Abstract
Necrotizing pneumonia caused by community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) isolates is increasingly common and frequently severe. The early inflammatory response in the lung after CA-MRSA infection remains largely undefined. Additionally, many workers have hypothesized that the Panton-Valentine leukocidin (PVL) is a key virulence determinant in CA-MRSA necrotizing pneumonia. We hypothesized that intratracheal inoculation of rats with a USA300 CA-MRSA isolate would result in early expression of genes involved in the immune response and that this would correlate with inflammation and tissue destruction characteristic of necrotizing pneumonia. In addition, we hypothesized that infection with a PVL deletion mutant would result in an attenuated early host response. Infection of rats with a sublethal inoculum of USA300 (strain LAC) resulted in rapid increased expression of most cytokine, chemokine, and inflammatory receptor gene transcripts studied, as assessed by quantitative real-time reverse transcriptase PCR (qRT-PCR). The increased gene transcription was followed by inflammation, increased bacterial survival in the lungs, and necrotizing pneumonia. Infection with strain LAC and infection with strain LAC Δpvl (lukSF-PV deletion mutant) resulted in indistinguishable diseases, as assessed by mortality, in vivo bacterial recovery, and pulmonary pathology. Assessment of the transcription of inflammatory genes by qRT-PCR also revealed little difference after infection with LAC and after infection with LAC Δpvl, either in animals that died or in animals that survived to 24 h after inoculation. We conclude that in a rat model of necrotizing pneumonia, there was an early, brisk inflammatory transcriptional response associated with neutrophil recruitment and tissue destruction. Deletion of lukSF-PV did not alter the early immune response to CA-MRSA in the lung.
Epidemic community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has been associated with several severe illnesses, including necrotizing pneumonia, that occur in healthy, immunocompetent children and young adults (1, 7, 10). CA-MRSA necrotizing pneumonia is characterized by an intense inflammatory response in the lung, resulting in obliteration of alveolar air spaces, severe pulmonary edema, intrapulmonary bacterial proliferation, and hemorrhage, with substantial mortality (1, 7).
The host immune response in CA-MRSA necrotizing pneumonia remains largely uncharacterized. The results of several recent studies employing animal models of S. aureus pneumonia demonstrated that there was an early influx of neutrophils into the alveolar air spaces (2, 20, 21). Ventura et al. recently employed a proteomics-based approach to demonstrate increased abundance of many proteins associated with the inflammatory response and the coagulation cascade in the bronchoalveolar lavage fluid from mice infected with a clinical blood isolate of S. aureus (21). However, transcription of chemokines and cytokines in lung tissue has not been studied.
The microbial virulence factors responsible for necrotizing pneumonia have been incompletely defined. A feature common to nearly all CA-MRSA isolates from patients with necrotizing pneumonia is the presence of the lukSF-PV genes encoding the Panton-Valentine leukocidin (PVL). Prior to the onset of epidemic CA-MRSA disease, limited data suggested that these genes were present in <5% of unselected S. aureus isolates (18). Driven by the epidemiologic association of the lukSF-PV genes with CA-MRSA isolates, many workers have hypothesized that PVL must play an important role in the pathogenesis of CA-MRSA necrotizing pneumonia (3). In support of this, the presence of PVL in patient isolates has been associated with increased illness severity; pneumonia caused by lukSF-PV-positive isolates was associated with sepsis, leukopenia, hemoptysis, pleural effusion, and death more frequently than was pneumonia caused by lukSF-PV-negative strains (7).
Despite this compelling epidemiologic association, demonstrating a role for PVL in virulence with animal models of pneumonia has proved to be difficult. Using wild-type USA300 and USA400 strains and corresponding isogenic lukSF-PV deletion mutants, Bubeck Wardenburg et al. demonstrated no role in virulence for PVL in a murine model (C57BL/6) of pneumonia (4). Indeed, they found that infection with the USA300 lukSF-PV deletion mutant in BALB/c mice resulted in higher mortality than infection with the wild-type parent isolate (5). In contrast, Labandeira-Rey et al. demonstrated that introduction of the lukSF-PV genes into a less virulent S. aureus isolate (RN6390) increased virulence and produced pneumonia after experimental inoculation (11). Other investigators, however, have found little or no role for PVL in virulence in a rabbit model of bacteremia and mouse models of bacteremia and skin infection (6, 23).
Using an established rat model of CA-MRSA necrotizing pneumonia (17), the present study aimed to define the transcription of genes mediating the early inflammatory response in the lung after CA-MRSA infection and to examine the role of PVL in modulating this response. We hypothesized that intratracheal inoculation of a CA-MRSA clinical isolate in the rat would be associated with a vigorous, time-dependent, innate immune response. Furthermore, we hypothesized that, given the possible role of PVL in evoking inflammation, infection with a lukSF-PV deletion mutant would result in an attenuated host response.
MATERIALS AND METHODS
Isolates.
USA300 CA-MRSA strain LAC (wild type) and an isogenic lukSF-PV deletion mutant, designated LAC Δpvl, were described previously (23). They were a generous gift from Michael Otto (NIH). Infection after experimental inoculation of strain LAC in the rat model was previously characterized (17).
Preparation of bacteria for inoculation.
To prepare an inoculum for animals, a frozen stock of S. aureus was subcultured onto tryptic soy agar and incubated at 37°C overnight. A single colony was inoculated into 5 ml tryptic soy broth, shaken at 250 rpm, and incubated at 37°C overnight. The overnight culture was diluted 1:100 and grown to early stationary phase (optical density at 600 nm, 3.0). The cells were centrifuged (3,200 × g, 15 min), washed in phosphate-buffered saline (PBS), and resuspended in PBS. All inocula were quantified by plating serial dilutions on tryptic soy agar and enumerating colonies. Inocula containing 2 × 108 or 5 × 108 CFU were prepared using a volume of 100 μl.
Rat model of necrotizing pneumonia.
Animal experiments were conducted in accordance with the regulations of the University of Chicago Animal Care and Use Committee. A rat model of necrotizing pneumonia was described previously (17). Briefly, male Sprague-Dawley rats (Harlan) weighing approximately 300 g were anesthetized with ketamine and xylazine, intratracheally inoculated with S. aureus or PBS (100 μl), and held upright for 15 s. Animals were returned to their cages and observed for signs of illness.
Two experiments were performed: (i) to examine the early transcription of host genes involved in the inflammatory response in pneumonia, rats were inoculated with a sublethal dose (2 × 108 CFU) of strain LAC (“time course experiment”); and (ii) to examine the influence of PVL on the host response, rats were inoculated with the 50% lethal dose (5 × 108 CFU) of LAC or LAC Δpvl (“PVL experiments”). In the time course experiment, infected (and PBS-inoculated) animals were sacrificed at 3, 6, 9, and 12 h after infection. In the PVL experiment, animals were observed until they died or became moribund (as determined by a preestablished illness severity score [17]), at which point they were sacrificed. All surviving animals were sacrificed 24 h after inoculation.
After death, the left lung was removed, washed twice in PBS, and homogenized (Tissuemiser; Fisher); serial dilutions of the homogenate were plated on mannitol salt agar for quantification of bacteria. The right upper lobe was placed in RNAlater (Ambion), a solution that inhibits tissue RNases. The remainder of the right lung was placed in formalin, sequentially infiltrated with increasing concentrations of ethanol and xylene, and embedded in paraffin. The tissues were sectioned and stained with hematoxylin and eosin. Pathological analysis was performed in a blinded fashion using a previously described scoring system with a maximum of 11 points (17).
RNA isolation and preparation of cDNA.
Lung tissue was stored in RNAlater for at least 24 h at 4°C to allow full penetration, following which it was removed and stored at −80°C, according to the manufacturer's instructions. Eukaryotic RNA was extracted from lung homogenates using an RNeasy tissue kit and treated with DNase to remove contaminating DNA (Qiagen). RNA quality and quantity were assessed using a Nanodrop spectrophotometer by measurement of A260/A280. RNA quality was confirmed by electrophoresis on a 1.2% agarose-0.66 M formaldehyde gel. One microgram of RNA was reverse transcribed to cDNA using a high-capacity cDNA archive kit (Applied Biosystems).
Quantitative real-time reverse transcriptase PCR analysis of cytokine expression.
Detection and quantification of gene expression in the lung were performed using an RT2 Profiler rat inflammatory cytokine and receptor kit (catalog no. PARN-011) according to the manufacturer's instructions (SABiosciences). This kit was chosen because it includes diverse genes important in the immune response, including genes encoding CC chemokines (n = 16), CXC chemokines (n = 9), interleukin cytokines (n = 14), other cytokines (n = 11), chemokine receptors (n = 15), and cytokine receptors (n = 11), as well as other genes involved in the inflammatory response (n = 8). Quantitative real-time reverse transcriptase PCR was performed using an ABI Prism 7300 series RT-PCR thermocycler (Applied Biosystems). The threshold cycle (CT) was calculated for each gene using the Sequence Detection software, version 1.2.2 (Applied Biosystems). The threshold and baseline were set manually according to the manufacturer's instructions. CT data were uploaded into the data analysis template on the manufacturer's website (http://www.sabiosciences.com/pcr/arrayanalysis.php). The relative expression of each gene compared with the expression in control animals was calculated on the website using the ΔΔCT method with five housekeeping genes as controls. In the time course experiment, data from animals sacrificed at each time point were pooled and compared with data from two animals inoculated with PBS that were sacrificed at the same time point. For the PVL experiment, the groups inoculated with LAC and LAC Δpvl were directly compared; each group was also compared with PBS-inoculated controls.
Data analysis.
A difference was considered significant if the P value was <0.05. Mortality data and data for the presence of necrotizing pneumonia were analyzed using Fisher's exact test. In vivo bacterial survival data were compared using Student's t test. Pulmonary pathology scores were compared with the Mann-Whitney U test. In the expression studies, a gene was considered differentially regulated if the difference was ≥3-fold in a comparison with the control and markedly differentially regulated if the difference was ≥10-fold. Expression of genes after inoculation with LAC was compared with expression of genes after inoculation with LAC Δpvl using Student's t test.
RESULTS
Necrotizing pneumonia after sublethal infection with CA-MRSA isolate LAC (time course experiment). (i) Clinical, microbiologic, and histopathologic features.
Each animal inoculated with 2 × 108 CFU of strain LAC became ill, which was characterized by decreased mobility, hunched posture, and labored breathing. Previous work has shown that infection with this inoculum is not lethal (data not shown). Five animals were sacrificed at each time point (i.e., at 3, 6, 9, or 12 h after infection). Two rats that were mock infected with sterile PBS were sacrificed at each time point.
The mean amount of bacteria recovered from the lung was 1 × 108 to 2 × 108 CFU at 3 and 6 h, after which it increased (Fig. 1A), an observation suggesting that bacterial replication occurred in the lung. The severity of histopathology (as assessed by the pathology score) increased in the 9 h after infection (Fig. 1B). Lungs from animals sacrificed at 3 h had minimal or no inflammatory infiltrate (Fig. 2B). At 6 h, there was more obvious inflammation with pulmonary edema; one of five animals had areas of necrosis and visible bacteria (Fig. 2C). At 9 and 12 h, there was severe pneumonia with marked pulmonary edema, necrosis, and multifocal bacterial aggregates in 60% (6/10) of the inoculated animals (Fig. 2D and E). The lungs of other animals sacrificed at 9 and 12 h after inoculation had mild or moderate inflammation.
FIG. 1.
Early events after infection with a sublethal inoculum of strain LAC. (A) In vivo recovery of bacteria from the lungs 3, 6, 9, and 12 h after infection. Each circle represents an individual animal. The bars indicate the means for the groups. (B) Pathology scores for lungs 3, 6, 9, and 12 h after infection. Each circle represents an individual animal. The bars indicate the medians for the groups.
FIG. 2.
Lung histopathology of animals infected with a sublethal inoculum of strain LAC 3, 6, 9, and 12 h after infection. Lung sections were stained with hematoxylin and eosin. Magnification, ×10. (A) Normal-appearing lung after inoculation with PBS. (B to E) Lungs from animals infected with LAC. (B) Minimal inflammation at 3 h. (C) At 6 h, bacterial colonies and inflammation were present. (D) At 9 h, diffuse pulmonary edema and inflammation were apparent. (E) At 12 h, multifocal bacterial colonies, pulmonary edema, and necrosis were visible.
(ii) Inflammatory response in the lung.
The expression of host inflammatory genes in animals inoculated with LAC was compared with the expression of these genes in PBS-inoculated animals sacrificed at the same time. Of the 84 genes included in the RT2 Profiler rat inflammatory cytokine and receptor array, 64 (76%) were differentially expressed in the infected animals at at least one time point (Fig. 3). The 54 genes with increased expression (≥3-fold) included genes encoding CC chemokines (14/16 genes studied), CXC chemokines (7/9 genes), interleukins (10/14 genes), other cytokines (6/11 genes), chemokine receptors (10/15 genes), and cytokine receptors (4/11 genes), as well as other genes involved in the inflammatory response (3/8 genes). The 31 genes with markedly increased expression (≥10-fold) included genes encoding CC chemokines (10/16 genes), CXC chemokines (6/9 genes), interleukins (9/14 genes), other cytokines (2/11 genes), chemokine receptors (3/15 genes), and cytokine receptors (1/11 genes). The 15 genes with decreased expression included genes encoding CXC chemokines (2/9 genes), interleukins (4/14 genes), other cytokines (1/11 genes), chemokine receptors (7/15 genes), and cytokine receptors (1/11 genes). Quantitative expression data for all genes included in the array are summarized in Fig. S1 in the supplemental material. In aggregate, there was widespread increased expression of genes mediating inflammation at 3 h (expression of 35 genes increased ≥3-fold, and expression of 7 genes increased ≥10-fold), 6 h (expression of 42 genes increased ≥3-fold, and expression of 30 genes increased ≥10-fold), 9 h (expression of 21 genes increased ≥3-fold, and expression of 14 genes increased ≥10-fold), and 12 h (expression of 28 genes increased ≥3-fold, and expression of 16 genes increased ≥10-fold) after inoculation. Decreased expression of genes was most common at 12 h postinoculation (12 genes).
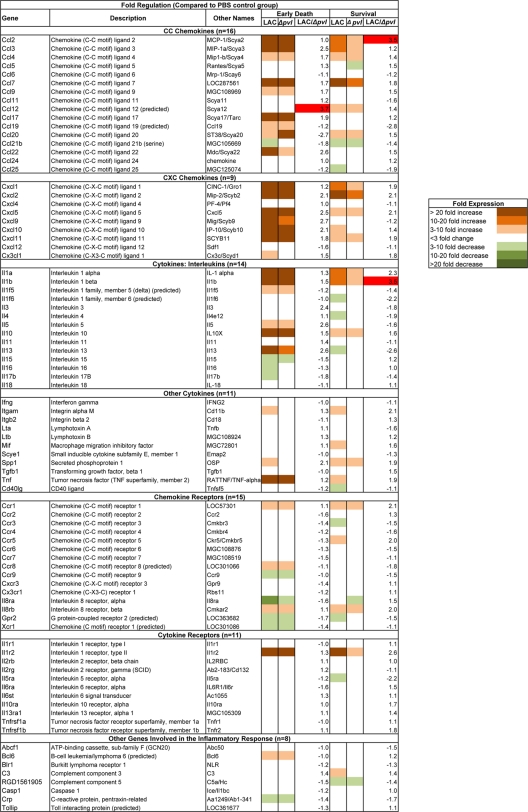
FIG. 3.
Transcription of inflammatory genes in the lung after sublethal infection with CA-MRSA isolate LAC. The level of expression is indicated by the change compared with PBS controls sacrificed at the same time point.
Role of PVL in experimental necrotizing pneumonia and the inflammatory response. (i) Deletion of lukSF-PV does not alter the course of CA-MRSA necrotizing pneumonia.
Two separate experiments were performed under identical conditions; there was no difference in mortality, bacterial recovery, or histopathology between the two experiments. Thus, data were pooled for analysis (21 animals in each group). There was no significant difference in mortality between inoculation with LAC (62%) and inoculation with LAC Δpvl (72%) (P = 0.44) (Fig. 4A). There also was no significant difference in the magnitude of bacterial recovery from the lung (P = 0.57) (Fig. 4B) or the severity of pulmonary pathology (P = 0.87) (Fig. 4C). Severe necrotizing pneumonia (a pathology score of ≥7) was present in 57% of the animals infected with LAC and in 55% of the animals infected with LAC Δpvl (P = 0.64) (Fig. 4D and E). The histopathologic appearance of the lungs of animals from the two groups was indistinguishable; the median pathology score for both groups was 8 (Fig. 4D and E). Seven animals infected with LAC and nine animals infected with LAC Δpvl died within 5 to 6 h after inoculation; these animals were classified as “early” deaths. Eight animals infected with LAC and six animals infected with LAC Δpvl survived for 24 h after inoculation; these animals were classified as “survivors” and were sacrificed at 24 h.
FIG. 4.
Comparison of inoculation with LAC and inoculation with LAC Δpvl in a rat model of pneumonia. (A) Mortality. (B) In vivo bacterial survival in the lung. Each circle represents an individual animal. The bars indicate the means for the groups. (C) Pulmonary pathology score. Each circle represents an individual animal. The bars indicate the medians for the groups. (D and E) Representative lung histopathology. The preparation was stained with hematoxylin and eosin. Magnification, ×10. (D) Lung from animal infected with LAC. (E) Lung from animal infected with LAC Δpvl. In each case, multifocal bacterial colonies and necrosis are visible.
(ii) Effect of PVL on the inflammatory response in CA-MRSA necrotizing pneumonia.
Four early deaths and six survivors were randomly selected from each group (LAC and LAC Δpvl) for comparative analysis of inflammatory gene expression. Comparison with the time course experiment revealed a similar pattern of altered expression, with a predominance of genes whose expression was increased after inoculation with LAC and LAC Δpvl compared with the PBS-inoculated controls. In the animals that died early after inoculation with LAC or LAC Δpvl, there was increased expression (≥3-fold) of multiple genes encoding chemokines, cytokines, and receptors compared with the PBS-inoculated controls (for the LAC group, 31 genes; for the LAC Δpvl group, 27 genes) (Fig. 5; see Fig. S2 in the supplemental material). In addition, there was decreased expression of several genes compared with the PBS-inoculated controls, and the majority of these genes encoded cytokines and chemokine receptors (for the LAC group, nine genes; for the LAC Δpvl group, six genes).
FIG. 5.
Expression of inflammatory genes after infection with LAC or LAC Δpvl. The results are expressed as changes compared with PBS-inoculated controls. Expression after inoculation with LAC and expression after inoculation with LAC Δpvl were also directly compared, and the results are expressed as the LAC/LAC Δpvl ratio. Genes for which there was a >3-fold difference between LAC-inoculated animals and LAC Δpvl-inoculated animals are highlighted with red.
Overall, the expression of fewer genes was altered in survivors than in early deaths; in survivors, there was increased expression (≥3-fold) of fewer genes (for the LAC group, 23 genes; for the LAC Δpvl group, 17 genes), and the expression of several genes was decreased (for the LAC group, 10 genes; for the LAC Δpvl group, 4 genes). Additionally, the number of genes whose expression was markedly altered was smaller for the survivors than for the early deaths; there was markedly increased expression (≥10-fold) of 17 genes in the early deaths irrespective of whether the animals were inoculated with LAC or with LAC Δpvl, compared with markedly increased expression of 8 and 2 genes in survivors inoculated with LAC and LAC Δpvl, respectively.
To examine the effects of PVL on the transcriptional response, scatter plots comparing the expression of genes after infection with LAC and the expression of genes after infection with LAC Δpvl were constructed for the early death and survivor groups (Fig. 6). We arbitrarily defined a difference to be present between expression after infection with LAC and expression after infection with LAC Δpvl if there was a threefold difference in expression for a given gene. The ratios of expression in animals infected with LAC to expression in animals infected with LAC Δpvl are shown in Fig. 5.
FIG. 6.
Scatter plots comparing the expression of genes involved in the inflammatory response in lungs of animals infected with LAC and the expression of these genes in lungs of animals infected with LAC Δpvl. (A) Animals that died within 5 to 6 h of inoculation. (B) Animals that survived to 24 h. Each circle represents an individual gene. The boundary lines indicate a threefold difference. Genes outside the boundary lines have ≥3-fold altered expression in animals inoculated with LAC compared with the expression in animals inoculated with LAC Δpvl. Genes with no change in regulation (<3-fold in either direction) are within the boundary lines.
Using this criterion, only the CCL12 gene had increased expression after infection with LAC compared with the expression after infection with LAC Δpvl in animals that died early (Fig. 5). No genes had decreased expression. In the survivors, two genes (the CCL2 and interleukin-1β [IL-1β] genes) had increased expression, and no gene had decreased expression in the LAC group compared with LAC Δpvl group. Statistical analysis revealed differential expression of the genes encoding CCL17, CCL22, CXCL9, CXCL12, RGD1561905, and Casp1 at the early time point and of the gene encoding IL-13ra1 at the later time point (P < 0.05), but the difference for none of these genes reached the predetermined threefold cutoff (Fig. 5; see Fig. S2 in the supplemental material). Thus, although there were some minor differences in transcription of a few genes, there was not any global change in the transcription of inflammatory genes in CA-MRSA necrotizing pneumonia that was attributable to the presence or absence of PVL.
DISCUSSION
Intratracheal infection of rats with a sublethal inoculum of USA300 CA-MRSA strain LAC resulted in necrotizing pneumonia with clinical and histopathologic features of clinical illness in humans. We observed a time-dependent increase in inflammation, predominantly infiltration of neutrophils, in the alveolar air spaces beginning as early as 6 h after infection. By 9 h, severe disease was present, with pulmonary edema, multifocal bacterial aggregates, and necrosis visible histologically in most animals. There was also a time-dependent increase in the number of bacteria recovered from the lungs. Moreover, there was early increased expression of genes encoding cytokines and chemokines and their receptors, which peaked at 6 h and began to decrease at 12 h after inoculation.
The presence of lukSF-PV did not alter the course of infection with CA-MRSA USA300 in the rat model, as mortality, in vivo bacterial survival, and pulmonary pathology were not affected. This finding supports the findings of other groups who found that lukSF-PV deletion had little or no effect on the course and severity of experimental infections in several animal models, including pneumonia, sepsis, bacteremia, and skin infection (4-6, 23).
Some workers have speculated that PVL may alter the host immune response. However, our results demonstrate that the presence of lukSF-PV did not change the transcription of a panel of genes reflecting the immune response in the lung. Infection with either LAC or LAC Δpvl generally resulted in a strong increase in inflammatory gene transcription, but only a few genes had significantly different expression in animals infected with LAC than in animals infected with LAC Δpvl. We believe that it is unlikely that these variations represent fundamentally different processes dependent on PVL. Thus, in this model, PVL did not appear to significantly affect the early host response in CA-MRSA necrotizing pneumonia.
The temporal pattern of altered inflammatory gene expression that we observed in the time course experiment is noteworthy; the expression of cytokine and chemokine genes was greatest 6 h after inoculation and decreased thereafter. Similarly, in the PVL experiments, we observed that the expression of chemokine and cytokine genes was greatest in animals that died early and decreased substantially by 24 h in survivors. Nearly all mortality in the rat model occurs in the 24 h following inoculation, during this phase of intense acute inflammation. We previously found that if an animal survived the early infection, inflammation subsided and S. aureus was cleared from the lungs by 72 to 96 h, even without treatment (unpublished observations). Although this feature of S. aureus pneumonia observed in rats does not regularly occur in patients, greater understanding of the mechanisms of bacterial clearance and modulation of inflammation may be of interest for understanding the pathophysiology of CA-MRSA disease.
There was markedly increased expression of the majority of the genes encoding chemokines included in the array that we employed. Several genes were upregulated ≥100-fold. The increased expression of chemokine genes was greatest prior to the onset of severe histopathologic inflammation. This suggests that the early host response to S. aureus in the lungs involves an increase in the expression of genes whose function is to recruit inflammatory cells, especially neutrophils, to the site of infection.
Experimental models of S. aureus infection have produced seemingly contradictory conclusions regarding the role of neutrophils in mediating the host response. For example, it has been well established that experimental depletion of neutrophils prior to inoculation with S. aureus increases disease severity in an animal brain abscess model (9) and increases mortality in pneumonia (19) and sepsis (22). Thus, neutrophils are essential for host defense against S. aureus. This concept may not be simple, however; the exuberance of the host inflammatory gene response has led us to speculate that excessive recruitment of neutrophils may lead to tissue destruction and facilitate bacterial access to the vasculature. In support of this, Gresham et al. found that intraperitoneal S. aureus inoculation of mice deficient in a gene (IAP) that regulates neutrophil migration resulted in less mortality, less neutrophil accumulation, and better bacterial clearance than infection of wild-type mice (8). These authors also demonstrated that mortality was decreased if mice were partially depleted of neutrophils by administration of antineutrophil antibodies prior to inoculation with S. aureus compared with either mice with complete neutrophil depletion or sham-treated animals (8). Furthermore, in models of S. aureus skin and wound infections, modulation of the neutrophilic response by CD4+ T cells resulted in less inflammation and more effective bacterial clearance (13). We hypothesize that CA-MRSA necrotizing pneumonia is analogous to models in which control of neutrophil-mediated inflammation is necessary for optimal host defense.
Most genes that had altered expression in our model have been implicated as genes that are important in other models of disease; their role in the pathogenesis of S. aureus pneumonia, however, has been undefined. Several of these genes have been studied in various models of disease caused by S. aureus. For example, the genes encoding the rat IL-8 homologs CXCL1 (KC) and CXCL2 (MIP-2), each of which is chemotactic for neutrophils, and their receptor, CXCR2 (IL-8Rβ), had markedly increased expression. These three genes were required for neutrophil recruitment and subsequent bacterial clearance in an S. aureus brain abscess model (9). CXCL1 and CXCL2 were also important in neutrophil recruitment in a model of S. aureus wound infection; however, exogenous administration of CXCL2 resulted in less effective clearance of S. aureus from the wound (13). Exogenous administration of CXCL1 and CXCL2 also increased mortality and the bacterial burden in a mouse model of S. aureus peritonitis (8). Conversely, blockade of CXCR2 resulted in improved bacterial clearance in wound infections (13).
Although they are required for neutrophil recruitment, CXCL1 and CXCL2 may contribute to persistence of S. aureus in neutrophils, perhaps by delayed neutrophil apoptosis and clearance of infected neutrophils by macrophages (8). Production of these chemokines has been attributed in part to IL-1β, IL-1R, and gamma interferon, all of which were also upregulated in this study (12, 14, 15). Of note, expression of IL-1β was greater in animals infected with LAC than in animals infected with LAC Δpvl; although this could be a mechanism by which PVL-positive isolates potentiate a more vigorous immune response, this possibility requires further study. Although CXCL1 and CXCL2 were present in bronchoalveolar lavage fluid (2) and pleural fluid (16) after infection with S. aureus, their role in the pathogenesis of CA-MRSA necrotizing pneumonia has not yet been established.
This study had several limitations. First, rodent models of S. aureus disease imperfectly mimic disease in patients. For example, rodents who survive the first 24 h after infection typically recover fully, as mentioned above. However, the pulmonary pathology that we have observed in the rat model bears a striking histologic resemblance to that seen in humans (1, 7, 17). Second, we measured gene transcription, not protein production. Thus, we did not take into account potential posttranscriptional and posttranslational modifications. There is some evidence that expression of chemokine genes may not always correlate with protein abundance (2). In addition, we assessed transcription of many cytokine and chemokine genes; therefore, in-depth analysis of the contribution of a single gene to the pathogenesis of CA-MRSA necrotizing pneumonia was limited.
In conclusion, this study demonstrated that there was an early increase in the expression of many genes involved in the inflammatory response in experimental CA-MRSA pneumonia, including genes encoding chemokines and cytokines and their receptors. This increased expression was followed by accumulation of neutrophils, increased bacterial recovery, and tissue destruction characteristic of CA-MRSA necrotizing pneumonia. Deletion of lukSF-PV did not significantly alter the course of CA-MRSA necrotizing pneumonia or the host inflammatory response.
Supplementary Material
Acknowledgments
R.S.D. has served on paid advisory boards for Clorox, Sanofi Pasteur, GlaxoSmithKline, Pfizer, Merck, and Wyeth; received lecture fees from Pfizer; and received grant support from Clorox, Pfizer, Sage Products, and Sanofi Pasteur.
This work was supported by the NICHD (Research Training in Pediatrics grant 5K12HD043387-04 to C.P.M.); the Children's Research Foundation, University of Chicago (C.P.M.); NIAID grants R01AI40481 and 1R01AI067584 to R.S.D.; Centers for Disease Control and Prevention grants R01CCR523379, R01CI000373, and U01-CI000384 to R.S.D.; and the Grant HealthCare Foundation (R.S.D.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 23 February 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adem, P. V., C. P. Montgomery, A. N. Husain, T. K. Koogler, V. Arangelovich, M. Humilier, S. Boyle-Vavra, and R. S. Daum. 2005. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N. Engl. J. Med. 3531245-1251. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, A. H., T. J. Foster, A. Hayashida, and P. W. Park. 2008. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J. Infect. Dis. 1981529-1535. [DOI] [PubMed] [Google Scholar]
- 3.Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 873-9. [DOI] [PubMed] [Google Scholar]
- 4.Bubeck Wardenburg, J., T. Bae, M. Otto, F. R. Deleo, and O. Schneewind. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 131405-1406. [DOI] [PubMed] [Google Scholar]
- 5.Bubeck Wardenburg, J., A. M. Palazzolo-Ballance, M. Otto, O. Schneewind, and F. R. DeLeo. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J. Infect. Dis. 1981166-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diep, B. A., A. M. Palazzolo-Ballance, P. Tattevin, L. Basuino, K. R. Braughton, A. R. Whitney, L. Chen, B. N. Kreiswirth, M. Otto, F. R. DeLeo, and H. F. Chambers. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS ONE 3e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359753-759. [DOI] [PubMed] [Google Scholar]
- 8.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 1643713-3722. [DOI] [PubMed] [Google Scholar]
- 9.Kielian, T., B. Barry, and W. F. Hickey. 2001. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 1664634-4643. [DOI] [PubMed] [Google Scholar]
- 10.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2981763-1771. [DOI] [PubMed] [Google Scholar]
- 11.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Hook, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 3151130-1133. [DOI] [PubMed] [Google Scholar]
- 12.McLoughlin, R. M., J. C. Lee, D. L. Kasper, and A. O. Tzianabos. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 1811323-1332. [DOI] [PubMed] [Google Scholar]
- 13.McLoughlin, R. M., R. M. Solinga, J. Rich, K. J. Zaleski, J. L. Cocchiaro, A. Risley, A. O. Tzianabos, and J. C. Lee. 2006. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. USA 10310408-10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, L. S., R. M. O'Connell, M. A. Gutierrez, E. M. Pietras, A. Shahangian, C. E. Gross, A. Thirumala, A. L. Cheung, G. Cheng, and R. L. Modlin. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 2479-91. [DOI] [PubMed] [Google Scholar]
- 15.Miller, L. S., E. M. Pietras, L. H. Uricchio, K. Hirano, S. Rao, H. Lin, R. M. O'Connell, Y. Iwakura, A. L. Cheung, G. Cheng, and R. L. Modlin. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 1796933-6942. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed, K. A., N. Nasreen, M. J. Ward, and V. B. Antony. 2000. Induction of acute pleural inflammation by Staphylococcus aureus. I. CD4+ T cells play a critical role in experimental empyema. J. Infect. Dis. 1811693-1699. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery, C. P., S. Boyle-Vavra, P. V. Adem, J. C. Lee, A. N. Husain, J. Clasen, and R. S. Daum. 2008. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J. Infect. Dis. 198561-570. [DOI] [PubMed] [Google Scholar]
- 18.Prevost, G., P. Couppie, P. Prevost, S. Gayet, P. Petiau, B. Cribier, H. Monteil, and Y. Piemont. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 42237-245. [DOI] [PubMed] [Google Scholar]
- 19.Robertson, C. M., E. E. Perrone, K. W. McConnell, W. M. Dunne, B. Boody, T. Brahmbhatt, M. J. Diacovo, N. Van Rooijen, L. A. Hogue, C. L. Cannon, T. G. Buchman, R. S. Hotchkiss, and C. M. Coopersmith. 2008. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J. Surg. Res. 150278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small, C. L., S. McCormick, N. Gill, K. Kugathasan, M. Santosuosso, N. Donaldson, D. E. Heinrichs, A. Ashkar, and Z. Xing. 2008. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 1805558-5568. [DOI] [PubMed] [Google Scholar]
- 21.Ventura, C. L., R. Higdon, L. Hohmann, D. Martin, E. Kolker, H. D. Liggitt, S. J. Skerrett, and C. E. Rubens. 2008. Staphylococcus aureus elicits marked alterations in the airway proteome during early pneumonia. Infect. Immun. 765862-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 652517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voyich, J. M., M. Otto, B. Mathema, K. R. Braughton, A. R. Whitney, D. Welty, R. D. Long, D. W. Dorward, D. J. Gardner, G. Lina, B. N. Kreiswirth, and F. R. DeLeo. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 1941761-1770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.