Abstract
Staphylococcus aureus can produce a wide variety of exotoxins, including toxic shock syndrome toxin 1 (TSST-1), staphylococcal enterotoxins, and staphylococcal enterotoxin-like toxins. These toxins share superantigenic activity. To investigate the β chain (Vβ) specificities of each of these toxins, TSST-1 and all known S. aureus enterotoxins and enterotoxin-like toxins were produced as recombinant proteins and tested for their ability to induce the selective in vitro expansion of human T cells bearing particular Vβ T-cell receptors (TCR). Although redundancies were observed between the toxins and the Vβ populations, each toxin induced the expansion of distinct Vβ subsets, including enterotoxin H and enterotoxin-like toxin J. Surprisingly, the Vβ signatures were not associated with a specific phylogenic group of toxins. Interestingly, each human Vβ analyzed in this study was stimulated by at least one staphylococcal superantigen, suggesting that the bacterium derives a selective advantage from targeting the entire human TCR Vβ panel.
Staphylococcus aureus produces a broad range of exoproteins, including staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1) (9). These toxins were initially implicated in staphylococcal food poisoning (SEs) and TSS (TSST-1) (39). Since the first characterization of SEA and SEB in 1959 to 1960 by Casman and Bergdoll, 18 different SEs have been described; they are designated SEA to SEV, in the chronological order of their discovery (2, 5, 41). Some were renamed SE-like toxins (SEl), because either no emetic properties were detected or because they were not tested in primate models (21, 41).
SEs, SEls, and TSST-1 share certain structural and biological properties. They have similar sizes (23 to 29 kDa), and their crystal structures, established for SEA, SEB, SEC, SED, SEH, SElI, SElK, and TSST-1, reveal significant homology in their secondary and tertiary conformations (26). However SEs, SEls, and TSST-1 can be divided into four phylogenic groups based on their primary amino acid sequences (41).
SEs, SEls, and TSST-1 share superantigenic activity (24). Superantigens (SAgs), unlike conventional antigens, do not need to be processed by antigen-presenting cells (APC) before being presented to T cells. They can directly stimulate T cells by cross-linking major histocompatibility complex class II molecules on APC with the variable portion of the T-cell antigen receptor β chain (TCR Vβ) or the T-cell antigen receptor α chain for SEH (TCR Vα), thereby inducing polyclonal cell proliferation (19, 36, 37). SAg binding sites lie outside the peptide-binding groove and therefore do not depend on T-cell antigenic specificity but rather on the Vβ and/or Vα region of the TCR (8, 19, 37). It was assumed that each SAg elicited a specific pattern of Vβ and/or Vα activation (24). As SAgs are active at very low concentrations (less than 1 pg/ml) (44), which are barely detectable in vivo, SAg-related diseases might theoretically be identified by determining TCR Vβ specificities in vitro. For example, an expansion of Vβ2 T cells on the one hand and of Vβ3, -14, and -17 T cells on the other hand, which correspond to TSST-1 and SEB superantigenic activities, respectively, has been detected in patients with TSS (6, 10, 25, 29). Such an approach would be particularly useful for investigating suspected SAg-related diseases, including some inflammatory disorders, Kawasaki disease, and atopy (11). However, the list of staphylococcal SAg (SSAg) Vβ specificities is not exhaustive, and different activation profiles have been obtained with different methods (41).
To determine the Vβ specificities of S. aureus SAgs, we produced all known SSAgs as recombinant proteins and investigated their Vβ TCR specificities in vitro, using commercial antibodies.
MATERIALS AND METHODS
Strains and plasmids.
The S. aureus strains listed in Table 1 were used to produce recombinant toxins. Escherichia coli M15 and S. aureus RN6390 and RN4220 were used for plasmid amplification and genetic manipulations.
TABLE 1.
Sequences of primers and bacterial strains used in this study
| Toxin | Strain | Reference or source | Strain and plasmid use for production | Primersa |
|---|---|---|---|---|
| SEA | A87 0502 | This study | S. aureus RN6390 | GAAGGCCTAA AAAAACAGCA TTTACATTAC TTTTATTCAT TG |
| pLUG 345 | CGCGGATCCG TTATTAATGA TGATGATGAT GATGAGAACC CCCACTTGTA TATAAATATA TATCAATATG CATGTTTTC | |||
| SEB | COL | 12 | S. aureus RN6390 | GAAGGCCTTA TAAGAGATTA TTTATTTCAC ATGTAATTTT G |
| pLUG 345 | CGCGGATCCG TTATTAATGA TGATGATGAT GATGAGAACC CCCCTTTTTC TTTGTCGTAA GATAAAC | |||
| SEC | FRI 137 | This study | E. coli M15 | GTAGGATCCG AGAGCCAACC AGACC |
| pQE30 | GGACTGCAGT TATCCATTCT TTGTTGTAAG GTGG | |||
| SED | FRI 137 | This study | E. coli M15 | GTTGGATCCA ATGAAAACAT TGATTCAGTA AAAGAG |
| pQE30 | TTGCTGCAGC TACTTTTCAT ATAAATAGAT GTCAATATG | |||
| SEE | FRI918 | 7 | E. coli M15 | CGCGGATCCA AAAAAACAGC ATTTATACTA CTTTTATTCA TTGC |
| pQE30 | CCCAAGCTTA GTTGTGTATA AATACAAATC AATATGGAGG | |||
| SEG | A99 0372 | This study | S. aureus RN6390 | GAAGGCCTAA GAAATTATCT ACTGTAATTA TTATTTTGAT TCTAGAAATA G |
| pLUG 345 | GGAAAGATCT TTTATTAATG ATGATGATGA TGATGAGAAC CCCCGTGAGT ATTAAGAAAT ACTTCC | |||
| SEH | MW2 | 1 | E. coli M15 | TCAGGATCCAAAGCAGAAG ATTTACACGA TAAAAG |
| pQE30 | TTTCTGCAGT TATACTTTTT TCTTAGTATA TAGATTTACA TC | |||
| SEI | A900322 | 18 | S. aureus RN6390 | GAAGGCCTAA AAAATTTAAA TATAGTTTTA TATTAGTTTT TATATTACTT TTTAACATTA AAG |
| pLUG 345 | CGCGGATCCG TTATTAATGA TGATGATGATGAT GAGAACCCCC GTTACTATCT ACATATGATA TTTCGAC | |||
| SEJ | Fukuoka 5 | 45 | E. coli M15 | TACGGATCCA GCAAAAATGA AACAATTAAA GAAAAGAATT TGCAC |
| pQE30 | TTACTGCAGC TACAGAACCA AAGGTAGACT TATTAATAC | |||
| SElK | HT2003 0702 | This study | E. coli M15 | CGCGGATCCA AAAAATTAAT AAGCATCTTA TTAATAAATA TAATAATTTT AGGTG |
| pQE30 | CCCAAGCTTT ATCGTTTCTT TATAAGAAAT ATCGACATC | |||
| SElL | HT2003 0702 | This study | S. aureus RN6390 | GAAGGCCTAA AAAAAGATTA TTATTTGTAA TTGTTATTAC TTTATTTATTTTTTCTTC |
| pLUG 345 | GGAAAGATCT TTTATTAATG ATGATGATGA TGATGAGAAC CCCCTCTTTTTGAAATTTCG ACATCTAGAT GAAATT | |||
| SElM | A900322 | 18 | S. aureus RN6390 | GAAGGCCTAA AAGAATACTT ATCATTGTTG TTTTATTGTT TTG |
| pLUG 345 | CGCGGATCCG TTATTAATGA TGATGATGAT GATGAGAACC CCCACTTTCG TCCTTATAAGA TATTTCGA | |||
| SElN | A900322 | 18 | E. coli M15 | GAAGGCCTAG ATTGTTCTAC ATAGCTGCAA TTATAATAAC |
| pQE30 | CGCGGATCCG TTATTAATGA TGATGATGAT GATGAGAACC CCCATCTTTA TATAAAAATA CATCAATATG ATAATTAG | |||
| SElO | A900322 | 18 | S. aureus RN6390 | GAAGGCCTAT TAAAAATAGT AAAGTAATGT TAAATGTATT ATTAATTTTA AATTTAATTG |
| pLUG 345 | CGCGGATCCG TTATTAATGA TGATGATGAT GATGAGAACC CCCTGTAAAT AAATAAACAT CAATATGATA GTCTG | |||
| SElP | HT2003 0702 | This study | S. aureus RN6390 | GAAGGCCTAG TAAAATAAAA AAAACAACAT TTATACTACT TTCATTTATT G |
| pLUG 345 | GGAAAGATCT TTTATTAATG ATGATGATGA TGATGAGAAC CCCCAGTTGT ATATAAATAT ATATCAATAT GCATATTTTT AGAC | |||
| SElQ | COL | 12 | E. coli M15 | GCAGGATCCGATGTAGGGG TAATCAATCT TA |
| pQE30 | AAACTGCAGT TATTCAGTTT TCTCATATGA AATCTC | |||
| SER | Fukuoka5 | 31 | E. coli M15 | GTAGGATCCAAACCAGATC CAAGGCCTG |
| pQE30 | CCGCTGCAGT CACATTGTAG TCAGGTGAAC TTC | |||
| SElU | A940624 | 42 | E. coli M15 | CA GGATCC ATG TTA AAT GGC AAT CCT AAA C CA |
| pQE30 | GC CTGCAG TTA TTT TTT GGT TAA ATG AAC TTC TAC ATT AAT AGA TTT A | |||
| SElV | A940624 | 42 | E. coli M15 | GCA GGATCC GAT GTC GGA GTT TTG AAT CTT AGG |
| pQE30 | TAA CTGCAG TTA GTT ACT ATC TAC ATA TGA TAT TTC GAC ATC | |||
| TSST-1 | N315 | Kuroda, 2001 | S. aureus RN6390 | GAAGGCCTAATAAAAAATTACTAATGAATTTTTTTATCGTAAGCCC |
| pLUG 345 | GGAAAGATCTTTTATTAATGATGATGATGATGATGAGAACCCCCATTAATTTCTGCTTCTATAGTTTTTATTTCATC |
Restriction sites are underlined (BamHI and PstI for pQE30 and BglII and StuI for pLUG345).
Toxin production and purification.
Primers were designed following the identification of suitable hybridization sites in the toxin genes (Table 1). DNA was extracted and used as a template for PCR amplification as previously described (18). The 5′ primers were chosen within the coding sequence of each gene, omitting the region predicted to encode the signal peptide, as determined by using the SignalP 3.0 World Wide Web prediction server (http://www.cbs.dtu.dk/services/SignalP/). The 3′ primers were chosen to overlap the stop codon of SSAg genes (Table 1). The PCR products were codigested with the appropriate restriction enzymes (Promega, Madison, WI), purified with the High Pure PCR product purification kit (Roche Applied Science, Meylan, France), and ligated using T4 DNA ligase (Roche Applied Science, Meylan, France) in either the pQE-30 expression vector (Qiagen, Courtaboeuf, France) or pLUG345 (4) digested with the same restriction enzymes (BamHI and PstI for pQE30 and BglII and StuI for pLUG345). The resulting pQE plasmids were transformed into E. coli strain M15. Open reading frame integrity was verified by sequencing the junctions between the plasmid and the insert. For toxin expression in S. aureus, plasmids were transferred by electroporation into RN4220, a nitrosoguanidine-induced mutant capable of accepting E. coli DNA, before transfer to RN6390. His-tagged recombinant toxins were purified by affinity chromatography on a nickel affinity column according to the supplier's instructions (New England Biolabs, Ipswich, MA). The protein purity was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lipopolysaccharide was removed from the toxin solutions by affinity chromatography with Detoxi-GEL endotoxin gel (Pierce, Rockford, IL). The QCL-1000 Limulus amebocyte lysate assay (Cambrex-BioWhittaker, Walkersville, MD) showed that the endotoxin content of the recombinant SSAg solutions was less than 0.005 units/ml.
Flow cytometry and CD69 assay.
Toxin activities were assessed by measuring CD69 surface expression by T cells upon toxin challenge (22). Briefly, 50 μl of whole blood was incubated with 1.0 and 0.1 μg/ml (final concentrations) of recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, and SElV in RPMI 1640 culture medium containing 5% heat-inactivated fetal calf serum (Gibco Invitrogen, Paisley, United Kingdom) for 24 h at 37°C in humidified air with 5% CO2. Culture medium and phytohemagglutinin (PHA; 10 μg/ml) were used as negative and positive controls, respectively. After ammonium chloride erythrocyte lysis, leukocytes were incubated with a mixture of anti-CD3 conjugated with cyanin-5-phycoerythrin (Dako, Glostrup, Denmark) and anti-CD69 conjugated with fluorescein isothiocyanate (Beckman Coulter, Miami, FL). The cells were then analyzed with a FACScan flow cytometer (Becton Dickinson Biosciences, San Jose, CA), and the results were expressed as the percentage of CD3+ lymphocytes expressing CD69. The experiments were done in triplicate with cells from three different blood donors.
Analysis of T-cell Vβ repertoires.
Peripheral blood mononuclear cells (PBMC) were isolated from the heparinized venous blood of healthy donors by Ficoll density gradient sedimentation (Pancoll; PAN Biotech GmbH, Aidenbach, Germany). The cells were washed three times in Hank's balanced salt solution (Sigma-Aldrich, St. Louis, MO) and suspended in RPMI 1640 medium supplemented with 5% heat-inactivated fetal calf serum, 20 mM HEPES buffer, 2 mM l-glutamine (Sigma-Aldrich), 100 IU/ml penicillin G, and 100 μg/ml streptomycin (Sigma-Aldrich) at a density of 2 × 106 to 5 × 106 cells per ml. The cells were stimulated with 500 ng/ml of recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, SElV, RPMI 1640 (negative control), or 10 μg/ml PHA (positive control) for three days at 37°C in humidified air with 5% CO2. In a previous kinetic study of PBMC stimulation with SAgs, we have observed that some Vβ expansion could be only detected later after stimulation, from day 6 to at least day 10 (42). Thus, to optimize the detection of Vβ expansion, we have decided to increase the length of the incubation by an additional 9 days of incubation with fresh culture medium. Thus, cells were then washed in Hank's balanced salt solution and incubated in culture medium with 20 to 100 U/ml hu-IL-2 (Eurobio, Courtaboeuf, France) for 9 days at 37°C in humidified air with 5% CO2. The Vβ profile was then determined by flow cytometry (FACScan; Becton Dickinson Biosciences, San Jose, CA) using the IOTest Beta Mark kit (Beckman Coulter, Miami, FL), according to the supplier's instructions. This kit is a multiparametric tool designed for the quantitative determination of the TCR Vβ repertoire of human T lymphocytes by flow cytometry (Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ5.3, Vβ7.1, Vβ7.2, Vβ8, Vβ9, Vβ11, Vβ12, Vβ13.1, Vβ13.2, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ18, Vβ20, Vβ21.3, Vβ22, and Vβ23). To complete the Vβ panel of IOTest Beta Mark, we performed additional staining and analysis with Vβ6.7 antibody (Pierce, Rockford, IL) as previously described (42). The multiparameter data files were analyzed with the Cellquest program (BD Biosciences, Le-Pont-de-Claix, France). The experiments were done in triplicate with cells from three different blood donors. Since the percentage of Vβ subsets varies between blood donors and after PBMC stimulation by PHA, we expressed the results as ratios of the percentage of TCR Vβ expansion induced by each toxin relative to that by PHA. The Vβ subsets that were more abundant with the toxin than with PHA among cells with the three donors were considered to be significantly expanded (Mann-Whitney test, P = 0.037).
Phylogenetic analysis.
The amino acid sequences of the mature toxins were deduced from the sequences obtained from GenBank and SignalP. The alignment was performed with ClustalX software (43). Evolutionary distances were determined by the Kimura method, and the values were used to construct a dendrogram by means of the neighbor-joining method using SplitsTree4 software (16). At least 1,000 bootstrap trees were generated to investigate the stability of the phylogenic relationships.
RESULTS
Toxin activities. (i) Induction of CD69 expression.
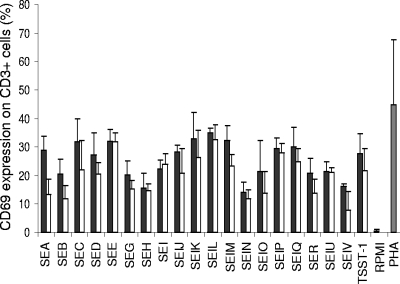
Challenge with 1.0 and 0.1 μg/ml purified recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, and SElV rapidly induced strong CD69 expression on CD3+ cells (Fig. 1). Depending on the toxin, CD69 was expressed by 8 to 32% of the cells and by 14 to 35% of the cells at concentrations of 0.1 and 1.0 μg/ml, respectively. By comparison, CD69 expression was below 1% when the cells were incubated with RPMI medium and 44% when they were incubated with PHA. These experiments showed that our recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, and SElV could activate T cells.
FIG. 1.
CD69 expression by T lymphocytes upon S. aureus SAg challenge. CD69 expression was measured on T lymphocytes (CD3+) after 24 h of incubation with 1.0 μg/ml of SSAgs (black bars) and 0.1 μg/ml of SSAgs (white bars) of whole blood, Eagle's minimal essential medium (negative control; RPMI [gray bar]), or 10 μg/ml PHA (positive control; gray bar). Results are means ± standard deviations (n = 3).
(ii) TCR Vβ repertoires.
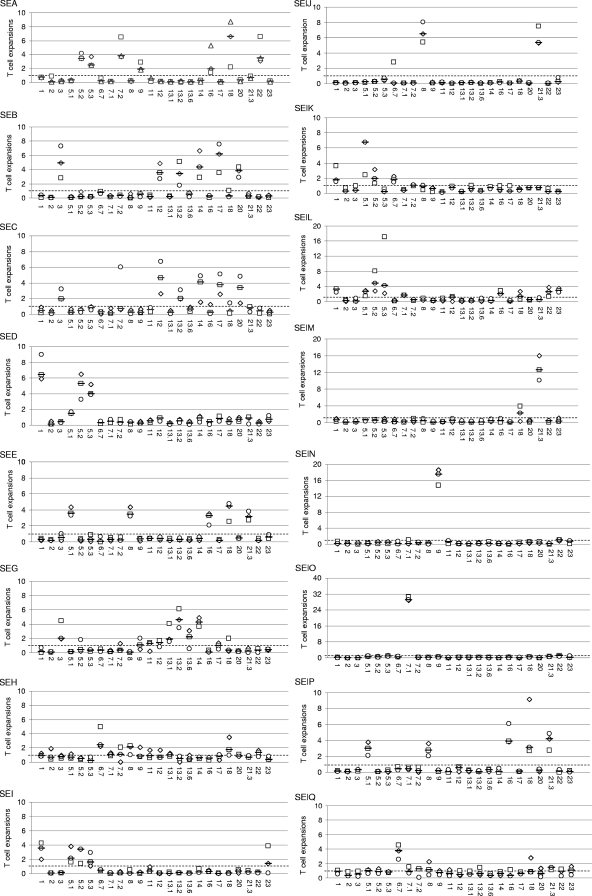
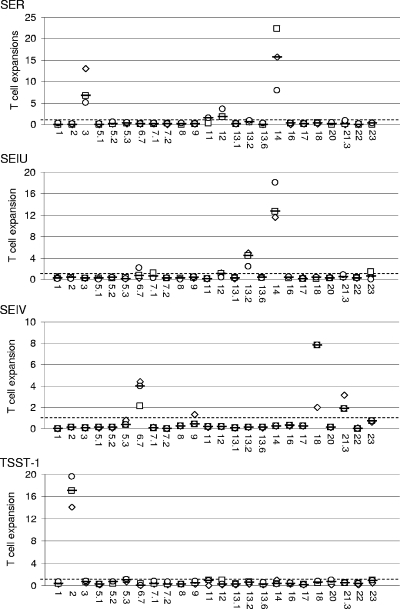
Purified recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, and SElV were studied for their ability to induce the selective expansion of T cells bearing particular TCR Vβ regions in PBMC culture. Vβ profiles were determined by flow cytometry using the IOTest Beta Mark kit completed with a Vβ6.7 antibody. The antibody against TCR Vβ4 generated nonspecific labeling, making it impossible to identify the TCR Vβ4 cells. Consequently, the TCR Vβ4 results were excluded from the study. As shown in Fig. 2, recombinant TSST-1, SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SER, SElU, and SElV induced the selective expansion of distinct Vβ subpopulations with various potencies. TSST-1, SEA, SEG, SEH, SElJ, SElK, SElL, SElM, SElN, SElO, SElQ, SER, SElU, and SElV induced unique expansion patterns. As summarized in Table 2, TSST-1 induced the expansion of Vβ2; SEA induced Vβ5.2, Vβ5.3, Vβ7.2, Vβ9, Vβ16, Vβ18, and Vβ22; SEG induced Vβ3, Vβ13.1, Vβ13.2, and Vβ14; SEH induced Vβ6.7 and Vβ8; SElJ induced Vβ8 and Vβ21.3; SElK induced Vβ1, Vβ5.1, Vβ5.2, and Vβ6.7; SElL induced Vβ1, Vβ5.1, Vβ5.2, Vβ5.3, Vβ7.1, Vβ16, Vβ22, and Vβ23; SElM induced Vβ21.3; SElN induced Vβ9; SElO induced Vβ7.1; SElQ induced Vβ6.7 and Vβ21.3; SER induced Vβ3, Vβ12, and Vβ14; SElU induced Vβ13.2 and Vβ14; and SElV induced Vβ6.7, Vβ18, and Vβ21.3. By contrast, similar patterns of Vβ expansion were observed with SEB and SEC (Vβ3, Vβ12, Vβ13.2, Vβ14, Vβ17, and Vβ20), SED and SEI (Vβ1, Vβ5.1, Vβ5.2, and Vβ5.3), and SEE and SElP (Vβ5.1, Vβ8, Vβ16, Vβ18, and Vβ21.3). When similar Vβ subpopulations were stimulated by more than one toxin, the potencies of the toxins sometimes differed. For example, the expansion of Vβ3 was stronger with SEB than with SEC, while Vβ12 expansion was stronger with SEC than with SEB.
FIG. 2.
Human Vβ expansion induced by S. aureus SAgs as detected with the IOTest Beta Mark kit. Purified recombinant toxins and PHA were studied for their ability to induce the selective expansion of T cells bearing particular TCR Vβ regions in PBMC culture. Vβ profiles were determined by flow cytometry using the IOTest Beta Mark kit completed with a Vβ6.7 antibody. Results are expressed as ratios of the percentage of TCR Vβ expansion induced by each toxin relative to that of PHA. The x axis label indicates Vβs. The data shown here are the representative ratios observed with each of the three blood donors (□, ⋄, ○) plus the medians of these ratios (−), while the horizontal dashed line represents a ratio of 1.
TABLE 2.
Human Vβ expansion induced by S. aureus superantigens, as detected with the IOTest Beta Mark kit
| Toxin | TCR Vβa |
|---|---|
| SEA | 5.2, 5.3, 7.2, 9, 16, 18, 22 |
| SEB | 3, 12, 13.2, 14, 17, 20 |
| SEC | 3, 12, 13.2, 14, 17, 20 |
| SED | 1, 5.1, 5.2, 5.3 |
| SEE | 5.1, 8, 16, 18, 21.3 |
| SEG | 3, 13.1, 13.2, 14 |
| SEH | 6.7, 8 |
| SEI | 1, 5.1, 5.2, 5.3 |
| SElJ | 8, 21.3 |
| SElK | 1, 5.1, 5.2, 6.7 |
| SElL | 1, 5.1, 5.2, 5.3, 7.1, 16, 22, 23 |
| SElM | 21.3 |
| SElN | 9 |
| SElO | 7.1 |
| SElP | 5.1, 8, 16, 18, 21.3 |
| SElQ | 6.7, 21.3 |
| SER | 3, 12, 14 |
| SElU | 13.2, 14 |
| SElV | 6.7, 18, 21.3 |
| TSST-1 | 2 |
Purified recombinant toxins were tested for their ability to induce the selective expansion of T cells bearing particular TCR Vβ regions in PBMC culture. Vβ profiles were determined by flow cytometry with the IOTest Beta Mark kit completed with a Vβ6.7 antibody. Experiments were done in triplicate with cells from three donors. Since the percentage of Vβ subsets varies between blood donors and after PBMC stimulation by PHA, we expressed the results as ratios of the percentage of TCR Vβ expansion induced by each toxin relative to that by PHA. Only Vβ subsets that were more abundant with the toxin than with PHA among cells with the three donors were considered to be significantly expanded (Mann-Whitney test, P = 0.037) and are indicated.
Phylogenic groups.
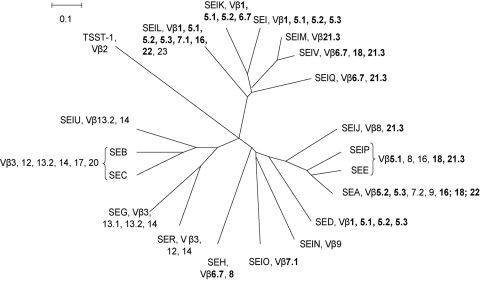
A phylogenetic tree was constructed from the deduced amino acid sequences of the mature TSST-1, the enterotoxins, and the enterotoxin-like toxins by using the neighbor-joining method (Fig. 3). The nodes were well supported (>70% bootstrap values) with four exceptions, namely, the nodes for TSST-1, SEH, the common branch SElO/SElN, and SElK/SEI/SElM/SElV. We identified four groups within the tree: SEA, SED, SEE, SEH, SElJ, SElN, SElO, and SElP; SEB, SEC, SEG, SER, and SElU; SEI, SElK, SElL, SElM, SElQ, and SElV; and TSST-1 alone. The Vβ specificity of each toxin is indicated in the tree (Fig. 3). Surprisingly, we observed no correlation between the phylogenic groups and the Vβ specificities. Similar Vβ subsets (e.g., Vβ1, Vβ5.1 to -5.3, Vβ6.7, Vβ7.1, Vβ16, Vβ18, Vβ21.3, and Vβ22) were elicited by toxins belonging to different monophyletic groups, such as SEA and SEI. For instance, SED (from the SEA phylum) and SEI both induced Vβ1, -5.1, -5.2, and -5.3 T-cell expansion. By contrast, all the Vβ subsets that were activated by most of the toxins belonging to the SEB group (except for SEB and SEC, which had the same Vβ specificity) and by TSST-1 were specific to these phylogenetic groups.
FIG. 3.
Reconstitution of the phylogenetic tree of S. aureus SAgs. Amino acid sequences of the mature toxins were deduced from sequences obtained from GenBank and SignalP analysis. Alignment was performed with ClustalX software. Evolutionary distances were determined by the Kimura method, and the values were used to construct a dendrogram by means of the neighbor-joining method using SplitsTree4 software. Vβ expansion induced by each toxin is indicated. Vβ expansions observed in response to several SSAg phylogenic groups are boldfaced.
DISCUSSION
To determine the Vβ specificities of SSAgs, we produced all known S. aureus SAgs as recombinant proteins and studied their ability to induce the selective expansion of T cells bearing particular TCR Vβ regions in PBMC culture. All the recombinant toxins induced T-cell activation, as shown by enhanced CD69 expression. Specific Vβ expansion was observed with all the toxins, confirming their SAg status. Importantly, SElJ, which induced strong Vβ8 and -21.3 expansions, and SEH, which induced significant Vβ6.7 and weak Vβ8 expansions, had not previously been shown to induce specific Vβ repertoires. We also found that SElQ induced the weak expansion of Vβ21.3.
The Vβ profiles observed with our recombinant toxins and with the IOTest Beta Mark kit completed with a Vβ6.7 antibody were similar to those described elsewhere (3). However, several of our results appear to conflict with published data. We did not detect the previously described Vβ1.1 expansion induced by SEA (27), Vβ8.1 and Vβ12.1 by SED (20), Vβ12 and Vβ13.6 by SEG (18), Vβ23 by SEI (18), Vβ18 by SElM, Vβ5.1 and Vβ22 by SElO (18), Vβ2.1 and Vβ5.1 by SElQ (35), or Vβ11 by SER (31). In contrast, we observed the specific expansion of Vβ1 by SElK (34), Vβ6.7 and Vβ8 by SEH, and Vβ1 and Vβ7 by SEL (33). These discrepancies may be explained by differences in the methods used to detect Vβ expansion (other sets of antibodies or reverse transcriptase PCR), the length of the incubation, and the use of different cutoff values used to define the significant enhancement of Vβ expansion. In several cases, Vβ expansion was only observed with two blood donors for Vβ13.6 and SEG, Vβ18 and SElM, and Vβ11 and SER.
Interestingly, our phylogenic analysis showed no correlation between the toxin phyla and Vβ specificity. Toxins from different phyla sometimes induced similar Vβ subsets, while several Vβ subsets were sometimes induced by more than one toxin. This suggests that only a few key amino acids in the toxin sequences are responsible for Vβ specificity. Only TSST-1, SEA, SEG, SEH, SElJ, SElK, SElL, SElN, SElM SElO, SElQ, SER, SElU, and SElV generated unique Vβ patterns. Thus, it would be risky to attempt to identify the SSAg involved in a given SAg-related disease simply by determining the Vβ repertoire of blood cells. Indeed, the results should be interpreted according to the toxin profile of the corresponding isolate. This would help to show which toxins are expressed in vivo and could provide pathophysiological insights, especially into putative SSAg-related diseases.
Depending on the S. aureus SAg, between one and eight (median, >3) Vβ T-cell subpopulations were activated, and each Vβ was induced by between one and six SSAgs (median, 3). These results emphasize the redundancy of S. aureus SSAgs. Vβ5.2 and Vβ14 were targeted by five SSAgs and Vβ5.1, Vβ18, and Vβ21.3 by six SSAgs, suggesting that S. aureus might derive a selective advantage by activating these particular T-cell subpopulations. It would be hazardous to assume that all SSAgs with similar Vβ specificities are biologically equivalent. At the level of APC/T-cell interaction, while SEB and SEC induced the expansion of similar T-cell subsets in our study, they bind preferentially to distinct major histocompatibility complex class II isotypes (14, 38). SEB shows higher affinity for human leukocyte antigen DR-like molecules, and SEC for human leukocyte antigen DQ-like molecules.
A given S. aureus strain harbors only a small number of SSAg genes (17). Sometimes, genes encoding toxins that share similar Vβ specificities can be found on the same genetic background (30). The expression of each of these genes by S. aureus is precisely controlled by several regulatory systems but in a different manner (28). As suggested by Grumann et al., we suspect that it is beneficial for S. aureus to extend the conditions when the immune system is triggered by SSAgs (13).
It is important to note that all the Vβ specificities tested were activated by at least one SSAg. Even other Vβs, such as Vβ15 (not tested with the IOTest Beta Mark kit) and Vβ4 are activated by SSAgs (3). It is widely thought that SSAgs benefit S. aureus by disrupting the immune system and notably by inducing immune anergy through T-cell suppressor activity, along with B-cell depression and the inhibition of antibody responses (15, 18, 23, 40). Our results are in keeping with this hypothesis, as they show that SSAgs interact with the entire Vβ repertoire in humans.
In conclusion, by examining the ability of all known SSAgs to induce selective TCR Vβ expansion, we found a certain redundancy among SSAg Vβ specificities. This clearly hinders SSAg identification based on elicited Vβ profiles. Interestingly, each human Vβ was stimulated by at least one SSAg, suggesting that the bacterium derives a selective advantage from targeting the entire TCR Vβ panel.
While the experimental work was finished, Ono et al. has reported the discovery of two novel staphylococcal enterotoxins, SES and SET (32). SES induced strong Vβ9 and -16 T-cell expansions, but no Vβ specificity was detected for SET.
Acknowledgments
We thank Grégoire Cozon for the preliminary characterization of the SSAgs; Martine Rougier, Annie Martra, Christine Courtier, Christine Gardon, Céline Spinelli, Caroline Bouveyron, and Florence Couzon for their technical advice; and David Young for editorial guidance.
The laboratory received research grants from the French Ministry of Health and Education, from the French medical research council (INSERM), and from Pfizer.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 2 March 2009.
REFERENCES
- 1.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 3591819-1827. [DOI] [PubMed] [Google Scholar]
- 2.Bergdoll, M. S., M. J. Surgalla, and G. M. Dack. 1959. Staphylococcal enterotoxin. I. Purification. Arch. Biochem. Biophys. 8562-69. [DOI] [PubMed] [Google Scholar]
- 3.Bohach, G. A. 2006. Staphylococcus aureus exotoxins, p. 464-477. In V. A. Fischetti, R. P. Novick, J. J. Ferreti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens, 2nd ed. ASM Press, Washington, DC.
- 4.Boisset, S., T. Geissmann, E. Huntzinger, P. Fechter, N. Bendridi, M. Possedko, C. Chevalier, A. C. Helfer, Y. Benito, A. Jacquier, C. Gaspin, F. Vandenesch, and P. Romby. 2007. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 211353-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casman, E. P. 1960. Further serological studies of staphylococcal enterotoxin. J. Bacteriol. 79849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, Y., J. A. Lafferty, J. R. Clements, J. K. Todd, E. W. Gelfand, J. Kappler, P. Marrack, and B. L. Kotzin. 1990. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J. Exp. Med. 172981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch, J. L., M. T. Soltis, and M. J. Betley. 1988. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J. Bacteriol. 1702954-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellabona, P., J. Peccoud, J. Kappler, P. Marrack, C. Benoist, and D. Mathis. 1990. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 621115-1121. [DOI] [PubMed] [Google Scholar]
- 9.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 1316-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferry, T., D. Thomas, T. Perpoint, G. Lina, G. Monneret, I. Mohammedi, C. Chidiac, D. Peyramond, F. Vandenesch, and J. Etienne. 2008. Analysis of superantigenic toxin Vß T-cell signatures produced during cases of staphylococcal toxic shock syndrome and septic shock. Clin. Microbiol. Infect. 14546-554. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, J., V. Arcus, P. Kong, E. Baker, and T. Proft. 2000. Superantigens—powerful modifiers of the immune system. Mol. Med. Today 6125-132. [DOI] [PubMed] [Google Scholar]
- 12.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 1872426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grumann, D., S. S. Scharf, S. Holtfreter, C. Kohler, L. Steil, S. Engelmann, M. Hecker, U. Volker, and B. M. Broker. 2008. Immune cell activation by enterotoxin gene cluster (egc)-encoded and non-egc superantigens from Staphylococcus aureus. J. Immunol. 1815054-5061. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann, T., R. S. Accolla, and H. R. MacDonald. 1989. Different staphylococcal enterotoxins bind preferentially to distinct major histocompatibility complex class II isotypes. Eur. J. Immunol. 192171-2174. [DOI] [PubMed] [Google Scholar]
- 15.Hu, H. L., W. D. Cornwell, T. J. Rogers, and Y. S. Lin. 1996. In vivo analysis of a superantigen-induced T cell suppressor factor. Cell. Immunol. 167285-292. [DOI] [PubMed] [Google Scholar]
- 16.Huson, D. H., and D. Bryant. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23254-267. [DOI] [PubMed] [Google Scholar]
- 17.Jarraud, S., C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne, and F. Vandenesch. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166669-677. [DOI] [PubMed] [Google Scholar]
- 19.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17435-466. [DOI] [PubMed] [Google Scholar]
- 20.Li, Y. F., L. Zhong, X. H. Zhu, and J. Yang. 2004. Study on the TCR Vbeta binding sites in the superantigen staphylococcal enterotoxin D. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 20757-759. [PubMed] [Google Scholar]
- 21.Lina, G., G. A. Bohach, S. P. Nair, K. Hiramatsu, E. Jouvin-Marche, and R. Mariuzza. 2004. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 1892334-2336. [DOI] [PubMed] [Google Scholar]
- 22.Lina, G., G. Cozon, J. Ferrandiz, T. Greenland, F. Vandenesch, and J. Etienne. 1998. Detection of staphylococcal superantigenic toxins by a CD69-specific cytofluorimetric assay measuring T-cell activation. J. Clin. Microbiol. 361042-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lussow, A. R., and H. R. MacDonald. 1994. Differential effects of superantigen-induced “anergy” on priming and effector stages of a T cell-dependent antibody response. Eur. J. Immunol. 24445-449. [DOI] [PubMed] [Google Scholar]
- 24.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248705-711. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda, Y., H. Kato, R. Yamada, H. Okano, H. Ohta, K. Imanishi, K. Kikuchi, K. Totsuka, and T. Uchiyama. 2003. Early and definitive diagnosis of toxic shock syndrome by detection of marked expansion of T-cell-receptor VBeta2-positive T cells. Emerg. Infect. Dis. 9387-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, D. T., D. G. Levitt, P. M. Schlievert, and D. H. Ohlendorf. 2000. Structural evidence for the evolution of pyrogenic toxin superantigens. J. Mol. Evol. 51520-531. [DOI] [PubMed] [Google Scholar]
- 27.Newton, D. W., M. Dohlsten, C. Olsson, S. Segren, K. E. Lundin, P. A. Lando, T. Kalland, and M. Kotb. 1996. Mutations in the MHC class II binding domains of staphylococcal enterotoxin A differentially affect T cell receptor Vbeta specificity. J. Immunol. 1573988-3994. [PubMed] [Google Scholar]
- 28.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 481429-1449. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi, R., J. Takaya, S. Tsuji, F. Yamato, M. Hasui, Y. Kinoshita, and Y. Kobayashi. 2005. Prognostic usefulness of lymphocyte V beta receptor determination in toxic shock syndrome. Eur. J. Pediatr. 164703-704. [DOI] [PubMed] [Google Scholar]
- 30.Omoe, K., D. L. Hu, H. Takahashi-Omoe, A. Nakane, and K. Shinagawa. 2005. Comprehensive analysis of classical and newly described staphylococcal superantigenic toxin genes in Staphylococcus aureus isolates. FEMS Microbiol. Lett. 246191-198. [DOI] [PubMed] [Google Scholar]
- 31.Omoe, K., K. Imanishi, D. L. Hu, H. Kato, H. Takahashi-Omoe, A. Nakane, T. Uchiyama, and K. Shinagawa. 2004. Biological properties of staphylococcal enterotoxin-like toxin type R. Infect. Immun. 723664-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono, H. K., K. Omoe, K. Imanishi, Y. Iwakabe, D. L. Hu, H. Kato, N. Saito, A. Nakane, T. Uchiyama, and K. Shinagawa. 2008. Identification and characterization of two novel staphylococcal enterotoxins, types S and T. Infect. Immun. 764999-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orwin, P. M., J. R. Fitzgerald, D. Y. Leung, J. A. Gutierrez, G. A. Bohach, and P. M. Schlievert. 2003. Characterization of Staphylococcus aureus enterotoxin L. Infect. Immun. 712916-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orwin, P. M., D. Y. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orwin, P. M., D. Y. Leung, T. J. Tripp, G. A. Bohach, C. A. Earhart, D. H. Ohlendorf, and P. M. Schlievert. 2002. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry 4114033-14040. [DOI] [PubMed] [Google Scholar]
- 36.Petersson, K., H. Pettersson, N. J. Skartved, B. Walse, and G. Forsberg. 2003. Staphylococcal enterotoxin H induces V alpha-specific expansion of T cells. J. Immunol. 1704148-4154. [DOI] [PubMed] [Google Scholar]
- 37.Pumphrey, N., A. Vuidepot, B. Jakobsen, G. Forsberg, B. Walse, and K. Lindkvist-Petersson. 2007. Cutting edge: evidence of direct TCR alpha-chain interaction with superantigen. J. Immunol. 1792700-2704. [DOI] [PubMed] [Google Scholar]
- 38.Rajagopalan, G., G. Polich, M. M. Sen, M. Singh, B. E. Epstein, A. K. Lytle, M. S. Rouse, R. Patel, and C. S. David. 2008. Evaluating the role of HLA-DQ polymorphisms on immune response to bacterial superantigens using transgenic mice. Tissue Antigens 71135-145. [DOI] [PubMed] [Google Scholar]
- 39.Schlievert, P. M., K. N. Shands, B. B. Dan, G. P. Schmid, and R. D. Nishimura. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143509-516. [DOI] [PubMed] [Google Scholar]
- 40.Sundstedt, A., S. Grundström, and M. Dohlsten. 1998. T cell- and perforin-dependent depletion of B cells in vivo by staphylococcal enterotoxin A. Immunology 9576-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, D., S. Chou, O. Dauwalder, and G. Lina. 2007. Diversity in Staphylococcus aureus enterotoxins. Chem. Immunol. Allergy 9324-41. [DOI] [PubMed] [Google Scholar]
- 42.Thomas, D. Y., S. Jarraud, B. Lemercier, G. Cozon, K. Echasserieau, J. Etienne, M. L. Gougeon, G. Lina, and F. Vandenesch. 2006. Staphylococcal enterotoxin-like toxins U2 and V, two new staphylococcal superantigens arising from recombination within the enterotoxin gene cluster. Infect. Immun. 744724-4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchiyama, T., Y. Kamagata, X. J. Yan, A. Kawachi, H. Fujikawa, H. Igarashi, and M. Okubo. 1989. Relative strength of the mitogenic and interleukin-2-production-inducing activities of staphylococcal exotoxins presumed to be causative exotoxins of toxic shock syndrome: toxic shock syndrome toxin-1 and enterotoxins A, B and C to murine and human T cells. Clin. Exp. Immunol. 75239-244. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, S., J. J. Iandolo, and G. C. Stewart. 1998. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 168227-233. [DOI] [PubMed] [Google Scholar]