Abstract
Airborne fungi, termed fungal bioaerosols, have received attention due to the association with public health problems and the effects on living organisms in nature. There are growing concerns that fungal bioaerosols are relevant to the occurrence of allergies, opportunistic diseases in hospitals, and outbreaks of plant diseases. The search for ways of preventing and curing the harmful effects of fungal bioaerosols has created a high demand for the study and development of an efficient method of controlling bioaerosols. However, almost all modern microbiological studies and theories have focused on microorganisms in liquid and solid phases. We investigated the thermal heating effects on fungal bioaerosols in a continuous-flow environment. Although the thermal heating process has long been a traditional method of controlling microorganisms, the effect of a continuous high-temperature, short-time (HTST) process on airborne microorganisms has not been quantitatively investigated in terms of various aerosol properties. Our experimental results show that the geometric mean diameter of the tested fungal bioaerosols decreased when they were exposed to increases in the surrounding temperature. The HTST process produced a significant decline in the (1→3)-β-d-glucan concentration of fungal bioaerosols. More than 99% of the Aspergillus versicolor and Cladosporium cladosporioides bioaerosols lost their culturability in about 0.2 s when the surrounding temperature exceeded 350°C and 400°C, respectively. The instantaneous exposure to high temperature significantly changed the surface morphology of the fungal bioaerosols.
Fungi are omnipresent in indoor and outdoor environments (2, 28, 39). Most fungi are dispersed through the release of spores into the air, a phenomenon known to be driven by two kinds of energy (17): the energy provided by the fungus itself and the energy provided by external sources, such as air currents, rain, gravity, or changes in temperature and nutritional sources. Of these various mechanisms of fungal particle release, dispersal by air currents is the most prevalent mechanism for indoor fungal particles (19, 31). These airborne fungal spores, termed fungal bioaerosols, are resistant to environmental stresses and are adapted to airborne transport.
Fungal bioaerosols constitute the major component of ambient airborne microorganisms (23, 50, 51). Several studies have reported that the concentration of fungal bioaerosols is relevant to the occurrence of human diseases and public health problems associated with acute toxic effects, allergies (3, 18), and asthma (4, 5, 13, 48). Fungal bioaerosols are of particular concern in healthcare facilities, where they can cause major infectious complications as opportunistic pathogens in patients with an immunodeficiency (9). For instance, invasive mycoses can affect patients undergoing high-dose chemotherapy for hematological malignancies associated with a prolonged period of neutropenia; they can also affect solid-organ transplant recipients. Despite all diagnostic and therapeutic efforts, the outcome of an invasive fungal infection is often fatal (with a mortality rate of around 50% for aspergillosis) (37). The main fungal genera responsible for these infections are as follows: Aspergillus spp., Fusarium spp., Scedosporium spp., and Mucorales spp. (10, 12, 20). However, virtually any filamentous fungus can be a pathogen (22, 41). In the hospital environment, possible sources of airborne nosocomial infection include ventilation or air-conditioning systems, decaying organic material, dust, water, food, ornamental plants, and building materials in and around hospitals (1).
One of the major bioaerosols of concern is (1→3)-β-d-glucans, which comprises up to 60% of the cell wall of most fungal organisms. The (1→3)-β-d-glucans are glucose polymers with a variable molecular weight and a degree of branching (49). The results of several studies about the exposure of subjects to airborne (1→3)-β-d-glucans suggest that these agents play a role in bioaerosol-induced inflammatory responses and resulting respiratory symptoms, such as a dry cough, phlegmy cough, hoarseness, and atopy (11, 44). In addition, given that many epidemiological studies have reported that (1→3)-β-d-glucan has strong immuno-modulating effects (42, 47), (1→3)-β-d-glucan is an important parameter for exposure assessment by itself and as a surrogate component for fungi (16).
To prevent the adverse health effects of fungal bioaerosols, we must ensure that control methods for airborne fungal spores are studied and developed. However, despite the necessity of controlling fungal bioaerosols, few studies have focused on such control mechanisms. The most common control methods are UV irradiation and electric ion emission. Given that UV irradiation is known to have a germicidal effect, several studies have examined how UV irradiation affects the viability of bioaerosols (35, 42). However, although UV irradiation can be easily applied by simply installing and turning on a UV lamp, the 254-nm-wavelength UV light produces ozone and radicals, which cause harmful effects to surrounding humans. Electric ion emission has also been studied as a means of controlling bioaerosols (21, 27). When the efficiency of the filter is increased, the efficacy of respiratory protection devices against bioaerosols can be enhanced. Although electric ions decrease the viability of airborne bacteria (25), the generation of the ions produces ozone, a pollutant, and also causes electric charges to accumulate on surrounding surfaces.
Recently, heat treatment of indoor air using thermal processes has been considered a safe, effective, and environment-friendly method; it does not produce ozone or use ion or filter media. A thermal heating process has long been considered a suitable and reliable method for controlling microorganisms. Two types of heat are generally used, moist heat and dry heat. Moist heat utilizes steam under pressure, whereas dry heat involves high-temperature exposure without additional moisture. Several types of heat treatment are currently used for killing microorganisms. The treatments include incineration, Tyndallization, pasteurization, and autoclaving (32). However, most of these technologies were originally limited to controlling microorganisms in liquid or on material surfaces. In addition, they may not be adequate for controlling bioaerosols because the continuous surrounding environment of bioaerosols is significantly different from the conditions in liquid and on solid surfaces. Therefore, it is necessary to find adequate and practical conditions for controlling bioaerosols. Thus far, several investigations regarding the use of thermal processes against bioaerosols have been reported. Some of these studies have targeted airborne bacteria spores widely used as surrogates for biological warfare agents (8, 34), while others have focused on environmental parameters for the culture and survival of various vegetative cells (14, 29, 46). However, in these studies novel techniques for aerosols, such as measuring and analyzing aerosol particle size, distributions, and concentrations, were not utilized. In addition, to the best of our knowledge, there has been no study on the use of a thermal process for controlling fungal bioaerosols in continuous airflow. Fungal bioaerosols were found to be very resistant to a thermal environment in previous studies.
In this study, we investigated the thermal heating effects on the physical, chemical, and biological properties of fungal bioaerosols using a high-temperature, short-time (HTST) sterilization process. The HTST process, a type of thermal heating process, is based on high-temperature stresses for very short periods. Although this thermal process has been used for the microbial decontamination of seeds and dried, powdered products, such as pharmaceuticals and heat-sensitive drink and food, it can be also applied to the control of an airborne microorganism in a continuous-flow system, such as a heating, ventilation, and air-conditioning system (15, 33, 38). When the fungal bioaerosol was passed through a thermal electric heating system, the fungal spores were exposed to various temperatures for short periods. Then, we examined the bioaerosol and aerosol characteristics, including aerosol size distribution, culturability, (1→3)-β-d-glucan production, and surface morphology, using a novel technique for sampling and measuring aerosols.
MATERIALS AND METHODS
The experimental system, shown schematically in Fig. 1, included four major components: (i) a system for generating fungal bioaerosols, (ii) an aerosol measurement system that consisted of a condensation particle counter and a particle size distribution analyzer, (iii) a sampling system for various analyses of fungal bioaerosols before and after exposure to the thermal environment, and (iv) a thermal electric heating system.
FIG. 1.
Experimental setup. The fungal bioaerosols generated from the mold surface were transported by air flowing from the top outlet of the generator to the inlet of the sampling chamber. The sampling chambers were positioned at the inlet and the outlet of the thermal electric heating system. An airflow containing fungal bioaerosols was sampled in order to measure the total particle number concentration (QCPC) and the aerodynamic particle size distribution (QPSD) and to analyze the viability of the fungal bioaerosol (QBIO). MFC, mass flow controller.
Fungal bioaerosol generation.
To generate the fungal bioaerosol, we used a fungal bioaerosol generator with multiorifice air jets and a rotating substrate. The production rates of fungal bioaerosols can be controlled in the bioaerosol generator by adjusting the entering airflow rate and the rotation speed of the substrate (26). The fungal bioaerosols are released from various mold surfaces on a petri plate inside the generator. In the generator, the petri plate, which has a surface area of 144.5 cm2, was placed on a rotating substrate connected to a speed-control motor. We selected a rotation period of 125 s per rotation. The air supply for the generator was controlled by means of a mass flow controller (FC-280S; Mykrolis Corp., MA). The use of dry-filtered compressed air at a constant flow rate of 35 liters/min yielded a high air velocity of approximately 46.4 m/s in each orifice of the generator. The relative humidity in the generator was about 30% ± 5% (SK-110TRH II type 1; Sato Corp., Tokyo, Japan). The details of the fungal bioaerosol generator are described by Jung et al. (26).
Thermal electric heating system.
The designed thermal system consisted of a quartz tube (inner diameter, 29 mm; length, 700 mm; thickness, 1 mm) and an electric heating controller (MC-P; Poong Lim Co., Seoul, South Korea). The thermal heating system used electric heating coils of the ohm resistance type because the heating coils can be installed easily in existing air-conditioning systems. The heating coils were located outside the quartz tube and were covered with insulating materials of ceramic powder and glass fiber fabric so that passing bioaerosols could be exposed to a high-temperature environment without any obstruction of the bioaerosol flow stream. For experimental temperature conditions from 20°C (normal temperature) to 700°C, the residence time of the airflow between the inlet and outlet of the quartz tube in the thermal system was estimated to be from about 0.29 s (room temperature) to 0.17 s (700°C), with allowance for the air volume expansion due to the increase in temperature. Figure 2 shows the details of the thermal electric heating system.
FIG. 2.
Detail and center temperature distributions of the thermal electric heating system. Temp, temperature.
Fungal species.
We chose the two fungal species Aspergillus versicolor (Korean Collection for Type Cultures (KCTC) 6987; Biological Resource Center, Korea) and Cladosporium cladosporioides (KCTC 16680). The Aspergillus spp. are frequently found in air and soil. Some species have harmful effects on human health, such as toxicity (aflatoxin, carcinogenic sterigmatocystin, and so on), and produce allergic reactions. This type of fungus is an indicator of moisture problems in buildings and is frequently isolated from water-damaged building materials such as wallpaper and fiber boards. Cladosporium spp. are not pathogenic for humans, except for immunocompromised patients. However, Cladosporium has the ability to trigger allergic reactions in sensitive individuals. Thus, prolonged exposure to elevated spore concentrations can cause chronic allergies and asthma. The fungi were grown on malt extract agar (MEA), which was prepared as described by Schmechel et al. (45). We harvested the spores from each matured sporulating culture by placing 1 g of dry autoclaved glass microbeads on each petri plate. The beads (Glastechnique Mfg., Germany), which had a diameter of 1 mm, were shaken gently across the sporulating culture and then transferred to a 50-ml sterile tube that contained a mixture of sterile deionized water and 0.05% Tween 80 (Sigma Chemicals Co., MO). The suspension of 0.1 ml was used to inoculate fungi on each fresh 2% MEA plate (20 g/liter dextrose, 20 g/liter malt extract, 20 g/liter agar, 1 g/liter peptone; Difco, Becton Dickinson, Sparks, MD). Before being used in the experiments, the fungal cultures were incubated at 20°C to 24°C with a relative humidity of 32% to 40% for a period of 4 to 6 weeks.
Aerosol measurement system.
The aerodynamic particle size distributions of the fungal bioaerosols were measured with a particle size distribution analyzer (PSD 3603; TSI Inc., MN). The PSD 3603 has 128 size channels ranging from 0.3 μm to 700 μm. While measuring the particle size distributions, we also used a condensation particle counter (CPC 4330; HCT Inc., South Korea) to measure the total particle number concentration of the fungal bioaerosols. The CPC 4330 has a maximum detectable concentration of 10,000 particles/cm3 and a minimum particle size of 15 nm, with 95% accuracy. Before the experiments with fungal spores, we used HEPA-filtered clean air to flush the system until the PSD 3603 and CPC 4330 could no longer detect any particles. The total fungal bioaerosol particle number concentrations in the inlet and outlet sampling chambers were used to calculate the viability of fungal bioaerosols.
Culturability determination.
A BioSampler (SKC Inc., PA) was used for fungal bioaerosol sampling before and after the thermal heating treatment. The fungal bioaerosols were collected into 20 ml of phosphate-buffered saline (pH 7.0) water at a nominal flow rate of 12.5 liters/min. The sampling time of the BioSampler was 15 min for each test. Stainless steel tubing, which can sufficiently cool the sampled air to avoid heat shock when hot spores reach a cold sampler medium, was used as sampling tubing. The sample from the BioSamplers was serially diluted and plated on 2% MEA culture plates. The plates were incubated at 25°C ± 2°C for 7 days. The number of colonies was enumerated, and the CFU concentrations (CFUInlet or CFUOutlet) were calculated per milliliter (CFU/ml) of sampled suspension by taking into account the measured fungal spore number concentration. The relative culturability of the bioaerosols was determined as the ratio of the culturability of the bioaerosols in the outlet BioSampler to the culturability of the bioaerosols in the inlet BioSampler, and the culturability loss was obtained from the relative culturability according to the following equations:
 |
(1) |
 |
(2) |
Assay of (1→3)-β-d-glucan.
A portion of the fungal particle suspension was analyzed for (1→3)-β-d-glucan by using the kinetic chromogenic Limulus amebocyte lysate method (Glucatell Associates of Cape Cod, East Falmouth, MA). For analysis, we added 0.5 ml of 0.6 M NaOH to each 0.5-ml suspension of fungal particles. This suspension was shaken with a mechanical shaker for 1 h so that we could extract the (1→3)-β-d-glucan from the suspended fungal particles by unwinding its triple-helix structure and making it water soluble. Next, we transferred 25-μl aliquots of the suspension samples to 96-microwell plates and added 50 μl of specific (1→3)-β-d-glucan lysate. The plates were incubated in an absorbance microplate reader (Infinite M200 microplate reader and Magellan, version 6, software; Tecan Ltd., Männedorf, Switzerland), and the kinetics of the ensuing color reaction were read at 405 nm. The (1→3)-β-d-glucan results were expressed as concentrations (ng/m3 of air) based on the sampling flow rate and the sampling time of the BioSampler. This ratio is also calculated by taking into account the fungal spore number concentrations of fungal bioaerosols during the sampling time. Finally, the (1→3)-β-d-glucan removal rate by the HTST process was obtained from the relative (1→3)-β-d-glucan ratio according to the following formulas:
 |
(3) |
 |
(4) |
SEM analysis.
The morphology of fungal bioaerosols was investigated with the aid of scanning electron microscope (SEM) analysis (XL30S FEG; Phillips, The Netherlands). For this purpose, fungal bioaerosols exposed to various temperatures were sampled onto a 13-mm mixed cellulose ester filter with a pore size of 1.2 μm (Millipore Corporation, MA) downstream from the outlet sampling chamber for 5 min. After the sampling process, the mixed cellulose ester filters were coated with an osmium coater by means of a chemical vapor deposition method (HPC-1SW; Vacuum Device Inc., Japan) and then analyzed with a SEM.
Statistical analysis.
All the experimental data were analyzed statistically in terms of analysis of variance, a t test, and linear regression by using the software package SAS, version 9.1, of Microsoft Windows.
RESULTS
Temperature characteristics.
Figure 2 shows the center temperature distributions of the inner thermal tube under the experimental set temperature (wall temperature) conditions. As shown in Fig. 2, the maximum temperature of each of the center temperature distributions was lower than the set temperature due to thermal heat transfer from the quartz tube wall to the airflow with rapid velocity (thus, the residence time was short). In contrast to the confined thermal systems for food and liquid, a thermal system for a continuous stream of airborne particles cannot, in practice, maintain a single constant temperature uniformly for an entire system because of the fluid flow and the continuous heat transfer in the system. That being the case, we regarded the set temperature as a possible parameter for describing the thermal heat treatment of the bioaerosols.
Aerodynamic size distribution and morphology.
Figure 3 shows the variations in the normalized aerodynamic particle size distributions of A. versicolor and C. cladosporioides fungal bioaerosols under conditions of elevated surrounding temperatures. The aerodynamic diameter, of an aerosol particle is equivalent to the diameter of standard density sphere particles that have the same gravitational settling velocities as the original particles (23). The number concentration of particles in the air, which was recorded for each channel size of the instrument (PSD 3603; TSI Inc., MN), was divided by the logarithmic interval of the corresponding particle size range and plotted as a function of the aerodynamic diameter for the experimental conditions of each fungus. The particle number concentrations were then normalized in terms of the values of the highest concentration under each condition. From this figure, the increase of the surrounding temperature inside the thermal heating tube was found to shift the aerosol particle size distributions of the fungal bioaerosols. As shown in Fig. 3 and 4, the geometric mean aerodynamic diameter (GMD) of the two species of fungal bioaerosols decreased as the surrounding temperature increased, and the geometric standard deviation (GSD) increased as the surrounding temperature increased. Compared with normal temperature conditions (17°C to 21°C), the GMD reduction ratio [1 − (outlet GMD/inlet GMD)] of the A. versicolor fungal bioaerosol was about 7.7% at 400°C and about 34.5% at 700°C. In the case of C. cladosporioides, the GMD reduction ratio was about 9.2% at 400°C and about 30.1% at 700°C. There was little variation in the GSD (<0.6% for A. versicolor and <1.5% for C. cladosporioides) of the fungal bioaerosols until 500°C, and their respective GSD values increased when the temperature exceeded 500°C.
FIG. 3.
Variation in the aerodynamic particle size distribution of A. versicolor and C. cladosporioides with surrounding temperature conditions. N, number concentration of particles in the air; da, aerodynamic diameter; Max, maximum. Temperature is in °C.
FIG. 4.
Variation in the GMD and GSD of A. versicolor (a) and C. cladosporioides (b) with surrounding temperature conditions. The error bars indicate standard deviations (n = 3).
An SEM method was used to observe the morphological changes in fungal particles exposed to high temperatures. Figure 5 shows microphotographs of A. versicolor and C. cladosporioides spores under temperature conditions of 20°C, 400°C, and 700°C. At normal temperature, the A. versicolor spores are spherical and have an echinulate surface structure; the diameters of the spores range from 1.8 μm to 3.5 μm. The C. cladosporioides spores are ellipsoidal to lemon shaped and also have an echinulate surface; they have a length of 2 μm to 5 μm and a width of 2 μm to 4 μm. With SEM analysis we found that the surface morphology of the spores became smoother when the temperature was greater than 400°C.
FIG. 5.
SEM images (magnification, ×30,000) at temperatures of 20°C (a), 400°C (b), and 700°C (c) of A. versicolor spores and at temperatures of 20°C (d), 400°C (e), and 700°C (f) of C. cladosporioides spores. The surface morphology of the sampled fungal particles becomes smoother at elevated temperature conditions.
Thermal inactivation of fungal bioaerosols.
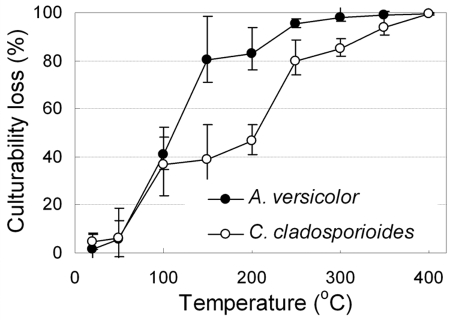
We measured culturability as a parameter of the viability of fungal bioaerosols. Figure 6 shows the culturability loss caused by exposure to high-temperature conditions inside the thermal tube for the fungal bioaerosols of A. versicolor and C. cladosporioides. As shown in Fig. 6, most fungal bioaerosols did not lose their culturability when they passed through the thermal tube at room temperature. This result implies that fungal bioaerosols maintain their culturability while passing through a thermal heating tube at room temperature and that there is negligible physical transport loss through the thermal heating tube.
FIG. 6.
Culturability loss of the A. versicolor and C. cladosporioides bioaerosols in relation to the surrounding temperature. The error bars indicate standard deviations (n = 5).
As the surrounding temperature increased, the culturability of fungal bioaerosols quickly decreased, and the culturability loss curve began to resemble an S-shaped curve. The rate of culturability loss for A. versicolor was the highest at nearly 100°C, representing a culturability loss of approximately 40% for each increase of 50°C. In the case of C. cladosporioides, the high rates of culturability loss were observed at 100°C and 250°C. More than 99% of the A. versicolor and C. cladosporioides bioaerosols lost their culturability at 350°C and 400°C, respectively.
Use of the HTST process to remove (1→3)-β-d-glucan.
Thermal heating is one of the most effective depyrogenation methods. We tested the efficiency of the thermal heating process on the removal of (1→3)-β-d-glucan in A. versicolor bioaerosols. As seen in Fig. 7, an increase in the surrounding temperature produced a significant decline in the A. versicolor (1→3)-β-d-glucan concentration. The removal ratio (equation 4) of (1→3)-β-d-glucan ranged from 4.8% ± 6.11% (200°C) to 32.2% ± 2.27% (700°C). We conducted a linear regression analysis, which showed that the total (1→3)-β-d-glucan removal ratio was significantly correlated with the combined GMD reduction ratio of the fungal particles (P value of <0.05; R2 = 0.9196) (Fig. 8).
FIG. 7.
Removal ratio (percent) of (1→3)-β-d-glucan from the thermal HTST process. The error bars indicate standard deviations (n = 3).
FIG. 8.
Linear regression analysis of the (1→3)-β-d-glucan removal ratio (percent) versus the GMD reduction ratio (percent) by exposure to high temperature conditions. The dashed line is 1:1.
DISCUSSION
The effects of fungal bioaerosols through continuous exposure to various temperature conditions in the HTST process were investigated experimentally. In terms of the variation of aerosol size distribution, while the GMDs of fungal bioaerosols decreased as the surrounding temperature increased, the GSD increased as the surrounding temperature increased, as shown in Fig. 3 and Fig. 4. This fact can be explained by several thermal effects on fungal bioaerosols (36). Specifically, the desiccation and oxidation effects on fungal bioaerosols can be major causes of lower GMD values under high-temperature and dry-air conditions. The evaporation of moisture in the cell membrane and the rapid oxidation process at temperatures greater than 500°C could shrink fungal bioaerosols and decrease the GMDs of fungal bioaerosols. These effects could also broaden the size distribution of fungal bioaerosols. In addition, the radial variation of the temperatures on one cross-section of the system could make a difference in thermal exposure conditions when fungal bioaerosols pass through. We think that this radial temperature variation can be the cause of the GSD increase with the variation in the aerodynamic diameters of fungal bioaerosols. The aerodynamic diameter of bioaerosols, which may differ from their physical size, determines their behavior and transport in the air. Therefore, the particle deposition in the human respiratory system or the filtration efficiency of the ventilation system can be affected seriously by this change in aerodynamic diameter (40). From SEM analysis, we found that the surface oxidation of the fungal spores can lead to a physical decrease in their aerodynamic GMD as well as a chemical reduction in the concentration of (1→3)-β-d-glucan because the oxidation process partially vaporizes and melts materials such as the (1→3)-β-d-glucan of fungal bioaerosols.
As seen in Fig. 6, more than 99% of the A. versicolor and C. cladosporioides bioaerosols lost their culturability at 350°C and 400°C, respectively, in this HTST process. The growth of a microorganism on a material surface can be inactivated by general dry heat treatment at 140°C for approximately 3 h (36), at 180°C for 15 min (6), or at 400°C for 20 s to 30 s (7). However, our results demonstrate that fungal bioaerosols in a continuous airflow can be inactivated by exposure to a high temperature in a thermal electric heating system at 350°C (A. versicolor) and 400°C (C. cladosporioides) for about 0.2 s. This result supports the view that the inactivation conditions for airborne microorganisms differ from those for microorganisms in food or water.
Prolonged exposure to conditions of dry air can affect the viability of microorganisms. The relative humidity in a continuous airflow with an atmospheric pressure condition drops to almost 0% when the temperature exceeds 100°C. Hong et al. (24) used the model to predict the effect of temperature and moisture on various conidia fungi. At a relative humidity of almost 0% and a temperature range of 4°C to 37°C, the time required to yield 50% survival is approximately 10 days to 1,000 days. In our HTST process, the exposure time was always less than 0.3 s. In addition, as shown in Fig. 6, the culturability loss data at 50°C for the two fungal species confirm much lower culturability losses of only 5.5% for A. versicolor and 6.12% for C. cladosporioides. Therefore, for these study conditions, we think the dry-air condition has little effect in terms of desiccation on the viability of fungal spores during brief exposure times; oxidation, on the other hand, has a dominant effect on the viability of fungal spores.
Fungi produce many agents that can be toxic with sufficient exposure. In general, there are two classes of these agents: secondary products of metabolism (e.g., mycotoxins, antibiotics, and volatile organic compounds) and structural components [e.g., (1→3)-β-d-glucan]. In this study, we showed that an increase in the surrounding temperature produced a significant decline in the A. versicolor (1→3)-β-d-glucan concentration. The standard depyrogenation treatment in the pharmaceutical industry for heat-stable materials has been to expose them to temperature conditions greater than 270°C for more than 30 min in a closed system (30). However, in our study, we obtained a (1→3)-β-d-glucan removal ratio of more than 30% by maintaining a temperature of 700°C for only about 0.2 s. Although we could not compare this result to that of previous studies because few studies have been published on the removal of (1→3)-β-d-glucan by an HTST process in a continuous-flow system, this result shows the possibility of rapid removal of (1→3)-β-d-glucan, which is a thermally stable component. In addition, the condition of either a high temperature of more than 700°C or a long exposure time of more than 0.2 s is needed for a (1→3)-β-d-glucan removal ratio of more than 30% in this system. In the data shown in Fig. 8, the correlation between the total (1→3)-β-d-glucan removal ratio and the combined GMD reduction ratio of the fungal bioaerosols shows that the particle GMD reduction ratio can be a sensitive indicator for determining the (1→3)-β-d-glucan removal ratio in the thermal inactivation process.
The presented thermal HTST process was found to be very effective for controlling fungal bioaerosols in continuous airflow. Parameters and mechanisms for the inactivation of airborne microorganisms are useful to meet the increasing need for developing extensive control methods for fungal bioaerosols.
Acknowledgments
This work was supported by a Korea Research Foundation grant funded by the Korean Government (MOEHRD) (KRF-2006-331-D00078) and the Seoul RNBD program. This work was also supported at the Korea Advanced Institute of Science and Technology by the Brain Korea 21 program of the South Korea Ministry of Education Science and Technology.
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Bouakline, A., C. Lacroix, N. Roux, J. P. Gangneux, and F. Derouin. 2000. Fungal contamination of food in hematology units. J. Clin. Microbiol. 38:4272-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge, H. 1990. Bioaerosols: prevalence and health effects in the indoor environment. J. Allergy Clin. Immunol. 86:687-701. [DOI] [PubMed] [Google Scholar]
- 3.Burge, H. A. 2001. Fungi: toxic killers or unavoidable nuisance? Ann. Allergy Asthma Immunol. 87:52-56. [DOI] [PubMed] [Google Scholar]
- 4.Bush, R. K., and J. M. Portnoy. 2001. The role and abatement of fungal allergens in allergic disease. J. Allergy Clin. Immunol. 107:S430-S440. [DOI] [PubMed] [Google Scholar]
- 5.Cooley, J. D., W. C. Wong, C. A. Jumper, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmady, E. M., K. E. A. Hughes, and J. D. Jones. 1958. Thermal death-time of spores in dry heat in relation to sterilization of instruments and syringes. Lancet 272:766-769. [DOI] [PubMed] [Google Scholar]
- 7.Darmady, E. M., K. E. A. Hughes, J. D. Jones, D. Prince, and W. Tuke. 1961. Sterilization by dry heat. J. Clin. Pathol. 14:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker, H. M., F. J. Citek, J. B. Hartsad. N. H. Gross, and F. J. Piper. 1954. Time temperature studies of spore penetration through an electric air sterilizer. Appl. Microbiol. 2:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning, D. W., E. G. V. Evans, C. C. Kibbler, M. D. Richardson, M. M. Roberts, T. R. Rogers, D. W. Warnock, and R. E. Warren. 1997. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 16:424-436. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, L. Gaztelurrutia, J. I. Villate Navarro, and J. L. Rodriguez Tudela. 2000. Genetic similarity among one Aspergillus flavus strain isolated from a patient who underwent heart surgery and two environmental strains obtained from the operating room. J. Clin. Microbiol. 38:2419-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douwes, J., P. Thorne, N. Pearce, and D. Heederik. 2003. Bioaerosol health effects and exposure assessment: progress and prospects. Ann. Occup. Hyg. 47:187-200. [DOI] [PubMed] [Google Scholar]
- 12.Dupont, B., M. Richardson, P. E. Verweij, and J. F. G. Meis. 2000. Invasive aspergillosis. Med. Mycol. 38:215-224. [PubMed] [Google Scholar]
- 13.Dutkiewicz, J. 1997. Bacteria and fungi in organic dust as potential health hazard. Ann. Agric. Environ. Med. 4:11-16. [Google Scholar]
- 14.Ehrlich, R., S. Miller, and R. L. Walker. 1970. Relationship between atmospheric temperature and survival of airborne bacteria. Appl. Microbiol. 19:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine, F., and P. Gervais. 2005. A new high temperature short time process for microbial decontamination of seeds and food powders. Powder Technol. 157:108-113. [Google Scholar]
- 16.Fogelmark, B., J. Thorn, and R. Rylander. 2001. Inhalation of (1→3)-beta-d-glucan causes airway eosinophilia. Mediators Inflamm. 10:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Górny, R. L., T. Reponen, S. A. Grinshpun, and K. Willeke. 2001. Source strength of fungal spore aerosolization from moldy building material. Atmos. Environ. 35:4853-4862. [Google Scholar]
- 18.Gravesen, S. 1979. Fungi as a cause of allergic disease. Allergy 34:135-154. [DOI] [PubMed] [Google Scholar]
- 19.Gregory, P. H. 1973. The microbiology of the atmosphere. Leonard Hill, London, United Kingdom.
- 20.Grigis, A., C. Farina, F. Symoens, N. Nolard, and A. Goglio. 2000. Nosocomial pseudo-outbreak of Fusarium verticillioides associated with sterile plastic containers. Infect. Control Hosp. Epidemiol. 21:50-52. [DOI] [PubMed] [Google Scholar]
- 21.Grinshpun, S. A., G. Mainelis, M. Trunov, A. Adhikari, T. Reponen, and K. Willeke. 2005. Evaluation of ionic air purifiers for reducing aerosol exposure in confined indoor spaces. Indoor Air 15:235-245. [DOI] [PubMed] [Google Scholar]
- 22.Guarro, J., M. Nucci, T. Akiti, J. Gené, J. Cano, C. B. M Da Gloria, and C. Auilar. 1999. Phialemonium fungemia: two documented nosocomial cases. J. Clin. Microbiol. 37:2493-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinds, W. C. 1999. Aerosol technology. John Wiley, New York, NY.
- 24.Hong, T. D., R. H. Ellis, and D. Moore. 1997. Development of a model to predict the effect of temperature and moisture on fungal spore longevity. Ann. Bot. 79:121-128. [Google Scholar]
- 25.Huang, R., I. Agranovski, O. Pyankov, and S. Grinshpun. 2008. Removal of viable bioaerosol particles with a low-efficiency HVAC filter enhanced by continuous emission of unipolar air ions. Indoor Air 18:106-112. [DOI] [PubMed] [Google Scholar]
- 26.Jung, J. H., C. H. Lee, J. E. Lee, J. H. Lee, S. S. Kim, and B. U. Lee. 2009. Design and characterization of a fungal bioaerosol generator using multi orifice air jets and a rotating substrate. J. Aerosol Sci. 40:72-80. doi: 10.1016/j.jaerosci.2008.09.002. [DOI] [Google Scholar]
- 27.Lee, B. U., M. Yermakov, and S. A. Grinshpun. 2004. Removal of fine and ultrafine particles from indoor air environments by the unipolar ion emission. Atmos. Environ. 38:4815-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, T., S. A. Grinshpun, D. Martuzevicius, A. Adhikari, C. M. Crawford, and T. Reponen. 2006. Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmos. Environ. 40:2902-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, Y. H., and B. U. Lee. 2006. Inactivation of airborne E. coli and B. subtilis bioaerosols utilizing thermal energy. J. Microbiol. Biotechnol. 16:1684-1689. [Google Scholar]
- 30.Macher, J. (ed.). 1999. Bioaerosols: assessment and control. American Conference of Governmental Industrial Hygenists, Cincinnati, OH.
- 31.Madelin, T. M. 1994. Fungal aerosol: a review. J. Aerosol Sci. 25:1405-1412. [Google Scholar]
- 32.Madigan, M. T., J. M. Martinko, and J. Parker. 2000. Brock biology of microorganisms. Prentice-Hall, Englewood Cliffs, NJ.
- 33.Mann, A., M. Kiefer, and H. Leuenberger. 2001. Thermal sterilization of heat-sensitive products using high-temperature short-time sterilization. J. Pharm. Sci. 90:275-287. [DOI] [PubMed] [Google Scholar]
- 34.Mullican, C. L., L. M. Buchanan, and R. K. Hoffman. 1971. Thermal inactivation of aerosolized Bacillus subtilis var. niger spores. Appl. Microbiol. 22:557-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicas, M., and S. L. Miller. 1999. A multi-zone model evaluation of the efficacy of upper-room air ultraviolet germicidal irradiation. Appl. Occup. Environ. Hyg. 14:317-328. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, J. J. 1956. Principles and methods of sterilization in health science. Charles C. Thomas, Springfield, IL.
- 37.Philpott-Howard, J. 1996. Prevention of fungal infections in haematology patients. Infect. Control Hosp. Epidemiol. 17:545-551. [DOI] [PubMed] [Google Scholar]
- 38.Piyasena, P., R. C. McKellar, and F. M. Bartlett. 2003. Thermal inactivation of Pediococcus sp. In simulated apple cider during high-temperature short-time pasteurization. Int. J. Food Microbiol. 82:25-31. [DOI] [PubMed] [Google Scholar]
- 39.Proctor, B. E. 1935. The microbiology of the upper air. J. Bacteriol. 30:363-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reponen, T., S. A. Grinshpun, K. L. Conwell, J. Wiest, and M. Anderson. 2001. Aerodynamic versus physical size of spores: measurement and implication for respiratory deposition. Grana 40:119-125. [Google Scholar]
- 41.Richter, S., M. G. Cormican, M. A. Pfaller, C. K. Lee, R. Gingrich, M. G. Rinaldi, and D. A. Sutton. 1999. Fatal disseminated Trichoderma longibrachiatum infection in an adult bone marrow transplant patient: species identification and review of the literature. J. Clin. Microbiol. 37:1154-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley, R. L., M. Knight, and G. Middlebrook. 1976. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. Am. Rev. Respir. Dis. 113:413-418. [DOI] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Rylander, R. 1999. Indoor air-related effects and airborne (1→3)-β-d-glucancan. Environ. Health Perspect. 107(Suppl. 3):501-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmechel, D., R. L. Górny, J. P. Simpson, T. Reponen, S. A. Grinshpun, and D. M. Lewis. 2003. Limitations of monoclonal antibodies for monitoring of fungal aerosols using Penicillium brevicompactum as a model fungus. J. Immunol. Methods 283:235-245. [DOI] [PubMed] [Google Scholar]
- 46.Theunissen, H. J. H., N. A. L. Toom, A. Burggraaf, E. Stoiz, and M. F. Michel. 1993. Influence of temperature and relative humidity on the survival of Chlamydia pneumoniae in aerosols. Appl. Environ. Microbiol. 59:2589-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorn, J., L. Beijer, and R. Rylander. 2001. Effects after inhalation of (1→3)-β-d-glucancan in healthy humans. Mediators Inflamm. 10:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhoeff, A. P., and H. A. Burge. 1997. Health risk assessment of fungi in home environments. Ann. Allergy Asthma Immunol. 78:544-556. [DOI] [PubMed] [Google Scholar]
- 49.Williams, D. L. 1997. Overview of (1→3)-β-d-glucan immunobiology. Mediators Inflamm. 6:247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, P. C., J. C. Tsai, F. C. Li, S. C. Lung, and H. J. Su. 2004. Increased levels of ambient fungal spores in Taiwan are associated with dust events from China. Atmos. Environ. 38:4879-4886. [Google Scholar]
- 51.Yeo, H., and J. Kim. 2002. SPM and fungal spores in the ambient air of west Korea during the Asian dust (yellow sand) period. Atmos. Environ. 36:5437-5442. [Google Scholar]