Abstract
Nanotechnology enables development and production of novel silver-based composite materials. We used in vitro tests to demonstrate the antimicrobial activity of a silver-silica nanocomposite compared to the activities of conventional materials, such as silver nitrate and silver zeolite. A silver-silica-containing polystyrene material was manufactured and shown to possess strong antimicrobial properties.
Many applications, including medicine and food production and storage, would benefit greatly from incorporation of safe and inexpensive long-lasting biocides into polymers, paints, or textiles (1, 5). The antimicrobial effect of silver additives is broadly used in various injection-molded plastic products, in textiles (13), and in coating-based applications, including air ducts, countertops, and food preparation areas (12). Some important advantages of silver-based antimicrobials are their excellent thermal stability and their health and environmental safety (19). However, like the use of all biocide products, the use of silver is strictly controlled by various national laws and control agencies. In the United States, the Environmental Protection Agency has regulated the use of silver as a biocide since 1954 (2) under the Federal Insecticide Fungicide and Rodenticide Act. In the European Union, a European biocide product directive (EU/BPD/98) imposes regulatory requirements on the use and claims associated with all biocide products (3).
In the past few years, there has been a tremendous push for development of inorganic nanoparticles with structures that exhibit novel physical, chemical, and biological properties (34). In particular, the potential benefits of nano-silver materials have been recognized by many industries due to the strong antimicrobial activity of silver against a broad spectrum of bacteria, viruses, and fungi and the low frequency of development of resistance (10, 30).
Generally, silver-based antimicrobial additives consist of silver ions integrated into inert matrices consisting of ceramic, glass, or zeolite. Other silver additives based on silver salts or metallic silver may be readily incorporated into thermoplastic polymers, such as polyethylene, polypropylene, polystyrene, or nylon (5). The bactericidal efficacy of silver-containing polymers is based on the release of silver ions (Ag+) through interaction with a liquid watery phase (19). Although the antimicrobial effects of silver ions and salts have been intensively studied, the mechanism of the inhibitory action of silver on microbes is still not fully understood. It has been proposed that silver ions interact with disulfide or sulfhydryl groups of enzymes, causing structural changes that lead to disruption of metabolic processes followed by cell death (8, 11). The inhibitory action of silver nanoparticles is also based on the release of Ag+ (20, 24). Exposure of microorganisms to silver nanoparticles was shown to result in strong antimicrobial activity (6, 9, 26, 33). In addition to the increased surface area and associated increased potential for the release of Ag+, when dispersed in liquid suspensions, silver nanoparticles may accumulate in the bacterial cytoplasmic membrane, causing a significant increase in permeability and cell death (33), and penetrate bacterial cells (28). Recently, it has been suggested that the antimicrobial mechanism of silver nanoparticles may also be related to membrane damage due to free radicals that are derived from the surface of the nanoparticles (16). This bactericidal activity also appears to be dependent on the size and shape of the silver nanoparticles (25).
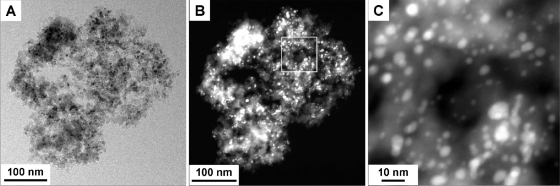
In this study, we evaluated the properties of a novel silver-silica nanocomposite material (HeiQ AGS-20; HeiQ Materials, Bad Zurzach, Switzerland) used as an antimicrobial additive and compared its efficacy to the efficacies of the conventional silver additives silver nitrate (AgNO3; 63.5% Ag) and silver zeolite (38% Ag bound to type A zeolite; Sigma-Aldrich, Buchs, Switzerland). The novel silver-silica nanocomposite material was produced using an industrial flame spray pyrolysis process. This process involves combustion of a flammable solvent containing homogeneously dissolved compounds as the source of components for the synthesis of the material (14, 21, 22). A representative transmission electron micrograph of the silver-silica material is shown in Fig. 1. The nanocomposite consists of silver nanoparticles embedded in a matrix of amorphous silicon dioxide (SiO2). The SiO2 fine structure consists of aggregate matrix particles with an average diameter of approximately 1 μm (Fig. 1A). Silver metal particles are located on the surface of the silica and are also embedded within the matrix (Fig. 1B). High-magnification scanning transmission electron microscopy imaging of a localized region of a nanoparticle indicated that each silica particle contains many small silver metal particles with a typical diameter between 1 and 10 nm (Fig. 1C). The specific surface area of the nanocomposite powder, as measured by nitrogen adsorption (7), is typically about 250 m2/g, a value which is consistent with the open structure of the silica aggregate shown in Fig. 1. It can be concluded that, upon contact with moisture, the pure silver particles act as a source that releases silver ions, which represent the active antimicrobial principle (27). Some key advantages of the novel nanocomposite are the dispersion of the discrete silver particles throughout the silica (which prevents agglomeration of the silver particles), the small diameter of the silver particles (which results in a large surface area and release of a large amount of Ag+, which results in high antimicrobial efficiency), and the small size of the silver-silica composite (ca. 1 μm) (which allows the material to be uniformly dispersed and readily incorporated into a variety of substrates, including synthetic fibers, plastics, and other thin or delicate materials). The silica structure acts as a convenient carrier for incorporating the fine silver particles into plastics, textiles, and coatings. A further advantage is that the immobilization of silver nanoparticles within the silica structure limits the potential for release and disposal of the nanoparticles themselves. This property may be highly desirable because of the possible abilities of nanoparticles to cross biological membranes and other barriers (31).
FIG. 1.
(A) Transmission electron micrograph showing an amorphous silicon dioxide aggregate particle (gray structure) together with numerous supported silver metal particles (dark spots). (B) Scanning transmission electron micrograph of the structure shown in panel A, providing better contrast between the silica structure (gray) and the silver metal particles (bright spots). (C) Higher magnification of the region in panel B enclosed in a box. The silver metal particles are typically between 1 and 10 nm in diameter. Transmission electron microscope and scanning transmission electron microscope images were obtained using an FEI Tecnai F30 FEG microscope operated at 300 kV.
The microorganisms and growth conditions used for antimicrobial testing are shown in Table 1. The MICs for all combinations of silver materials and microorganisms were determined by preparing twofold serial dilutions of the additives in an appropriate growth medium (Table 1). The tubes were then inoculated with 107 CFU/ml from overnight cultures of the bacteria or 106 CFU/ml for Candida albicans and incubated on a shaker (180 rpm) for 24 h. The MIC was defined as the lowest concentration of the silver additive at which no visual turbidity of the growth medium developed. The minimal bactericidal concentration (MBC) was determined by surface plating 0.2-ml aliquots from the nonturbid tubes, followed by incubation at 37°C for 24 h. The MBC was defined as the lowest concentration of silver additive resulting in less than 200 colonies per plate (corresponding to a killing rate of more than 4 logs). Aspergillus niger spores were harvested by floating the spores in densely grown lawns on malt extract agar plates in an extraction buffer (0.1% [vol/vol] Tween 20, 145 mM sodium chloride, 20 mM sodium phosphate; pH 7.4) and removing them. The MIC was determined by spreading approximately 200 spores on malt extract agar plates containing serial dilutions of the silver additives (Table 1) and was defined as the lowest concentration that prevented visible growth after 72 h of incubation at 30°C.
TABLE 1.
Comparison of the antimicrobial activities of silver nanocomposite powder, silver nitrate, and silver zeolite
| Microorganism | Silver nanocomposite
|
Silver nitrate
|
Silver zeolite
|
|||
|---|---|---|---|---|---|---|
| MIC (μg/ml)a | MBC or MFC (μg/ml)b | MIC (μg/ml)a | MBC or MFC (μg/ml)b | MIC (μg/ml)a | MBC or MFC (μg/ml)b | |
| Escherichia coli ATCC 2732c | 62.5 | 125 | 7.8 | 15.6 | 3.9 | 15.6 |
| Klebsiella pneumoniae ATCC 4352c | 62.5 | 125 | 3.9 | 7.8 | 7.8 | 31.2 |
| Pseudomonas fluorescens LME 2333d | 62.5 | 250 | 7.8 | 7.8 | 15.6 | 31.2 |
| Salmonella enterica serovar Enteritidis D1c | 62.5 | 250 | 3.9 | 7.8 | 15.6 | 62.5 |
| Salmonella enterica serovar Typhimurium DB 7155c | 62.5 | 250 | 3.9 | 15.6 | 15.6 | 31.2 |
| Enterococcus faecalis ATCC 19433e | 62.5 | 250 | 3.9 | 7.8 | 7.8 | 7.8 |
| Bacillus cereus ATCC 14579e | 250 | 500 | 31.2 | 31.2 | 62.5 | 250 |
| Listeria monocytogenes Scott Af | 500 | 1,000 | 31.2 | 31.2 | 31.2 | 62.5 |
| Staphylococcus aureus ATCC 29213f | 250 | 1,000 | 15.6 | 15.6 | 15.6 | 125 |
| Candida albicans ATCC 10259g | 125 | 2,000 | 31.2 | 250 | 62.5 | 250 |
| Aspergillus niger ATCC 9642h | 2,000 | NDi | 15.6 | ND | 125 | ND |
The MIC was determined at least in duplicate.
The MBC or minimum fungicidal concentration (MFC) was determined at least in duplicate.
Cultured in Luria-Bertani broth (10 g/liter peptone from casein, 5 g/liter yeast extract, 10 g/liter NaCl; Merck, Darmstadt, Germany) at 37°C.
Cultured in Biotone tryptose broth (Biolife, Milan, Italy) at 30°C.
Cultured in Biotone tryptose broth (Biolife, Milan, Italy) at 37°C.
Cultured in half-strength brain heart infusion broth (Biolife, Milan, Italy) at 37°C.
Cultured in malt extract broth (Merck, Darmstadt, Germany) at 37°C.
Cultured on malt extract agar (Merck, Darmstadt, Germany) at 30°C. The MIC for A. niger was defined as the lowest concentration not associated with visible growth on malt extract agar after 72 h of incubation at 30°C.
ND, not determined.
MICs and MBCs for the silver additives tested are shown in Table 1. All experiments were performed at least in duplicate. For bacteria, the MICs of the nanocomposite material ranged from 62.5 to 500 μg/ml, corresponding to 12.5 to 100 μg pure Ag/ml. The MICs of silver nitrate varied from 3.9 to 31.2 μg/ml (corresponding to 2.4 to 19.8 μg Ag/ml), and the MICs of silver zeolite ranged from 3.9 to 31.2 μg/ml (corresponding to 2 to 12 μg Ag/ml). The MBCs determined were in the ranges from 125 to 1,000 μg/ml for the nanocomposite powder, from 7.8 to 31.2 μg/ml for silver nitrate, and from 7.8 to 125 μg/ml for silver zeolite. Growth of C. albicans was inhibited by 125 μg/ml silver nanocomposite, and the minimal fungicidal concentration was 2 mg/ml. Development of visible colonies of A. niger on agar plates was also completely inhibited by 2 mg silver nanocomposite per ml agar.
In general, gram-positive bacteria appeared to be more tolerant to silver than gram-negative cells (Table 1), except for Enterococcus faecalis, for which the MICs and MBCs were similar to those for gram-negative bacteria. It has previously been reported that gram-positive bacteria are less susceptible to the antimicrobial activity of silver (15, 16, 29). It was speculated that this may be due to differences in the cell wall structure (15). The cell wall of gram-positive bacteria contains multiple layers of peptidoglycan compared to the cell wall of gram-negative bacteria. Peptidoglycan is a complex structure and often contains teichoic acids or lipoteichoic acids which have a strong negative charge, which may contribute to sequestration of free Ag+ ions. Thus, gram-positive bacteria may allow less Ag+ to reach the cytoplasmic membrane than gram-negative bacteria allow (15) and may therefore be less susceptible.
Susceptibility tests using different silver compounds in previous studies revealed that the MICs of silver particles for Escherichia coli ranged from 2 to 75 μg/ml (24, 32). However, because corresponding silver concentrations were not specified, it is not possible to compare these values to our results. For silver zeolite containing 1.9% (wt/wt) Ag, the previously reported MICs determined by using a similar protocol ranged from 256 to 2,048 μg/ml, corresponding to 4.8 to 38.4 μg/ml of Ag (15). Here we used silver zeolite containing 38% (wt/wt) Ag to determine the MIC for E. coli. The MIC determined (1.9 to 3.9 μg/ml) was much lower than the previously reported MICs of the silver zeolite containing 1.9% Ag. The nanocomposite material had an MIC of 62.5 μg/ml (12.5 μg Ag/ml) for E. coli. Not considering the relative Ag content, silver nitrate and silver zeolite (38% Ag) resulted in inhibition that was approximately 10 times more effective than the inhibition observed with the nanocomposite. This can be explained by the fact that in aqueous systems silver nitrate dissolves completely and the silver is completely available in its biologically active ionic form. The silver ions held in the zeolite structure are also relatively rapidly released into solution. In contrast, the silver nanoparticles embedded within the silica matrix release Ag+ in a more gradual, controlled manner and at a much lower rate (18). Thus, although silver nitrate and silver zeolite are more effective in applications where high Ag+ concentrations are required immediately, the effect is only short lived. In contrast, the nanocomposite powder allows slow and controlled release of Ag+, resulting in long-term antimicrobial activity. This should be a clear advantage in any long-term antimicrobial applications (e.g., contact surfaces, fibers, plastics, medical devices, food-manufacturing equipment, cutting boards, etc.).
To examine the antimicrobial properties of a typical application product, silver-containing polystyrene plates were manufactured from commercially available polystyrene polymer (clear, unfilled) using a thermoplastic injection-molding process (17, 23). Test coupons that were 50 by 50 by 1.5 mm and contained the nanocomposite material (0.25% [wt/wt], corresponding to approximately 500 ppm Ag) were produced by dry blending the polystyrene polymer with the required amount of polymer concentrate containing the silver nanocomposite additive, which was followed by injection molding. The antimicrobial activity was determined by using the Japanese industrial standard test (JIS Z 2801:2000) (4). In brief, the test samples were placed in petri dishes and inoculated with 0.4 ml of a bacterial culture containing 105 to 106 CFU/ml. The inoculum was covered with a polyester film (X-131 transparent copier film; Folex Imaging), and the petri dishes were incubated at 37°C for 24 h in a humid chamber to prevent desiccation. After the incubation period 20 ml of extraction solution (0.1% [vol/vol] Tween 20, 145 mM sodium chloride, 20.5 mM sodium phosphate; pH 7.4) was added to the petri dishes and shaken for 2 min. Subsequently, serial dilutions of the extraction solution were spread on agar plates in triplicate and incubated at 37°C overnight. Colonies were counted visually, and the numbers of CFU per sample were determined. The activity value was calculated from the mean value for the individual samples by subtraction of the log value determined for the test sample from the log value determined for the control. The results for viable counts determined for the control and the silver nanocomposite-containing samples are shown in Table 2. The activity values determined by the JIS Z 2801:2000 method (4) were 4.4 for E. coli and 2.1 for Staphylococcus aureus (P < 0.05, Student's t test [n = 3]). The results demonstrate that the silver-silica nancomposite-containing polystyrene material has significant antibacterial activity against both E. coli and S. aureus.
TABLE 2.
Antimicrobial activity of silver nanocomposite-containing polystyrene platesa
| Organism | No. of cells (CFU/sample)b
|
Activity | ||
|---|---|---|---|---|
| Polystyrene control after inoculation | Polystyrene control after 24 h | Polystyrene with silver after 24 h | ||
| Escherichia coli | 1.7 × 105 ± 4.9 × 104 | 2.6 × 106 ± 6.5 × 104 | <100 | 4.4 |
| Staphylococcus aureus | 1.7 × 105 ± 7.9 × 104 | 1.8 × 105 ± 6.9 × 104 | 1.4 × 103 ± 9.0 × 102 | 2.1 |
Activity was tested by using Japanese industrial standard JIS Z 2801:2000 (4).
The values are means ± standard deviations for measurements obtained for three independent sample pieces. The values for the polystyrene control after 24 h and polystyrene with silver after 24 h were significantly different according to the Student t test (P < 0.05; n = 3).
In this study, a silver-silica nanocomposite material with a novel structure and composition was investigated to determine its antimicrobial properties. The material exhibited very good antimicrobial activity against a wide range of microorganisms. The inhibition of microbial growth due to surface contact with the silver-silica nanocomposite-containing polystyrene demonstrated that materials functionalized with the silver nanocomposite have excellent antimicrobial properties. Further studies of the mode of action of the silver-silica nanocomposite material with gram-positive and gram-negative bacteria and also with yeasts and molds are required to fully evaluate its potential for use as an antimicrobial additive in various materials.
Acknowledgments
We are grateful to Joos Kiener for excellent technical assistance and to Elisabeth Müller Gubler from the Electron Microscopy Center of ETH Zürich for the electron microscopy images. M.J.H. thanks S. E. Pratsinis for his suggestions and ideas concerning synthesis of the material and the Particle Technology Laboratory, Department of Mechanical and Process Engineering at the Swiss Federal Institute of Technology (ETH Zurich), for supporting initial development of the material.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Adams, A. P., E. M. Santschi, and M. A. Mellencamp. 1999. Antibacterial properties of a silver chloride-coated nylon wound dressing. Vet. Surg. 28:219-225. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1993. EPA reregistration eligibility document for silver, case 4082. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/oppsrrd1/REDs/old_reds/silver-pdf.
- 3.Anonymous. 1998. Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market. Off. J. Eur. Communities L123/1. http://ec.europa.eu/environment/biocides/pdf/dir_98_8_biocides.pdf.
- 4.Anonymous. 2000. JIS Z 2801:2000. Antimicrobial products—test for antimicrobial activity and efficacy. Japanese Standards Association, Tokyo, Japan.
- 5.Appendini, P., and J. H. Hotchkiss. 2002. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 3:113-126. [Google Scholar]
- 6.Baker, C., A. Pradhan, L. Pakstis, D. J. Pochan, and S. Ismat Shah. 2005. Synthesis and antimicrobial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 5:244-249. [DOI] [PubMed] [Google Scholar]
- 7.Brunauer, S., P. H. Emmett, and E. Teller. 1938. Adsorption of gases in multimolecular layer. J. Am. Chem. Soc. 60:309-319. [Google Scholar]
- 8.Butkus, M. A., L. Edling, and M. P. Labare. 2003. The efficacy of silver as a bactericidal agent: advantages, limitations and considerations for future use. J. Water Supply Res. Technol. AQUA 52:407-416. [Google Scholar]
- 9.Cho, K.-H., J.-E. Park, T. Osaka, and S.-G. Park. 2005. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 51:956-960. [Google Scholar]
- 10.Damm, C., M. Neumann, and H. Münstedt. 2006. Properties of nanosilver coatings on polymethyl methacrylate. Soft Mater. 3:71-88. [Google Scholar]
- 11.Feng, Q. L., J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim, and J. O. Kim. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 52:662-668. [DOI] [PubMed] [Google Scholar]
- 12.Galeano, B., E. Korff, and W. L. Nicholson. 2003. Inactivation of vegetative cells, but not spores, of Bacillus anthracis, B. cereus, and B. subtilis on stainless steel surfaces coated with an antimicrobial silver- and zinc-containing zeolite formulation. Appl. Environ. Microbiol. 69:4329-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, Y., and R. Cranston. 2008. Recent advances in antimicrobial treatment of textiles. Text. Res. J. 78:60-72. [Google Scholar]
- 14.Height, M. J., and S. E. Pratsinis. February 2006. Antimicrobial and antifungal powders made by flame spray pyrolysis. European patent EP1846327.
- 15.Kawahara, K., K. Tsuruda, M. Morishita, and M. Uchida. 2000. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 16:452-455. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. S. K., E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong, and M.-H. Cho. 2007. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 3:95-101. [DOI] [PubMed] [Google Scholar]
- 17.Ku, P. L. 1988. Polystyrene and styrene copolymers. I. Their manufacture and application. Adv. Polym. Technol. 8:177-196. [Google Scholar]
- 18.Kumar, R., S. Howdle, and H. Münstedt. 2005. Polyamide/silver antimicrobials: effect of filler types on the silver ion release. J. Biomed. Mater. Res. Part B 75:311-319. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, R., and H. Münstedt. 2005. Polyamide/silver antimicrobials: effect of crystallinity on the silver ion release. Polym. Int. 54:1180-1186. [Google Scholar]
- 20.Lok, C.-N., C.-M. Ho, R. Chen, Q.-Y. He, W.-Y. Yu, H. Sun, P. K.-H. Tam, J.-F. Chiu, and C.-M. Che. 2007. Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 12:527-534. [DOI] [PubMed] [Google Scholar]
- 21.Mädler, L., H. K. Kammler, R. Mueller, and S. E. Pratsinis. 2002. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 33:369-389. [Google Scholar]
- 22.Mädler, L., W. Stark, and S. E. Pratsinis. 2003. Simultaneous deposition of gold nanoparticles during flame synthesis of titania and silica. J. Mater. Res. 18:115-120. [Google Scholar]
- 23.Martin, M. F., J. P. Viola, and J. R. Wuensch. 2003. Preparation, properties and applications of high-impact polystyrene, p. 247-280. In J. Scheirs and D. B. Priddy (ed.), Modern styrenic polymers: polystyrenes and styrenic copolymers. John Wiley & Sons, New York, NY.
- 24.Morones, J. R., J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez, and M. J. Yacaman. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346-2353. [DOI] [PubMed] [Google Scholar]
- 25.Pal, S., Y. K. Tak, and J. M. Song. 2007. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73:1712-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panáček, A., L. Kvítek, R. Prucek, M. Kolár, R. Večeřová, N. Pizúrová, V. K. Sharma, T. Nevěčná, and R. Zbořil. 2006. Silver colloid nanoparticles: synthesis, characterization, and their antimicrobial activity. J. Phys. Chem. B 110:16248-16253. [DOI] [PubMed] [Google Scholar]
- 27.Radeshkumar, C., and H. Münstedt. 2006. Antimicrobial polymers from polypropylene/silver composites—Ag+ release measured by anode stripping voltammetry. React. Funct. Polym. 66:780-788. [Google Scholar]
- 28.Raffi, M., F. Hussain, T. M. Bhatti, J. I. Akhter, A. Hameed, and M. M. Hasan. 2008. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 24:192-196. [Google Scholar]
- 29.Rhim, J.-W., S.-I. Hong, H.-M. Park, and P. K. W. Ng. 2006. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 54:5814-5822. [DOI] [PubMed] [Google Scholar]
- 30.Russell, A. D., and W. B. Hugo. 1994. Antimicrobial activity and action of silver. Prog. Med. Chem. 31:351-370. [DOI] [PubMed] [Google Scholar]
- 31.Sanvicens, N., and M. P. Marco. 2008. Multifunctional nanoparticles—properties and prospects for their use in human medicine. Trends Biotechnol. 26:425-433. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar, S., A. D. Jana, S. K. Samanta, and G. Mostafa. 2007. Facile synthesis of silver nano particles with highly efficient anti-microbial property. Polyhedron 26:4419-4426. [Google Scholar]
- 33.Sondi, I., and B. Salopek-Sondi. 2004. Silver nanoparticles as antimicrobial agent: a case study of E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 275:177-182. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, J., P. Takhistov, and D. J. McClements. 2006. Functional materials in food nanotechnology. J. Food Sci. 71:R107-R116. [Google Scholar]