Abstract
In this study, we determined the effects of incubation temperature and prior heat treatment on the lag-phase kinetics of individual spores of nonproteolytic Clostridium botulinum Eklund 17B. The times to germination (tgerm), one mature cell (tC1), and two mature cells (tC2) were measured for individual unheated spores incubated at 8, 10, 15, or 22°C and used to calculate the tgerm, the outgrowth time (tC1 − tgerm), and the first doubling time (tC2 − tC1). Measurements were also made at 22°C of spores that had previously been heated at 80°C for 20 s. For unheated spores, outgrowth made a greater contribution to the duration and variability of the lag phase than germination. Decreasing incubation temperature affected germination less than outgrowth; thus, the proportion of lag associated with germination was less at lower incubation temperatures. Heat treatment at 80°C for 20 s increased the median germination time of surviving spores 16-fold and greatly increased the variability of spore germination times. The shape of the lag-time (tC1) and outgrowth (tC1 − tgerm) distributions were the same for unheated spores, but heat treatment altered the shape of the lag-time distribution, so it was no longer homogeneous with the outgrowth distribution. Although heat treatment mainly extended germination, there is also evidence of damage to systems required for outgrowth. However, this damage was quickly repaired and was not evident by the time the cells started to double. The results presented here combined with previous findings show that the stage of lag most affected, and the extent of any effect in terms of duration or variability, differs with both historical treatment and the growth conditions.
Clostridium botulinum is a group of four physiologically and phylogenetically distinct anaerobic spore-forming bacteria (known as groups I, II, III, and IV) that produce the highly toxic botulinum neurotoxin (12). The severity of the intoxication, botulism, ensures considerable effort is directed at preventing the growth of this pathogen in food. Nonproteolytic (group II) C. botulinum is one of the two groups most frequently associated with food-borne botulism. It forms heat-resistant spores and can germinate, grow, and produce toxin at 3°C (8); thus, nonproteolytic C. botulinum is a particular concern in mild heat-treated chilled foods (16, 17).
Spores formed by pathogens such as C. botulinum are a significant food safety issue since they are able to resist many of the processes, such as cooking, used to kill vegetative cells. Understanding the transformation from a dormant spore to active vegetative cells is an important part of quantifying the risk associated with such organisms. Considerable effort has been targeted at measuring and relating the kinetic responses of populations of C. botulinum to environmental conditions and such data have been used to create predictive models, for example, ComBase (www.combase.cc). Such approaches have made a considerable contribution to ensuring food safety but problems with using population based predictions may arise when an initial inoculum is very small or additional information beyond point values is required. Spores typically contaminate foods at low concentrations so that growth of C. botulinum, when it occurs, is likely to initiate from just a few spores. In these circumstances the distribution of times to growth in packs will reflect the heterogeneity of times to growth from the contaminating individual spores. There is an intrinsic variability between individual spores within a population, and the relationship between population lag and individual lag is complex. Consequently, individual lag times cannot be predicted from population measurements (3). Knowledge of the underlying distribution would allow greater refinement of risk assessments.
The lag period between a spore being exposed to conditions suitable for growth and the start of exponential growth will reflect the combined times of germination, emergence, elongation, and first cell division. Currently, very little is known about the variability and duration of these stages and any relationships between them. Measuring the kinetics of spore germination is usually achieved by measuring a population to identify time to percent completion. Such germination curves represent the summation of responses by individual spores. Some authors have measured the biovariability associated with individual spores, but most studies have examined only germination (4-7, 11, 22) and not subsequent outgrowth. More recently, we have used phase-contrast microscopy and image analysis to follow individual spores of nonproteolytic C. botulinum from dormancy, through germination and emergence, to cell division (21, 23). These experiments showed there is very little, or no, relationship between the time spent in each stage by individual spores. We have now extended this work to determine distributions of times for different stages in lag phase as affected by heat treatment and incubation temperature.
MATERIALS AND METHODS
Spore preparation.
Spores of nonproteolytic C. botulinum type B strain Eklund 17B (NCIB 10642) were produced using a two-phase medium prepared under a headspace of 5% CO2, 10% H2, and 85% N2. The solid phase was 250 ml of Robertson's cooked-meat medium (Southern Group Laboratories, London, United Kingdom) with 4.5 g of agar, and the liquid phase was 100 ml of deoxygenated distilled water. The liquid phase of the sporulation medium was inoculated with 500 μl of culture (grown overnight in peptone-yeast extract-glucose-starch [PYGS] broth [20] at 30°C) and incubated for 7 days at 30°C. Spores were harvested from the liquid phase by centrifugation (6,000 × g, 4°C, 15 min), and the resulting pellet was washed five times by centrifugation (as described above) in 0.85% (wt/vol) sterile saline (20 ml). After each wash, the spore suspension was sonicated for 5 min by immersion in an ultrasonic cleaning bath to disperse any spore clumps. Spores were separated from cell debris by discontinuous density gradient centrifugation, as described previously (21). The resulting phase bright spores were washed a further four times by centrifugation (6,000 × g, 4°C, 15 min) in 0.85% (wt/vol) saline (20 ml) that had been passed through a 0.2-μm-pore-size filter. Cleaned spores were resuspended in 1 ml of 0.85% (wt/vol) saline, exposed to 5 min sonication in an ultrasonic cleaning bath to disperse clumps, and then stored at 1°C until required. The viable count was determined on PYGS agar incubated for 2 days at 30°C under a headspace of 10% CO2-90% H2 in an anaerobic jar. Purity and the absence of significant proteolytic activity were checked on Viande-Levure blood agar and reinforced clostridial medium containing 5% (wt/vol) skim milk, respectively, incubated in the same conditions.
Heat treatment.
The times for germination and subsequent growth from individual spores after a heat treatment were measured. It was not possible to distinguish between viable and nonviable dormant spores using phase-contrast microscopy. Therefore, to measure the same number of germinating and outgrowing spores as in the unheated experiments, the total number of spores observed had to be increased as the percentage of viable spores decreased. A heat treatment that would reduce the number of viable spores by a factor of 10 was chosen as a compromise between causing spore damage and having a measurable number of viable spores per slide remaining after heat treatment. Preliminary studies had determined that a 10-fold reduction in the number of CFU on PYGS agar was achieved by using a heat treatment of 20s at 80°C. The viability of the heat treated spores used in the experiments was measured by most-probable-number method in PYGS broth.
Spores were heated at 80°C for 20 s using a submerged tube method based on that of Kooiman and Geers (10). Briefly, glass tubes fitted with a septum-containing cap and filled with 9.9 ml of Sorenson's phosphate buffer (pH 7.0) were preheated fully submerged in a water bath at 80°C. A 100-μl aliquot of concentrated spore suspension (containing 7.8 × 108 spores ml−1) was injected into the submerged tube through the septum using a needle and Hamilton syringe. After 20 s, the inoculated tube was transferred to an ice-water bath and shaken vigorously. Once cool, the spores were used to prepare slides in the same way as unheated spores.
Slide preparation and microscopic observation.
Slides were prepared with spores attached to a defined area. Press-to-Seal adhesive silicon isolators (Grace Bio-Labs, Bend, OR) were used to create one well per slide on the surface of electrostatically charged Superfrost Plus microscope slides (VWR International, Lutterworth, United Kingdom). Wells were filled with spore suspension equivalent to 1.5 × 103 to 3.0 × 103 spores mm−2 of unheated spores or 1.4 × 104 to 1.8 × 104 mm−2 of heat-treated spores and then sealed with a plastic coverslip. The slides were incubated overnight at 1°C to allow spore attachment. The silicon gasket was then removed, and the slide was immersed in distilled water to remove any unattached spores. Slides were dried briefly at 22°C in a circulating air incubator and then stored at 22°C in an anaerobic cabinet under an atmosphere of 5% CO2, 10% H2, and 85% N2. All slides were stored for at least 16 h before use to ensure traces of oxygen had been removed. To initiate the experiment, the deoxygenated spores on a prepared slide were overlaid with molten anaerobic PYGS medium containing 0.5% (wt/vol) agar. Assuming all spores had remained attached during slide preparation, spore concentrations would have been between 5 × 104 ml−1 and 5 × 106 ml−1. A coverslip was pressed onto the molten agar to create a thin agar film and sealed in place with aluminum tape to prevent desiccation and oxygen ingress. Once prepared, the sealed slide was quickly transferred from the anaerobic cabinet to the microscope stage and measurement started. Measurement began between 3 and 9 min after addition of PYGS.
Microscopy.
Spore germination and outgrowth was measured at 8, 10, 15, and 22°C with the temperature maintained using a stage-mounted Peltier device (Linkham Scientific Instruments, Tadworth, United Kingdom) with a temperature controller (Linkham PE60). Individual bacteria were observed by using phase-contrast microscopy at ×40 magnification (Leica ×40/0.70 numerical aperture, PL FLUOTAR objective) on a Leica DMRB optical microscope. The microscope was fitted with an XYZ stage (Marzhauser, Wetzlar-Steindorf, Germany) with a H128 motor controller (Prior Scientific Instruments, Cambridge, United Kingdom), controlled by Image-Pro Plus image analysis software (Media Cybernetics, Silver Spring, MD), which allowed images of multiple set fields to be captured by using a JVC KY-F703 charge-coupled device color digital camera at regular intervals throughout each experiment. Images of unheated spores were acquired every 5 min for 15 h at 22°C. At 15°C, the images were acquired every 5 min for 4 h and then every 10 min for 44 h. At 10°C, they were acquired every 6 min for 4 h and then every 30 min for 68 h. At 8°C, they were acquired every 10 min for 4 h, followed by every 30 min for 44 h, and then every 60 min for 43 h. Heat-treated spores were incubated at 22°C, and images were captured every 10 min for 8 h and then every 20 min for 66 h. Individual images were compiled to give a sequence of frames from each field of view.
Quantification of germination and outgrowth events.
The compiled image sequences allowed individual spores to be followed through dormancy, germination, emergence, elongation. and eventually cell division. Maximum pixel intensity and object length were measured for each spore/cell in each frame of each sequence of images and used to calculate times to germination (tgerm), emergence (tem), the time to a length equivalent to one mature cell (tC1), and the time equivalent to two mature cells (tC2) as described previously (21). Germination time (tgerm) was taken as the time to the midpoint of the fall in pixel intensity as a spore changed from phase bright to phase dark. Emergence (tem) was defined as the time at which a new cell was first observed breaking out of its spore coats. Time to a mature cell (tC1) was measured as the time it took the cell to reach the length equivalent of a single, newly divided exponential cell in PYGS broth culture at 22°C which was 5.5 μm. If lag time is defined as the time between a dormant spore being exposed to conditions suitable for growth and the formation of a mature vegetative cell, then lag time can be represented by tC1. Similarly, outgrowth will be tC1 − tgerm, the time taken for a germination spore to grow to a size equivalent to a mature cell. Doubling time (tC2 − tC1) was defined as the time taken for a cell to increase from the length of one to two cells.
At least three replicate slides were processed for each test condition. Statistical comparisons of the data were made using the data analysis tools in a Microsoft Excel spreadsheet. Homogeneity between the shape of pairs of distribution curves was determined by using a chi-square test. For this test, data were first centered about the same mean and normalized with regard to variability using the formula: normalized value = (value − mean)/standard deviation; before the observed and expected values of the data bins were calculated. Curves were considered homogeneous when the significance level was described above 95% (P > 0.05).
RESULTS
The number of spores observed, percentage reaching each stage, median times, interquartile range, mean times, standard deviations and coefficient of variation for times to germination (tgerm), emergence (tem), the length equivalent to one mature cell (tC1), length equivalent to two mature cells (tC2), outgrowth (tC1 − tgerm), and first doubling time (tC2 − tC1) for each of the test conditions are shown in Table 1. Lowering the incubation temperature did not reduce the proportion of spores able to germinate, emerge, or initiate outgrowth but did increase the time for germination and subsequent outgrowth events.
TABLE 1.
Data for germination and subsequent outgrowth from spores at various incubation temperatures
| Parameter | Incubation temp (°C)
|
||||
|---|---|---|---|---|---|
| 8 | 10 | 15 | 22 | 22 | |
| Spore heat treatment | None | None | None | None | 20 s at 80°C |
| No. of spores observed | 370 | 1,163 | 1,130 | 1,739 | 5,348 |
| % Germination | 95.1 | 94.6 | 96.9 | 83.2 | 30.6 |
| % Emerged | 85.7 | 85.0 | 86.5 | 67.5 | 8.8 |
| % Growing to one cell length (5.5 μm) | 79.0 | 82.3 | 83.7 | 52.2 | 6.4 |
| % Growing to two cells in length (11 μm) | 74.2 | 78.6 | 76.3 | 40.4 | 5.0 |
| Median tgerm (IQR) | 1.43 (1.06) | 1.26 (0.89) | 0.96 (0.92) | 0.93 (2.97) | 15.20 (13.32) |
| Median tem (IQR) | 15.59 (5.95) | 11.59 (3.68) | 6.65 (3.51) | 4.90 (3.48) | 20.31 (6.30) |
| Median tC1 (IQR) | 24.61 (8.09) | 18.92 (5.41) | 11.10 (3.90) | 6.98 (3.46) | 21.87 (5.75) |
| Median tC2 (IQR) | 29.73 (7.59) | 23.07 (5.10) | 13.29 (4.11) | 8.18 (3.31) | 22.22 (6.11) |
| Median tC1 − tgerm (IQR) | 22.96 (7.55) | 17.39 (4.77) | 9.92 (3.37) | 5.83 (2.08) | 8.53 (4.44) |
| Median tC2 − tC1 (IQR) | 5.29 (1.24) | 4.36 (1.07) | 2.45 (0.66) | 1.32 (0.61) | 1.21 (0.45) |
| Mean tgerm ± SD (CV) | 1.93 ± 1.51 (0.78) | 1.96 ± 2.63 (1.34) | 2.12 ± 3.77 (1.26) | 2.51 ± 3.17 (1.26) | 13.47 ± 7.92 (0.59) |
| Mean tem ± SD (CV) | 16.65 ± 5.18 (0.31) | 12.60 ± 4.14 (0.33) | 7.90 ± 4.14 (0.52) | 5.82 ± 2.92 (0.50) | 20.20 ± 4.37 (0.22) |
| Mean tC1 ± SD (CV) | 26.07 ± 5.77 (0.22) | 20.10 ± 4.96 (0.25) | 12.18 ± 4.54 (0.37) | 7.60 ± 2.61 (0.34) | 21.90 ± 4.20 (0.19) |
| Mean tC2 ± SD (CV) | 30.82 ± 5.31 (0.17) | 24.07 ± 4.58 (0.19) | 14.29 ± 3.83 (0.27) | 8.71 ± 2.65 (0.31) | 22.33 ± 4.20 (0.19) |
| Mean tC1 − tgerm ± SD (CV) | 24.25 ± 5.35 (0.22) | 18.36 ± 4.50 (0.24) | 10.74 ± 3.53 (0.33) | 6.12 ± 1.76 (0.29) | 9.31 ± 3.61 (0.39) |
| Mean tC2 − tC1 ± SD (CV) | 5.28 ± 1.00 (0.19) | 4.45 ± 0.99 (0.22) | 2.75 ± 1.17 (0.43) | 1.51 ± 0.78 (0.52) | 1.31 ± 0.47 (0.23) |
aThe number of spores observed, percentage reaching each stage, median times, interquartile ranges (IQR), mean times, standard deviations, and coefficients of variation (CV) of events occurring during germination of, and subsequent outgrowth from, spores of nonproteolytic C. botulinum Eklund 17B as affected by incubation temperature and prior heat treatment are given. All times are in hours.
Effect of incubation temperature on lag phase of individual unheated spores.
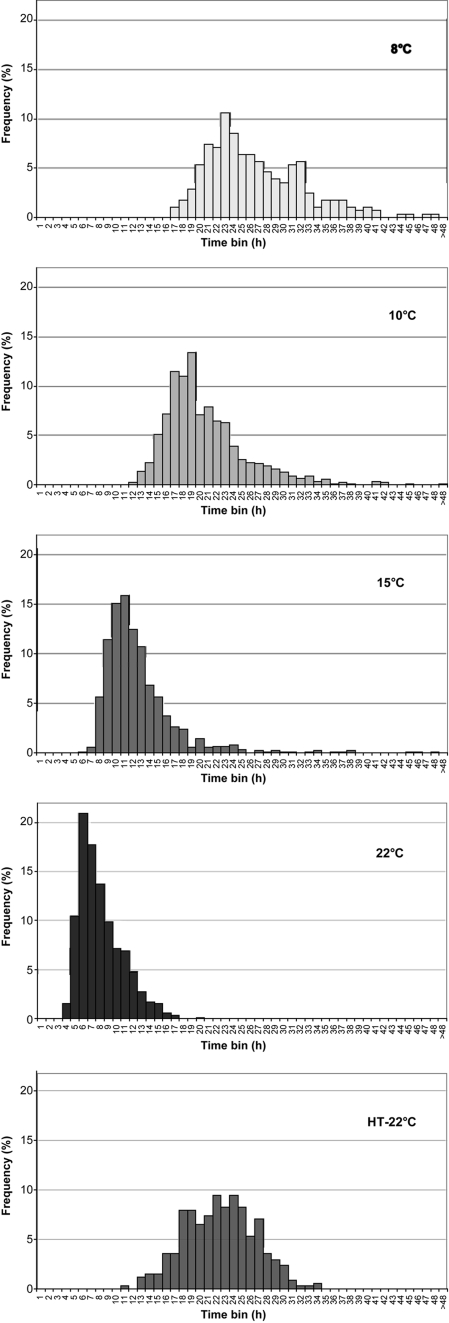
The distribution of tC1 for individual spores under different incubation temperatures is shown in Fig. 1. Lowering the incubation temperature increased both the lag time and the associated standard deviation of the spore population but did not increase the coefficient of variation (Table 1) showing that, relatively, variability had not been increased. The smaller coefficients of variation observed at lower temperature also indicates the cultures were more synchronous at lower temperatures.
FIG. 1.
Frequency distributions of lag time (tC1) for single spores of nonproteolytic C. botulinum Eklund 17B. Spores were unheated and incubated in PYGS medium at 22, 15, 10, and 8°C or were heated at 80°C for 20 s (∼10-fold reduction) and incubated in PYGS medium at 22°C (HT-22°C).
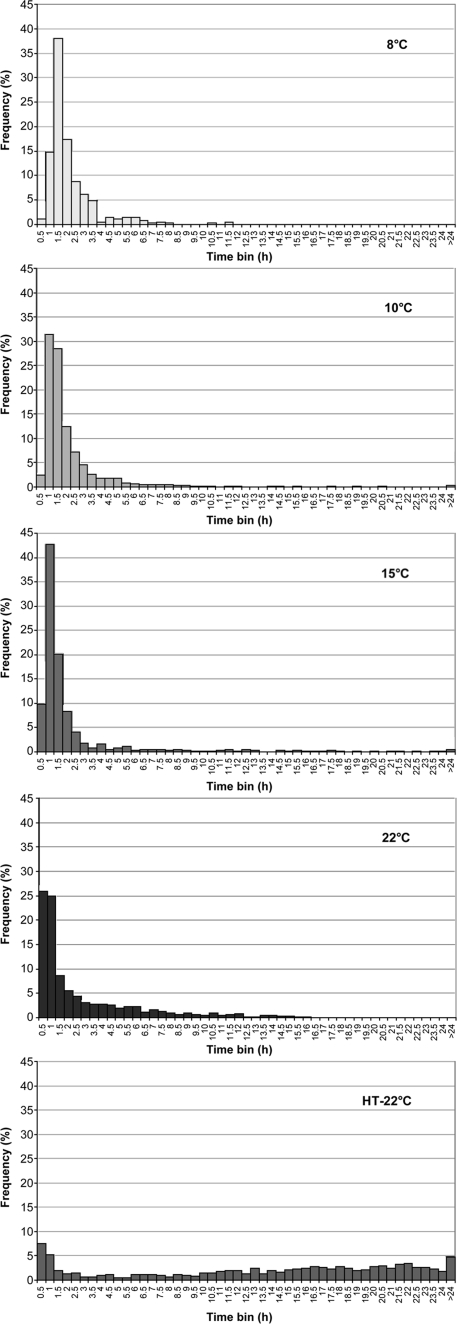
The time required for stages within lag was also measured. The effect of incubation temperature on times for germination (tgerm), outgrowth (tC1 − tgerm), and first doubling time (tC2 − tC1) are shown in Fig. 2, 3, and 4, respectively. Germination times were not normally distributed at any incubation temperature, with large numbers of spores germinating relatively rapidly, followed by a tail formed from slower-germinating spores (Fig. 2). The median germination time was extended at lower temperatures, being 0.93, 0.96, 1.26, and 1.43 h at 22, 15, 10, and 8°C, respectively. However, the mean germination times were shorter at lower incubation temperatures (Table 1). This relates to an increased number of late-germinating spores at the higher temperatures, leading to greater tailing and biasing the mean.
FIG. 2.
Frequency distributions of germination times (tgerm) for single spores of nonproteolytic C. botulinum Eklund 17B. Spores were unheated and incubated in PYGS medium at 22, 15, 10, and 8°C or were heated at 80°C for 20 s (∼10-fold reduction) and incubated in PYGS medium at 22°C (HT-22°C).
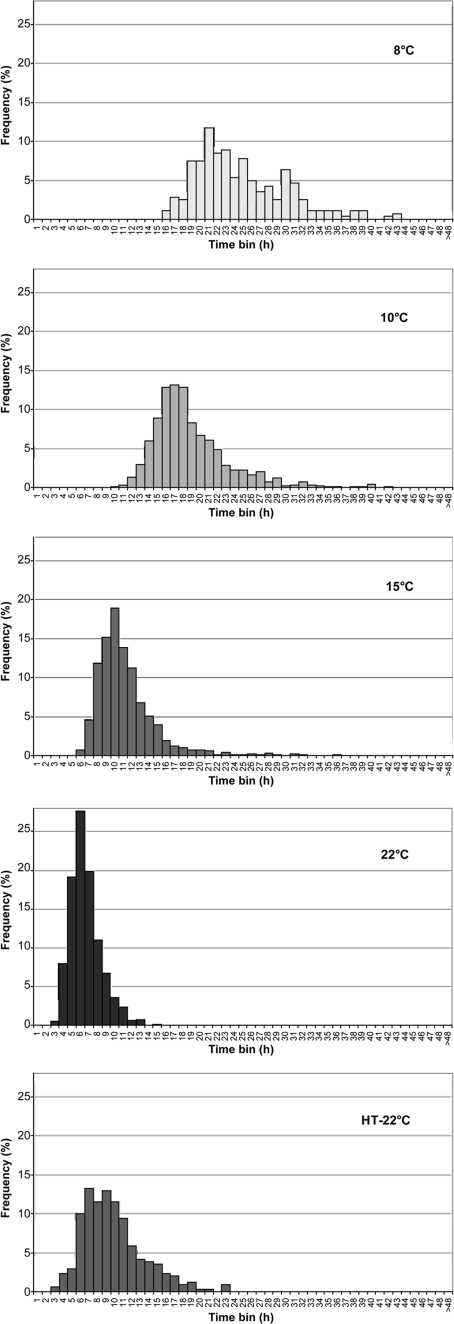
FIG. 3.
Frequency distributions of times for outgrowth (tC1 − tgerm) for single spores of nonproteolytic C. botulinum Eklund 17B. Spores were unheated and incubated in PYGS medium at 22, 15, 10, and 8°C or were heated at 80°C for 20 s (∼10-fold reduction) and incubated in PYGS medium at 22°C (HT-22°C).
FIG. 4.
Frequency distributions of doubling times (tC2 − tC1) for single spores of nonproteolytic C. botulinum Eklund 17B. Spores were unheated and incubated in PYGS medium at 22, 15, 10, and 8°C or were heated at 80°C for 20 s (∼10-fold reduction) and incubated in PYGS medium at 22°C (HT-22°C).
Lowering the incubation temperature increased both the mean and the median outgrowth (tC1 − tgerm) time, with the median time at 8°C four times longer than that at 22°C. However, it did not increase the coefficient of variation (Table 1). The distributions of time for outgrowth (Fig. 3) were shown to be homogeneous (chi-square test, P > 0.05) with their equivalent lag time distributions (tC1) at the same temperature (Fig. 1). This shows that the lag time distributions for unheated cells are dominated by the time required for outgrowth rather than that for germination. Lowering the incubation temperature also increased the mean and median first doubling time (tC2 − tC1). Again, the median time at 8°C was four times longer than that at 22°C (Table 1).
Figure 5 shows the effect of incubation temperature on the median time for lag-phase events. Changes in incubation temperature induced less change in times for germination than in times for outgrowth or doubling. At all four test temperatures, the median duration of outgrowth from unheated spores was much greater than that of cell doubling, which was longer than the median germination time (Fig. 5).
FIG. 5.
Median times for germination (tgerm), outgrowth (tC1 − tgerm), and doubling (tC2 − tC1) obtained from replicate experiments at different temperatures and the best-fit regression line.
Effect of prior heat treatment on the lag phase of individual spores.
Most-probable-number counts on spores before and after heat treatment were 4.7 × 108 and 3.3 × 107 spores ml−1, respectively; 7% of the initial viable population were still viable after heat treatment. Heating spores at 80°C for 20 s reduced the percentage able to germinate at 22°C from 83 to 31% and the percentage subsequently outgrowing to one cell from 52% to 6% (Table 1). The lag time for heated spores that were able to lead to growth was greatly extended compared to unheated spores grown in the same conditions (Table 1 and Fig. 1). The median time to germination was 16 times longer in heated spores than unheated spores incubated at 22°C, and germination accounted for 70% of the median tC1 as opposed to 13% for the unheated spores. Heat treatment had a large affect on the distribution of times to germination, with the curves becoming very broad with extended tails as germination occurred over a long period (Fig. 2). Heat treatment also slightly extended the median time for outgrowth (ca. 50% longer). However, by the time cells started to double the effect of previous heat treatment had been overcome (Fig. 4). The doubling time (tC2 − tC1) distributions from unheated or heat-treated spores were shown to be homogeneous (chi-square test, P > 0.05) without any transformation, confirming these distributions were the same.
Unlike unheated spores, the shapes of the lag (tC1) and outgrowth (tC1 − tgerm) distributions were not found to be homogeneous for the heat-treated spores. This is presumably because outgrowth formed a far larger proportion of the total lag time for unheated spores than for the heat-treated spores. Germination had a far larger effect on lag time distribution for heat-treated spores.
DISCUSSION
Predicting the growth response of a microorganism to an environment depends on knowledge of both the lag phase and the growth rate. The lag phase for a spore inoculum is a multistage process encompassing germination processes, conversion to a vegetative cell, and adaptation to the environment. Germination differs from later growth events since it is a series of degradative reactions using preformed enzymes, whereas subsequent growth stages require macromolecular synthesis (13). Previous studies of lag times have shown individual spores are highly heterogeneous with all stages of lag contributing to variability (21, 23). Changes in environmental conditions, such as growth temperature, are likely to affect all stages of lag but may not necessarily affect all stages to the same extent.
The asymmetric shape of the germination distribution curves, with a majority of spores germinating within a short time interval followed by a long tail of late-germinating spores, is similar to that reported in previous germination-only studies of individual spores of bacillus or clostridia (4, 5, 6, 7, 11, 21, 22, 23). Billon et al. examined the effect of incubation temperature on germination times for individual spores of proteolytic C. botulinum 62A (4). They found that germination started later and went on for longer and that the standard deviation was increased and observed that the germination distribution curves become less peaky as the temperature was reduced from 37 to 20°C. The measurements obtained for nonproteolytic C. botulinum Eklund 17B in the present study similarly showed that lowering the incubation temperature increased the median germination time but did not increase the standard deviation or interquartile range, showing that lowering the temperature did not broaden the distributions of germination times. The germination times observed for unheated C. botulinum Eklund 17B spores at 8 to 22°C were also all more rapid than those previously described for proteolytic C. botulinum 62A at 20°C (4). Differences between proteolytic and nonproteolytic C. botulinum are not surprising since they are physiologically distinct organisms (12), with differences in their germination mechanisms (1, 18): the species C. botulinum is classified primarily by its ability to produce botulinum toxin.
Although reducing the growth temperature increased the median time to germination, the mean time appeared to decrease. The raw data shown in Fig. 2 do not indicate that reducing growth temperature decreases the incubation time: the apparent increase in mean germination time relates to increased tailing, which biases the mean value. This highlights that mean values should be used with caution for comparing the effects of different treatments on germination. The median value is the preferred comparator.
Nonproteolytic C. botulinum is psychotropic with a growth optimum at 25°C (14) and a minimum growth temperature of 3°C (8). Previous population based studies on spores of nonproteolytic C. botulinum have variously reported the extent of germination to be maximum at 9°C (9), 20°C (18), 30°C (19), or 37°C (2). The rate of germination has been reported as maximum at 30°C (19), 37°C (2), and 35°C (18) in germination mixtures but 20°C in PYGS growth medium (18). Our experiments found the extent of germination to be similar at 8, 10, or 15°C and lower at 22°C, but the initial rate of germination increased, and the median time for germination decreased with increasing temperature up the maximum temperature tested of 22°C. After observing the maximum extent of germination occurring at 9°C, Grecz and Arvay (9) suggested that germination was a psychrophilic process. This was based on observations made at 2, 4, 9, 14, 37, and 50°C. Since all reports have indicated that the maximum rate of germination occurs at temperatures greater than 20°C, germination should be considered a mesophilic process. The maximum extent of germination appeared to occur over a wide range of temperatures from 8°C (the lowest tested) to 15°C, suggesting a borderline psychrophilic process, although the maximum could have occurred between 15 to 22°C. The observed changes in the rate of germination, without an associated change in the extent of germination, is also consistent with decreasing temperature simply reducing the rate of the germination process rather than altering the germination mechanism at temperatures down to 8°C. This agrees with previous studies on nonproteolytic C. botulinum that failed to detect more than one type of spore germination system (18).
Although most studies of individual spores have concentrated solely on germination, this stage is not the only, or even necessarily the major, source of lag phase variability. Environmental conditions, such as growth temperature, could have different effects on the different components of lag. Previous studies have shown there was very little relationship between the times spent in each stage of lag phase for each individual spores/cell (21, 23). The same lack of correlation between germination, outgrowth, and doubling time was found for individual spores/cells at each of the test incubation temperatures in the present study. The individual spores that spent the least time in outgrowth were not necessarily those that had germinated first or subsequently had the quickest first doubling times. This does not mean there is not a relationship between the average times spent in different stages of lag. The mean times for outgrowth and doubling were related since both were strongly correlated to temperature.
The shape of the distribution curves for time to outgrowth from unheated spores were found to be homogeneous (P > 0.05), with equivalent distributions for total time to one cell at each incubation temperature. This suggests that for unheated spores, although germination must occur and contributes to the total lag time, outgrowth is the more significant source of variability between individual spore/cells. This is presumably a reflection of a greater proportion of lag time being spent in outgrowth than in germination. Changes in incubation temperature in the range 8 to 22°C induced greater changes in later growth-related events than in germination. Consequently, germination accounted for a smaller proportion of lag time as the temperature was lowered and contributed proportionally less to the lag variability. The rate and extent of germination did not seem to be limiting at these temperatures, and the distributions of lag time for unheated spores were almost identical to those for outgrowth. The same is not true in all conditions since germination time was an important component of the lag from heat-treated spores.
Heat treatment at 80°C for 20 s reduced the number of spores able to outgrow by 93% and extended the lag time and increased the lag variability of the surviving spores. While lowering the incubation temperature extended the lag rate mostly by extending the time for outgrowth, germination time was the major target of heat treatment, with the median germination time being extended 16-fold, from 0.9 to 15.2 h. Heat treatment also had a major effect on the shape of the germination distribution curve, inducing a greater degree of tailing, which is consistent with heat treatment adversely affecting the germination mechanism rather than a simple shift of the rate of chemical reaction.
Heat treatment is thought to inactivate the germination system of spores of nonproteolytic C. botulinum (15). Although most of the extended lag observed after heat treatment seemed to relate to damage to the germination system, there is also evidence of heat damage to other areas of the spore. Heat treatment extended the median time for outgrowth by approximately 3 h compared to unheated spores. It also increased the tail of the distributions curve such that the distributions of time for outgrowth from unheated and heat-treated spores were not homogeneous. Thus, heat damage is not restricted solely to the spore germination apparatus, with further repair taking place during outgrowth. By the time the cells started doubling, any damage was repaired and distributions for unheated and heat-treated spores were the same (P > 0.05).
The results from the present study and work published previously (23) show that both the duration of lag time and the shape of the lag time distribution curve depend on both the historical treatment of a spore and the conditions at the time of germination and outgrowth. All stages in lag contribute to both lag duration and variability, but the relative contribution of each stage varies with the treatment applied. Lowering growth temperature had proportionally greater effect on outgrowth and doubling time than germination time. Heat treatment extended germination to a much greater extent than the later growth stages, whereas previous work (23) showed the times for germination, outgrowth, and first doubling were all extended when unheated spores were grown at 22°C in medium containing 2% (wt/vol) NaCl compared to growth in the absence of added NaCl. The data on how environmental factors and pretreatments affect lag time duration and variability can be used by risk assessors to improve prediction of the food poisoning risk posed by nonproteolytic C. botulinum. Currently, most approaches to risk assessment are based on studies of populations and, although they may combine thermal death and growth models, they usually fail to take account of the effect of sublethal injury. The data documented in the present study help characterize and quantify growth from sublethally damaged spores and may be useful in developing alternative approaches that include estimates of variability and the effect of sublethal injury.
Acknowledgments
This research was funded by the EU program Quality of Life and Management of Living Resources, by grant QLK1-CT-2001-01145 (BACANOVA), and by a Competitive Strategic Grant from the UK Biotechnology and Biological Sciences Research Council.
We thank Carmen Pin for useful discussions.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Alberto, F., V. Broussolle, D. R. Mason, F. Carlin, and M. W. Peck. 2003. Variability in spore germination response by strains of proteolytic Clostridium botulinum types A, B, and F. Lett. Appl. Microbiol. 36:41-45. [DOI] [PubMed] [Google Scholar]
- 2.Ando, Y., and H. Iida. 1970. Factors affecting the germination of spores of Clostridium botulinum type E. Jpn. J. Microbiol. 14:361-370. [DOI] [PubMed] [Google Scholar]
- 3.Baranyi, J. 1998. Comparison of stochastic and deterministic concepts of bacterial lag. J. Theor. Biol. 192:403-408. [DOI] [PubMed] [Google Scholar]
- 4.Billon, C. M. P., C. J. McKirgan, P. J. McClure, and C. Adair. 1997. The effect of temperature on the germination of single spores of Clostridium botulinum 62A. J. Appl. Microbiol. 82:48-56. [DOI] [PubMed] [Google Scholar]
- 5.Chea, F. P., Y. H. Chen, T. J. Montville, and D. W. Schaffner. 2000. Modeling the germination kinetics of Clostridium botulinum 56A spores as affected by temperature, pH, and sodium chloride. J. Food Prot. 63:1071-1079. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D., S. S. Huang, and Y. Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936-6941. [DOI] [PubMed] [Google Scholar]
- 7.Coote, P. J., C. M. P. Billon, S. Pennell, P. J. McClure, D. P. Ferdinando, and M. B. Cole. 1995. The use of confocal scanning laser microscopy (CSLM) to study the germination of individual spores of Bacillus cereus. J. Microbiol. Methods 21:193-208. [Google Scholar]
- 8.Graham, A. F., D. R. Mason, F. J. Maxwell, and M. W. Peck. 1997. Effect of pH and NaCl on growth from spores of non-proteolytic Clostridium botulinum at chill temperature. Lett. Appl. Microbiol. 24:95-100. [DOI] [PubMed] [Google Scholar]
- 9.Grecz, N., and L. H. Arvay. 1982. Effect of temperature on spore germination and vegetative cell growth of Clostridium botulinum. Appl. Environ. Microbiol. 43:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooiman, W. J., and J. M. Geers. 1975. Simple and accurate technique for the determination of heat resistance of bacterial spores. J. Appl. Bacteriol. 38:185-189. [DOI] [PubMed] [Google Scholar]
- 11.Leuschner, R. G. K., and P. J. Lillford. 1999. Effects of temperature and heat activation on germination of individual spores of Bacillus subtilis. Lett. Appl. Microbiol. 29:228-232. [DOI] [PubMed] [Google Scholar]
- 12.Lund, B. M., and M. W. Peck. 2000. Clostridium botulinum, p. 1057-1109. In B. M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food, vol. II. Aspen Publishers, Gaithersburg, MD. [Google Scholar]
- 13.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 14.Peck, M. W. 2006. Clostridium botulinum and the safety of minimally heated, chilled foods: an emerging issue? J. Appl. Microbiol. 101:556-570. [DOI] [PubMed] [Google Scholar]
- 15.Peck, M. W., D. A. Fairbairn, and B. M. Lund. 1992. Factors affecting growth from heat-treated spores of nonproteolytic Clostridium botulinum. Lett. Appl. Microbiol. 15:152-155. [DOI] [PubMed] [Google Scholar]
- 16.Peck, M. W., K. E. Goodburn, R. P. Betts, and S. C. Stringer. 2008. Assessment of the potential for growth and neurotoxin formation by non-proteolytic Clostridium botulinum in short shelf-life commercial foods designed to be stored chilled. Trends Food Sci. Technol. 19:207-216. [Google Scholar]
- 17.Peck, M. W., and S. C. Stringer. 2005. The safety of pasteurised in-pack chilled meat products with respect to the food-borne botulism hazard. Meat Sci. 70:461-475. [DOI] [PubMed] [Google Scholar]
- 18.Plowman, J., and M. W. Peck. 2002. Use of a novel method to characterize the response of spores of non-proteolytic Clostridium botulinum types B, E, and F to a wide range of germinants and conditions. J. Appl. Microbiol. 92:681-694. [DOI] [PubMed] [Google Scholar]
- 19.Strasdine, G. A. 1967. Rapid germination of Clostridium botulinum type E spores. J. Fish. Board Can. 24:595-605. [Google Scholar]
- 20.Stringer, S. C., N. Haque, and M. W. Peck. 1999. Growth from spores of nonproteolytic Clostridium botulinum in heat-treated vegetable juice. Appl. Environ. Microbiol. 65:2136-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringer, S. C., M. D. Webb, S. M. George, C. Pin, and M. W. Peck. 2005. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 71:4998-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vary, J. C., and H. O. Halvorson. 1965. Kinetics of germination of Bacillus spores. J. Bacteriol. 89:1340-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb, M. D., C. Pin, M. W. Peck, and S. C. Stringer. 2007. Historical and contemporary NaCl concentrations affect the duration and distribution of lag times from individual spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 73:2118-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]