylum, within which they form a distinct order (5). At present, the genus Bifidobacterium includes 32 species and 9 subspecies, many of which have been isolated from fecal sources (60). The species most commonly isolated from samples obtained from breast-fed or formula-fed infants is Bifidobacterium breve, followed by B. longum subsp. infantis, B. longum, and B. bifidum (34). In addition, B. catenulatum, B. adolescentis, B. pseudolongum, and B. dentium have been detected but less frequently (20). It has been reported previously that the postnatal maturation of a balanced immune system requires constant microbial stimulation from the developing intestinal microbiota (9, 23). Moreover, the intestinal microbiota has been claimed to have many beneficial effects, and specifically, the bifidobacteria have been implicated in protection against pathogens (14), the normal development and maintenance of a balanced immune system (9, 23, 55), and the exertion of positive nutritional effects on the intestinal cells and the host (43). In spite of the numerous studies on the diversity of bifidobacteria in the human intestine, insight into the specific activities and functions of bifidobacteria in the gastrointestinal tract remains very sparse. Most studies have focused on molecular techniques targeting the 16S rRNA genes, such as PCR-denaturing gradient gel electrophoresis (PCR-DGGE) (52), fluorescent in situ hybridization (22), quantitative real-time PCR (qPCR) (20), and more recently, DNA microarrays (71), to identify and quantify the different intestinal inhabitants of the gut. However, a new era has started with the sequence characterization of bifidobacterial genomes (61). In silico analysis of the total genome sequence of B. longum NCC2705 predicted this bacterium to be adapted to a special colonic niche (54). Several genes are predicted to encode transcriptional regulators, which allow quick and stringent responses to environmental changes. Moreover, some genes are predicted to code for proteins that show homology to glycoprotein-binding fimbriae, structures that may be involved in adhesion and persistence in the gastrointestinal tract (54). Unfortunately, only a few complete bifidobacterial genome sequences have been reported, and only the full annotations of the genomes of B. longum NCC2705 (54), B. longum DJO10A (30), B. adolescentis ATCC 15703 (60), B. longum subsp. infantis ATCC15697 (56), and Bifidobacterium animalis subsp. lactis (26) have been made publicly available. A significant portion of the genome of B. adolescentis differs from the genome of B. longum, reflecting the evolutionary difference between these species based on 16S rRNA genes. Genes coding for lacto-N-biose (LNB) phosphorylase, 1,2-α-l-fucosidase, and endo-α-N-acetylgalactosamidase, which are associated with host-bacterial interaction, are absent in the B. adolescentis genome, suggesting an alternative strategy for this species to interact with the host (60).
Genomic sequences, however, provide only a static view, and insight into the expression levels of the predicted genes in the intestinal tract is not yet available. In the present study, we investigated the feasibility of using rapidly processed fecal samples from infants to determine the bifidobacterial transcriptome with a DNA microarray based on 6,000 clones from a shotgun library, thereby bypassing the need for genome sequence information (45). This mixed-species microarray was recently developed and used to study the transcriptome of B. longum grown in vitro in the presence of human milk (17). To improve our understanding of the interaction between bifidobacteria and the host, fecal microbiotas of infants receiving solely human breast milk and those receiving an infant formula containing a mixture of galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS) (3, 37) were studied at the transcriptional and diversity levels. The results reveal the expression of a selection of genes that may be related to the adaptive responses of these species to the physiological gastrointestinal conditions.
MATERIALS AND METHODS
Subjects.
In a preliminary study, the transcriptomes of fecal microbiotas from four infants ranging in age from 1 week to 10 months were evaluated to test the feasibility of the selected approach for detecting the expression of protein-encoding genes with the clone-based microarray described previously (15). High-quality RNA preparations obtained from infants of various ages contained only a low number of sequences complementary to rRNA (less than 10% of the total hybridizing clones; data not shown). Hence, we systematically monitored two breast-fed infants (infants 1 and 2) and three formula-fed infants (infants 3 to 5) over time in a controlled dietary intervention. During the trial, the formula-fed infants received a milk-based formula containing a mixture of GOS and long-chain FOS (lcFOS) (9:1 [wt/wt]) (3, 37) that was provided by Danone Research, Centre for Specialised Nutrition, Wageningen, The Netherlands. All infants were healthy Caucasians delivered normally. Their ages at the start of the intervention were on average 8 months (breast-fed infants 1 and 2 were between 6 and 7 months old, and formula-fed infants 3, 4, and 5 were between 6 and 10 months old). They received solely breast or formula milk prior to and during the sampling, in addition to small amounts of solid food after 6 months, as is common practice in The Netherlands. The formula-fed infants received a standard formula without GOS-lcFOS prior to the trial. The infants were monitored for up to 8 weeks, and periodically, fecal samples were collected and processed as described below. Written consent was obtained from the parents of each infant.
PCR-DGGE analysis and qPCR.
DNA was isolated from mechanically disrupted cells (68) from stool samples stored at −20°C and was purified using the QIAamp DNA stool minikit (Qiagen Sciences, MD). PCR and DGGE analyses of the V6-to-V8 regions of the 16S rRNA genes of the total microbial community were performed as described previously using 16S rRNA gene-targeted primers 968-f/1401-r (40). The V6-to-V8 regions of the 16S rRNA genes of the bifidobacterial population were targeted using Im26-f/Im3-r, followed by Bif164-f/Bif662-rGC (52, 69).
qPCR analysis of DNA extracts was performed in triplicate as described previously (20) to obtain percentages for the total bifidobacterial community as well as specifically B. adolescentis, B. angulatum, B. animalis, B. bifidum, B. breve, B. catenulatum, B. dentium, B. longum subsp. infantis, and B. longum.
Total RNA isolation.
Fecal samples were collected at three time points and immediately mixed with RNAlater (Ambion Inc., Austin, TX) at a ratio of 1:2 (wt/vol), and the mixtures were incubated overnight at 4°C and stored at −20°C until further processing as described previously (70).
Design and processing of a clone library-based DNA microarray.
Glass spotted microarrays were constructed as described previously (15) and contained approximately 5,000 spots of 1- to 2-kb PCR amplicons that were derived from plasmid inserts in genomic libraries of B. longum LMG 13197 (48), B. pseudolongum subsp. pseudolongum LMG 11571 (68), B. adolescentis LMG 10502 (48), B. animalis subsp. animalis LMG 10508 (36), B. bifidum LMG 11041 (42), and B. catenulatum LMG 11043 (53). Slides were stored in the dark under dust-free conditions until further use. Before the addition of the hybridization mix, the slides were prehybridized for 45 min at 42°C in a solution of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% sodium dodecyl sulfate and 10 mg/ml bovine serum albumin, washed in MilliQ water and isopropanol, and then dried. Labeling, hybridization, and washing were performed as described previously (46). In all instances, Cy3-labeled DNA sequences from the bifidobacterial strains used to create the clone library were employed as a reference.
Microarray analysis.
The hybridized microarrays were analyzed with a ScanArray Express 4000 scanner (Perkin-Elmer). Fluorescent images were captured in a multi-image-tagged image file format and analyzed with ImaGene software (BioDiscovery, Marina del Rey, CA). As expected, labeled chromosomal DNA of all bifidobacterial species on the microarray showed hybridization to virtually all spots on the array. Spots that were flagged by the ImaGene software (BioDiscovery) were not included in the data analysis. In total, 5,314 spots met the set quality criteria and were included in the analysis. For each spot, the mean signal intensity was divided by the local background intensity and considered to indicate positive hybridization when it was at least 1.25 times higher than the background. The hybridization intensity values were normalized to the total intensity to enable the comparison of results from different microarrays. Spots that did not show positive hybridization to any of the cDNA samples were removed from further analyses. Approximately 500- to 800-bp regions of both the 3′ and 5′ ends of the inserts in selected clones were subsequently sequenced using primers SL1 and SR2, based on pSMART (GATC Biotech, Germany) (16). The Smith and Waterman algorithm (57) was applied to evaluate the sequences against different data sets. All sequences were analyzed against the complete protein and nucleotide sequences for all genomes with complete sequences present in the NCBI repository (as of February 2009). In addition, specific searches were performed using the complete genome sequences of B. longum NCC2705, B. adolescentis ATCC 15703, B. longum subsp. infantis, and B. animalis subsp. lactis. Following these searches, all matches with the genome sequences were analyzed for annotated features by using the protein and RNA annotation files for the genomes.
Principal component analysis.
Principal component analysis was performed using Canoco software package 4.5 (Biometris, Wageningen, The Netherlands) to assess the impact of diet on the microarray hybridization patterns of the total RNAs isolated from the fecal microbiotas. Redundancy analysis (RA) was applied in specific cases to explain the contribution of the diet (breast milk or formula) to the hybridization data (calculated as the hybridization intensity divided by the local background intensity for each clone). Community similarities were graphed by using ordination plots with scaling focused on the dietary difference. The ordination plot for species and environmental variables is characterized by biplots that approximate the weighted averages for each species with respect to the environmental variable (a diet of either breast milk or formula). To test the significance of the relationship of the hybridization results with the dietary group, unrestricted Monte Carlo permutation tests were performed with 499 random permutations and a significance level (P value) of 0.05.
qPCR.
Primers were designed using Primer Express 1.5a (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands), which also takes the presence of secondary structures, including possible primer-dimers, into account. All primers were designed to have melting temperatures of 60 to 70°C and amplicon sizes between 70 and 130 bp. The specificities of the primers to bifidobacteria were evaluated by nucleotide similarity searches with the BLAST algorithm for short, nearly exact matches at the NCBI website (http://www.ncbi.nlm.nih.gov) (35). In silico comparisons and PCR amplification products confirmed that primer sets were specific for both B. longum NCC2705 and B. longum LMG 13197 but did not target other organisms, including Lactobacillus plantarum WCFS1 and Escherichia coli (data not shown). Target genes were the LNB phosphorylase (BL1641) gene, with primer set AAC CGT ACA AGG ACG GAT TCG/CGG AAT ATC GGC GAT CAT GC; the α-l-arabinosidase (BL0544) gene, with primer set TAC ACG CAA CGG CCA AGG/CCA GCA GGA CCA TCT GAC C; and the thymidylate synthase (BL1665) gene, with primer set CAC GTG CAT ATT TGG GAT GAG TG/CCA GGA ACG CCA CTG CAC. iQ Sybr green supermix (Bio-Rad) was used in all reactions. The iQ5 real-time PCR detection system (Bio-Rad) was used for all qPCR analyses. Each reaction was carried out in a solution containing 5.0 μl of cDNA, 12.5 μl of power Sybr green master mix (Applied Biosystems), the forward and reverse primers (2 μM each), and 6.5 μl of distilled water. The PCR thermal protocol applied consisted of a 2-min 95°C denaturation step, followed by 45 repeats of a 15-s denaturation step at 95°C, a 30-s annealing step (at a temperature defined for each primer set), and a 30-s extension step at 72°C. A melting curve analysis was performed after the final amplification period by using a temperature gradient from 60 to 95°C. Standard curves for quantification were based on dilution series of DNA of B. longum NCC2705. PCR products were sent to GATC Biotech (Germany) for purification and sequencing to confirm the specific amplification of the target gene.
Microarray data.
Raw microarray data are provided in File S2 in the supplemental material.
RESULTS AND DISCUSSION
Temporal stability and diversity of the predominant bacterial community as determined by PCR-DGGE.
The fecal microbiotas of infants are influenced by transitions between breast milk, formula, and solid foods (4, 13, 21). Hence, prior to gene expression studies, we compared the diversities of the microbiotas, including bifidobacteria, in five partly age-matched infants that were breast fed (infants 1 and 2) or subjected to a diet of formula containing prebiotic oligosaccharides (infants 3, 4, and 5). Both the expression of bifidobacterial genes (see below) and the bacterial diversity were analyzed over a period of 2 to 7 weeks, and samples were taken periodically.
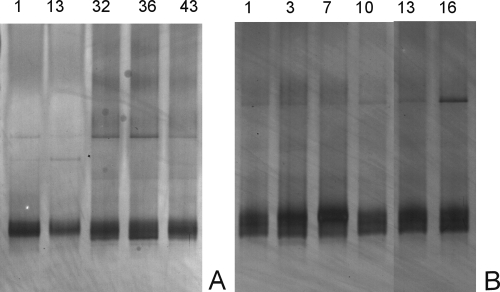
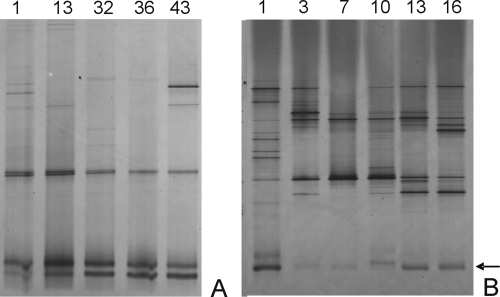
The diversities of the total fecal microbiotas and the bifidobacterial populations were determined by DGGE analyses of the 16S rRNA amplicons. Analyses of the diversities of the bifidobacterial communities revealed relatively simple and stable patterns for both breast- and formula-fed infants. This finding is illustrated for representative infants 2 and 5, of the same age (6 to 7 months), who received breast milk and formula, respectively, and showed the same dominant bifidobacterial species for several weeks (Fig. 1). In contrast, the DGGE profiles representing the diversities of the total bacterial communities from the breast-fed infants were much more stable than those from the formula-fed infants. This finding is illustrated for the same infants whose results are presented in Fig. 2. This stability is especially notable when the sample time is considered, as the community in the formula-fed infant fluctuated considerably in a period of 2 weeks while that in the breast-fed infant showed virtually the same microbial composition for 6 weeks. Similar observations were made with the fecal samples from the other infants (data not shown).
FIG. 1.
DGGE profiles of the fecal bifidobacteria from breast-fed infant 2 (A) and formula-fed infant 5 (B) at different time points (sampling days are indicated at the top).
FIG. 2.
DGGE profiles of the fecal microbiotas of breast-fed infant 2 (A) and formula-fed infant 5 (B) at different sampling points (sampling days are indicated at the top). The arrow identifies bands in the gel that correspond to the bifidobacterial populations.
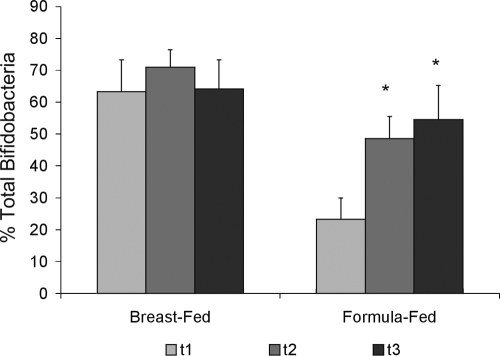
As DGGE analysis of PCR amplicons is qualitative rather than quantitative (71), a qPCR approach was used to determine the temporal development of the total number of bifidobacteria (Fig. 3). This analysis showed that the total bifidobacterial numbers in the breast-fed infants were higher than those in the formula-fed infants at the start of the intervention but that the numbers in the formula-fed infants increased significantly over time, due possibly to the intake of the oligosaccharide-containing formula (Fig. 3). qPCR analyses of specific Bifidobacterium species indicated that there were few significant differences between the breast- and formula-fed infants and that numbers of these species in the breast-fed infants were initially higher (Fig. 3). In samples from all infants, B. animalis and B. dentium were not detectable and B. angulatum was present in very low numbers. In addition, B. longum subsp. infantis, B. breve, B. bifidum, and B. longum were detected in samples from all infants, with B. longum subsp. infantis being the major species found. B. adolescentis, which is most commonly found in adults, was detected at a low proportion in samples from the formula-fed infants but not at all in those from infants receiving breast milk. In conclusion, these analyses indicated that the samples from the formula-fed infants contained fewer bifidobacteria than those from the breast-fed infants but showed higher levels of diversity among bifidobacterial species. During the period in which the infants received the oligosaccharide-containing formula, the fecal bifidobacterial diversities and quantities changed and approached those in the samples from the breast-fed infants, which is in line with previous findings (29, 37).
FIG. 3.
Total percentages of bifidobacteria in fecal samples from infants who were breast fed (infants 1 and 2) or fed a standard formula supplemented with GOS-lcFOS (infants 3, 4, and 5) as determined by qPCR. The bars represent the standard errors of the means. The percentages of bifidobacteria in the formula-fed but not in the breast-fed group increased significantly compared to the baseline over time (t1, start of analysis; t2, approximately 3 weeks later for breast-fed and 1 week later for formula-fed infants; and t3, an additional 1 to 2 weeks later) as determined by Student's t test (*, P < 0.05).
Transcriptomes of the fecal bifidobacterial populations.
To gain insight into the activities of the bifidobacterial populations within the fecal microbiotas, transcripts were profiled using a mixed-species microarray of bifidobacteria (7). In parallel to the fecal microbial diversity analysis, samples from the same five partly age-matched infants (infants 1 to 5; see Materials and Methods) with specified diets (breast milk or formula) were compared using the microarray at three time points in a 2- to 7-week period. A total of 4,524 clones showed positive hybridization of cDNA to at least one of the samples on the microarray, and the total sums of the fluorescent signals were comparable between arrays (data not shown). A selection of the positively hybridized clones was sequenced and showed a wide range of protein-encoding genes, indicating the metabolic activities of the fecal bifidobacterial communities and the power of this mixed-species clone library-based microarray.
It is important that the microarray consisted of cloned genomic fragments derived from a mixture of six Bifidobacterium spp. and that the hybridization took place under stringent conditions—hence, the expression of only the cloned genes of these specific strains or genes that have significant (generally more than 80%) sequence similarity and, therefore, are predicted to have the same function as their cloned homolog could be monitored.
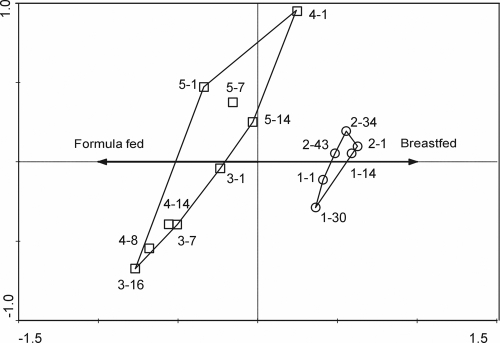
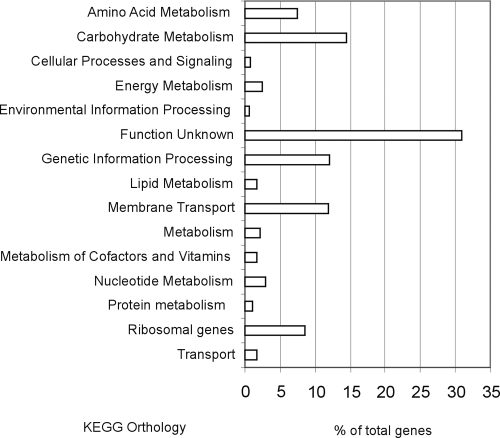
Statistical analysis with the Canoco software package was performed to identify whether host, environmental, or stochastic effects explained the grouping of the studied samples. The RA showed that 44% of the difference could be explained significantly (P value of 0.004) by the difference in diet, either breast milk or formula. The RA ordination plot for the hybridizations of the three samples from infants 2 to 5 visualizes the effects of the different diets (Fig. 4). The formula-fed infants differed from one another more than the breast-fed infants differed from each other, possibly because one of the formula-fed infants was 3 months older than the other infants in the group. However, all individuals within one diet group did not differ significantly from one another (P values above 0.05). A set of approximately 250 significantly hybridizing clones was selected for sequencing to predict the functional identities of the genes carried on the inserts. Most of the sequences obtained (500 to 800 bp) did not include complete genes, but the vast majority of the inserts (90%) matched closely (E value ≤ 10−4) to already described bifidobacterial genes, and all ribosomal genes matched closely to bifidobacterial rRNA genes. Less than 1% of the sequences were predicted to be noncoding or could not be identified (data not shown). The sequences appeared to encode a wide range of functions, such as those of components of transport systems, energy metabolism, and carbohydrate metabolism (Fig. 5). Like the taxonomic assignments, the identities of transcripts were inferred from the closest matches. These assignments are only as good as the existing database, and genes that are rare in genomes because they code for unusual or specialized traits are particularly susceptible to poor database coverage. The largest fraction of transcripts (31%) was categorized as hypothetical, and these transcripts were partly unclassified (typically having known functions but not readily placed into a role category during annotation). Among the corresponding genes may be those encoding novel factors that allow the colonization of the human intestine by bifidobacterial species.
FIG. 4.
RA ordination plot comparing the total hybridization patterns of fecal bifidobacterial transcriptomes obtained from breast-fed infants (○; infants 1 and 2) and formula-fed infants (□; infants 3, 4, and 5) at different time points. Each sample is labeled with the identification number of the infant followed by the day of sampling. The vector represents the diet variable.
FIG. 5.
Classification of the predicted functions of the 250 sequenced bifidobacterial inserts present on the microarray and showing significant hybridization to the labeled total RNAs extracted from infant fecal samples. KEGG, Kyoto Encyclopedia of Genes and Genomes.
The inserts' sequences present in the hybridizing clones on the microarrays were functionally annotated and grouped according to the contribution of the gene expression values (signal over background) to the RA, as a measure for the impact of each gene on the difference between the dietary groups (see the supplemental material). The clones are listed in order of importance for the clustering of the transcriptomes of the infants' microbiotas according to diet (formula or breast milk) by using the hybridization signals for samples from infants 1 to 5 (Table 1). This means that the list starts with genes that have a large influence on the deviation of the infants' samples according to the dietary group. The signals for the hybridization to the clones at the end of the list do not indicate any impact on the difference between the two dietary groups.
TABLE 1.
Hybridization values for glycobiome-related genes with significant hybridization signals in samples from infants 1 to 5a
| Locus tag(s) | Function(s) or identity(ies) of gene product(s) | RA result (%) | Hybridization value for sample:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-14 | 1-30 | 2-1 | 2-34 | 2-43 | 3-1 | 3-7 | 3-16 | 4-1 | 4-8 | 4-14 | 5-1 | 5-7 | 5-14 | |||
| BAD_0708 | Pullulanase | 12.89 | 39.61 | 41.89 | 33.60 | 67.83 | 92.57 | 64.86 | 12.05 | 2.82 | ND | 39.58 | 4.16 | 6.27 | ND | 37.04 | 2.28 |
| BAD_1412 | Probable sugar kinase | 12.25 | 1.89 | 1.89 | ND | 1.51 | 1.84 | 1.50 | 1.34 | 1.32 | ND | 2.02 | 1.36 | 1.33 | ND | 3.20 | 1.53 |
| BAD_1527 | Endo-1,4-β-xylanase | 9.74 | ND | 1.74 | ND | 1.31 | 1.58 | ND | 1.58 | ND | ND | ND | ND | ND | ND | 2.28 | 1.29 |
| BAD_1605 | β-Galactosidase | 3.01 | ND | 1.36 | ND | ND | 1.36 | ND | ND | ND | 1.28 | ND | ND | ND | 1.65 | 1.79 | 1.80 |
| BL0146 | Possible arabinosidase | 12.39 | ND | 2.43 | ND | 1.59 | 1.36 | 1.30 | 1.26 | ND | ND | ND | ND | ND | 2.16 | ND | 1.45 |
| BL0421 | Narrowly conserved HP possibly involved in xylan degradation | 12.81 | ND | 1.53 | ND | ND | 1.31 | 1.39 | 1.28 | ND | ND | ND | 1.32 | ND | ND | 1.33 | 1.45 |
| BL0529 | Probable α-1,4-glucosidase; maltase-like enzyme | 22.79 | 1.58 | 1.90 | ND | ND | 2.60 | ND | 2.08 | ND | ND | ND | ND | ND | 2.54 | 2.82 | 1.68 |
| BL0536 | Sucrose phosphorylase | 0.05 | 2.08 | 1.95 | 1.27 | 2.21 | 2.50 | 2.08 | 1.32 | 2.03 | ND | 1.36 | ND | 1.68 | 3.46 | 4.63 | ND |
| BL0544 | α-l-Arabinosidase | 24.40 | ND | ND | 1.37 | 1.30 | 1.46 | 1.10 | 1.38 | ND | ND | 1.27 | ND | ND | 0.99 | 1.59 | 2.40 |
| BL0597 | Glycogen phosphorylase | 86.10 | 245.91 | 208.05 | 200.41 | 217.36 | 150.31 | 166.54 | 80.33 | 34.42 | ND | 5.77 | 8.25 | 26.81 | 1.94 | 97.23 | 39.30 |
| BL0715 | Transaldolase | 1.23 | 12.99 | 10.18 | 3.94 | 8.64 | 22.69 | 36.03 | 9.99 | 2.45 | ND | 24.63 | 1.37 | 3.33 | 46.41 | 63.17 | 25.69 |
| BL0716 | Transketolase | 5.75 | 1.33 | 1.27 | ND | ND | 1.73 | ND | ND | ND | ND | 1.46 | ND | ND | 1.29 | ND | ND |
| BL0978 | Glycosyl hydrolase (LacZ) | 90.9 | 30.91 | 46.13 | 43.51 | 41.34 | 59.71 | 51.23 | 10.01 | 1.99 | ND | 7.47 | ND | 2.95 | ND | 10.41 | 6.60 |
| BL1104 | Possible glycosyltransferase | 12.77 | 2.93 | 1.60 | 1.86 | 5.92 | 4.10 | 2.65 | 1.73 | ND | ND | 1.28 | 1.63 | 3.90 | 4.85 | 2.43 | 34.88 |
| BL1308 | Lactate dehydrogenase | 0.17 | 1.49 | 1.69 | ND | 1.26 | 1.52 | ND | 1.49 | ND | ND | ND | ND | 1.27 | ND | 4.10 | 1.26 |
| BL1518 | α-Galactosidase | 28.8 | ND | 1.29 | ND | ND | ND | ND | 1.42 | ND | 1.33 | ND | ND | ND | 1.71 | 1.25 | ND |
| BL1638 | Solute-binding protein of ABC transporter for sugars | 4.28 | 1.33 | 1.44 | ND | ND | 1.73 | ND | 1.52 | ND | ND | ND | ND | ND | 1.52 | 1.82 | ND |
| BL1639 | Permease of ABC transporter for sugars | 14.37 | ND | ND | 1.37 | ND | 1.38 | 1.27 | 1.38 | ND | ND | 1.27 | ND | ND | 1.82 | 2.92 | 3.16 |
| BL1641 | LNB phosphorylase | 5.61 | ND | ND | ND | ND | ND | ND | ND | ND | 2.30 | ND | ND | ND | ND | ND | ND |
| BL1643 and BL1644 | Galactose-1-phosphate uridylyltransferase and UDP-glucose 4-epimerase | 0.42 | ND | ND | ND | ND | 1.39 | ND | 1.26 | ND | ND | ND | ND | ND | 1.31 | 1.36 | ND |
| BL1674 | Probable glycosyltransferase | 1.76 | 1.64 | 1.35 | ND | 1.26 | 2.76 | 1.34 | 2.21 | 1.31 | ND | ND | ND | 1.39 | 2.87 | 4.27 | 1.56 |
Significant hybridization signals were those for which signal/background ratios were >1.25. Infants 1 and 2, for which sample designations are identified in bold, were breast fed. Sample designations indicate the infant identification number and the day of sampling. ND, not detected (no significant hybridization signal detected); HP, hypothetical protein.
Active carbohydrate metabolism.
The genome of B. longum NCC2705 includes numerous genes for carbohydrate transport and metabolism (54). These are collectively termed the glycobiome and show a preference for the metabolism of di-, tri-, and oligosaccharides, pointing toward biased utilization for complex oligosaccharides complemented with transporters for a variety of disaccharides and oligosaccharides (44). The importance of carbohydrate metabolism for the activity of bifidobacteria in the intestinal tract is also evident from the results of this study, as carbohydrate metabolism functions are the most important category of predicted functions (Fig. 5), corresponding to 14% of the genes, a proportion greater than that determined by the sequence-based classification of carbohydrate-active enzymes (8%) (60). Here, the most salient features of the predicted bifidobacterial genes involved in intestinal carbohydrate metabolism (the glycobiome) are discussed briefly, with specific attention to those that are highly expressed, have the greatest impact on the RA, or are organized into coexpressed operons (Table 1).
Taking the RA into account, the DNA fragment that had the greatest impact on the difference between the two diet groups included the B. longum lacZ gene (BL0978) that shows significant homology to the B. longum subsp. infantis HL96 gene encoding β-galactosidase I, which degrades lactose and other sugars containing a β-d-anomer-linked galactoside (24). Recent growth studies with laboratory media showed the induction of the B. longum lacZ gene by lactose, maltose, and FOS (44). In the present study, the lacZ-like gene showed greater expression in breast-fed infants, though a clone with a very similar gene, BAD_1605, showed much lower hybridization signals than those for the lacZ-like gene, indicating the significance of the encoded β-galactosidase in intestinal sugar degradation. Similarly, the RA value for the fragment including BL0597 (coding for a glycogen phosphorylase) is high, and this gene is involved in the breakdown of starch into glucose units. The α-1,6 bonds in amylopectin, glycogen, and pullulan can be hydrolyzed by so-called pullulanases (encoded by BAD_0708), which were previously shown to be expressed in several bifidobacterial species growing on these sugar polymers in laboratory media (41, 49). The latter genes show higher hybridization levels in breast-fed infants than in formula-fed infants.
A bifidobacterial α-l-arabinosidase-like gene (BL0544, or abfB) was found to be expressed in all of the infants. The abfB gene encodes a hemicellulose-degrading enzyme that hydrolyzes terminal α-l-arabinofuranosyl groups from arabinose-containing oligosaccharides and polysaccharides such as arabinans, arabinoxylans, and xylans, major components of plant cell walls (50). It is known that α-arabinofuranosidases, together with other hydrolases with endo- and exoactivities, are required for the complete degradation of polymeric carbohydrates. These substrates are poorly digested by the host or other intestinal microbes. The sequencing of the B. longum genome (54) allowed the annotation of at least 14 different enzymes with hypothetical roles in the catabolism of arabinose-containing polymers, which corroborated the importance of these types of enzymes and polysaccharides in the metabolisms of some bifidobacteria. The expression of the abfB-like gene and the influences of inducers and repressors in B. longum NIZO B667 have been studied, and the findings have indicated transcriptional regulation. Degenerate primers revealed the widespread presence of the α-arabinofuranosidase enzyme family (family 51 of glycoside hydrolases) in B. longum, B. longum subsp. infantis, B. animalis, and B. bifidum (19). A different gene, possibly coding for an arabinosidase (BL0146), was found to have hybridization levels similar to those of abfB-like gene and may also be involved in the breakdown of arabinose-containing saccharides. AbfB activity indicates a selective advantage for bifidobacteria for nutritional competition in and the colonization of the human gastrointestinal tract, as arabinose-containing polymers, such as hemicellulose and pectin, are abundant in the human diet and are known to reach the colon. The expression of arabinosidases by the bifidobacterial community may give these species an advantage to survive and colonize the human colon. As the infants that received only a breast milk-based diet also showed the expression of an abfB-like gene, it is possible either that breast milk contains arabinose-like sugars or arabinose-decorated glycoproteins or that it contains related sugars that act as inducers of abfB-like gene expression. An alternative explanation is that milk serves as a substrate for the growth of microbes that produce arabinose-like compounds.
The novel putative operon for galactose metabolism (BL1638 to BL1644) was detected in the B. longum clone library by the hybridization of RNAs from both breast-fed and formula-fed infants. This operon is involved in the breakdown of structures present in mucin sugars (10, 39, 63), indicating a way for bifidobacteria to colonize the intestine. Additionally, the complex mixture of human milk oligosaccharides contains LNB structures (27, 65) broken down by the products of the genes in this operon; BL1638 to BL1640 genes are annotated as encoding component proteins of the ABC-type sugar transporter, and the BL1641 gene is annotated as encoding LNB phosphorylase. The cluster of BL1642, BL1643, and BL1644 genes, which were annotated as encoding mucin desulfatase, galactose-1-phosphate uridylyltransferase, and UDP-glucose 4-epimerase, respectively, likely codes for a metabolic pathway for mucin sugars, because galacto-N-biose is the core structure of mucin-type sugars (10). In this pathway, galactose 1-P, formed by the phosphorolysis of LNB/galacto-N-biose, is converted into UDP-glucose. Other genes involved in carbohydrate metabolism are predicted to encode an α-1,4-glucosidase (BL0529) and an α-galactosidase (BL1518), both of which are expressed to a greater degree in formula-fed infants than in breast-fed infants. Finally, a lactate dehydrogenase gene (BL1308), involved in the final step in anaerobic glycolysis and the formation of lactate, was found to hybridize to RNAs isolated from both formula- and breast-fed infants, reflecting the activity of bifidobacterial sugar metabolism.
An endo-1,4-β-xylanase gene (BAD_1527) and a hypothetical gene involved in xylan degradation (BL0421) may be involved in the breakdown of xylans by the hydrolysis of 1,4-β-d-xylosidic linkages in xylans. The two genes were detected in samples from all infants and show similar hybridization signals. The BAD_1412 product, a probable sugar kinase, has high levels of similarity to xylose kinases and may be involved in the breakdown of xylans as well. Sucrose phosphorylase (encoded by the BL0536 gene) breaks down sucrose and is involved in energy metabolism.
Overall, the expression of the glycobiome (Table 1), especially the genes encoding pullulanases, α-1,4-glucosidase, and the glycogen phosphorylase, indicates a higher potential for carbohydrate metabolism in breast-fed infants than in formula-fed infants. This finding may very well be explained by the high degree of diversity of complex oligosaccharides in human milk (6, 38) that activate and/or increase the abundance of species expressing these genes.
Colonization factors.
Streptococcus mutans 20381 is known to use glycosyltransferases to form polysaccharides, which are involved in the formation of biofilms and have been shown previously to be the major contributors to adherence (67). In this study, the expression of two putative glycosyltransferases, the BL1104 and BL1674 products, was detected in most of the samples, suggesting the in vivo production of polysaccharides that may also play a role in biofilm formation and the colonization of the host's intestine by bifidobacteria. A possible penicillin-binding protein (encoded by BAD_1336) is predicted to be involved in the synthesis of peptidoglycan, is the major component of bacterial cell walls (31), and may be involved in the recognition of the bacteria by the host. Only samples from formula-fed babies showed hybridization to the clone containing BAD_1336 (see File S1 in the supplemental material).
Activity of bifidobacteria within the human intestine.
Several other genes and operons are worth mentioning, as these are biologically relevant and may explain functions of the intestinal bifidobacteria related to host interaction and colonization and competition within the intestinal microbiota. The gene for transaldolase (BL0715), which takes part in the nonoxidative phase of the pentose phosphate route, was expressed in samples from all infants. Its translation product was also detected in the metaproteomes from infant feces (28) and the proteome of B. longum subsp. infantis BI07 grown in a laboratory medium (62). The gene for transketolase (BL0716), also involved in the pentose phosphate route characteristic of bifidobacterial metabolism, was expressed at lower levels than the gene for transaldolase.
Hybridization to the clone containing BTH_II0919, coding for a glutamine-dependent NAD+ synthetase (EC 6.3.5.10), indicates the presence of a glutamine-rich substrate. A previous study showed increased intestinal bifidobacterial numbers upon the intake of prebiotics containing glutamine-rich protein by healthy adults (25). Glutamine is known to be among the nutrient requirements for infant gut maturation, as the endogenous capacity to synthesize glutamine from glutamate is not fully developed. The lower hybridization levels for samples from formula-fed infants may be due to lower glutamine levels in the formula than in breast milk (1).
Several genes predicted to be involved in folate biosynthesis pathways were found to be expressed, namely, those encoding dihydrofolate reductase (BL1666) and thymidylate synthase (BL1665), involved in the last step of the production of folate. Folate is involved in many metabolic pathways, such as methyl group biogenesis and the synthesis of nucleotides, vitamins, and some amino acids. It has been demonstrated previously that folate synthesized by bacteria in the human intestine is absorbed and used by the host (51). Moreover, several bifidobacterial species have been found to produce folate in laboratory media (47). The expression of genes encoding folate in the infants' intestinal tracts indicates the in vivo production of this vitamin, which is beneficial to the host and can be absorbed through the large intestine (47). Remarkably, the levels of hybridization of folate biosynthesis-like genes in samples from formula-fed infants were found to be slightly higher, but not significantly so, than those in samples from breast-fed infants.
The gene for a putative copper-transporting ATPase (BL0409) was expressed mostly in samples from formula-fed infants, and its translation product was also found in the proteome of B. longum subsp. infantis (62). In a recent study, a transcriptome analysis of L. plantarum indicated significant expression of a gene for an orthologous putative copper-transporting protein in intestinal samples from humans (11) and mice (8), but the function is not confirmed.
qPCR and sequencing analyses.
Using the clone insert sequences and matching sequences in the database, primer sets for qPCR were designed to target the thymidylate synthase (BL1665) gene, BL1641, and the α-l-arabinosidase (BL0544) gene, and qPCR analysis of cDNAs was performed to confirm the specific hybridization of transcripts to the microarray. Melting curves showed specific amplification of one product for each sample primer set combination (data not shown).
The quantification of gene activity can be complicated because it is not known how many active bifidobacteria with sequences that match the amplicons spotted onto the microarray are present in the samples. The yield of RNA per sample unit was found to differ among samples and individuals because of transit time, diet, and other host-related conditions. The sizes of the gene fragments on the microarray can also cause variability in hybridization among samples from different origins. However, the relative copy numbers obtained with qPCR showed trends similar to those demonstrated by the relative signal-to-background ratios obtained with the microarray hybridizations, confirming the presence of target mRNA (data not shown). Moreover, sequence analyses of the amplicons from qPCR confirmed the amplification of the target genes BL1665, BL1641, and BL0544.
Concluding remarks.
This study revealed that bifidobacterial species undergo dynamic changes in infant feces at the level of persistence as well as their functional complements. To aid the detection of the gene transcript, the numbers and diversities of bifidobacteria in the infants' feces were determined using molecular tools. This approach was complemented by transcriptome analysis using mixed-bifidobacterial-species microarrays that showed a significant impact of diet on the transcriptional responses of bifidobacteria in breast- and formula-fed infants.
Genome-wide transcript analyses using DNA microarrays provide opportunities for comprehensive and integrative views of bacterial activities occurring within the intestinal tract. The potential of this approach was exemplified by previous studies reporting full-genome transcriptome profiles for Bacteroides thetaiotaomicron and B. longum residing in the ceca of germfree mice (58, 59), as well as for L. plantarum in conventional mice (32). In the present study, the hybridization profile could be used to assess the influence of the diet on the transcriptomes of fecal bifidobacterial communities from infants. Particularly, the use of a mixed-species microarray made it possible to simultaneously target several bifidobacterial species in the complex fecal microbial ecosystem. By using this array, it was possible to compare fecal bifidobacterial gene expression profiles for individuals despite the unique fecal microbial compositions.
Samples from breast-fed infants with stable total microbiotas and bifidobacterial populations showed variety in transcripts at different time points, indicating active bifidobacterial populations. As expected, the transcriptomes of microbiotas in the formula-fed infants, with more unstable microbial diversity, also showed differences over time. The observed differences may have been caused by the variation of activities of individual bifidobacterial species and/or environmental factors such as diet. Possibly, host factors and the addition of solid food to the milk diet also had an influence, and hence, more individuals should be compared. It is likely that specific bifidobacterial species may be a target of modulation by prebiotics, but this study did not reveal a direct link between the expressed genes and the species. The genome sequence of B. longum NCC2705 revealed that the chromosome includes numerous genes for carbohydrate utilization, specifically those for over 30 glycosyl hydrolases that are predicted to be involved in the degradation of higher-order oligosaccharides (54), and 19 carbohydrate transport systems were proven to be active in laboratory media (44). B. adolescentis MB 239 prefers lactose, FOS, and raffinose over glucose and fructose in laboratory media, which was explained by α-galactosidase, β-galactosidase, and β-fructofuranosidase activities (2). The findings of this study show that at least a portion of these genes are being transcribed in vivo in the infant intestinal tract. This gene expression may give these bifidobacteria advantages in colonizing the infant gut and explains the growth-promoting effect of oligosaccharides which are described as prebiotics.
The sequencing of an extensive metagenomic library from the human distal gut revealed a high degree of diversity in bifidobacterial genes compared to the B. longum NCC2705 genome (15). This finding suggests the presence of multiple and related bifidobacterial strains. The presently known and expected sequences of several bifidobacterial genomes will open up a new era of comparative genomics and provide a basis for the setup of a molecular model that can predict the bifidobacterium-host interaction. This progress will improve the molecular studies of the functional complement with tools such as transcriptomics, described here, as well as metaproteomics (28) and metabolomics (18). The further development of high-throughput sequencing techniques will allow the detection and quantification of total cDNAs without the limitation of relevant probes on a microarray (33, 64).
Ultimately, this advancement will lead to a detailed understanding of the nutritional lifestyle of bifidobacteria and its impact on the host. Linking the functional activities of intestinal bifidobacteria to specific groups of healthy or diseased individuals or special diets will open up leads for the modulation of the intestinal bifidobacterial community, exerting health benefits.
Supplementary Material
Acknowledgments
This work was supported by the Dutch Ministry of Economic Affairs through the Innovation Oriental Research Program on Genomics (IOP-IGE01016).
We thank Aldwin Vriesema and Kaouther Ben Amor and coworkers from Danone Research for excellent experimental help. We thank Michiel Wells for database searching. We very are grateful for DGGE analysis by Rocio Martin.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Agostoni, C., B. Carratu, C. Boniglia, E. Riva, and E. Sanzini. 2000. Free amino acid content in standard infant formulas: comparison with human milk. J. Am. Coll. Nutr. 19:434-438. [DOI] [PubMed] [Google Scholar]
- 2.Amaretti, A., E. Tamburini, T. Bernardi, A. Pompei, S. Zanoni, G. Vaccari, D. Matteuzzi, and M. Rossi. 2006. Substrate preference of Bifidobacterium adolescentis MB 239: compared growth on single and mixed carbohydrates. Appl. Microbiol. Biotechnol. 73:654-662. [DOI] [PubMed] [Google Scholar]
- 3.Arslanoglu, S., G. E. Moro, and G. Boehm. 2007. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 137:2420-2424. [DOI] [PubMed] [Google Scholar]
- 4.Benno, Y., K. Sawada, and T. Mitsuoka. 1984. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol. Immunol. 28:975-986. [DOI] [PubMed] [Google Scholar]
- 5.Biavati, B., and P. Mattarelli. 2001. The family Bifidobacteriaceae, p. 1-70. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes. Springer, New York, NY.
- 6.Boehm, G., and B. Stahl. 2007. Oligosaccharides from milk. J. Nutr. 137(3 Suppl. 2):847S-849S. [DOI] [PubMed] [Google Scholar]
- 7.Boesten, R. J., F. Schuren, and W. M. de Vos. 2009. A Bifidobacterium mixed-species microarray for high resolution discrimination between intestinal bifidobacteria. J. Microbiol. Methods 76:269-277. [DOI] [PubMed] [Google Scholar]
- 8.Bron, P. A., D. Molenaar, W. M. de Vos, and M. Kleerebezem. 2006. DNA micro-array-based identification of bile-responsive genes in Lactobacillus plantarum. J. Appl. Microbiol. 100:728-738. [DOI] [PubMed] [Google Scholar]
- 9.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051. [DOI] [PubMed] [Google Scholar]
- 10.Derensy-Dron, D., F. Krzewinski, C. Brassart, and S. Bouquelet. 1999. Beta-1,3-galactosyl-N-acetylhexosamine phosphorylase from Bifidobacterium bifidum DSM 20082: characterization, partial purification and relation to mucin degradation. Biotechnol. Appl. Biochem. 29:3-10. [PubMed] [Google Scholar]
- 11.de Vries, M. C. 2006. Analyzing global gene expression of Lactobacillus plantarum in the human gastro-intestinal tract. Ph.D. thesis. Wageningen University, Wageningen, The Netherlands.
- 12.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favier, C. F., E. E. Vaughan, W. M. de Vos, and A. D. L. Akkermans. 2002. Molecular monitoring of succession of bacterial communities in human neonates. Appl. Environ. Microbiol. 68:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson, G. R., and X. Wang. 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77:412-420. [DOI] [PubMed] [Google Scholar]
- 15.Gill, S. R., M. Pop, R. T. DeBoy, P. B. Eckburg, P. J. Turnbaugh, B. S. Samuel, J. I. Gordon, D. A. Relman, C. M. Fraser-Liggett, and K. E. Nelson. 2006. Metagenomic analysis of the human distal gut microbiome. Science 312:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez, R., E. S. Klaassens, E. Malinen, W. M. de Vos, and E. E. Vaughan. 2008. Differential transcriptional response of Bifidobacterium longum to human milk, formula milk, and galactooligosaccharide. Appl. Environ. Microbiol. 74:4686-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González, R., E. S. Klaassens, E. Malinen, W. M. de Vos, and E. E. Vaughan. 2008. Differential transcriptional response of Bifidobacterium longum to human milk, formula milk and galactooligosaccharide. Appl. Environ. Microbiol. [DOI] [PMC free article] [PubMed]
- 18.Goodacre, R. 2007. Metabolomics of a superorganism. J. Nutr. 137:259S-266S. [DOI] [PubMed] [Google Scholar]
- 19.Gueimonde, M., L. Noriega, A. Margolles, and C. G. de los Reyes-Gavilan. 2007. Induction of alpha-L-arabinofuranosidase activity by monomeric carbohydrates in Bifidobacterium longum and ubiquity of encoding genes. Arch. Microbiol. 187:145-153. [DOI] [PubMed] [Google Scholar]
- 20.Haarman, M., and J. Knol. 2005. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen, H. J., A. C. Wildeboer-Veloo, G. C. Raangs, A. A. Wagendorp, N. Klijn, J. G. Bindels, and G. W. Welling. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61-67. [DOI] [PubMed] [Google Scholar]
- 22.Harmsen, H. J. M., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper, L. V. 2004. Bacterial contributions to mammalian gut development. Trends Microbiol. 12:129-134. [DOI] [PubMed] [Google Scholar]
- 24.Hung, M. N., Z. Xia, N. T. Hu, and B. H. Lee. 2001. Molecular and biochemical analysis of two beta-galactosidases from Bifidobacterium infantis HL96. Appl. Environ. Microbiol. 67:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanauchi, O., Y. Fujiyama, K. Mitsuyama, Y. Araki, T. Ishii, T. Nakamura, Y. Hitomi, K. Agata, T. Saiki, A. Andoh, A. Toyonaga, and T. Bamba. 1999. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int. J. Mol. Med. 3:175-179. [DOI] [PubMed] [Google Scholar]
- 26.Kim, J. F., H. Jeong, D. S. Yu, S. H. Choi, C. G. Hur, M. S. Park, S. H. Yoon, D. W. Kim, G. E. Ji, H. S. Park, and T. K. Oh. 2009. Genome sequence of the probiotic bacterium Bifidobacterium animalis subsp. lactis AD011. J. Bacteriol. 191:678-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaoka, M., J. Tian, and M. Nishimoto. 2005. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 71:3158-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaassens, E. S., W. M. de Vos, and E. E. Vaughan. 2007. Metaproteomics approach to study the functionality of the microbiota in the human infant gastrointestinal tract. Appl. Environ. Microbiol. 73:1388-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knol, J., P. Scholtens, C. Kafka, J. Steenbakkers, S. Gro, K. Helm, M. Klarczyk, H. Schopfer, H. M. Bockler, and J. Wells. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 40:36-42. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J. H., V. N. Karamychev, S. A. Kozyavkin, D. Mills, A. R. Pavlov, N. V. Pavlova, N. N. Polouchine, P. M. Richardson, V. V. Shakhova, A. I. Slesarev, B. Weimer, and D. J. O'Sullivan. 2008. Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth. BMC Genomics 9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovering, A. L., L. H. de Castro, D. Lim, and N. C. J. Strynadka. 2007. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science 315:1402-1405. [DOI] [PubMed] [Google Scholar]
- 32.Marco, M. L., R. S. Bongers, W. M. de Vos, and M. Kleerebezem. 2007. Spatial and temporal expression of Lactobacillus plantarum genes in the gastrointestinal tracts of mice. Appl. Environ. Microbiol. 73:124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marioni, J. C., C. E. Mason, S. M. Mane, M. Stephens, and Y. Gilad. 2008. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 18:1509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marteau, P., P. Pochart, J. Dore, C. Bera-Maillet, A. Bernalier, and G. Corthier. 2001. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl. Environ. Microbiol. 67:4939-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGinnis, S., and T. L. Madden. 2004. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 32:W20-W25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsuoka, T. 1969. Vergleichende untersuchungen uber die Bifidobakterien aus dem verdauungstrakt von menschen and tieren. Zentralbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 210:52-64. [PubMed] [Google Scholar]
- 37.Moro, G., S. Arslanoglu, B. Stahl, J. Jelinek, U. Wahn, and G. Boehm. 2006. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 91:814-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ninonuevo, M. R., Y. Park, H. Yin, J. Zhang, R. E. Ward, B. H. Clowers, J. B. German, S. L. Freeman, K. Killeen, R. Grimm, and C. B. Lebrilla. 2006. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 54:7471-7480. [DOI] [PubMed] [Google Scholar]
- 39.Nishimoto, M., and M. Kitaoka. 2007. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol. 73:6444-6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nubel, U., B. Engelen, A. Felske, J. Snaidr, A. Wieshuber, R. I. Amann, W. Ludwig, and H. Backhaus. 1996. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 178:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell Motherway, M., G. F. Fitzgerald, S. Neirynck, S. Ryan, L. Steidler, and D. van Sinderen. 2008. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 74:6271-6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orla-Jensen, S. 1924. La classification des bactéries lactiques. Lait 4:468-474. [Google Scholar]
- 43.O'Sullivan, D. J. 2001. Screening of intestinal microflora for effective probiotic bacteria. J. Agric. Food Chem. 49:1751-1769. [DOI] [PubMed] [Google Scholar]
- 44.Parche, S., J. Amon, I. Jankovic, E. Rezzonico, M. Beleut, H. Barutcu, I. Schendel, M. P. Eddy, A. Burkovski, F. Arigoni, and F. Titgemeyer. 2007. Sugar transport systems of Bifidobacterium longum NCC2705. J. Mol. Microbiol. Biotechnol. 12:9-19. [DOI] [PubMed] [Google Scholar]
- 45.Parro, V., and M. Moreno-Paz. 2003. Gene function analysis in environmental isolates: the nif regulon of the strict iron oxidizing bacterium Leptospirillum ferrooxidans. Proc. Natl. Acad. Sci. USA 100:7883-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pieterse, B., E. Quirijns, F. Schuren, and M. van der Werf. 2005. Mathematical design of prokaryotic clone-based microarrays. BMC Bioinform. 6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pompei, A., L. Cordisco, A. Amaretti, S. Zanoni, D. Matteuzzi, and M. Rossi. 2007. Folate production by bifidobacteria as a potential probiotic property. Appl. Environ. Microbiol. 73:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reuter, G. 1963. Vergleichende untersuchunge úber die bifidus-flora im sáuglings-und erwachsenenstuhl. Zentralbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig.1 91:486-507. [PubMed] [Google Scholar]
- 49.Ryan, S. M., G. F. Fitzgerald, and D. van Sinderen. 2006. Screening for and identification of starch-, amylopectin-, and pullulan-degrading activities in bifidobacterial strains. Appl. Environ. Microbiol. 72:5289-5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saha, B. C., and R. J. Bothast. 1998. Purification and characterization of a novel thermostable α-l-arabinofuranosidase from a color-variant strain of Aureobasidium pullulans. Appl. Environ. Microbiol. 64:216-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Said, H. M., and Z. M. Mohammed. 2006. Intestinal absorption of water-soluble vitamins: an update. Curr. Opin. Gastroenterol. 22:140-146. [DOI] [PubMed] [Google Scholar]
- 52.Satokari, R. M., E. E. Vaughan, A. D. L. Akkermans, M. Saarela, and W. M. de Vos. 2001. Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scardovi, V., and F. Crociani. 1974. Bifidobacterium catenulatum, Bifidobacterium dentium, and Bifidobacterium angulatum: three new species and their deoxyribonucleic acid homology relationships. Int. J. Syst. Bacteriol. 24:21-28. [Google Scholar]
- 54.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M.-C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiffrin, E. J., and S. Blum. 2002. Original communication: Interactions between the microbiota and the intestinal mucosa. Eur. J. Clin. Nutr. 56:S60-S64. [DOI] [PubMed] [Google Scholar]
- 56.Sela, D. A., J. Chapman, A. Adeuya, J. H. Kim, F. Chen, T. R. Whitehead, A. Lapidus, D. S. Rokhsar, C. B. Lebrilla, J. B. German, N. P. Price, P. M. Richardson, and D. A. Mills. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 105:18964-18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith, T. F., and M. S. Waterman. 1981. Identification of common molecular subsequences. J. Mol. Biol. 147:195-197. [DOI] [PubMed] [Google Scholar]
- 58.Sonnenburg, J. L., J. Xu, D. D. Leip, C.-H. Chen, B. P. Westover, J. Weatherford, J. D. Buhler, and J. I. Gordon. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955-1959. [DOI] [PubMed] [Google Scholar]
- 59.Souza, D. G., A. T. Vieira, A. C. Soares, V. Pinho, J. R. Nicoli, L. Q. Vieira, and M. M. Teixeira. 2004. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J. Immunol. 173:4137-4146. [DOI] [PubMed] [Google Scholar]
- 60.Ventura, M., C. Canchaya, A. Tauch, G. Chandra, G. F. Fitzgerald, K. F. Chater, and D. van Sinderen. 2007. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71:495-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ventura, M., S. O'Flaherty, M. J. Claesson, F. Turroni, T. R. Klaenhammer, D. van Sinderen, and P. W. O'Toole. 2009. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7:61-71. [DOI] [PubMed] [Google Scholar]
- 62.Vitali, B., V. Wasinger, P. Brigidi, and M. Guilhaus. 2005. A proteomic view of Bifidobacterium infantis generated by multi-dimensional chromatography coupled with tandem mass spectrometry. Proteomics 5:1859-1867. [DOI] [PubMed] [Google Scholar]
- 63.Wada, J., T. Ando, M. Kiyohara, H. Ashida, M. Kitaoka, M. Yamaguchi, H. Kumagai, T. Katayama, and K. Yamamoto. 2008. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl. Environ. Microbiol. 74:3996-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, Z., M. Gerstein, and M. Snyder. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward, R. E., M. Ninonuevo, D. A. Mills, C. B. Lebrilla, and J. B. German. 2007. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 51:1398-1405. [DOI] [PubMed] [Google Scholar]
- 66.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiater, A., A. Choma, and J. Szczodrak. 1999. Insoluble glucans synthesized by cariogenic streptococci: a structural study. J. Basic Microbiol. 39:265-273. [DOI] [PubMed] [Google Scholar]
- 68.Yaeshima, T., T. Fujisawa, and T. Mitsuoka. 1992. Bifidobacterium globosum, subjective synonym of Bifidobacterium pseudolongum, and description of Bifidobacterium pseudolongum subsp. pseudolongum comb. nov. and Bifidobacterium pseudolongum subsp. globosum comb. nov. Syst. Appl. Microbiol. 15:280-385. [Google Scholar]
- 69.Zoetendal, E. G., C. C. G. M. Booijink, E. S. Klaassens, H. G. H. J. Heilig, M. Kleerebezem, H. Smidt, and W. M. de Vos. 2006. Isolation of DNA from bacterial samples of the human gastrointestinal tract. Nat. Protoc. 1:870-873. [DOI] [PubMed] [Google Scholar]
- 70.Zoetendal, E. G., C. C. G. M. Booijink, E. S. Klaassens, H. G. H. J. Heilig, M. Kleerebezem, H. Smidt, and W. M. de Vos. 2006. Isolation of RNA from bacterial samples of the human gastrointestinal tract. Nat. Protoc. 1:954-959. [DOI] [PubMed] [Google Scholar]
- 71.Zoetendal, E. G., M. Rajilic-Stojanovic, and W. M. de Vos. 2008. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 57:1605-1615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.