Abstract
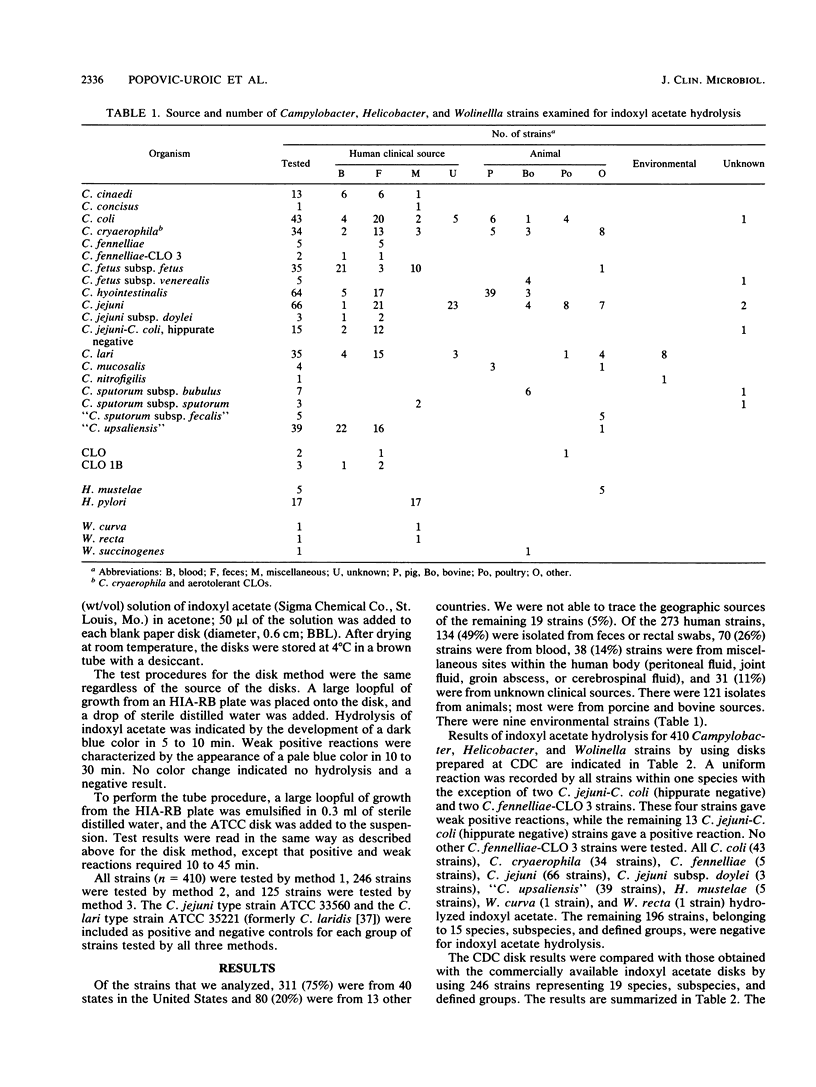
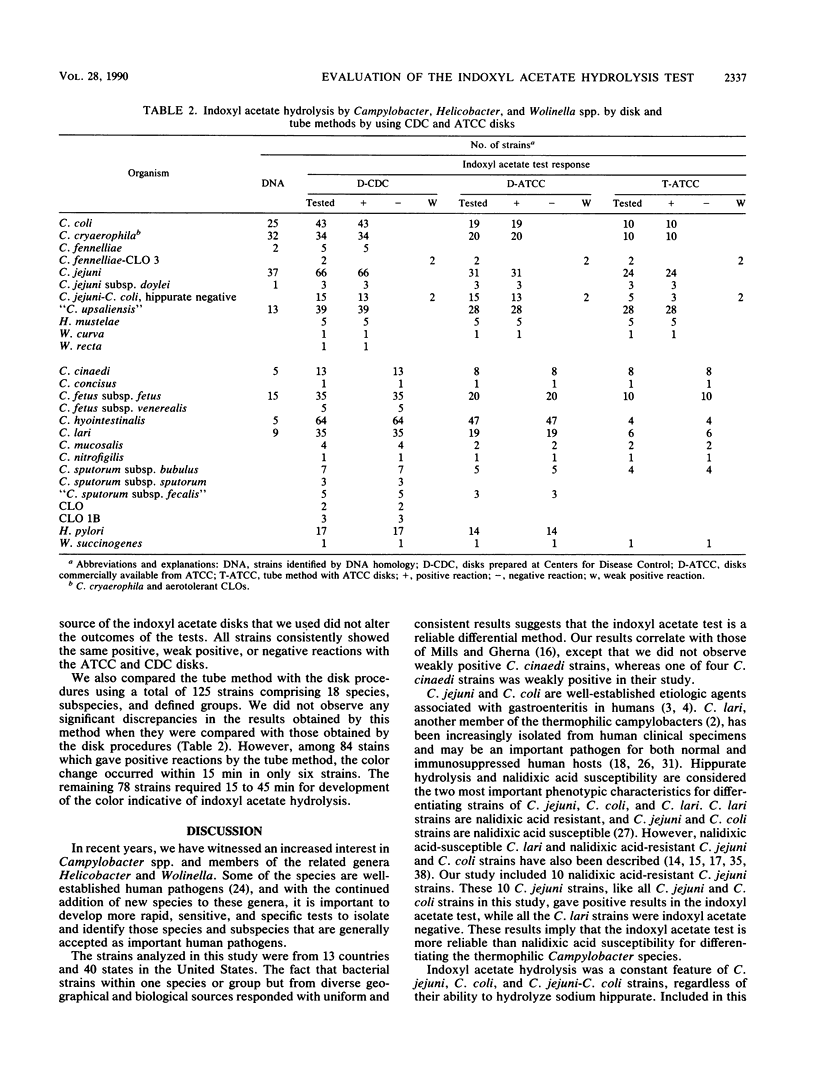
A total of 410 well-defined Campylobacter, Helicobacter, and Wolinella strains, comprising 26 named species, subspecies, and defined groups, were tested for indoxyl acetate hydrolysis by a disk method by using disks prepared at the Centers for Disease Control, Atlanta, Ga. All C. coli (43 strains), C. cryaerophila (34 strains), C. fennelliae (5 strains), C. fennelliae-Campylobacter-like organism 3 (2 strains), C. jejuni (66 strains), C. jejuni subsp. doylei (3 strains), hippurate-negative C. jejuni-C. coli (15 strains), "C. upsaliensis" (39 strains), H. mustelae (5 strains), W. curva (1 strain), and W. recta (1 strain) hydrolyzed indoxyl acetate. Four strains gave weak positive reactions, and the remaining 196 strains, which belonged to 15 species, subspecies, and defined groups, gave negative reactions. Of the 410 study strains, 246 and 125 strains were tested for indoxyl acetate hydrolysis by a disk method and a tube method, respectively, by using commercially produced disks. The disk method, regardless of source, required less time and interpretation than the tube method did. Better differentiation between Campylobacter spp. was obtained with the indoxyl acetate test than with the trimethylamine N-oxide test. The indoxyl acetate disk distinguished C. lari from C. jejuni and C. coli, C. cinaedi from C. fennelliae, and H. pylori from H. mustelae and suggested that W. succinogenes could be differentiated from W. recta and W. curva. The indoxyl acetate disk method could be performed in 5 to 30 min, was easy to read and interpret, and should be useful as a routine diagnostic test for identification of Campylobacter spp.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Cimolai N., Gill M. J., Jones A., Flores B., Stamm W. E., Laurie W., Madden B., Shahrabadi M. S. "Campylobacter cinaedi" bacteremia: case report and laboratory findings. J Clin Microbiol. 1987 May;25(5):942–943. doi: 10.1128/jcm.25.5.942-943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealler S. F., Abbott M., Croughan M. J., Hawkey P. M. Identification of Branhamella catarrhalis in 2.5 min with an indoxyl butyrate strip test. J Clin Microbiol. 1989 Jun;27(6):1390–1391. doi: 10.1128/jcm.27.6.1390-1391.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealler S. F., Hawkey P. M., Millar M. R. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988 Oct;26(10):2152–2156. doi: 10.1128/jcm.26.10.2152-2156.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle G. J., Ley A. Rapid detection of Escherichia coli in urine samples by a new chromogenic beta-glucuronidase assay. J Clin Microbiol. 1989 Apr;27(4):778–779. doi: 10.1128/jcm.27.4.778-779.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. C., Finegold S. M. Uncommonly encountered, motile, anaerobic gram-negative bacilli associated with infection. Rev Infect Dis. 1987 Nov-Dec;9(6):1150–1162. doi: 10.1093/clinids/9.6.1150. [DOI] [PubMed] [Google Scholar]
- Kokeguchi S., Kato K., Kurihara H., Murayama Y. Cell surface protein antigen from Wolinella recta ATCC 33238T. J Clin Microbiol. 1989 Jun;27(6):1210–1217. doi: 10.1128/jcm.27.6.1210-1217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. N., Bowers R. J., Wolfe S. Indoxyl-beta-D-glucuronide, a novel chromogenic reagent for the specific detection and enumeration of Escherichia coli in environmental samples. Can J Microbiol. 1988 May;34(5):690–693. doi: 10.1139/m88-115. [DOI] [PubMed] [Google Scholar]
- Lior H. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli, and "Campylobacter laridis". J Clin Microbiol. 1984 Oct;20(4):636–640. doi: 10.1128/jcm.20.4.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. K., Gherna R. L. Hydrolysis of indoxyl acetate by Campylobacter species. J Clin Microbiol. 1987 Aug;25(8):1560–1561. doi: 10.1128/jcm.25.8.1560-1561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. K., el Sherbeeny M. R., Patton C. M., Kodaka H., Lombard G. L., Edmonds P., Hollis D. G., Brenner D. J. Comparison of four hippurate hydrolysis methods for identification of thermophilic Campylobacter spp. J Clin Microbiol. 1985 Nov;22(5):714–718. doi: 10.1128/jcm.22.5.714-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F., Chevrier D., Desplaces N., Sedallian A., Guesdon J. L. Urease-positive thermophilic Campylobacter (Campylobacter laridis variant) isolated from an appendix and from human feces. J Clin Microbiol. 1988 May;26(5):1050–1051. doi: 10.1128/jcm.26.5.1050-1051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Stowell C., Skalina D., Jones A. M., Hoop R. M., 2nd, Smibert R. M. Campylobacter laridis causing bacteremia in an immunosuppressed patient. Ann Intern Med. 1984 Jul;101(1):55–57. doi: 10.7326/0003-4819-101-1-55. [DOI] [PubMed] [Google Scholar]
- Ng V. L., Hadley W. K., Fennell C. L., Flores B. M., Stamm W. E. Successive bacteremias with "Campylobacter cinaedi" and "Campylobacter fennelliae" in a bisexual male. J Clin Microbiol. 1987 Oct;25(10):2008–2009. doi: 10.1128/jcm.25.10.2008-2009.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Gibbons R. J. Chemotactic response to formate by Campylobacter concisus and its potential role in gingival colonization. Infect Immun. 1986 May;52(2):378–383. doi: 10.1128/iai.52.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Smibert R. M., Johnson J. L., Krieg N. R. Differential characteristics of catalase-positive campylobacters correlated with DNA homology groups. Can J Microbiol. 1984 Jul;30(7):938–951. doi: 10.1139/m84-147. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Wilcox L. Enteritis associated with Campylobacter laridis. J Clin Microbiol. 1987 Jan;25(1):10–12. doi: 10.1128/jcm.25.1.10-12.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Dzink J. L., Ebersole J. L., Socransky S. S. Wolinella recta, campylobacter concisus, bacteroides gracilis, and Eikenella corrodens from periodontal lesions. J Periodontal Res. 1987 Jul;22(4):327–330. doi: 10.1111/j.1600-0765.1987.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Patton C. M., Edmonds P., Barrett T. J., Brenner D. J., Blake P. A. Illness associated with Campylobacter laridis, a newly recognized Campylobacter species. J Clin Microbiol. 1985 Feb;21(2):222–225. doi: 10.1128/jcm.21.2.222-225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Baird R., Dyall-Smith M., Dwyer B. Campylobacter cryaerophila isolated from a human. J Clin Microbiol. 1988 Dec;26(12):2469–2473. doi: 10.1128/jcm.26.12.2469-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Fennell C. L., Tenover F. C., Wezenberg J. M., Perine P. L., Stamm W. E., Holmes K. K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985 Jan;151(1):131–139. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Patton C. M., Tenover F. C., Barrett T. J., Stamm W. E., Steigerwalt A. G., Lin J. Y., Holmes K. K., Brenner D. J. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J Clin Microbiol. 1987 Sep;25(9):1747–1752. doi: 10.1128/jcm.25.9.1747-1752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P., Falsen E., Pot B., Hoste B., Kersters K., De Ley J. Identification of EF group 22 campylobacters from gastroenteritis cases as Campylobacter concisus. J Clin Microbiol. 1989 Aug;27(8):1775–1781. doi: 10.1128/jcm.27.8.1775-1781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins W. D., Rippey S. R., Clavet C. R., Kelley-Reitz D. J., Burkhardt W., 3rd Novel compound for identifying Escherichia coli. Appl Environ Microbiol. 1988 Jul;54(7):1874–1875. doi: 10.1128/aem.54.7.1874-1875.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graevenitz A. Revised nomenclature of Campylobacter laridis, Enterobacter intermedium, and "Flavobacterium branchiophila". Int J Syst Bacteriol. 1990 Apr;40(2):211–211. doi: 10.1099/00207713-40-2-211. [DOI] [PubMed] [Google Scholar]



