Abstract
For evaluating N2 fixation of diazotrophic bacteria, nitrogen-poor liquid media supplemented with at least 0.5% sugar and 0.2% agar are widely used for acetylene reduction assays. In such a soft gel medium, however, many N2-fixing soil bacteria generally show only trace acetylene reduction activity. Here, we report that use of a N2 fixation medium solidified with gellan gum instead of agar promoted growth of some gellan-preferring soil bacteria. In a soft gel medium solidified with 0.3% gellan gum under appropriate culture conditions, bacterial microbiota from boreal forest bed soils and some free-living N2-fixing soil bacteria isolated from the microbiota exhibited 10- to 200-fold-higher acetylene reduction than those cultured in 0.2% agar medium. To determine the N2 fixation-activating mechanism of gellan gum medium, qualitative differences in the colony-forming bacterial components from tested soil microbiota were investigated in plate cultures solidified with either agar or gellan gum for use with modified Winogradsky's medium. On 1.5% agar plates, apparently cryophilic bacterial microbiota showed strictly distinguishable microbiota according to the depth of soil in samples from an eastern Siberian Taiga forest bed. Some pure cultures of proteobacteria, such as Pseudomonas fluorescens and Burkholderia xenovorans, showed remarkable acetylene reduction. On plates solidified with 1.0% gellan gum, some soil bacteria, including Luteibacter sp., Janthinobacterium sp., Paenibacillus sp., and Arthrobacter sp., uniquely grew that had not grown in the presence of the same inoculants on agar plates. In contrast, Pseudomonas spp. and Burkholderia spp. were apparent only as minor colonies on the gellan gum plates. Moreover, only gellan gum plates allowed some bacteria, particularly those isolated from the shallow organic soil layer, to actively swarm. In consequence, gellan gum is a useful gel matrix to bring out growth potential capabilities of many soil diazotrophs and their consortia in communities of soil bacteria.
In 1967, Schöllhorn and Burris discovered that nitrogenase from an N2-fixing rhizobium of soybean can reduce acetylene to produce ethylene (C2H4) (32), a reaction analogous to the conversion of the natural substrate N2 into ammonia. Shortly afterwards, it was shown that this acetylene reduction activity parallels N2 reduction by nitrogenase (13), and since then, acetylene reduction assays have been widely used in the evaluation of biological N2 fixation. An acetylene reduction assay is generally performed under the following conditions: precultured bacterial cells are suspended into N-free or -deficient liquid medium containing a carbon source, usually d-glucose or d-mannitol (35) at 0.5 to 2.0%, and exposed for 24 h or less at a representative room temperature, e.g., 25°C (2). However, this method is not applicable to free-living, microaerobic N2-fixing bacteria, which have been regarded as notoriously difficult to culture. To solve this problem, Döbereiner and her group developed a soft gel method (7), which used 0.2% agar as a gel matrix for the medium. Due to a vertical gradient of dissolved oxygen concentrations, these microaerobes formed a thin layer at the particular depth of the medium that contained an ideal level of dissolved oxygen (10). Also, significant activities in acetylene reduction assays were observed for N2-fixing microaerobes, particularly those from the rhizoplane of monocotyledonous crop plants (e.g., Azospirillum and Herbaspirillum spp.) (1, 9, 40). To date, these soft gel media solidified with 0.2% agar have been widely used as the most basic method for the screening of free-living or difficult-to-culture N2-fixing bacteria (2, 16).
In an agar composed of soft gel, however, the layer formation of highly transparent colony-forming bacteria is often obscured and is more difficult to observe than comparable layer formation in water due to the higher turbidity of the agar gel, and some members of the soil bacterial community do not show any positive response in acetylene reduction assays under these conditions. These drawbacks to the usage of agar as a soft gel matrix delayed the recognition that free-living N2 fixers make a potent contribution to the support of ecosystems under adverse soil conditions. Hashidoko et al. developed an improved soft gel medium for growth of N2-fixing bacteria in 2002 (15). In their study, 0.2% agar was replaced with 0.3% gellan gum, a bacterial extracellular polysaccharide (EPS) produced by Sphingomonas elodea (a synonym of Sphingomonas paucimobilis) ATCC 31461 (12, 17, 18). Initially, gellan gum was used for the purpose of preparing a highly transparent soft gel medium that was better for culturing microaerobic N2-fixing rhizobacteria. It had other favorable physical properties: when 0.3% gellan gum containing Winogradsky's mineral mixture was autoclaved, the medium remained in a liquid form over a period of several hours while cooling to room temperature. Even after the gellan gum had been solidified, the soft gel was easily liquefied upon mechanical agitation. The liquefied medium was able to resolidify after a short period of time, so it was easy to uniformly disperse inoculants into the soft gel medium. The outstanding transparency (14) and other properties of this gel matrix enable easy visualization of transparent colony-forming N2-fixing bacteria and also allow observation of their responses to various concentrations of dissolved oxygen and cell motilities (15).
In many preliminary experiments, nitrogen-poor gellan gum media allowed high growth of diazotrophs, but this study was needed to compare gellan gum with agar as a gel matrix for N2 fixation. Because Siberian boreal forest soils have been noted for their low N2-fixing capability (3), we first cultured bacterial microbiota from the eastern Siberian Taiga forest bed in gellan gum medium. A quantitative comparison of N2 fixation behaviors of free-living soil bacteria was attempted to investigate gellan gum as a potential N2 fixation-promoting soft gel matrix. We here first report on the efficacy of gellan gum as a soft gel matrix for monitoring acetylene reduction by the use of free-living N2-fixing soil bacteria.
MATERIALS AND METHODS
Soil sampling method and sites.
Larch forest bed soils were collected in mid-July 2006 from the eastern Siberian Taiga near Yakutsk, Republic of Russia. At the Vilyuy site (located 52 km southwest from the city of Yakutsk) (62°.03′N, 129°.49′E), an organic layer (at a depth of 5 cm) and different depths of defrosted sandy soils (at depths of 10, 20, and 30 cm) were sampled, in accordance with soil differentiation based on compositions and concentrations of organic substances, and these four samples were coded V5, V10, V20, and V30, respectively. Supernatants of soil suspensions (ca. 1 g of soil per 3 ml of sterilized water) were used as inoculants and were kept in 15-ml Falcon tubes stored at −20°C until use.
Inoculation of microfloral communities derived from soil microbiota.
To evaluate the functionality of gellan gum as a soft gel matrix for N2 fixation, a uniform inoculant was prepared as follows: 1 ml of supernatant from the soil suspension described above was inoculated into 100 ml of Winogradsky's mineral mixture base containing 0.3% gellan gum and 0.05% d-mannitol and then incubated at 15°C. After a 14-day incubation, the soft gel medium in which microfloral communities were suitably grown was agitated well with a vortex mixer, and 1 ml of the resulting culture was placed into a 1.5-ml serum tube for storage at −80°C. Approximately 50 tubes of the same culture stock thus prepared were used as a uniform inoculant. For inoculation, the frozen stock was immediately defrosted and 100 μl was used as an inoculant.
Media and vials for acetylene reduction measurement.
A stock of Winogradsky's nitrogen-poor mineral medium was prepared of the following composition: KH2PO4 (50.0 g liter−1); MgSO47H2O (25.0 g liter−1); NaCl (25.0 g liter−1); FeSO4 7H2O (1.0 g liter−1); Na2MoO4 2H2O (1.0 g liter−1); and MnSO4 4H2O (1.0 g liter−1) (plus NaOH to adjust the pH to 7.2) (39 ). Five milliliters of this mixed mineral solution was added to 1,000 ml of a sugar (sucrose, d-mannitol, d-glucose, or dl-malic acid) (0.5 g) and CaCO3 (powder type) (0.1 g) solution. The pH of the solution was adjusted to 6.2 with 2 M H2SO4 (15), and the solution was filtered through a hydrophilic 0.45-μm-pore-size polytetrafluoroethylene membrane (Millipore, Billerica, MA). Gellan gum powder (plant tissue culture grade; Wako Pure Chemical Industries Ltd., Osaka, Japan) (0.3% [wt/vol]) or agar powder (medium grade; Wako Pure Chemical Industries Ltd.) (0.2% [wt/vol]) was also added as a gelling matrix. The medium was then heated to dissolve the gelling material and cooled. The glass vial used for the acetylene reduction assay was a 30-ml gas chromatographic vial (Nichiden-Rika Glass Co., Kobe, Japan) that seals headspace gas with a top-opening screw cap and an inner butyl-rubber plug. After cooling, the resulting medium was poured into the vial to gain exactly 10.00 g (= 10.00 ml) of weight, with the medium intended to be used as a soft gel. The headspace volume (in milliliters) of the glass vials was measured according to the average weight of water (in grams) that filled the headspace of the plugged vials. The headspace volume was almost constant (22.57 ml ± 0.06 standard error; n = 20) and was used to calculate the absolute concentration of C2H4 gas produced in each vial. In this study, the pH of the medium was measured with a Horiba F-22 portable pH meter (Horiba, Kyoto, Japan) equipped with an Orion 8103BN glass electrode (Orion, Beverly, MA) both before and after autoclaving.
Basic conditions for the acetylene reduction assay.
In the acetylene reduction assay, the test bacteria or bacterial mixture was preincubated for 2 weeks at 15°C in the culture vial. After 14 days of preincubation, a 10% volume of acetylene gas was injected into the headspace (22.57 ml) of the glass vial by the use of a gas-tight syringe (Hamilton, Bonaduz, Switzerland) (5 ml), at which point the culture medium (initially pH 6.2) was incubated for an additional 7 days without agitation. The incubation period was determined by measuring the concentration of C2H4 gas in the headspace (every day for 11 days and then every 2 days up to 28 days) by the use of Klebsiella pneumoniae IFO 3318. The passage of linear C2H4 flux into the headspace continued for 20 days in the absence of agitation after the injection of acetylene gas into the vials. In contrast, incubation with agitation before or after the acetylene injection resulted in a fivefold-faster increase of C2H4 production than incubation with no agitation for up to 5 days. After incubation for 8 days, C2H4 levels showed no more fluctuation until the 28-day time point (data not shown). Therefore, reliable culturing conditions and the duration of incubation were set at 7 days without agitation, unless otherwise mentioned.
Fifty microliters of headspace gas containing C2H4 produced from the injection of acetylene gas was analyzed using a Hitachi G-5000 capillary gas chromatograph (Hitachi, Japan) connected to a CP-PoraPLOT U 10-m glass capillary column (Chrompack, Middelburg, The Netherlands) (0.32-mm inside diameter). The analytical conditions consisted of injection at 150°C, detection at 250°C, use of a flame ionization detector for detection, head gas pressure at 60 kPa, and column temperature at 50°C. Representative peak retention times for C2H4 gas and acetylene were 0.90 min and 1.30 min, respectively.
A standard curve used for quantification of C2H4 was generated by an absolute calibration method using a series of absolute concentrations (0.125, 0.25, 0.5, 1.0, 2.5, 5.0, and 10 μl per 50 μl) of standard C2H4 gas. The resulting standard curve equation for the volume of C2H4 gas in the headspace was y = 62x, where peak intensity (y) was correlated to the volume (in nanoliters) of C2H4 (x). By calculating the whole headspace volume in the vial, the absolute nanomole volume of C2H4 is then shown by the equation of z (in nanomoles) = 20.2x. Hence, from the peak intensity calculation, the absolute nanomole volume of C2H4 in the headspace (z) is z = 0.326y, with a regression coefficient of 0.9998. We expressed the acetylene reduction values in micromoles day−1 in 10 ml of medium, unless otherwise mentioned.
Media for bacterial isolation and preculture for acetylene reduction assay.
For the purification of soil bacteria, colonies were streaked repeatedly on MWA (modified Winogradsky's agar medium [Winogradsky's mineral mixture with 1.0% sucrose, 0.005% yeast extract, and 1.5% agar]) plates and incubated at 15°C. For the preculture of each isolate, either plate culture or shaking culture was used. On this MWA, supplemented yeast extract was increased to 0.05%. For rapid growth, a 10-fold-higher amount of yeast extract was added. Isolated N2-fixing bacteria that formed colonies after a 3- or 4-day incubation at 15°C were suspended in sterilized Milli-Q water (Millipore), and the optimal density (OD) was adjusted to 0.5 to 0.7 at 665 nm by the use of a CENios microplate reader (Tecan, Männedorf, Switzerland). Of this bacterial cell suspension, 100 μl was subsequently inoculated into soft gel media for the series of acetylene reduction assays. For quick preculture in liquid medium, bacteria inoculated into 50 ml of a 0.05% yeast extract-containing Winogradsky's mineral solution in which carbon source was replaced with 0.05% d-glucose were precultured for 24 h at 25°C as a rotation-shaken culture (120 rpm). In order to remove residual nitrogen from the medium, the bacterial cell culture was centrifuged (3,500 × g for 10 min); the pellet was resuspended in sterilized water and the OD was adjusted to 0.5 to 0.6 at 665 nm for the inoculant as described above. Beijerinckia indica subsp. indica IFO 3744 was used as a reference bacterium (15).
Identification of isolated bacteria.
Chromosomal DNA was extracted from each pure isolate by the use of an Isoplant II DNA extraction kit (Nippon Gene, Toyama, Japan). With the extracted DNA used as the template, 16S rRNA gene regions were amplified with HotStarTaq (Qiagen, Bothell, WA) or an LA Taq Hot Start version (TaKaRa, Kyoto, Japan) and sequenced by a direct PCR method. The first amplification for the 16S rRNA gene region used universal forward (27f) and reverse (1525r) primers (20). The PCR conditions were as follows: preheating at 97°C for 15 min and 30 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 1 min. For direct PCR sequence analysis, an ABI Prism 310 genetic analyzer was used with a BigDye Terminator version 3.1 cycle sequencing ready-reaction kit (Applied Biosystems, Foster City, CA). Three forward (357f, 926f, and 1112f) and five reverse (327r, 518r, 803r, 1080r, and 1389r) primers were utilized for the direct PCR method (41). Sequence homology for the 1.5-kbp amplicons was searched using the BLAST database program (http://www.ddbj.nig.ac.jp/top-j.html) provided by the DDBJ (DNA Data Bank of Japan; National Institute of Genetics, Mishima, Japan). After analysis, tentatively identified soil bacteria were registered using Sakura, a nucleotide sequence data submission system, through the World Wide Web server at DDBJ. The bacterial isolates obtained from culturing on MWA and for which a 1.5-kbp sequence of the 16S rRNA gene region was fully determined were analyzed by the Clustal W program to determine the molecular phylogeny (21).
Appropriate conditions for the acetylene reduction assay.
As appropriate bacterial culturing conditions for the acetylene reduction assay, we set the other conditions as follows: for the initial medium, pH 6.2, 15°C, and a 7-day incubation period with five replicates unless otherwise mentioned. To determine these conditions, a range of incubation temperatures (5, 10, 15, 20, and 25°C) was generated by the use of a temperature gradient chamber (Nippon Medical & Chemical, Osaka, Japan) and an MLR-350H temperature-integrated growth incubator (Sanyo, Moriguchi, Japan). The various temperature conditions were used in acetylene reduction assays of the Siberian soil bacterial communities and of a reference strain, B. indica subsp. indica, with gellan gum medium containing 0.05% d-mannitol as the carbon source (see Fig. S1 in the supplemental material). Furthermore, 0.05% d-sucrose, 0.05% d-glucose, and 0.05% dl-malic acid were tested with the gellan gum medium as alternative carbon sources.
Three different types of media were prepared to investigate the effects of the use of gellan gum gel on the acetylene reduction of the tester soil microfloral communities. Winogradsky's mineral mixture containing 0.05% mannitol was prepared as (i) liquid medium, (ii) soft gel medium solidified with 0.2% agar, and (iii) soft gel medium solidified with 0.3% gellan gum. Both of the solidified concentrations for the gel matrix were set as previously reported and were generally used in the standard manner (9, 10, 15). These assays were performed with or without the use of the gel matrix under conditions identical to those described above.
Soil bacteria isolated on MWA containing 1.0% sucrose (see Table 2) were precultured on MWA plates to prepare a bacterial cell suspension, and each isolate was assayed for acetylene reduction in the presence of an alternative carbon source (0.05% of d-glucose, d-sucrose, dl-malic acid, or d-mannitol) containing gellan gum medium. The incubation conditions were as follows: after 14 days of preincubation at 15°C in the dark, 10% acetylene gas was injected and the cultures were further incubated for 7 days without agitation. After the second incubation, the C2H4 concentration in the headspace gas was measured by gas chromatographic analysis.
TABLE 2.
Identification of soil bacteria cultured on MWA containing 1.0% sucrosea
| Isolate | Bacterial species isolated from soil sample:
|
|||
|---|---|---|---|---|
| V5 | V10 | V20 | V30 | |
| A1 | Pseudomonas sp. | Pseudomonas sp.b | Burkholderia sp. | Pseudomonas sp. |
| A2 | Burkholderia sp. | Pseudomonas sp.b | NIc | Pseudomonas sp.b |
| A3 | Pseudomonas sp. | Rhizobium sp.b | Pseudomonas sp.b | Burkholderia sp.b |
| A4 | Pseudomonas sp. | Pseudomonas sp.b | Pseudomonas sp.b | Burkholderia sp.b |
| A5 | Pseudomonas sp. | Pseudomonas sp.b | Pseudomonas syringaeb | Pseudomonas fluorescens sp.b |
| A6 | Pseudomonas sp. | Pseudomonas sp.b | Pseudomonas sp.b | Burkholderia xenovoransb |
| A7 | Pseudomonas sp. | Rhizobium sp.b | Pseudomonas sp.b | Burkholderia xenovoransb |
| A8 | NI | Pseudomonas sp.b | Burkholderia xenovoransb | Burkholderia sp. |
| A9 | Rhizobium sp. | Pseudomonas sp.b | Burkholderia sp.b | |
| A10 | Pseudomonas sp. | Pseudomonas syringaeb | Burkholderia sp. | |
| A11 | NI | Janthinobacterium sp.b | ||
| A12 | Actinobacteria sp.b | |||
| A13 | Actinobacteria | |||
| A14 | NI | |||
| A15 | Pseudomonas sp.b | |||
| A16 | Pseudomonas sp. | |||
Individual isolates from each of the four soil samples examined were numbered independently. For purification, modified Winogradsky's medium solidified with 1.5% agar was used. As many bacteria as possible that were distinguishable in the culture were isolated from plates incubated at 15°C in the dark.
The designated isolates have had 1.5 kbp of their 16S rRNA gene regions sequenced, while unmarked isolates have had approximately 400 bp containing the V6 region of their 16S rRNA gene sequenced.
NI, not identified.
Plate culture of soil bacteria on gels composed of agar or gellan gum.
Winogradsky's mineral solution supplemented with 1.0% sucrose (Wako Pure Chemical Industries Ltd.) and 0.005% yeast extract (Difco, Sparks, MD) was solidified with 1.5% agar (MWA) or 1.0% gellan gum (MWG). As an alternative carbon source for the solid media, dl-malic acid plus d-glucose (both 0.25%) instead of 1.0% sucrose (MWA′ and MWG′) was tested. Precultured soil bacteria in gellan gum medium maintained at −80°C were directly plated as a 100-μl aliquot, and the resulting plates were incubated at 15°C for 7 days in the dark. A total of 10 to 18 major and distinguishable colonies grew on MWA, and these bacterial colonies newly obtained from several depths of soil were subjected to the isolation process. A resulting single colony from each sample was purified again on new plates. In contrast, MWG′ plates allowed the growth of slimy and transparent bacterial colonies. Hence, the slime was spread over new MWG′ plates. Single colonies that had appeared on the plates were subsequently repurified on MWG′ plates. This purification process was repeated several times until uniform colonies were finally obtained. We gave the pure isolates appearing on MWA (MWA-culturable bacteria) the code A with a number following (e.g., A1, A2, and A3), while those that primarily spread on MWG (MWG-culturable bacteria) that were then purified on MWG were coded G with a number following (e.g., G1, G2, and G3). When the alternative carbon source (0.25% d-glucose plus 0.25% dl-malic acid) was used, the bacterial isolates were identified by the use of a prime (e.g., G′1, G′2, and G′3).
Swarming assay on MWA and MWG.
A swarming assay was done for all the bacterial isolates from different depths of Siberian soil by the use of MWA or MWG with agar or gellan. To examine the effect of the gel matrix and its concentrations on bacterial swarming, the gel matrix concentration was set at 1.0 or 1.5% with 1.0% sucrose as the common carbon source in the test media. A 1-μl bacterial cell suspension of each isolate was inoculated by the use of a 10-μl micropipette onto MWA or MWG as 38 to 45 spots per plate and then incubated at 15°C for 36 h. The maximal edge-to-edge dimensions of the colony were measured on the dish with a CD-15GS electric vernier caliper (Mitutoyo, Kanagawa, Japan), and then a digital photograph was taken. The original inoculation spots were approximately 2.6 mm in diameter.
RESULTS
Evaluation of gellan gum as a soft gel matrix for acetylene reduction assays.
In an acetylene reduction assay using two different gel matrices (gellan gum and agar) or no matrix (an unsolidified liquid), gellan gum gel matrix (at a 0.3% concentration) showed the effects on acetylene reduction of the V5, V10, V20, and V30 soil microbial biome (Table 1). A 10-fold increase in acetylene reduction in the gellan gum medium was observed even with B. indica subsp. indica IFO 3744, which was used as a reference bacterium. Despite its similar physicochemical properties and its wide acceptance as a gel matrix for acetylene reduction, agar (0.2%) did not show any significantly different effect on acetylene reduction compared to liquid medium. The effect of the use of gellan gum is not specific to Siberian soil, because Shizunai (Hokkaido, Japan) farmland soil showed a similar tendency (see Table S1 in the supplemental material).
TABLE 1.
Effect of gellan gum on acetylene reduction of bacterial microbiota from Siberian soila
| Source | Activity (nmol day−1 vial−1) of C2H4 on indicated medium (0.05% d-mannitol-containing Winogradsky's mineral mix)
|
||
|---|---|---|---|
| Liquid | 0.2% agar soft gel | 0.3% gellan gum soft gel | |
| V5 | 4.8 ± 0.4 | 5.1 ± 0.5 | 47.1 ± 46.1 |
| V10 | 4.6 ± 2.5 | 4.6 ± 1.5 | 283.0 ± 27.4 |
| V20 | 5.3 ± 0.5 | 4.9 ± 1.5 | 482.6 ± 47.1 |
| V30 | 5.0 ± 1.2 | 4.3 ± 1.1 | 1041.4 ± 216.4 |
| B. indica subsp. indica | 5.4 ± 1.8 | 11.2 ± 2.6 | 117.9 ± 7.7 |
After being subjected to preculturing conditions for 14 days, acetylene gas was injected to the headspace of each culture vial at a 10% (vol/vol) concentration, and the culture was further incubated for 7 days at 15°C. Three different conditions (liquid, 0.2% agar, 0.3% gellan gum) for Winogradsky's medium (with 0.05% d-mannitol as the carbon source) were tested. B. indica subsp. indica IFO3744 was used as a positive reference. Values represent the means and standard deviations of C2H4 production determined for five replicates.
Isolation, identification, and acetylene reduction assay of bacterial isolates from boreal soil.
Samples V5, V10, V20, and V30, all of which displayed high levels of acetylene reduction when cultured on gellan gum medium, were spread over MWA plates. After incubation at 15°C, colonies of several oligotrophic and diazotrophic bacteria appeared on the plates. From the master plates of V5, V10, V20, and V30, a total of 8, 10, 16, and 11 distinguishable bacterial isolates were obtained, respectively. These bacterial isolates from sandy Siberian soil were identified by means of sequencing of their 16S rRNA gene regions (Tables 2, 3, and 4) and were diverse in bacterial species, as determined on the basis of their clustering in a phylogenetic tree made using Clustal W software (21). Almost all colonies isolated from MWA were composed of either Pseudomonas or Burkholderia species, but DNA sequence alignments for each group showed that each exhibited high diversity at the species level (see Fig. S1 in the supplemental material).
TABLE 3.
Identification of soil bacteria cultured on MWG containing 1.0% sucrosea
| Isolate | Bacterial species isolated from soil sample:
|
|||
|---|---|---|---|---|
| V5 | V10 | V20 | V30 | |
| G1 | Pseudomonas sp. | NI | Arthrobacter sp. | Pseudomonas sp.b |
| G2 | Pseudomonas sp. | NI | Pseudomonas sp.b | Pseudomonas sp.b |
| G3 | Pseudomonas sp. | Pseudomonas sp. | NI | Pseudomonas sp.b |
| G4 | Pseudomonas sp. | NI | Pseudomonas sp.b | Pseudomonas sp.b |
| G5 | Pseudomonas sp. | NI | Arthrobacter sp.b | Pseudomonas sp.b |
| G6 | Pseudomonas sp. | NI | Paenibacillus sp.b | Janthinobacterium sp.b |
| G7 | Pseudomonas sp.b | Pseudomonas sp. | Arthrobacter sp. | Janthinobacterium sp.b |
| G8 | Pseudomonas sp.b | Pseudomonas sp. | Arthrobacter sp.b | Pseudomonas sp.b |
| G9 | Pseudomonas sp. | Pseudomonas sp.b | Pseudomonas sp.b | |
| G10 | Pseudomonas sp. | Pseudomonas sp. | ||
| G11 | NI | |||
Conditions and methods for isolation of bacteria on MWG were the same as for Table 2, except that 1.0% gellan gum was used for gel matrix. As many bacteria as possible that were distinguishable in the culture were isolated from plates incubated at 15°C in the dark.
The designated isolates have had 1.5 kbp of their 16S rRNA gene regions sequenced, while unmarked isolates have had approximately 400 bp containing the V6 region of their 16S rRNA gene sequenced. NI, not identified.
TABLE 4.
Identification of soil bacteria cultured on MWG′ containing 0.25% d-glucose and 0.25% dl-malic acida
| Isolate | Bacterial species isolated from soil sample:
|
|||
|---|---|---|---|---|
| V5 | V10 | V20 | V30 | |
| G′1 | Stenotrophomonas sp.b | Paenibacillus pabulib | Paenibacillus pabulib | Luteibacter rhizovicinusb |
| G′2 | Pseudomonas sp.b | Pseudomonas sp.b | Paenibacillus borealisb | Luteibacter rhizovicinusb |
| G′3 | Pseudomonas sp.b | Paenibacillus pabuli | Arthrobacter sp. | Luteibacter rhizovicinusb |
| G′4 | Janthinobacterium agaricidamnosumb | Pseudomonas sp.b | Arthrobacter sp.b | Actinobacterium sp. |
| G′5 | Pseudomonas collierea sp.b | Pseudomonas sp.b | Paenibacillus borealisb | Burkholderia xenovoransb |
| G′6 | Janthinobacterium agaricidamnosumb | Pseudomonas sp.b | Paenibacillus borealisb | Luteibacter rhizovicinus |
| G′7 | Cupriavidus campinensisb | Paenibacillus pabulib | Paenibacillus sp.b | Burkholderia xenovoransb |
| G′8 | Pseudomonas sp. | Pseudomonas sp.b | Paenibacillus sp. | Luteibacter sp.b |
| G′9 | Pseudomonas sp.b | Pseudomonas sp.b | Luteibacter rhizovicinusb | |
| G′10 | Pseudomonas sp.b | Pseudomonas sp.b | Luteibacter rhizovicinusb | |
| G′11 | Janthinobacterium lividumb | Paenibacillus pabulib | Luteibacter sp.b | |
| G′12 | Janthinobacterium agaricidamnosumb | Paenibacillus pabulib | Arthrobacter sp. | |
| G′13 | Janthinobacterium agaricidamnosumb | |||
Conditions and methods for isolation of bacteria on MWG were the same as those described for Table 3, except 0.25% d-glucose plus 0.25% dl-malic acid was used as the carbon source. As many bacteria as possible that were distinguishable in the culture were isolated from plates incubated at 15°C in the dark.
The designated isolates have had 1.5 kbp of their 16S rRNA gene regions sequenced, while unmarked isolates have had approximately 400 bp containing the V6 region of their 16S rRNA gene sequenced.
Forty-five isolates from MWA were individually tested for preliminary acetylene reduction in gellan gum medium with four different carbon sources at a 0.05% concentration. In the 0.05% d-glucose-supplemented medium, of the 45 resulting bacterial isolates, those coded V20-A8, V20-A9, V20-A10, V30-A5, V30-A6, and V30-A7 showed particularly clear positive responses comparable with the positive-control results in the acetylene reduction assay (0.1 to 0.6 μmol day−1).
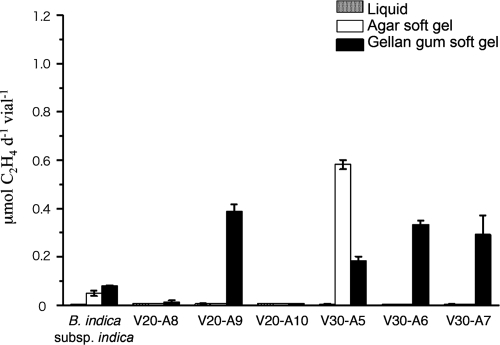
In dl-malic acid-containing medium, isolate V30-A2 uniquely showed acetylene reduction. Subsequent acetylene reduction assays for V20-A8, V20-A9, V20-A10, V30-A5, V30-A6, and V30-A7 were performed in 0.05% d-glucose-containing media with or without matrices, with five replicates (Fig. 1). Four isolates, V20-A9, V30-A5, V30-A6, and V30-A7, showed a significant level of acetylene reduction in 0.3% gellan gum. These measured activities were all higher than that seen with B. indica subsp. indica IFO 3744 grown under the same conditions. V30-A5 was the single exception of a N2-fixing bacterium that showed acetylene reduction in agar approximately 2.5-fold higher than that seen with gellan gum medium.
FIG. 1.
Acetylene reduction of some isolated bacteria in soft gel media. Isolated bacteria that showed acetylene reduction in the preliminary assay represented by Fig. 1 were precultured in liquid medium to prepare each inoculant. Culturing and assay conditions were the same with those as described for Table 1, except glucose was used as the carbon source. Five replicates were performed for the acetylene reduction. B. indica subsp. indica IFO3744 was used as a positive reference. Error bars at the top of each column indicate the standard deviations. d, day.
Among these acetylene reduction assay-positive isolates, three Burkholderia sp. isolates (V20-A8, V30-A6, and V30-A7) closely resembled Burkholderia xenovorans (99.8% homology with Burkholderia xenovorans LB400 and B2-5, of accession no. CP000270 and EF467847 in GenBank, respectively). On the other hand, isolates V20-A9, V20-A10, V30-A2, and V30-A5 were tentatively identified as Pseudomonas spp. (Table 2). In particular, V20-A10 and V30-A2 were closely related (98.5% homology) to some P. syringae pathovars (e.g., DDBJ accession no. AB001439 and GenBank accession no. AE016853) but are more likely new species. They corresponded highly (with 99.5% homology) to a cryophilic Antarctic bacterium of unknown species, Pseudomonas sp. strain R-9113 (EMBL accession no. AJ441004). The accession numbers of isolates V20-A8 (Burkholderia xenovorans), V20-A9 (Pseudomonas sp.), V20-A10 (P. syringae), V30-A2 (Pseudomonas sp.), V30-A5 (P. fluorescens), V30-A6 (Pseudomonas sp.), and V30-A7 (Pseudomonas sp.), as characterized by 1.5 kbp of a determined sequence, are AB379686, AB379687, AB379688, AB379689, AB379690, AB379691, and AB379692, respectively.
Along with acetylene reduction characteristics, the cell growth performance of some representative N2-fixing isolates (V20-A8, V20-A9, V30-A5, V30-A6, and V30-A7) in the soft gel media was also measured, using a microplate reader at 665 nm (see Fig. S3 in the supplemental material). The results for some bacteria, particularly those cultured in gellan gum, exhibited a slight positive correlation between OD and acetylene reduction. V20-A9 (Pseudomonas sp.) showed a relatively low OD (<0.07 in 10 mm), but it showed relatively high acetylene reduction in gellan gum medium (0.39 μmol day−1 vial−1). In contrast, V30-A6 (Burkholderia xenovorans), which grew uniquely well in agar and gellan gum media, exhibited the highest acetylene reduction results among the tested bacteria.
The results for bacteria grown on MWA′ plates on which 1.0% sucrose was replaced with 0.25% glucose plus 0.25% dl-malic acid are shown in Table S2 in the supplemental material. On this agar plate, many actinobacteria appeared, particularly from samples V20 and V30, along with Pseudomonas spp. of gammaproteobacteria from V5 and V10 and Burkholderia spp. of betaproteobacteria from V10 and V30.
Comparisons of microbial biomes grown on plates solidified with gellan gum and agar.
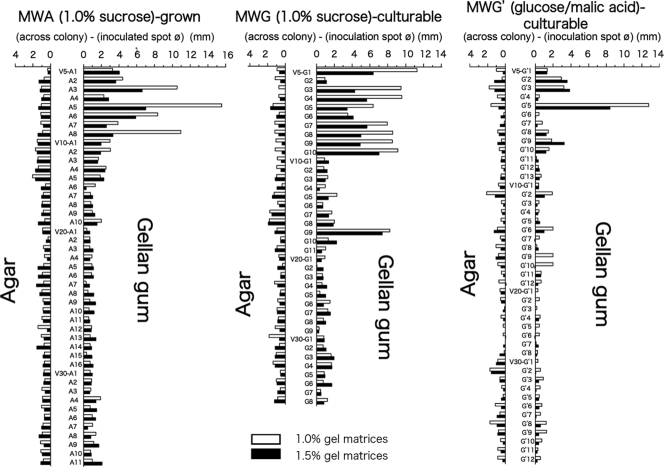
Soil microfloral samples of samples V5, V10, V20, and V30 precultured in gellan gum medium were spread over 1.0% sucrose-containing MWG plates on which the 1.5% agar was replaced with 1.0% gellan gum and were isolated using the same procedures used for those prepared on MWA. After incubation at 15°C for 48 h, hundreds of colonies of oligotrophic bacteria appeared. From the master plates of V5, V10, V20 and V30, respectively, 10, 11, 9, and 8 distinguishable bacterial colonies were obtained as isolates. All the isolates that had emerged on the gellan gum plates were also identified by partial (over 400-bp) sequence determination of the 16S rRNA gene, including the V6 region, and it became obvious that the bacteria grown on the agar plates and on the gellan plates were utterly different from each other despite the use of the same composition of the medium, except for the gelling material used for solidification (Table 3). In particular, none of the Burkholderia species grew and formed visible colonies, but two Janthinobacterium spp. were obtained as new bacterial components from the MWG plates. In V20, two Arthrobacter species of the actinobacteria uniquely appeared, while many unidentified bacteria, including some actinobacteria, were obtained from sample V10 from among those culturable on gellan gum plates.
On the other hand, MWG′ in which the 1.0% sucrose was replaced with a mixture of 0.25% dl-malic acid and 0.25% glucose allowed the emergence of different bacterial components. The numbers of distinct bacterial colonies isolated from the master plates of samples V5, V10, V20, and V30 were 13, 12, 8, and 12, respectively. By means of sequencing the 16S rRNA gene, it was found that these MWG′-culturable bacteria were much more diverse than those grown with 1.0% sucrose as the single carbon source (see Table 4 and Fig. S1B in the supplemental material). In fact, gellan gum plates allowed several unique bacterial species, including Luteibacter spp. from V30 and Janthinobacterium spp. from V5, to grow on the solid medium consisting of MWG with 1.0% sucrose. Unlike the soil bacteria grown on MWA plates and identified as either Pseudomonas spp. or Burkholderia spp. as shown in Table 2, soil samples from four different depths and cultured on gellan gum plates appeared to include clearly segregated microbiota (e.g., Janthinobacterium spp. and another group of Pseudomonas sp. bacteria from V5, more different groups of Pseudomonas spp. from V10, Paenibacillus spp. from V10 and V20, an Arthrobacter sp. from V20, and a Luteibacter sp. from V30). All the isolates were individually tested for acetylene reduction in gellan gum medium with 0.05% of alternative carbon sources, e.g., glucose, sucrose, mannitol, or dl-malic acid. Only Burkholderia sp. strain V30-G′7 showed a weak positive response (0.1 μmol day−1) among MWG′-culturable bacteria in 0.05% glucose-containing medium, which was comparable with the positive-control results seen in this acetylene reduction assay.
Swarming ability of bacterial isolates on MWA and MWG.
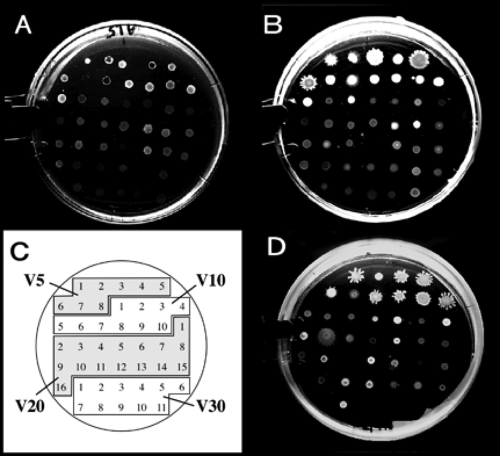
As shown in Fig. 2 and 3, swarming induction was observed only for bacterial isolates from samples V5 and V10 among the MWA-grown, MWG-grown, and MWG′-culturable bacteria. In particular, eight out of nine isolates of MWG′-culturable bacteria from V5 displayed active swarming ability on MWG plates solidified with 1.0 and 1.5% gellan gum. Colonies of swarming bacteria tended to spread more effectively on a softer gellan gum plate (1.0% gellan gum) than on 1.5% gellan gum. In contrast, bacteria that had exhibited swarming ability on MWG showed no swarming on MWA plates, even under conditions that included 1.0% agar. Among the MWA-grown bacteria, four Pseudomonas isolates out of eight from V5 showed potent swarming properties (Fig. 3, left panel). In the swarming assay, MWG-grown Pseudomonas isolates from V5 showed a similar swarming reaction only on MWG plates (Fig. 3, center panel). Bacteria isolated from glucose- and malic acid-supplemented MWG′ also tended to show similar activity; in particular, some Pseudomonas spp. showed a characteristic swarming reaction specifically on the assay plates solidified with 1.0% and 1.5% gellan gum (Fig. 3, right panel).
FIG. 2.
Colonies of the tester MWA-grown and MWG-culturable bacteria on agar or gellan plates. (A and B) Swarming assay after a 40-h incubation of MWA-grown bacteria on a 1.5% agar plate (A) and on a 1.5% gellan gum plate (B) of modified Winogradsky's mineral mixture supplemented with 1.0% sucrose. Note that the bacteria represented by panel B grew better than those represented by panel A. (C) Key showing V5-A1 and other isolates spotted as shown in panels A and B. (D) Bacteria culturable on 1.0% sucrose-containing MWG after inoculation on a 1.5% gellan gum plate containing 1.0% sucrose. The plate surfaces were not wet, so swarming on MWG seemed to be caused by the gellan gum. The assay conditions were the same as for panels A and B.
FIG. 3.
Swarming activity of soil bacteria on 1.0% sucrose-containing MW plates solidified with agar or gellan gum. Tester bacteria isolated from soils and grown on MWA and MWG supplemented with 1.0% sucrose (Tables 2 and 3) or MWG′ supplemented with 0.25% glucose plus 0.25% dl-malic acid (Table 4) were precultured on MWA for preparation of a bacterial cell suspension in a small volume of sterilized water. A 1-μl aliquot of the suspension was inoculated onto a 1.0% sucrose- and 0.005% yeast extract-containing Winogradsky's mineral mixture solidified with a 1.0% or 1.5% gel matrix (agar or gellan gum). The inoculated plates were cultured for 36 h at 15°C in the dark. The spotted diameter of the inoculants was 2.6 mm, and swarming after the incubation period was measured according to the maximum width of the colony that developed. Values represent the differences (in millimeters) of the developed-colony diameters from the spotted diameters of the inoculants.
DISCUSSION
Gellan gum has advantageous physical properties in culturing free-living N2-fixing bacteria, as reported previously (15), and rarely prevents DNA extraction or subsequent PCRs of isolates from colonies grown in the gelled medium, unlike agar (11, 29). Furthermore, some soil bacteria that were difficult to culture or that grew slowly from sediment microbiota became culturable on gellan gum medium, and the colonies grew until they became visible (38). Such a growth-promoting effect of gellan gum on the tester bacterial isolates and communities gave rise to the idea that the drastic increase in acetylene reduction of the N2-fixing bacteria cultured in gellan gum is simply due to better growth (4, 36, 37). In fact, B. indica subsp. indica IFO 3744 and Burkholderia sp. isolate V30-A5, which both showed high acetylene reduction in 0.05% glucose-containing agar soft gel media, also grew well in the agar medium (see Fig. S3 in the supplemental material). These observations gave rise to speculation about the functionality of gellan gum. However, Burkholderia xenovorans V20-A8, which exhibited low acetylene reduction (<0.02 μmol day−1 vial−1), showed rather high growth (approximately the same level as V30-A5 in agar medium); hence, the results seen with this bacterial isolate likely indicate that not only high cell growth but also another factor is a necessary condition for the higher acetylene reduction of N2-fixing bacteria.
As the MWG′-culturable bacteria were diversified in genera and were characteristic of each soil depth, these bacteria are surely part of the majority of the soil bacterial microbiota. Thus, gellan gum conclusively exhibited growth-promoting activity for some environmental bacteria that were hardly culturable on agar plates, as reported in previous bacterial studies (4, 37). Bacteria grow on an MWA′ plate supplemented with 0.25% glucose plus 0.25% dl-malic acid showed a different composition pattern from those maintained on MWG′ containing the different gel matrix and were also distinguishable in bacterial composition from those grown on MWA with 1.0% sucrose as an alternative carbon source. This result showed that gellan gum, a gel material for solid plates, contributed much more to the diversity of emerging bacteria than the alternative carbon sources in solid media. In contrast, almost none of the MWG′-culturable isolates showed acetylene reduction under the same assay conditions as those used for MWA-grown bacteria. This result seemed to be contradictory in light of the aforementioned effect of gellan gum on N2-fixing bacteria, because while the use of gellan gum enhanced the acetylene reduction of N2-fixing bacteria, those species that grew preferably on gellan gum plates did not show any acetylene reduction.
Another important aspect of the findings is that N2-fixing Pseudomonas spp. that had grown well on MWA plates were also able to grow well on the gellan gum plates, while most of the MWG′-culturable bacteria grew slowly on MWA and some were unable to grow on the solid medium (Fig. 2). It was therefore suggested that some MWG′-culturable bacteria comprising bacterial microbiota of V5 and V30 soils play a role as helper or stimulus-generating bacteria for the free-living N2-fixing bacteria in soil microbiota (25, 28, 32). We subsequently hypothesized that it is not a single bacterial isolate but is rather rather bacterial consortia that exhibit capabilities of N2 fixation in the soil, as is similar to other cases of bacterial consortia previously reported (14, 25, 38). Some of these MWG′-culturable bacteria may provide a good model of bacterial consortia for efficient N2 fixation in bulk soil.
Among the bacteria isolated on MWA and MWG′, only Pseudomonas spp. isolated from V5 soil clearly showed swarming induction on gellan gum plates (Fig. 3, left and right panels). None of the N2-fixing bacteria that were isolated from V20 and V30 soils showed any swarming activity, even on MWG plates. The mechanisms controlling why and how gellan gum induces swarming are not yet known. In some reports (5, 24), it was indicated that swarming induction is highly linked to the bacterial quorum sensing system, signals of which also trigger biofilm formation, so certain growth-sensing signal responses may be involved in this phenomenon. However, it is true that use of the biogel material led to direct or indirect acceleration of N2 fixation. Swarming induction by gellan gum is therefore likely to be a key factor for understanding the functionality of this gel material. Since bacterial mixtures often tended to exhibit higher acetylene reduction than pure isolates derived from them in our experiments, cell-to-cell signaling probably plays an important role in N2 fixation of the bacterial consortium.
In general, EPS or other extracellular polymeric substances of bacteria are often a basic matrix for bacterial biofilms (6, 27, 34), and indeed, gellan gum from Sphingomonas elodea ATCC 31461 is one of them (18). In the process of biofilm development, fibrous EPS on the bacterial cell surface make bacterial cells combine, and then the excess volume of EPS gels enfolds the bacterial cell aggregates to form an immature biofilm (6). Mature biofilms generally exert specified functionalities in bacterial communities, such as antibiotic production and xenobiotic degradation (5, 6, 24), and in studies of biofilms of free-living Bradyrhizobium japonicum, activation of N2 fixation has also been reported (33). Recently, it has been reported that a linear type (R)-3-hydroxybutyrate tetramer produced by a Sphingomonas sp. showed a growth-promoting effect on some bacterial cells that was similar to the effect seen with gellan gum (28). It is widely known that (R)-3-hydroxybutyrate polymer is also an extracellular polymeric substance produced by many N2-fixing bacteria (23, 31). Therefore, the growth-promoting activity of gellan gum and (R)-3-hydroxybutyrate tetramer may act on cell growth and promotion for eubacteria by similar mechanisms.
The “missing link of nitrogen” in boreal forests (8) has been discussed for a long time, since boreal forest soil generally exhibits an unexpectedly low level of acetylene reduction (30). However, tester soil bacteria cultured in gellan gum medium exhibited higher acetylene reduction under specified culture conditions. This phenomenon suggested that appropriate environmental conditions allow some soil bacteria to exert potent activity linked to their proper contribution to nitrogen cycling in a vast terrestrial ecosystem, the Taiga forest in eastern Siberia. Gellan gum may provide a microenvironment in the culture medium similar to the soil environment for bacterial consortia of cryophilic N2-fixing bacteria and N2 fixation helpers. Further studies are hence needed to reveal the functionalities of gellan gum and MWG′-culturable bacteria. Thus, a gellan gum gel matrix providing appropriate conditions for N2 fixation to soil microfloral communities would be a powerful tool for investigation of the real potential of such climatically adverse soil and bacterial microbiota (22).
A trend in microfloral analysis in soil is to use denaturing gradient gel electrophoresis (19, 26, 42) and one or more of the many available useful kits to extract DNA from soils. In this study, we investigated and obtained culturable bacteria from the soil samples. Complex effects of bacterial combinations on N2 fixation could be demonstrated by using some characteristic and representative isolated bacteria. Optimal combinations of these isolated soil bacteria will likely assist in construction of a model for the cryophilic soil ecosystem and in understanding the nitrogen cycle in a boreal forest ecosystem.
Supplementary Material
Acknowledgments
We thank F. Takakai and A. Desyatkin (Laboratory of Soil Science, Graduate School of Agriculture, Hokkaido University) for their technical advice and assistance with the field investigation in Yakutsk, Republic of Russia.
This research work was supported by Grants-in-Aid for Scientific Research A (16208032 and 20255002 to Y.H.) from the Japan Society for the Promotion of Science and also by the CTC (core-to-core) program between Hokkaido University and Martin Luther University, Halle-Wittenberg, Germany (project 17001, granted to R.H.).
Footnotes
Published ahead of print on 13 March 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baldani, J. I., L. Caruso, V. L. D. Baldani, S. R. Goi, and J. Döbereiner. 1997. Recent advances in BNF with non-legume plants. Soil Biol. Biochem. 29:911-922. [Google Scholar]
- 2.Barraquio, W. L., J. K. Ladha, and I. Watanabe. 1983. Isolation and identification of N2-fixing Pseudomonas associated with wetland rice. Can. J. Microbiol. 29:867-873. [DOI] [PubMed] [Google Scholar]
- 3.Binkley, D., Y. Son, and D. W. Valentine. 2000. Do forests receive occult inputs of nitrogen? Ecosystems 3:321-331. [Google Scholar]
- 4.Chandrasekaran, R., and A. Radha. 1995. Molecular architectures and functional-properties of gellan gum and related polysaccharides. Trends Food Sci. Technol. 6:143-148. [Google Scholar]
- 5.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 6.Crossman, L., and J. M. Dow. 2004. Biofilm formation and dispersal in Xanthomonas campestris. Microb. Infect. 6:623-629. [DOI] [PubMed] [Google Scholar]
- 7.Day, J. M., and J. Döbereiner. 1976. Physiological aspects of N2-fixation by a Spirillum from Digitaria roots. Soil Biol. Biochem. 8:45-50. [Google Scholar]
- 8.DeLuca, T. H., O. Zackrisson, M.-C. Nilsson, and A. Sellstedt. 2002. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917-919. [DOI] [PubMed] [Google Scholar]
- 9.Döbereiner, J. 1988. Isolation and identification of root associated diazotrophs. Plant Soil 110:207-212. [Google Scholar]
- 10.Döbereiner, J. 1995. Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants, p. 134-141. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, London, United Kingdom.
- 11.Gibb, A. P., and S. Wong. 1998. Inhibition of PCR by agar from bacteriological transport media. J. Clin. Microbiol. 36:275-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding, N. E., Y. N. Patel, and R. J. Coleman. 2004. Organization of genes required for gellan polysaccharide biosynthesis in Sphingomonas elodea ATCC 31461. J. Ind. Microbiol. Biotechnol. 31:70-82. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, R. W. F., R. D. Holsten, E. K. Jackson, and R. C. Burns. 1968. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 43:1185-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashidoko, Y., Y. Gotou, M. Osaki, E. Purnomo, S. H. Limin, and S. Tahara. 2006. Characterization and ecological role of free-living nitrogen-fixing bacteria isolated from the rhizoplane of Melastoma malabathricum inhabiting acidic plain lands in Kalimantan. Tropics 15:365-369. [Google Scholar]
- 15.Hashidoko, Y., M. Tada, M. Osaki, and S. Tahara. 2002. Soft gel medium solidified with gellan gum for preliminary screening for root-associating, free-living nitrogen-fixing bacteria inhabiting the rhizoplane of plants. Biosci. Biotechnol. Biochem. 66:2259-2263. [DOI] [PubMed] [Google Scholar]
- 16.Jeffrey, J. S., A. Hunter, and E. R. Atwill. 2000. A field-suitable, semisolid aerobic enrichment medium for isolation of Campylobacter jejuni in small numbers. J. Clin. Microbiol. 38:1668-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, K. S., and G. T. Veeder. March 1983. Polysaccharide S-60 and bacterial fermentation process for its preparation. U.S. patent 4,377,636.
- 18.Kang, K. S., G. T. Veeder, P. J. Mirrasoul, T. Kaneko, and I. W. Cottrell. 1982. Agar-like polysaccharide produced by Pseudomonas species: production and basic properties. Appl. Environ. Microbiol. 43:1086-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalchuk, G. A., J. R. Stephen, W. De Boer, J. I. Prosser, T. M. Embley, and J. W. Woldendorp. 1997. Analysis of ammonia-oxidizing bacteria of the β subdivision of the class proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-136. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY.
- 21.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 22.Limmer, C., and H. L. Drake. 1996. Non-symbiotic N2-fixation in acidic and pH-neutral forest soils: aerobic and anaerobic differentials. Soil Biol. Biochem. 28:177-183. [Google Scholar]
- 23.Lopez, N. I., J. A. Ruiz, and B. S. Mendez. 1998. Survival of poly-3-hydroxyrate-producing bacteria in soil microcosmos. World J. Microbiol. Biotechnol. 14:681-684. [Google Scholar]
- 24.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 25.Minamisawa, K., K. Nishioka, T. Miyaki, B. Ye, T. Miyamoto, M. You, A. Saito, M. Saito, W. L. Bamaquio, N. Teaumroong, T. Sein, and T. Sato. 2004. Anaerobic nitrogen-fixing consortia consisting of clostridia isolated from gramineous plants. Appl. Environ. Microbiol. 70:3096-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muyzer, G., E. C. De Waal, and A. G. Uitierlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakhamchik, A., C. Wilde, and D. A. Rowe-Magnus. 2008. Cyclic-di-GMP regulates extracellular polysaccharide production, biofilm formation, and rugose colony development by Vibrio vulnificus. Appl. Environ. Microbiol. 74:4199-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogita, N., Y. Hashidoko, S. H. Limin, and S. Tahara. 2006. Linear 3- hydroxybutyrate tetramer (HB4) produced by Sphingomonas sp. is characterized as a growth stimulating factor of some rhizobacterial composers. Biosci. Biotechnol. Biochem. 70:2325-2329. [DOI] [PubMed] [Google Scholar]
- 29.Rath, P. M., and D. Schmidt. 2001. Gellan gum as a suitable gelling agent in microbiological media for PCR applications. J. Med. Microbiol. 50:108-109. [DOI] [PubMed] [Google Scholar]
- 30.Rennie, R. J. 1981. A single medium for the isolation of acetylene-reducing (dinitrogen-fixing) bacteria from soils. Can. J. Microbiol. 27:8-14. [DOI] [PubMed] [Google Scholar]
- 31.Reusch, R. 1992. Biological complexes of poly-β-hydroxybutyrate. FEMS Microbiol. Rev. 103:119-129. [DOI] [PubMed] [Google Scholar]
- 32.Schöllhorn, R., and R. H. Burris. 1967. Acetylene as a competitive inhibitor of N2 fixation. Proc. Natl. Acad. Sci. USA 58:213-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seneviratne, G., and H. S. Jayasinghearachchi. 2005. A rhizobial biofilm with nitrogenase activity alters nutrient availability in a soil. Soil Biol. Biochem. 37:1975-1978. [Google Scholar]
- 34.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 35.Stowers, M. D. 1985. Carbon metabolism in Rhizobium species. Annu. Rev. Microbiol. 39:89-108. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, S., T. Okuda, and S. Komatsubara. 1999. Selective isolation and distribution of Sporichthya strain in soil. Appl. Environ. Microbiol. 65:1930-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, S., K. Takahashi, T. Okuda, and S. Komatsubara. 1998. Selective isolation of Actinobispora on gellan gum plates. Can. J. Microbiol. 44:1-5. [Google Scholar]
- 38.Tamaki, H., Y. Sekiguchi, S. Hanada, K. Nakamura, N. Nomura, M. Matsumura, and Y. Kamagata. 2005. Comparative analysis of bacterial diversity in freshwater sediment of a shallow eutrophic lake by molecular and improved cultivation-based techniques. Appl. Environ. Microbiol. 71:2162-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tchan, Y.-T., and P. B. New. 1984. Genus 1: Azotobacter Beijerinck 1907 567AL, p. 220-229. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 8th ed., vol. 1. Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 40.Tran, V. V., S, Ngoke, O. Berge, D. Faure, R. Bally, P. Hebbar, and T. Heulin. 1997. Isolation of Azospirillum lipoferum from the rhizosphere of rice by a new, simple method. Can. J. Microbiol. 43:486-490. [Google Scholar]
- 41.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, X. Y., J. Lubeck, and J. J. Kilbane II. 2003. Characterization of microbial communities in gas industry pipelines. Appl. Environ. Microbiol. 69:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.