Abstract
Some strains of Vibrio anguillarum, the causative agent of vibriosis in a variety of marine animals, produce a catechol-type siderophore named vanchrobactin. The biosynthetic pathway and regulation of vanchrobactin are quite well understood. However, aspects concerning its entry into the cell have remained uncharacterized. In the present study we characterized two genes, fvtA and orf13, encoding potential TonB-dependent ferric-vanchrobactin receptors in serotype O2 V. anguillarum strain RV22. We found that an fvtA mutant was defective for growth under iron limitation conditions and for utilization of vanchrobactin, suggesting that fvtA encodes the vanchrobactin receptor of V. anguillarum. Interestingly, an orf13 mutant was not significantly affected, and results of reverse transcriptase PCR, as well as analysis of outer membrane proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggested that this gene is not expressed. Furthermore, fatA, a plasmid gene coding for the anguibactin receptor in plasmid pJM1-harboring strains, is also present in the chromosome of RV22, although it is inactivated by insertion of transposases. In addition, we found that FvtA is the route of entry for vanchrobactin analogues, and there is evidence that it recognizes primarily the catechol-iron center. These analogues are potential candidate vectors for a Trojan horse strategy aimed at generating antimicrobial compounds exploiting the same route of entry for native siderophores. We found that fvtA and vanchrobactin biosynthesis genes are ubiquitous in both vanchrobactin- and anguibactin-producing V. anguillarum strains, which reinforces the utility of the vanchrobactin route of entry for the design of future strategies for the control of vibriosis.
Vibrio anguillarum strains possess an array of specific virulence factors that enable them to cause a hemorrhagic septicemia called vibriosis in a variety of marine animals (41). There are more than 20 recognized serotypes, but serotypes O1 and O2 are the serotypes predominantly implicated in vibriosis outbreaks (43). Although the virulence mechanisms of V. anguillarum are not fully understood, it is known that the ability to scavenge iron through utilization of siderophores contributes significantly to the virulence of this bacterium (11, 20, 45). Currently, two clearly different siderophore-mediated iron uptake systems in V. anguillarum are known. One of them is the vanchrobactin-mediated system encoded by a chromosomal gene cluster. The chemical structure of the siderophore in this system and its biosynthesis and regulation pathways were recently established (3, 4, 36). The other system is a plasmid pJM1-encoded system called the anguibactin system that is found only in pJM1-containing serotype O1 strains (10, 40), whose synthesis requires additional chromosomal genes that are also involved in vanchrobactin production (1, 4).
Uptake of ferric iron-siderophore complexes into the cell requires a specific outer membrane (OM) receptor protein connected to a TonB-ExbB-ExbD complex that produces the energy necessary for active transport (6, 12). Two functional tonB systems have been identified in V. anguillarum, and the tonB2 system is essential for transport of siderophores and virulence (39). The ferric iron-anguibactin complex is transported via the OM receptor FatA, the periplasmic binding protein FatB, and the inner membrane proteins FatC and FatD (ABC transporter) (40). Although the genetic basis of anguibactin transport has been well characterized, little is known about how V. anguillarum vanchrobactin-producing strains transport the ferric iron-vanchrobactin complexes into the cell.
Increasing antibiotic-mediated selective pressure has led to the emergence of multiresistant strains of many bacterial pathogens, and fish pathogens are no exception. To facilitate penetration of antibiotics into bacterial cells, the so-called “Trojan horse strategy” can be employed, where antimicrobial drugs are transported across the bacterial membranes by exploiting the iron uptake pathways (7, 8, 24, 26, 34, 38). The vanchrobactin chemical structure was recently determined, and a series of vanchrobactin analogues which have functionality (an amino group) appropriate for use as antibiotic vectors and keep their siderophore activity have been synthesized and evaluated (36, 37). Similar approaches have been used and have provided promising results with the pyoverdine-mediated iron uptake system (18) and conjugated siderophore-β-lactamase inhibitors (9).
In previous studies, a gene cluster (vab cluster) encoding the functions involved in the biosynthesis of vanchrobactin and its regulation was widely characterized (3, 4). The fvtA gene, linked to the biosynthetic genes, was initially postulated to encode a vanchrobactin receptor (3), and it is known that V. anguillarum senses ferric-vanchrobactin in the extracellular environment, resulting in upregulation of the fvtA gene (3). However, the actual role of fvtA in the vanchrobactin uptake process has not been demonstrated yet. In this paper we functionally characterize the role of fvtA in acquisition of ferric-vanchrobactin and the possible utility of using the vanchrobactin acquisition pathway as a strategy for therapy against vibriosis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The strains and plasmids used, including those derived in this study, are listed in Tables 1 and Table 3. V. anguillarum strains were grown at 25°C on tryptic soy agar (Difco) supplemented with 1% NaCl and in tryptic soy broth (Difco) supplemented with 1% NaCl, as well as in M9 minimal medium (25) supplemented with 0.2% Casamino Acids (Difco) (CM9). Escherichia coli strains were routinely grown at 37°C in Luria-Bertani medium (Pronadisa) supplemented with the appropriate antibiotics. Antibiotics (Sigma-Aldrich) were used at the following final concentrations: ampicillin (sodium salt) and kanamycin, 50 μg ml−1; and gentamicin, 10 μg ml−1. Ethylenediamine-di-(o-hidroxyphenyl acetic acid) (EDDA) and 2,2′-dipyridyl were used as iron chelators at appropriate concentrations.
TABLE 1.
Bacterial strains and plasmids
| Plasmid or strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Plasmids | ||
| pGEMT-Easy | PCR cloning vector, Ampr | Promega |
| pWKS30 | Low-copy-number cloning vector, Ampr | 44 |
| pNidKan | pir-dependent suicide plasmid, pCVD442 derivative, sacB, Kanr | 27 |
| pHRP309 | Low-copy-number vector, Gmr | 30 |
| MBcos167 | Cosmid containing orf13 | 3 |
| MBcos69 | Cosmid containing vabD | 3 |
| pMB54 | fvtA gene and promoter cloned in pHRP309, Gmr | This study |
| V. anguillarum strains | ||
| RV22 | Serotype O2 strain isolated from diseased turbot | 20 |
| MB11 | RV22 ΔvabB | 4 |
| MB84 | RV22 ΔfvtA | This study |
| MB70 | RV22 Δorf13 | This study |
| MB90 | RV22 ΔfvtA Δorf13 | This study |
| MB102 | RV22 ΔvabB ΔfvtA | This study |
| MB104 | RV22 ΔvabB Δorf13 | This study |
| MB107 | RV22 ΔvabB ΔfvtA Δorf13 | This study |
| E. coli S17-1-λpir | Tpr Smr, recA thi pro hsdR-M+RP4::2-Tc::Mu::Km Tn7 λpir | 19 |
TABLE 3.
Presence of vab cluster genes in a collection of V. anguillarum strains as determined by PCR and Southern blot hybridization
| Strain | Source | Presence of:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanchrobactin biosynthesis and transport genes
|
RS1a | ||||||||||
| vabA | vabB | vabC | vabE | vabF | vabS | vabH | fvtA | orf13 | |||
| Serotype O1 | |||||||||||
| TM14 | Oncorhynchus mykiss, Spain | + | + | + | + | + | + | + | + | + | + |
| ATCC 43305 | Oncorhynchus mykiss, Denmark | + | + | + | + | + | + | + | + | + | + |
| SE56.1 | Salmo spp., Spain | + | + | + | + | + | + | + | + | + | + |
| SO121.1 | Salmo spp., Spain | + | + | + | + | + | + | + | + | + | + |
| RI33.1 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | + |
| SE145.1 | Salmo spp., Spain | + | + | + | + | + | + | + | + | + | − |
| PC933.1 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | + |
| 96F | Morone saxatilis, United States | + | + | + | + | + | + | + | + | + | − |
| R82 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | + |
| 775 | Oncorhynchus kisutch, United States | + | + | + | + | + | + | + | + | + | + |
| Serotype O2 | |||||||||||
| ATCC 14181 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| PC640.1 | Solea solea, Spain | + | + | + | + | + | + | + | + | + | − |
| CA3.1/04 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | − |
| AZ215.1 | Solea solea, Spain | + | + | + | + | + | + | + | + | + | − |
| CA13.1 | Pollachius pollachius, Spain | + | + | + | + | + | + | + | + | + | − |
| ACC4.1 | Scophthalmus maximus, Portugal | + | + | + | + | + | + | + | + | + | − |
| PC628.1 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | − |
| ATCC 43306 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| RV22 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | − |
| 43F | Morone saxatilis, United States | + | + | + | + | + | + | + | + | + | − |
| CA1.1/04 | Solea solea, Spain | + | + | + | + | + | + | + | + | + | − |
| Serotype O3 | |||||||||||
| PT-493 | Plecoglossus altivelis, Japan | + | − | + | + | + | + | + | + | + | − |
| 11008 | Dicentrarchus labrax, France | + | − | + | + | + | + | + | + | + | − |
| ATCC 43307 | Oncorhynchus mykiss, Denmark | + | − | + | + | + | + | + | + | + | − |
| ET-208 | Anguilla japonica, Japan | + | + | + | + | + | + | + | + | + | − |
| Serotype O4 | |||||||||||
| RPM41.11 | Scophthalmus maximus, Spain | + | + | + | + | + | + | + | + | + | − |
| ATCC 43308 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O5 | |||||||||||
| ATCC 43309 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O6 | |||||||||||
| ATCC 43310 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O7 | |||||||||||
| ATCC 43311 | Anguilla japonica, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O8 | |||||||||||
| ATCC 43312 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O9 | |||||||||||
| ATCC 43313 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
| Serotype O10 | |||||||||||
| ATCC 43314 | Gadus morhua, Denmark | + | + | + | + | + | + | + | + | + | − |
RS1 is an IS that disrupts the vabF gene.
DNA manipulations.
Total genomic DNA from V. anguillarum was purified with an Easy-DNA kit (Invitrogen). Plasmid DNA purification and extraction of DNA from agarose gels were carried out using kits from Qiagen (Qiagen). DNA probe labeling and Southern blot analyses were performed with the ECL DNA labeling and detection system (Amersham Biosciences). DNA probes for gene screening by Southern blotting were obtained by PCR amplification using suitable primer pairs (see Table S1 in the supplemental material). PCRs were routinely carried out using a T-Gradient thermal cycler (Biometra) with Taq polymerase BioTaq (Bioline).
DNA sequencing and bioinformatics tools.
DNA sequences were determined by the dideoxy chain termination method for either cosmid, plasmid, or PCR products using a GenomeLab DTCS quick start kit with a CEQ 8000 DNA sequencer (Beckman Coulter). Sequences were examined and assembled using BioEdit, version 7.0.4.1 (16). The European Bioinformatics Institute and the NCBI services were used to consult DNA and protein sequence databases with FASTA3 and BLAST algorithms.
Construction of fvtA and orf13 mutants by allelic exchange.
In-frame deletions of fvtA and orf13 in V. anguillarum RV22 were constructed by using PCR amplification of two fragments of each gene and flanking regions, which, when ligated together, would result in an in-frame (nonpolar) deletion. The oligonucleotides used to amplify the upstream and downstream ends of each gene are shown in Table S1 in the supplemental material. Each deleted allele construction was ligated into the suicide vector pNidKan (27). As a pCVD442 derivative, pNidKan contains R6K ori, requiring the pir gene product for replication, and the sacB gene, conferring sucrose sensitivity. The resulting plasmids were mated from E. coli S17-1-λpir into V. anguillarum wild-type strain RV22 and into previously constructed mutant strains, and exconjugants with the plasmid (conferring kanamycin resistance) integrated into the chromosome by homologous recombination were selected. A second recombination event involved selecting for sucrose (10%) resistance and further checking for plasmid loss and for allelic exchange. This process led to the generation of V. anguillarum single mutant strains MB84 (ΔfvtA) and MB70 (Δorf13) and mutants MB102 (ΔvabB ΔfvtA), MB104 (ΔvabB Δorf13), and MB107 (ΔvabB ΔfvtA Δorf13). Deletion of the parental gene was checked by Southern blot hybridization, and DNA sequencing of the region involved in the deletion was carried out to ensure that all mutations were in frame.
Complementation of V. anguillarum fvtA mutants.
The fvtA gene, along with its promoter sequence, was PCR amplified from the V. anguillarum RV22 chromosome using specific primers (see Table S1 in the supplemental material), cloned into the pHRP309 vector (30), and subsequently transformed into E. coli strain S17-1-λpir. The resulting plasmid (Table 1) was mated from E. coli S17-1-λpir into the V. anguillarum fvtA mutant, and transformants were selected on agar medium containing gentamicin (resistance conferred by pHRP309) and ampicillin (to select for V. anguillarum).
Growth under iron-limited conditions and test for siderophore production.
To test the ability of V. anguillarum deletion mutants to grow under iron-limited conditions, the optical densities at 600 nm (OD600) of overnight cultures in Luria-Bertani medium of the parental and mutant strains were adjusted to 0.5, and the cultures were diluted 1:15 in CM9 containing 10 μM EDDA. In the case of the complemented fvtA mutant, gentamicin was added to avoid loss of plasmid pMB54. Cultures were incubated at 25°C with shaking at 150 rpm, and growth (OD600) was measured after 12 h of incubation. Siderophore production was measured using the chrome azurol S (CAS) liquid assay (35), which detects the presence of iron-chelating compounds. For siderophore production, strains were grown with 5 μM EDDA instead of 10 μM EDDA to allow cultures to grow enough to make siderophore secretion detectable. A noninoculated sample of CM9 containing EDDA at an appropriate concentration and a sample of the vabB mutant (MB11) were used as a spectrophotometric blank and as a negative control for the CAS liquid assay, respectively. Growth curve and CAS assays were carried out in triplicate, and the results shown below are the means of three independent experiments.
OM protein analysis.
V. anguillarum strains were grown in CM9 supplemented with either 10 μM Fe2(SO4)3 or 5 μM EDDA (iron-sufficient or iron-restricted conditions, respectively). Cells were centrifuged, and OM proteins were obtained as previously described (42). The protein concentration was adjusted for all the samples, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein bands were visualized by staining with Coomassie brilliant blue.
Cross-feeding assays.
The biological activities of vanchrobactin and analogues of this compound were determined by using bioassays. Strains MB11 (ΔvabB), MB102 (ΔvabB ΔfvtA), MB104 (ΔvabB Δorf13), and MB107 (ΔvabB ΔfvtA Δorf13), used as indicator strains, were inoculated into CM9 containing the iron chelator 2,2′-dipyridyl at a concentration of 120 μM, a concentration higher than the MIC for the wild-type strain. Paper disks were loaded with 25 μg of each compound and put on the surfaces of the plates. The compounds tested were synthetic vanchrobactin (2,3-dihydroxybenzoic acid [DHBA]-Arg-Ser) and several synthetic derivatives with known siderophore activity, including DHBA-Orn, DHBA-Orn-Ser, DHBA-Ser, DHBA-Ser-Orn, DHBA-Ser-Arg, and DHBA-Arg (37), that could be the vanchrobactin esterase product. The results were considered positive when a compound promoted the growth of indicator strains. A 10 μM Fe2(SO4)3 solution was used as a positive growth control.
RNA purification and RT-PCR.
V. anguillarum cultures (5 ml) were grown until exponential phase in low-iron CM9 containing 5 μM EDDA, and total RNA was isolated with the RNA isolation reagent RNAwiz (Ambion) by following the manufacturer's recommendations. Reverse transcriptase (RT) PCRs were performed with 0.5 to 3 μg of RNA pretreated with RQ1 RNase-free DNase (Promega) by using the Moloney murine leukemia virus RT (Invitrogen). Negative controls for PCR were performed with total RNA without Moloney murine leukemia virus RT to confirm the lack of genomic DNA contamination in each reaction mixture. A primer pair flanking a 639-bp internal fragment of the fvtA gene was used to PCR amplify the cDNA from fvtA transcripts (see Table S1 in the supplemental material).
Nucleotide sequence accession number.
The nucleotide sequence reported in this study has been deposited in the EMBL database under accession number AM168450.
RESULTS AND DISCUSSION
The vanchrobactin gene cluster includes two genes encoding predicted TonB-dependent ferric iron-siderophore receptors.
The vanchrobactin biosynthesis gene cluster (vab cluster) includes a gene designated fvtA encoding a protein with high levels of similarity to TonB-dependent siderophore receptors. Expression of fvtA is known to be iron regulated via the Fur repressor, and fvtA is cotranscribed with vabD, a gene encoding a phosphopantetheinyl transferase essential for vanchrobactin biosynthesis. In addition, expression of fvtA was found to be vanchrobactin dependent (3). These findings, together with homology data, suggested that FvtA could be the vanchrobactin receptor, although no direct experimental data confirmed this hypothesis.
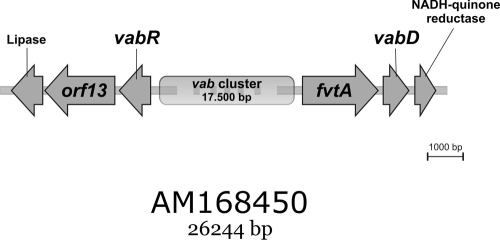
Using V. anguillarum RV22 cosmid clones identified in a previous study (3), we extended DNA sequencing on both sides of the vab cluster and identified a previously undescribed gene designated orf13 downstream of vabR and transcribed from the same strand (Fig. 1). VabR is a predicted LysR family regulator that activates expression of vabG, a gene encoding a 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase involved in vanchrobactin biosynthesis (3). Downstream of orf13 we found a putative lipase gene. On the other side of the vab cluster, sequencing downstream of vabD yielded a gene encoding a putative NADH-quinone reductase (Fig. 1). We believe that the vab cluster sequence might now be complete, since the proteins encoded by the two new genes at the borders of this cluster (lipase and NADH-quinone reductase) do not show any known relationship with siderophore synthesis or transport proteins, as deduced from protein database homology searches. Interestingly, the predicted protein encoded by orf13 showed homology with, among other proteins, FepA, the OM receptor for ferrienterochelin and colicins of Vibrio alginolyticus (accession no. ZP_01260504; 59% identity and 77% similarity), and IrgA, the enterobactin receptor of Vibrio cholerae (accession no. ZP_01950374; 44% identity and 60% similarity) (14).
FIG. 1.
Physical map of the V. anguillarum RV22 vab cluster, showing newly sequenced genes at the borders of the previously sequenced vab genes (4). Flanking genes encoding a predicted lipase and an NADH-quinone reductase are thought to be not part of the vab cluster.
The extended vab cluster, as described here, includes two genes which encode potential OM receptors for vanchrobactin. Alignment of these two protein sequences with the sequences of described TonB-dependent receptors (data not shown) showed that there was conservation of some residues near the N termini of both FvtA (DETVVVVGE) and ORF13 (METLVVTAS) (residues conserved in other TonB-dependent ferric siderophore receptors are underlined), which might be involved in direct interaction with the TonB protein (the so-called TonB box) (15, 22, 29). In addition, various OM proteins possess a highly conserved C-terminal sequence, which was proposed to form an amphipathic β-sheet important for correct assembly of the protein in the OM (13). This sequence motif also is present in ORF13 and FvtA and in other OM TonB-dependent siderophore and heme receptors of other Vibrio species.
FvtA is directly involved in ferric-vanchrobactin uptake.
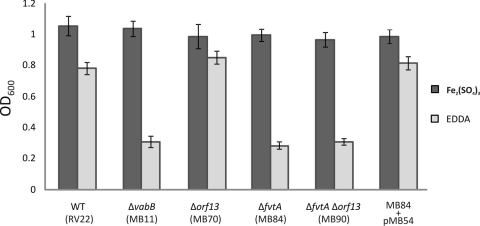
Since fvtA and orf13 are part of the vab cluster and are closely linked to other genes whose role in vanchrobactin biosynthesis has already been demonstrated, these two genes are candidates for the genes that encode the ferric-vanchrobactin OM receptor. In order to assess this possibility, in-frame deletion mutants were constructed by allelic exchange, and their ability to grow under iron limitation conditions was evaluated. As a control, we also included in these experiments the vanchrobactin biosynthesis mutant MB11 (ΔvabB) (3). Under iron-sufficient conditions (CM9 plus 10 μM ferric sulfate), no significant differences in growth rates between the mutants and parental strain RV22 were observed (Fig. 2). However, when the same strains were cultured under iron-restricted conditions (CM9 with 10 μM EDDA), the growth of the ΔfvtA mutant (MB84) was significantly impaired (Fig. 2), resulting in growth levels similar to those of the vanchrobactin-deficient mutant, whereas the growth of the Δorf13 mutant (MB70) was not affected. As expected, a ΔfvtA Δorf13 double mutant (MB90) had a phenotype similar to that of the ΔfvtA single mutant (Fig. 2). Interestingly, analysis of the culture supernatants by the CAS assay showed an increase in the siderophore concentration in the ΔfvtA mutant compared with the parental strain and the Δorf13 mutant; at an OD600 of approximately 0.6 in CM9 plus 5 μM EDDA, the parental and Δorf13 strains showed CAS values (A630) of ca. −0.2, while the ΔfvtA mutant showed CAS assay values (A630) of −0.3 or less (lower values indicate higher siderophore concentrations [35]). Under these conditions, the ΔvabB mutant showed mean CAS assay values (A630) of −0.015. These results demonstrate that the impaired-growth phenotype of the ΔfvtA mutant is not due to the lack of vanchrobactin production. The increase in siderophore concentration could be explained by the extracellular accumulation of vanchrobactin, which is not transported back into the cell. When the ΔfvtA mutant was complemented with a plasmid harboring an intact fvtA gene (pMB54), the growth and CAS assay values were restored to wild-type levels (Fig. 2). All these results clearly suggest that FvtA plays a crucial role in the transport of ferric-vanchrobactin.
FIG. 2.
Growth (OD600) after 12 h of incubation of V. anguillarum RV22, ΔvabB (MB11), Δorf13 (MB70), ΔfvtA (MB84), and ΔfvtA Δorf13 (MB90) mutants, and the MB84 mutant complemented with plasmid pMB54 in CM9 supplemented with Fe2(SO4)3 (10 μM) or the iron chelator EDDA (10 μM). WT, wild type.
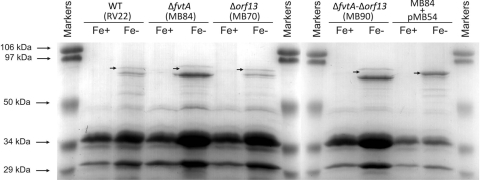
The OM protein profiles under iron-sufficient and iron-deficient conditions were analyzed for V. anguillarum RV22, for three mutants, and for the complemented ΔfvtA mutant (Fig. 3). The molecular masses of most of the TonB-dependent OM proteins involved in iron acquisition in gram-negative bacteria fall in the range from 70 to 80 kDa. Several protein bands at molecular masses in this range could be visualized for the parental strain under iron limitation conditions but not under iron-sufficient conditions. However, one of these iron-regulated protein bands (Fig. 3, RV22 Fe− lane) was absent in the lane containing the ΔfvtA mutant, and it presumably corresponded to the FvtA protein (the predicted molecular mass of FvtA, based on its amino acid sequence, is 78 kDa). This band was also absent in the lane containing the double mutant but not in the lane containing the Δorf13 mutant, while it was present in the lane containing the complemented ΔfvtA mutant (Fig. 3). Interestingly, the Δorf13 mutant showed a pattern identical to that of the parental strain, and similarly, the pattern of the ΔfvtA Δorf13 double mutant was the same as that of the ΔfvtA mutant. Together, these results suggest that orf13 is not expressed (the predicted molecular mass of ORF13, based on its amino acid sequence, is 71 kDa). This hypothesis was reinforced by the negative results obtained in repeated attempts to detect the presence of orf13 transcripts by RT-PCR (data not shown). The other iron-regulated protein bands whose sizes are close to that of the FvtA band can be attributed to other iron-regulated OM iron transporters that have molecular masses in the range from 70 to 80 kDa, like the heme receptor HuvA (ca. 79 kDa) (23), and molecular masses like those of uncharacterized receptors for exogenous siderophores like ferrichrome (see below).
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel of OM proteins obtained from cultures of V. anguillarum RV22 (wild type [WT]), ΔfvtA (MB84), Δorf13 (MB70), and ΔfvtA Δorf13 (MB90) mutants, and the MB84 mutant complemented with plasmid pMB54 under iron-sufficient (Fe+) and iron-deficient (Fe−) conditions. The arrows indicate the locations of the bands corresponding to the iron-regulated putative FvtA protein. The numbers on the left indicate the molecular masses of the protein markers.
Synthetic vanchrobactin and vanchrobactin analogues use FvtA as the sole route of entry.
As mentioned above, the route of entry of Fe-siderophore complexes into bacterial cells can be exploited by using a Trojan horse strategy for introduction of modified molecules with antimicrobial activity (24, 26). In a previous study, a series of vanchrobactin analogues were found to promote growth of V. anguillarum (RV22 and 775 strains) under iron-deficient conditions, indicating that they are indeed internalized and utilized as siderophores (37). However, the route of entry for these analogues into the V. anguillarum cell remained unknown. In addition, we ignored which part of the siderophore mediated the specific recognition by its cognate receptor, information that would be of great interest for selecting which region of the siderophore can be modified without altering its recognition. Bacteria often possess multiple OM receptors, each of which provides the bacterium with specificity for different siderophores, and some receptors have been found to efficiently transport derivatives of their cognate siderophores (12, 17, 32). We therefore wanted to assess the role of FvtA and ORF13 in the uptake of vanchrobactin analogues, using agar plate bioassays. For this purpose, a vabB mutant strain (MB11) (mutation of vabB abolishes vanchrobactin production) (4) was used as the parental strain to construct ΔfvtA and Δorf13 mutants to specifically assay the transport of synthetic vanchrobactin and the most relevant vanchrobactin analogues. Using a ΔvabB strain, we made sure that the growth halo was directly related to the ability to use the compound on the paper disk rather than to the utilization of endogenous vanchrobactin. The double mutants MB102 (ΔvabB ΔfvtA) and MB104 (ΔvabB Δorf13), as well as the triple mutant MB107 (ΔvabB ΔfvtA Δorf13), were used as indicator strains (Table 2), and the vabB single mutant was used as a positive control. Although orf13 seems not to be expressed, we cannot rule out the possibility that expression of this gene was induced under other conditions (e.g., when a cognate ligand was present). Therefore, orf13 mutant strains were included in the bioassay experiments as well. The compounds tested are shown in Table 2.
TABLE 2.
Results of bioassays using vanchrobactin analogues and purified siderophores
| Compound | Promotion of the growth of V. anguillarum indicator strainsa
|
||||
|---|---|---|---|---|---|
| MB11 (ΔvabB) | MB102 (ΔvabB ΔfvtA) | MB104 (ΔvabB Δorf13) | MB107 (ΔvabB ΔfvtA Δorf13) | MB102 (pMB54) | |
| Vanchrobactin (DHBA-Arg-Ser) | + | − | + | − | + |
| DHBA-Orn | + | − | + | − | + |
| DHBA-Orn-Ser | + | − | + | − | + |
| DHBA-Ser | + | − | + | − | + |
| DHBA-Ser-Arg | + | − | + | − | + |
| DHBA-Arg | + | − | + | − | + |
| Amonabactin | − | − | − | − | − |
| Anguibactin | − | − | − | − | − |
| Enterobactin | + | (+) | + | (+) | + |
| Ferrichromeb | + | + | + | + | + |
| Aerobactinb | − | − | − | − | − |
The abilities of the different compounds to promote the growth of each indicator strain are indicated as follows: +, positive; −, negative; (+), weakly positive.
Hidroxamate-type siderophore.
As expected, synthetic vanchrobactin and its analogues promoted growth of MB11 (ΔvabB) and MB104 (ΔvabB Δorf13) (Table 2). However, MB102 (ΔvabB ΔfvtA) and MB107 (ΔvabB ΔfvtA Δorf13) were unable to use any of these compounds, which indicates that FvtA is the transporter for vanchrobactin, as well as for the analogues tested. When MB102 (ΔvabB ΔfvtA) was complemented with plasmid pMB54 (harboring the fvtA gene and its promoter), transport of vanchrobactin and its analogues was restored (Table 2). An interesting finding is that all of the analogues tested share the same moiety, the DHBA molecule. This suggests that the specificity of the V. anguillarum vanchrobactin receptor FvtA could be mediated by the iron-catecholate center, whereas the d-Arg-l-Ser backbone could play a minor role in the recognition. A similar situation has been described for the chrysobactin receptor FctA, where recognition is mediated by the DHBA moiety (31).
Together, our results indicate that FvtA is the only route of entry for vanchrobactin (DHBA-Arg-Ser), as well as for its analogue DHBA-Orn-Ser. Elucidation of the route of entry for the latter molecule was of special interest, since this molecule possess an amino group that could be used to link antibacterial agents in the Trojan horse strategy (37).
We also tested the utilization of siderophores from other bacterial species. Results of these bioassays (Table 2) demonstrated that ferrichrome can be utilized by the parental strain and by all mutants, indicating that in V. anguillarum RV22 a ferrichrome receptor distinct from FvtA and ORF13 is required. Interestingly, enterobactin supported growth of the ΔfvtA and Δorf13 mutant strains, which implies that an unknown V. anguillarum receptor is able to transport enterobactin, a catechol siderophore whose functional relationship with vanchrobactin has been suggested previously (10, 20). It is frequently found that bacteria utilize exogenous siderophores since in this way they can pirate siderophores of their competitors and escape the bacteriostatic effects caused by these compounds (33). E. coli K-12 possesses at least six OM receptors that enable acquisition of eight different iron-chelator complexes, four of which are exogenously produced (2).
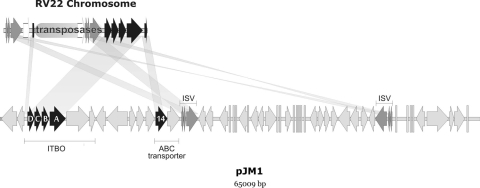
However, amonabactin, aerobactin, and anguibactin (a siderophore produced by V. anguillarum strains that carry pJM1 or pJM1-like plasmids) are three siderophores that cannot be utilized by V. anguillarum RV22. These results are in agreement with previous observations that plasmidless V. anguillarum strains cannot utilize anguibactin and do not express FatA, the anguibactin OM receptor (10, 20). Surprisingly, the presence of gene sequences with high levels of similarity to fatA and fatD (the receptor and ABC transporter genes of the anguibactin system, respectively) (1, 40) was recently reported in strains that contained either no plasmids or only small plasmids (5). Interestingly, we found that the chromosome of V. anguillarum RV22 actually contains fatDCBA homologues, as well as other genes present in the pJM1 plasmid, although it has a different gene arrangement (Fig. 4). However, the expression of fatDCBA is likely abolished, since insertion of ca. 9 kb of transposases disrupts the first gene of the operon, from which transcription of the four genes is driven. This finding is supported by the negative results in all attempts to detect fatDCBA transcripts by RT-PCR (data not shown). Furthermore, Naka et al. (28) detected FatA in the OM of strain RV22 only when it was transformed with a pJM1 plasmid. These findings not only demonstrate that the FatA-mediated anguibactin acquisition system is inappropriate for use in the Trojan horse strategy but also bring up interesting questions about the origin and evolution of the vanchrobactin and anguibactin systems in V. anguillarum.
FIG. 4.
Comparative analysis of the RV22 chromosomal fatDCBA gene cluster and genes of the pJM1 plasmid. The predicted open reading frames in RV22 showed levels of identity near 100% to different pJM1 regions corresponding to fatDCBA genes (DCBA), an uncharacterized putative ABC transporter gene (orf14) (labeled 14), and transposases (ISV), which have a different organization in RV22 and pJM1. In pJM1, fatDCBA form an operon (ITBO) together with the anguibactin biosynthesis genes angR and angT (11, 40) but not with the ABC transporter gene orf14. The open arrows indicate transposases in RV22 that are absent from pJM1. The light gray arrows indicate genes specific to one of the two molecules compared.
Vanchrobactin biosynthesis and transport genes are ubiquitous in V. anguillarun strains.
It is not known if the vanchrobactin transport system is widely distributed in V. anguillarum strains. This question is of special interest because in order for the vanchrobactin analogues to be employed in the development of novel antimicrobials, their route of entry into the cell should be widespread in the species. PCR and Southern hybridization were used to assay the presence of the fvtA, orf13, and vab genes in a collection of 33 strains that represented the 10 main serotypes (serotypes O1 to O10) of V. anguillarum. The results indicate that all strains contain fvtA and orf13 and that vabB is absent from some serotype O3 isolates (strains PT-4933, 11008, and ATCC 4330) (Table 3). Thus, all V. anguillarum strains seem to harbor a copy of the fvtA gene.
It appears from the present results that the vanchrobactin biosynthesis and transport gene system is ubiquitous in V. anguillarum strains. A question that arises is why not all of these strains produce vanchrobactin. The presence of the pJM1 plasmid, containing most genes necessary for anguibactin production and transport, has been associated with the lack of production of vanchrobactin. Naka et al. (28) showed that vabF in the V. anguillarum 775 (a strain carrying pJM1) chromosome is disrupted by the insertion sequence (IS) RS1. In the present study we detected the presence of RS1, an insertion that abolishes vanchrobactin biosynthesis, in vabF in all strains that carry pJM1 or pJM1-like plasmids (Table 3). RS1 encodes a transposase that is 100% identical to the RS1 (orf21) transposase originally described for the pJM1 plasmid (11), suggesting that this IS could have transposed from the plasmid to the chromosome. The genes encoding the proteins of the anguibactin-iron uptake system on pJM1-like plasmids are flanked by ISs ISV-A1 and ISV-A2 in a transposon-like structure, and transposition of ISV-A2 at a frequency of 7.2 × 10−6 was recently demonstrated (21).
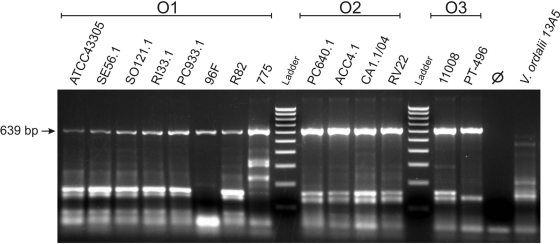
The vanchrobactin system was proposed to be the ancestral siderophore in V. anguillarum (28), a hypothesis reinforced by our finding that vanchrobactin biosynthesis genes are present in all strains of V. anguillarum tested, as well as by the dependence of anguibactin biosynthesis on some vanchrobactin biosynthesis elements that complement pJM1 pseudogenes (1). fvtA is cotranscribed with vabD as a polycistronic mRNA, and we know that these genes are essential for vanchrobactin transport and biosynthesis, respectively (3). The sequence of fvtA homologues in anguibactin-producing strains is 100% identical to the sequence of RV22 fvtA. In addition, V. anguillarum 775 (an anguibactin-producing strain) can also use vanchrobactin (37), and in this strain vabD is a functional gene (28). These two observations clearly suggest that fvtA (from which promoter vabD gene transcription is driven) is expressed not only in vanchrobactin-producing strains but also in anguibactin-producing strains. To verify this hypothesis, we used RT-PCR to detect the presence of fvtA transcripts in RNA samples from V. anguillarum strains grown under low-iron conditions. Figure 5 shows the expression of fvtA in 14 selected strains representing the three most relevant pathogenic serotypes (serotypes O1, O2, and O3) (Table 3). Sequencing of the PCR amplicons confirmed that the 639-bp band detected corresponded to fvtA (data not shown).
FIG. 5.
Detection of fvtA transcripts using RT-PCR. Fourteen V. anguillarum strains belonging to serotypes O1, O2, and O3 were analyzed (strain designations are indicated at top). The arrow on the left indicates the expected size of the fvtA RT-PCR product. The same RT-PCR carried out with Vibrio ordalii 13A5 was negative. Negative controls using DNase-treated RNA as the PCR template to rule out the presence of contaminating DNA were negative in all cases (an example is the control for RV22 [⊘]). The ladder is composed of 10 fragments whose sizes range from 100 to 1,000 bp in 100-bp increments.
Therefore, when siderophore uptake machinery is used to internalize siderophore analogues coupled to antimicrobial agents against V. anguillarum, the strategy of choice should be to use the vanchrobactin entry pathway, since it is the only pathway present in all strains, especially strains of the three main pathogenic serotypes.
Conclusions.
In this work we have determined that FvtA is the OM transporter for vanchrobactin and its analogues in V. anguillarum. In addition, we have demonstrated that fvtA is present in both vanchrobactin- and anguibactin-producing strains, where it is a functional gene. The vanchrobactin analogue DHBA-Orn-Ser, of special interest for attaching antimicrobial ligands, is optimally transported by FvtA. FvtA would be an optimum route of entry for new antibacterial compounds using the Trojan horse strategy, due to its ubiquity in this species and to the wide range of vanchrobactin analogues transported.
Supplementary Material
Acknowledgments
We thank Carlos Jiménez from the University of A Coruña (Spain) for supplying purified vanchrobactin and vanchrobactin analogues used in this work.
This work was supported by grant AGL2006-00697 from the Ministry of Science and Innovation of Spain (cofunded by the FEDER Programme from the European Union) to M.L.L. M.B. acknowledges the Ministry of Science and Innovation of Spain for a predoctoral FPI fellowship.
Footnotes
Published ahead of print on 6 March 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alice, A. F., C. S. Lopez, and J. H. Crosa. 2005. Plasmid- and chromosome-encoded redundant and specific functions are involved in biosynthesis of the siderophore anguibactin in Vibrio anguillarum 775: a case of chance and necessity? J. Bacteriol. 187:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Balado, M., C. R. Osorio, and M. L. Lemos. 2008. Biosynthetic and regulatory elements involved in the production of the siderophore vanchrobactin in Vibrio anguillarum. Microbiology 154:1400-1413. [DOI] [PubMed] [Google Scholar]
- 4.Balado, M., C. R. Osorio, and M. L. Lemos. 2006. A gene cluster involved in the biosynthesis of vanchrobactin, a chromosome-encoded siderophore produced by Vibrio anguillarum. Microbiology 152:3517-3528. [DOI] [PubMed] [Google Scholar]
- 5.Bay, L., J. L. Larsen, and J. J. Leisner. 2007. Distribution of three genes involved in the pJM1 iron-sequestering system in various Vibrio anguillarum serogroups. Syst. Appl. Microbiol. 30:85-92. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., K. Hantke, and W. Koster. 1998. Bacterial iron transport: mechanisms, genetics, and regulation. Met. Ions Biol. Syst. 35:67-145. [PubMed] [Google Scholar]
- 7.Brochu, A., N. Brochu, T. I. Nicas, T. R. Parr, Jr., A. A. Minnick, Jr., E. K. Dolence, J. A. McKee, M. J. Miller, M. C. Lavoie, and F. Malouin. 1992. Modes of action and inhibitory activities of new siderophore-beta-lactam conjugates that use specific iron uptake pathways for entry into bacteria. Antimicrob. Agents Chemother. 36:2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budzikiewicz, H. 2001. Siderophore-antibiotic conjugates used as Trojan horses against Pseudomonas aeruginosa. Curr. Top. Med. Chem. 1:73-82. [DOI] [PubMed] [Google Scholar]
- 9.Buynak, J. D. 2004. The discovery and development of modified penicillin- and cephalosporin-derived beta-lactamase inhibitors. Curr. Med. Chem. 11:1951-1964. [DOI] [PubMed] [Google Scholar]
- 10.Conchas, R. F., M. L. Lemos, J. L. Barja, and A. E. Toranzo. 1991. Distribution of plasmid- and chromosome-mediated iron uptake systems in Vibrio anguillarum strains of different origins. Appl. Environ. Microbiol. 57:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Lorenzo, M., M. Stork, M. E. Tolmasky, L. A. Actis, D. Farrell, T. J. Welch, L. M. Crosa, A. M. Wertheimer, Q. Chen, P. Salinas, L. Waldbeser, and J. H. Crosa. 2003. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J. Bacteriol. 185:5822-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, A. D., and J. Deisenhofer. 2004. Metal import through microbial membranes. Cell 116:15-24. [DOI] [PubMed] [Google Scholar]
- 13.Funahashi, T., K. Moriya, S. Uemura, S. Miyoshi, S. Shinoda, S. Narimatsu, and S. Yamamoto. 2002. Identification and characterization of pvuA, a gene encoding the ferric vibrioferrin receptor protein in Vibrio parahaemolyticus. J. Bacteriol. 184:936-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, M. B., S. A. Boyko, J. R. Butterton, J. A. Stoebner, S. M. Payne, and S. B. Calderwood. 1992. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol. Microbiol. 6:2407-2418. [DOI] [PubMed] [Google Scholar]
- 15.Gudmundsdottir, A., P. E. Bell, M. D. Lundrigan, C. Bradbeer, and R. J. Kadner. 1989. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J. Bacteriol. 171:6526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 17.Hantke, K. 1990. Dihydroxybenzoylserine: a siderophore for E. coli. FEMS Microbiol. Lett. 55:5-8. [DOI] [PubMed] [Google Scholar]
- 18.Hennard, C., Q. C. Truong, J. F. Desnottes, J. M. Paris, N. J. Moreau, and M. A. Abdallah. 2001. Synthesis and activities of pyoverdin-quinolone adducts: a prospective approach to a specific therapy against Pseudomonas aeruginosa. J. Med. Chem. 44:2139-2151. [DOI] [PubMed] [Google Scholar]
- 19.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemos, M. L., P. Salinas, A. E. Toranzo, J. L. Barja, and J. H. Crosa. 1988. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J. Bacteriol. 170:1920-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, N., H. Wu, J. Ye, P. Xu, Y. Zhang, and H. Zhang. 2007. Molecular cloning of an insertion sequence-like element from Vibrio anguillarum and its functional identification in E. coli. Biotechnol. Lett. 29:1951-1957. [DOI] [PubMed] [Google Scholar]
- 22.Lundrigan, M. D., and R. J. Kadner. 1986. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J. Biol. Chem. 261:10797-10801. [PubMed] [Google Scholar]
- 23.Mazoy, R., C. R. Osorio, A. E. Toranzo, and M. L. Lemos. 2003. Isolation of mutants of Vibrio anguillarum defective in haeme utilisation and cloning of huvA, a gene coding for an outer membrane protein involved in the use of haeme as iron source. Arch. Microbiol. 179:329-338. [DOI] [PubMed] [Google Scholar]
- 24.Miethke, M., and M. A. Marahiel. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71:413-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Miller, M. J., H. Zhu, Y. Xu, C. Wu, A. J. Walz, A. Vergne, J. M. Roosenberg, G. Moraski, A. A. Minnick, J. McKee-Dolence, J. Hu, K. Fennell, E. Kurt Dolence, L. Dong, S. Franzblau, F. Malouin, and U. Mollmann. 2009. Utilization of microbial iron assimilation processes for the development of new antibiotics and inspiration for the design of new anticancer agents. Biometals 22:61-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouriño, S., C. R. Osorio, and M. L. Lemos. 2004. Characterization of heme uptake cluster genes in the fish pathogen Vibrio anguillarum. J. Bacteriol. 186:6159-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naka, H., C. S. Lopez, and J. H. Crosa. 2008. Reactivation of the vanchrobactin siderophore system of Vibrio anguillarum by removal of a chromosomal insertion sequence originated in plasmid pJM1 encoding the anguibactin siderophore system. Environ. Microbiol. 10:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nau, C. D., and J. Konisky. 1989. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J. Bacteriol. 171:1041-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parales, R. E., and C. S. Harwood. 1993. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for gram− bacteria. Gene 1:23-30. [DOI] [PubMed] [Google Scholar]
- 31.Persmark, M., D. Expert, and J. B. Neilands. 1992. Ferric iron uptake in Erwinia chrysanthemi mediated by chrysobactin and related catechol-type compounds. J. Bacteriol. 174:4783-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabsch, W., U. Methner, W. Voigt, H. Tschape, R. Reissbrodt, and P. H. Williams. 2003. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect. Immun. 71:6953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 34.Roosenberg, J. M., II, Y. M. Lin, Y. Lu, and M. J. Miller. 2000. Studies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents. Curr. Med. Chem. 7:159-197. [DOI] [PubMed] [Google Scholar]
- 35.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 36.Soengas, R. G., C. Anta, A. Espada, V. Paz, I. R. Ares, M. Balado, J. Rodriguez, M. L. Lemos, and C. Jimenez. 2006. Structural characterization of vanchrobactin, a new catechol siderophore produced by the fish pathogen Vibrio anguillarum serotype O2. Tetrahedron Lett. 47:7113-7116. [Google Scholar]
- 37.Soengas, R. G., M. Larrosa, M. Balado, J. Rodriguez, M. L. Lemos, and C. Jimenez. 2008. Synthesis and biological activity of analogues of vanchrobactin, a siderophore from Vibrio anguillarum serotype O2. Org. Biomol. Chem. 6:1278-1287. [DOI] [PubMed] [Google Scholar]
- 38.Stojiljkovic, I., V. Kumar, and N. Srinivasan. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 31:429-442. [DOI] [PubMed] [Google Scholar]
- 39.Stork, M., M. Di Lorenzo, S. Mouriño, C. R. Osorio, M. L. Lemos, and J. H. Crosa. 2004. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect. Immun. 72:7326-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stork, M., M. Di Lorenzo, T. J. Welch, L. M. Crosa, and J. H. Crosa. 2002. Plasmid-mediated iron uptake and virulence in Vibrio anguillarum. Plasmid 48:222-228. [DOI] [PubMed] [Google Scholar]
- 41.Toranzo, A. E., and J. L. Barja. 1990. A review of the taxonomy and seroepizootiology of Vibrio anguillarum, with special reference to aquaculture in the northwest of Spain. Dis. Aquat. Org. 9:73-82. [Google Scholar]
- 42.Toranzo, A. E., J. L. Barja, S. A. Potter, R. R. Colwell, F. M. Hetrick, and J. H. Crosa. 1983. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect. Immun. 39:1220-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toranzo, A. E., Y. Santos, and J. L. Barja. 1997. Immunization with bacterial antigens: Vibrio infections. Dev. Biol. Stand. 90:93-105. [PubMed] [Google Scholar]
- 44.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 45.Wolf, M. K., and J. H. Crosa. 1986. Evidence for the role of a siderophore in promoting Vibrio anguillarum infections. J. Gen. Microbiol. 132:2949-2952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.