Abstract
Antibody recognition force microscopy showed that OmcA and MtrC are expressed on the exterior surface of living Shewanella oneidensis MR-1 cells when Fe(III), including solid-phase hematite (Fe2O3), was the terminal electron acceptor. OmcA was localized to the interface between the cell and mineral. MtrC displayed a more uniform distribution across the cell surface. Both cytochromes were associated with an extracellular polymeric substance.
Shewanella oneidensis MR-1 is a dissimilatory metal-reducing bacterium that is well known for its ability to use a variety of anaerobic terminal electron acceptors (TEAs), including solid-phase iron oxide minerals, such as goethite and hematite (8, 10). Previous studies suggest that S. oneidensis MR-1 uses outer membrane cytochromes OmcA and MtrC to catalyze the terminal reduction of Fe(III) through direct contact with the extracellular iron oxide mineral (2, 8, 10, 15, 16, 20, 21, 23). However, it has yet to be shown whether OmcA or MtrC is actually targeted to the external surface of live S. oneidensis MR-1 cells when Fe(III) serves as the TEA.
In the present study, we used atomic force microscopy (AFM) to probe the surface of live S. oneidensis MR-1 cells, using AFM tips that were functionalized with cytochrome-specific polyclonal antibodies (i.e., anti-OmcA or anti-MtrC). This technique, termed antibody recognition force microscopy (Ig-RFM), detects binding events that occur between antibodies (e.g., anti-OmcA) on an AFM tip and antigens (e.g., OmcA) that are exposed on a cell surface. While this is a relatively new technique, Ig-RFM has been used to map the nanoscale spatial location of single molecules in complex biological structures under physiological conditions (5, 9, 11, 13).
Anti-MtrC or anti-OmcA molecules were covalently coupled to silicon nitride (Si3N4) cantilevers (Veeco or Olympus) via a flexible, heterofunctional polyethylene glycol (PEG) linker molecule. The PEG linker consists of an NHS (N-hydroxysuccinimide) group at one end and an aldehyde group at the other end (i.e., NHS-PEG-aldehyde). AFM tips were functionalized with amine groups, using ethanolamine (6, 7). The active NHS ester of the NHS-PEG-aldehyde linker molecule was then used to form a covalent linkage between PEG-aldehyde and the amine groups on the AFM tips (6, 7). Next, anti-MtrC or anti-OmcA molecules were covalently tethered to these tips via the linker molecule's aldehyde group. This was accomplished by incubating the tips with antibody (0.2 mg/ml) and NaCNBH3 as described previously (7). The cantilevers were purchased from Veeco and had spring constant values between 0.06 and 0.07 N/m, as determined by the thermal method of Hutter and Bechhoefer (12).
Prior to conducting the Ig-RFM experiments, the specificity of each polyclonal antibody (i.e., anti-OmcA and anti-MtrC) for OmcA or MtrC was verified by Western blot analysis as described previously (24, 28). Proteins were resolved by both denaturing and nondenaturing polyacrylamide gel electrophoresis (PAGE). Briefly, 2.5 μg of purified OmcA or MtrC (23) was resolved by sodium dodecyl sulfate-PAGE or native PAGE, transferred to a polyvinylidene difluoride membrane, incubated with either anti-OmcA or anti-MtrC, and then visualized using the Amersham ECL Plus Western blotting detection kit. Anti-OmcA bound exclusively to OmcA, anti-MtrC bound exclusively to MtrC, and neither antibody showed cross-reactivity with the other cytochrome. Antibody specificities of anti-OmcA and anti-MtrC were also validated by immunoblot analysis of S. oneidensis whole-cell lysate (28).
To determine if MtrC or OmcA was expressed on the external surface of live bacteria when Fe(III) served as the TEA, Ig-RFM was conducted on wild-type versus ΔomcA ΔmtrC double mutant cells. For these experiments, bacteria were cultivated anaerobically with Fe(III), in the form of Fe(III) chelated to nitrilotriacetic acid (NTA), serving as the TEA (19, 23). Growth conditions have been described elsewhere (3, 15) and were based on previous studies (3, 15, 16, 18) that suggest that S. oneidensis MR-1 targets OmcA and MtrC to the cell surface when Fe(III) serves as the TEA.
An Asylum Research MFP-3D-BIO AFM or a Digital Instruments Bioscope AFM (16, 17) was used for these experiments. The z-piezoelectric scanners were calibrated as described previously (17). Cells were deposited on a hydrophobic glass coverslip and immersed in imaging buffer (i.e., phosphate-buffered saline [pH 7.4]). The hydrophobic glass coverslips were made as described previously (17) using a self-assembling silane compound called octadecyltrichlorosilane (OTS; Sigma-Aldrich). S. oneidensis MR-1 cells readily adsorbed onto OTS glass coverslips and remained attached to the coverslips during the entire experiment. No lateral cell movement was observed during the experiment, consistent with previous studies that used OTS glass to immobilize bacteria (15, 17, 18, 27).
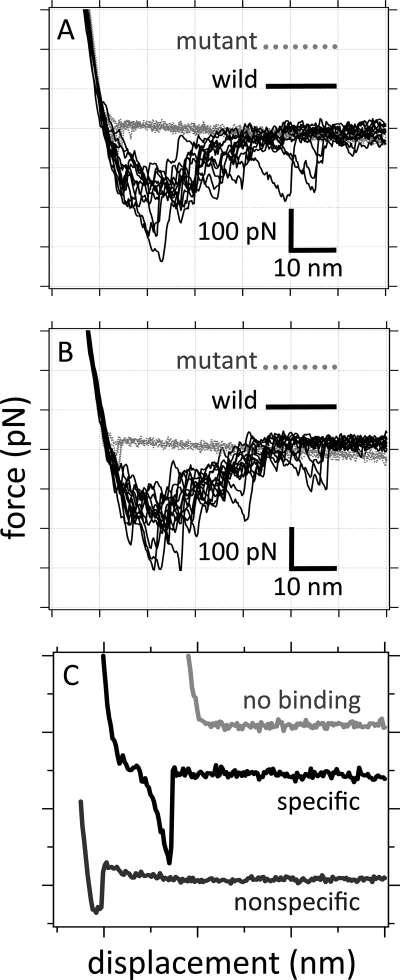
The AFM tip was brought into contact with the surface of a bacterium, and the antibody-functionalized tip was repeatedly brought into and out of contact with the sample, “fishing” for a binding reaction with cytochrome molecules that were exposed on the external cell surface. Binding events were observed upon separating anti-OmcA- or anti-MtrC-functionalized tips from wild-type S. oneidensis MR-1 cells (Fig. 1). For the wild-type cells, we observed both nonspecific and specific interactions (Fig. 1).
FIG. 1.
Retraction force curves for anti-MtrC-functionalized tips (A) and anti-OmcA-functionalized tips (B) that are being pulled away from the surface of living ΔomcA ΔmtrC double mutant (gray dotted line) or wild-type (solid black line) S. oneidensis MR-1. These bacteria were adsorbed onto OTS glass coverslips. (C) Retraction curves exhibiting nonspecific binding, specific binding, or no binding between the AFM tip and the cell surface.
The distinction between “specific” and “nonspecific” adhesion is made by observing the change in slope of the force curve during the retraction process (26). During specific binding (Fig. 1C), the cantilever is initially relaxed as it is pulled away from the sample. Upon further retraction, the ligand-receptor complex becomes stretched and unravels, resulting in a nonlinear force profile as noted in references 26 and 16. On the other hand, nonspecific adhesion (Fig. 1C) maintains the same slope during the retraction process because only the cantilever flexes (26).
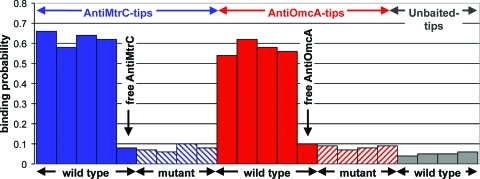
Figure 2 summarizes the frequency or probability of observing a binding event for both anti-OmcA and anti-MtrC tips. Each bar in Fig. 2 represents one experiment in which 500 to 1,000 force curves were collected between one AFM tip and two to four live bacterial cells. This figure does not make a distinction between specific and nonspecific binding. It simply shows the frequency of observing an attractive interaction as the antibody-functionalized tip was pulled away from the surface of S. oneidensis MR-1. Binding events occurred with roughly the same frequency when wild-type S. oneidensis MR-1 cells were probed with anti-MtrC-functionalized tips as when they were probed with anti-OmcA-functionalized tips (Fig. 2).
FIG. 2.
Histograms showing the frequency of observing a binding event for anti-MtrC-functionalized (blue) or anti-OmcA-functionalized (red) AFM tips on live wild-type S. oneidensis MR-1 (solid bars) or ΔomcA ΔmtrC double mutant (diagonally hatched bars) cells. The downward arrows designate injection of free antibody into the imaging buffer. The solid gray bars correspond to results obtained with unbaited AFM tips.
A number of control experiments were performed to verify the detection of OmcA and MtrC on the surface of wild-type S. oneidensis MR-1. First, 0.1 μM of free anti-OmcA (or anti-MtrC) was added to the imaging fluid to block binding between the antibody-functionalized AFM tip and surface-exposed cytochromes (11, 16). This decreased the adhesion that was observed between the antibody-functionalized tip and the cell surface (Fig. 2).
Second, we performed force measurements on ΔomcA ΔmtrC double mutant S. oneidensis MR-1 cells. This mutant is deficient in both OmcA and MtrC (19, 23, 24) but produces other proteins native to the outer surface of S. oneidensis MR-1. The resulting force spectra showed a noticeable reduction in binding events for the ΔomcA ΔmtrC double mutant cells (Fig. 2). The binding events that were observed for the double mutant were only nonspecific in nature (Fig. 1). This indicates that the antibodies on the tip do not participate in specific interactions with other proteins on the surface of S. oneidensis MR-1 cells.
As a final control experiment, force measurements were conducted on wild-type S. oneidensis MR-1 cells, using Si3N4 tips conjugated with the PEG linker but not functionalized with polyclonal antibody (unbaited tips). Like the results with the double mutant, the unbaited tips were largely unreactive with the surface of the bacteria (Fig. 2). Those binding events that were observed were nonspecific in nature. Taken together, these results demonstrate that the antibody-coated tips have a specific reactivity with OmcA and MtrC molecules. Furthermore, these force measurements show that MtrC and OmcA are present on the external cell surface when Fe(III) serves as the TEA.
To map the distribution of cytochromes on living cells, Ig-RFM was conducted on living S. oneidensis MR-1 cells that were growing on a hematite (α-Fe2O3) thin film. The conditions for these experiments were as follows. A hematite film was grown on a 10-mm by 10-mm by 1-mm oxide substrate via oxygen plasma-assisted molecular beam epitaxy (14, 16). The cells were grown anaerobically to mid-log phase with Fe(III)-NTA serving as the TEA. Cells were deposited onto the hematite thin film along with anaerobic growth medium that lacked Fe(III)-NTA. The cells were allowed to attach to the hematite surface (without drying) overnight in an anaerobic chamber. The following day, the liquid was carefully removed and immediately replaced with fresh anaerobic solution (pH 7.4). Ig-RFM was performed on the cells by raster scanning an antibody-functionalized AFM tip across the sample surface, thereby creating an affinity map (1). Force curves were collected for a 32-by-32 array. The raw pixilated force-volume data were deconvoluted using a regularized filter algorithm. The total time to acquire a complete image was approximately 20 min.
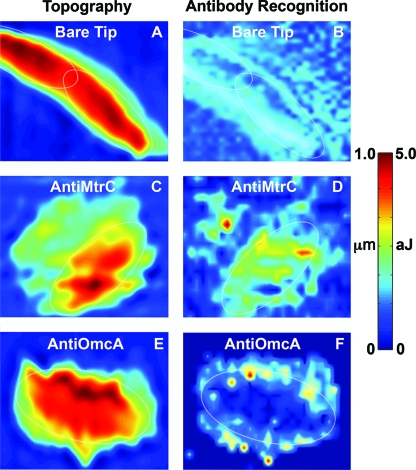
As noted above, attractive interactions between an antibody tip and cell resulted in relatively short-range, nonspecific and longer-range, specific adhesive forces (Fig. 1C). To distinguish between these two interactions, we integrated each force curve beginning at >20 nm and ending at the full retraction of the piezoelectric motor (∼1,800 nm). This integration procedure quantifies the work of binding, measured in joules, between the antibody tip and a particular position on the sample. While this integration procedure does not totally exclude nonspecific binding, it does select for those events associated primarily with specific antibody-antigen binding. Figure 3 is the antibody-cytochrome recognition images for MtrC and OmcA. The corresponding height (or topography) images of the bacterial cells are also shown in Fig. 3.
FIG. 3.
Ig-RFM of live S. oneidensis MR-1 cells deposited on a hematite (α-Fe2O3) thin film. Height image (A) and corresponding Ig-RFM image (B) for a bare unfunctionalized Si3N4 tip. Height and corresponding Ig-RFM image for a tip functionalized with anti-MtrC (C and D) or anti-OmcA (E and F). Each panel contains a thin white oval showing the approximate location of the bacterium on the hematite surface. A color-coded scale bar is shown on the right (height in micrometers [μm], and the work required to separate the tip from the surface in attojoules [aJ]).
OmcA molecules were concentrated at the boundary between the bacterial cell and hematite surface (Fig. 3E and F). MtrC molecules were also detected at the edge of a cell (Fig. 3C and D). Some MtrC, unlike OmcA, was observed on the cell surface distal from the point of contact with the mineral (Fig. 3C and D). Both OmcA and MtrC were also present in an extracellular polymeric substance (EPS) on the hematite surface (Fig. 3D and F), which is consistent with previous results showing MtrC and OmcA in an EPS produced by cells under anaerobic conditions (19, 24). This discovery is interesting in light of the research by Rosso et al. (22) and Bose et al. (4), who found that Shewanella can implement a nonlocal electron transfer strategy to reduce the surface of hematite at locations distant from the point of cell attachment. Rosso et al. (22) proposed that the bacteria utilize unknown extracellular factors to access the most energetically favorable regions of the Fe(III) oxide surface. The Ig-AFM results (Fig. 3) suggest the possibility that MtrC and/or OmcA are the “unknown extracellular factors” that are synthesized by Shewanella to reduce crystalline Fe(III) oxides at points distal from the cell. Additional experiments showing reductive dissolution features coinciding with the extracellular location of MtrC and/or OmcA would need to be performed to test this hypothesis.
It is important to note that these affinity maps were collected on only a few cells because it so challenging to produce large numbers of quality images. Future work should be conducted on a population of cells. Until this time, these affinity maps can be used to provide a crude, lowest-order estimate of the number of cytochromes on the outer surface of living S. oneidensis MR-1. For example, there were 236 force curves collected on the bacterium shown in Fig. 3D. Thirty-eight of these curves exhibited a distinct, sawtooth-shaped, antibody-antigen binding event. In other words, MtrC molecules were detected in one out of every six force curves (16%) that were collected on the cell surface.
This probability can be compared to other independent studies that estimated the density and size of MtrC and OmcA molecules from S. oneidensis MR-1. Lower et al. (16) estimated that S. oneidensis has 4 × 1015 to 7 × 1015 cytochromes per square meter by comparing AFM measurements for whole cells to force curves on purified MtrC and OmcA molecules. Wigginton et al. (25) used scanning tunneling microscopy to determine that the diameter of an individual cytochrome is 5 to 8 nm. These values can be used to create a simple, geometric, close-packing arrangement of MtrC or OmcA molecules on a surface. Using this approach, cytochromes could occupy 8 to 34% of the cell surface.
This estimate is consistent with the observed number of putative MtrC molecules shown in Fig. 3D. Therefore, it appears that these affinity maps can be used as a lowest-order estimate for the number of cytochromes on S. oneidensis MR-1 even though we do not know a priori the exact configuration of the antibody tip (e.g., the concentration of antibody on the tip, the exact shape of the tip, the binding epitopes within the antibody).
In summary, the data presented here show that S. oneidensis MR-1 localizes OmcA and MtrC molecules to the exterior cell surface, including an EPS, when Fe(III) is the TEA. Here, the cytochromes presumably serve as terminal reductases that catalyze the reduction of Fe(III) through direct contact with the extracellular iron-oxide mineral.
Acknowledgments
B.H.L. acknowledges the support of the DOE-OBES Geosciences Research Program, L.S. the support of the DOE-OBER Genomics-Genomes to Life Program, and S.K.L. the support of the National Science Foundation (EAR-0745808). A portion of this research was performed as part of the Biogeochemistry Grand Challenge project at the W. R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE-OBER Program and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated for the U.S. DOE by Battelle Memorial Institute under contract number DE-AC05-76RLO1830.
We are grateful to J. K. Fredrickson and J. M. Zachara for helpful discussions, the suggestions of the anonymous reviewers, and the efforts and comments of the editor. We are forever grateful for the advice given to us by Terry J. Beveridge.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Almqvist, N., R. Bhatia, G. Primbs, N. Desai, S. Banerjee, and R. Lal. 2004. Elasticity and adhesion force mapping reveals real-time clustering of growth factor receptors and associated changes in local cellular rheological properties. Biophys. J. 86:1753-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 3.Biju, V., D. Pan, Y. A. Gorby, J. Fredrickson, J. McLean, D. Saffarini, and H. P. Lu. 2007. Combined spectroscopic and topographic characterization of nanoscale domains and their distributions of a redox protein on bacterial cell surfaces. Langmuir 23:1333-1338. [DOI] [PubMed] [Google Scholar]
- 4.Bose, S., M. F. Hochella, Jr., Y. A. Gorby, D. W. Kennedy, D. E. McCready, A. S. Madden, and B. H. Lower. 2009. Bioreduction of hematite nanoparticles by the dissimilatory iron reducing bacterium Shewanella oneidensis MR-1. Geochim. Cosmochim. Acta 73:962-976. [Google Scholar]
- 5.Chtcheglova, L. A., J. Waschke, L. Wildling, D. Drenckhahn, and P. Hinterdorfer. 2007. Nano-scale dynamic recognition imaging on vascular endothelial cells. Biophys. J. 93:L11-L13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebner, A., P. Hinterdorfer, and H. J. Gruber. 2007. Comparison of different aminofunctionalization strategies for attachment of single antibodies to AFM cantilevers. Ultramicroscopy 107:922-927. [DOI] [PubMed] [Google Scholar]
- 7.Ebner, A., L. Wildling, A. S. Kamruzzahan, C. Rankl, J. Wruss, C. D. Hahn, M. Holzl, R. Zhu, F. Kienberger, D. Blaas, P. Hinterdorfer, and H. J. Gruber. 2007. A new, simple method for linking of antibodies to atomic force microscopy tips. Bioconjug. Chem. 18:1176-1184. [DOI] [PubMed] [Google Scholar]
- 8.Fredrickson, J. K., and J. M. Zachara. 2008. Electron transfer at the microbe-mineral interface: a grand challenge in biogeochemistry. Geobiology 6:245-253. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert, Y., M. Deghorain, L. Wang, B. Xu, P. D. Pollheimer, H. J. Gruber, J. Errington, B. Hallet, X. Haulot, C. Verbelen, P. Hols, and Y. F. Dufrene. 2007. Single-molecule force spectroscopy and imaging of the vancomycin/d-Ala-d-Ala interaction. Nano Lett. 7:796-801. [DOI] [PubMed] [Google Scholar]
- 10.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 11.Hinterdorfer, P., W. Baumgartner, H. J. Gruber, K. Schilcher, and H. Schindler. 1996. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl. Acad. Sci. USA 93:3477-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutter, J., and J. Bechhoefer. 1993. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64:1868-1873. [Google Scholar]
- 13.Kienberger, F., H. Mueller, V. Pastushenko, and P. Hinterdorfer. 2004. Following single antibody binding to purple membranes in real time. EMBO Rep. 5:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y. J., Y. Gao, and S. A. Chambers. 1997. Selective growth and characterization of pure, epitaxial alpha-Fe2O3(0001) and Fe3O4(001) films by plasma-assisted molecular beam epitaxy. Surf. Sci. 371:358-370. [Google Scholar]
- 15.Lower, B. H., M. F. Hochella, Jr., and S. K. Lower. 2005. Putative mineral-specific proteins synthesized by a metal reducing bacterium. Am. J. Sci. 305:687-710. [Google Scholar]
- 16.Lower, B. H., L. Shi, R. Yongsunthon, T. C. Droubay, D. E. McCready, and S. K. Lower. 2007. Specific bonds between an iron oxide surface and outer membrane cytochromes MtrC and OmcA from Shewanella oneidensis MR-1. J. Bacteriol. 189:4944-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lower, B. H., R. Yongsunthon, F. P. Vellano III, and S. K. Lower. 2005. Simultaneous force and fluorescence measurements of a protein that forms a bond between a living bacterium and a solid surface. J. Bacteriol. 187:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lower, S. K., M. F. Hochella, Jr., and T. J. Beveridge. 2001. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and alpha-FeOOH. Science 292:1360-1363. [DOI] [PubMed] [Google Scholar]
- 19.Marshall, M. J., A. S. Beliaev, A. C. Dohnalkova, D. W. Kennedy, L. Shi, Z. Wang, M. I. Boyanov, B. Lai, K. M. Kemner, J. S. McLean, S. B. Reed, D. E. Culley, V. L. Bailey, C. J. Simonson, D. A. Saffarini, M. F. Romine, J. M. Zachara, and J. K. Fredrickson. 2006. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 4:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers, C. R., and J. M. Myers. 2004. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett. Appl. Microbiol. 39:466-470. [DOI] [PubMed] [Google Scholar]
- 22.Rosso, K. M., J. M. Zachara, J. K. Fredrickson, Y. A. Gorby, and S. C. Smith. 2003. Nonlocal bacterial electron transfer to hematite surfaces. Geochim. Cosmochim. Acta 67:1081-1087. [Google Scholar]
- 23.Shi, L., B. Chen, Z. Wang, D. A. Elias, M. U. Mayer, Y. A. Gorby, S. Ni, B. H. Lower, D. W. Kennedy, D. S. Wunschel, H. M. Mottaz, M. J. Marshall, E. A. Hill, A. S. Beliaev, J. M. Zachara, J. K. Fredrickson, and T. C. Squier. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 188:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi, L., S. Deng, M. J. Marshall, Z. Wang, D. W. Kennedy, A. C. Dohnalkova, H. M. Mottaz, E. A. Hill, Y. A. Gorby, A. S. Beliaev, D. J. Richardson, J. M. Zachara, and J. K. Fredrickson. 2008. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J. Bacteriol. 190:5512-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigginton, N. S., K. M. Rosso, B. H. Lower, L. Shi, and M. F. Hochella, Jr. 2007. Electron tunneling properties of outer-membrane decaheme cytochromes from Shewanella oneidensis. Geochim. Cosmochim. Acta 71:543-555. [Google Scholar]
- 26.Willemsen, O. H., M. M. Snel, K. O. van der Werf, B. G. de Grooth, J. Greve, P. Hinterdorfer, H. J. Gruber, H. Schindler, Y. van Kooyk, and C. G. Figdor. 1998. Simultaneous height and adhesion imaging of antibody-antigen interactions by atomic force microscopy. Biophys. J. 75:2220-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yongsunthon, R., V. G. Fowler, Jr., B. H. Lower, F. P. Vellano III, E. Alexander, L. B. Reller, G. R. Corey, and S. K. Lower. 2007. Correlation between fundamental binding forces and clinical prognosis of Staphylococcus aureus infections of medical implants. Langmuir 23:2289-2292. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, H., X. Tang, G. R. Munske, N. Zakharova, L. Yang, C. Zheng, M. A. Wolff, N. Tolic, G. A. Anderson, L. Shi, M. J. Marshall, J. K. Fredrickson, and J. E. Bruce. 2008. In vivo identification of the outer membrane protein OmcA-MtrC interaction network in Shewanella oneidensis MR-1 cells using novel hydrophobic chemical cross-linkers. J. Proteome Res. 7:1712-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]