Abstract
B lymphocytes play an important role in the immune response induced by mucosal adjuvants. In this study we investigated the in vitro antigen-presenting cell (APC) properties of human B cells upon treatment with cholera toxin (CT) and Escherichia coli heat-labile enterotoxin (LT) and nontoxic counterparts of these toxins, such as the B subunit of CT (CT-B) and the mutant of LT lacking ADP ribosyltransferase activity (LTK63). Furthermore, forskolin (FSK), a direct activator of adenylate cyclase, and cyclic AMP (cAMP) analogues were used to investigate the role of the increase in intracellular cAMP caused by the A subunit of CT and LT. B lymphocytes were cultured with adjuvants and polyclonal stimuli necessary for activation of B cells in the absence of CD4 T cells. Data indicated that treatment with CT, LT, FSK, or cAMP analogues, but not treatment with CT-B or LTK63, upregulated surface activation markers on B cells, such as CD86 and HLA-DR, and induced inhibition of the proliferation of B cells at early time points, while it increased cell death in long-term cultures. Importantly, B cells treated with CT, LT, or FSK were able to induce pronounced proliferation of both CD4+ and CD8+ allogeneic T cells compared with untreated B cells and B cells treated with CT-B and LTK63. Finally, only treatment with toxins or FSK induced antigen-specific T-cell proliferation in Mycobacterium tuberculosis purified protein derivative or tetanus toxoid responder donors. Taken together, these results indicated that the in vitro effects of CT and LT on human B cells are mediated by cAMP.
The development of effective mucosal vaccines has been hindered by the lack of useful adjuvants and our limited knowledge of their modes of action. Cholera toxin (CT) from Vibrio cholerae and Escherichia coli heat-labile enterotoxin (LT) are potent immunological adjuvants, as indicated by mouse vaccine studies, although their mechanisms of action are not fully understood. These toxins are holotoxins composed of an enzymatically active A subunit that is noncovalently linked to a pentamer of B subunits binding a variety of galactose-containing molecules present in the plasma membranes of eukaryotic cells. CT binds mostly to the ganglioside GM1, which is believed to be the major toxin receptor, whereas LT binds not only to GM1 but also to other glycosphingolipids. Once internalized, the A subunit ADP ribosylates the α subunit of the GTP-binding regulatory protein Gs, thereby inducing permanent adenylate cyclase activation, resulting in an increase in the level of intracellular cyclic AMP (cAMP) (reviewed in reference 34).
The potentiation of antigen-presenting cell (APC) function is a major aspect of adjuvant action, and it has been shown that CT and LT induce maturation of both murine dendritic cells (DC) (26, 36) and human DC (5, 14, 15). Several studies demonstrated the ability of these toxins to promote B-cell isotype switch differentiation in mice (19, 27) and upregulation of activation markers in both murine and human B cells (2-4). While these toxins are potent adjuvants, their toxicity makes them unsuitable for human use. For this reason, a number of investigators have tried to develop nontoxic derivatives of CT and LT that retain adjuvanticity either by removing the A domain or by rendering it enzymatically inactive by site-directed mutagenesis (34). Although the current data suggest that the enzymatic activity of CT and LT holotoxins is responsible for the most potent adjuvant activity, a number of reports proposed that there are multiple immune modulating pathways that are triggered by CT and LT, including mechanisms independent of ADP ribosyltransferase activity (11, 13, 30, 33, 42). Numerous studies have suggested that engagement of the ganglioside GM1, the major receptor for CT and LT, is required for the ability of these molecules to modulate immune responses (22, 31). Recently, workers demonstrated that in the absence of the toxic A subunit, the B subunit of CT (CT-B) induces intracellular signaling associated with the in vitro activation of murine B cells and macrophages (37).
The majority of these studies have been performed with murine cells and have confirmed the in vivo adjuvanticity of nontoxic compounds, such as CT-B and LTK63, a mutant of LT lacking the ADP ribosyltransferase enzymatic activity, when they were mucosally delivered into animals, even if the immune responses observed in the in vivo studies were usually weaker than those induced by the wild-type toxins (6, 11, 20, 36, 40, 41). In order to develop a mucosal adjuvant for human vaccine, the mechanism(s) of action of potential nontoxic adjuvants should be investigated in vitro by using human APC. It has been shown that the B-cell antigen-presenting functions may be important for the induction of optimal vaccine-induced responses (10, 35). Moreover, B cells are present in mucosa-associated lymphoid tissues (8), and their function in these sites is related not only to immunoglobulin (Ig) production but also to their antigen-presenting properties (24). To elucidate the mechanisms by which enterotoxins modulate antigen-presenting properties, we decided to carry out a comprehensive and comparative analysis of the effects of the toxins and their nontoxic derivatives on the APC function of human B cells. Here we present evidence that CT and LT, as well as forskolin (FSK) and cAMP analogues, but not CT-B and LTK63, increase the activation of human B cells and induce improvement in their APC capability, indicating that the presence of the enzymatic subunit is critical for their adjuvanticity.
MATERIALS AND METHODS
Recombinant enterotoxins.
CT and CT-B were purchased from List Biological Laboratories (Campbell, CA); E. coli LT was purchased from Swiss Serum Vaccine Institute (Berne, Switzerland); LTK63 was provided by Novartis (Siena, Italy); and FSK, dibutyryl-cAMP (Db-cAMP), and 8-bromo-cAMP (8Br-cAMP) were purchased from Sigma Chemical Co. (St. Louis, MO). Endotoxin contamination in adjuvant preparations was evaluated by Limulus amoebocyte lysate analysis (Pyrochrome; Associates of Cape Cod, Falmouth, MA). The concentration of endotoxin was less than 0.09 endotoxin unit /μg in all of the preparations utilized in this study.
Isolation and activation of B cells.
Human B cells were isolated from peripheral blood mononuclear cells (PBMC) from healthy donors by positive selection using anti-CD19 microbeads and the manufacturer's suggested protocol (Miltenyi Biotec S.r.l., Bologna, Italy). The cells obtained were >95% CD19 positive, as assessed by flow cytometry analysis. B cells were cultured in 24-well plates or in 96-well plates at a concentration of 1.5 × 106 to 2 × 106 cells/ml in RPMI 1640 medium (GIBCO Invitrogen, Paisley, United Kingdom) supplemented with 100 U/ml of penicillin-streptomycin-glutamine (GIBCO Invitrogen, Paisley, United Kingdom), 10% heat-inactivated fetal bovine serum (Euroclone, Life Sciences Division, Pero, Italy), sodium pyruvate (Euroclone), and nonessential amino acids (Euroclone). In order to obtain polyclonal stimulation, B cells were cultured in the presence of 2.5 μg/ml of CpG ODN 2006 (MWG Biotech, M-Medical, Milan, Italy), 50 U/ml of interleukin-2 (IL-2) (BD Biosciences, San Diego, CA), and 2 μg/ml of anti-Ig monoclonal antibody (MAb) (Jackson ImmunoResearch Laboratories, Suffolk, United Kingdom). In addition, together with the stimuli, on day zero B cells were either treated with 3 μg/ml of CT, 10 μg/ml of CT-B, 0.1 μg/ml of LT, 10 μg/ml of LTK63, 50 μM of FSK, 0.5 mM Db-cAMP, or 8Br-cAMP or left untreated. In some experiments CD27+ and CD27− B cells were isolated by sorting total B cells with FACSAria (BD Biosciences, San Diego, CA). Briefly, B cells were isolated by using anti-CD19 microbeads, as described above. CD27+ and CD27− B-cell subsets were purified based on CD27 cell surface expression by FACSAria after staining with phycoerythrin-conjugated anti-CD27 MAb (Immunological Sciences, Rome, Italy). Dead cells were excluded on the basis of propidium iodide (PI) (5 μg/ml; BD Biosciences) fluorescence intensity. The two subpopulations were stimulated with polyclonal stimuli and treated with adjuvants as described above for the unfractionated B cells.
Determination of intracellular cAMP content.
B cells (2 × 105 cells in 200 μl, seeded in duplicate) were stimulated and treated for 24 h with adjuvants or FSK or left untreated in the presence of 100 μM 3-isobutyl-1-methylxanthine (Sigma Chemical Co., St. Louis, MO), which inhibits cAMP-hydrolyzing phosphodiesterases, in order to avoid cAMP degradation. The culture medium was removed after 10 min of centrifugation at 1,300 rpm, and cold 0.1 N HCl was used to lyse the cells. The intracellular cAMP content was measured by an enzyme-linked immunoassay by following the manufacturer's instructions (Biotrak EIA, GE Healthcare).
Flow cytometric immunofluorescence analysis of surface markers and apoptotic cells.
B cells were stained with the following mouse anti-human MAbs obtained from Becton Dickinson (BD Biosciences, San Diego, CA): phycoerythrin-labeled anti-CD86, anti-HLA class I, anti-CD80, and anti-CD40 and peridinin chlorophyll protein-labeled anti-CD20 and anti-HLA class II. Isotype-matched mouse IgG MAbs were used as controls. To evaluate B-cell death, stimulated B cells, treated as indicated above for 3 and 5 days, were stained with Annexin V-fluorescein isothiocyanate (FITC) plus PI (Annexin V-FITC apoptosis detection kit II; BD PharMingen, San Diego, CA) by following the manufacturer's instructions. Flow cytometric analysis of the cells was performed using a FACSCalibur and CellQuest software (BD Biosciences).
B-cell proliferation assay.
PBMC or purified B cells were labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) in phosphate-buffered saline containing 1% fetal bovine serum for 10 min at 37°C, washed in complete RPMI 1640 medium, and then seeded in 24-well or 96-well culture plates (1.5 × 106 cells/ml) containing polyclonal stimuli (see above) and subjected to different treatments. After 3 and 5 days of culture, the cells were washed and stained with MAbs against human CD4, CD8, or CD20 from Becton Dickinson. The amounts of cell proliferation in the cell populations were quantified by monitoring the sequential loss of fluorescence intensity of the CFSE-labeled cells using a FACSCalibur.
Mixed allogeneic cultures and antigen-specific presentation assay.
Purified, stimulated B cells treated as indicated above or left untreated for 3 days, carefully washed, and irradiated (3,000 rads) were cocultured with allogeneic PBMC labeled with CFSE (see above). A total of 1 × 105 PBMC per well were seeded onto 96-well plates (Sigma-Aldrich S.r.l., Milan, Italy) with titrated numbers of irradiated B cells (the B cell/PBMC ratio ranged from 1:16 to 1:1). After 3 days of coculture, cells were collected and stained with anti-human CD4 or anti-human CD8 MAbs. The levels of PBMC, CD4+, and CD8+ T-cell proliferation were evaluated by fluorescence-activated cell sorting (FACS) analysis. To examine the antigen-specific response, B cells isolated from Mycobacterium tuberculosis purified protein derivative (PPD) responder donors or tetanus toxoid (TT) responder donors were stimulated with CpG, IL-2, and PPD (Statens Serum Institute, Copenhagen, Denmark) or with TT (Novartis, Siena, Italy) at day zero. Toxins, CT-B, LTK63, and FSK were added on the same day. PPD and TT were also added on day 2. On day 3, cells were extensively washed, irradiated, and cocultured with autologous PBMC previously labeled with CFSE. CD4+ and CD8+ T-cell proliferation was evaluated after 5 days of coculture using a FACSCalibur and CellQuest software.
In some experiments anti-human CD86 and/or anti-human HLA-DR MAbs were used in order to block the interaction between APC and T cells in a mixed leukocyte reaction (MLR) assay. Briefly, B cells were incubated for 2 h at 4°C with 20 μg/ml of anti-CD86 (BU63; mouse IgG1; Ancell Immunology Research Products, Bayport, MN) and/or anti-HLA-DR (G46-6; mouse IgG2a; BD Biosciences, San Diego, CA) MAbs, extensively washed to remove free MAbs, and then added to CFSE-labeled PBMC.
Cytokine production.
Cytokine concentrations in supernatants collected from stimulated B cells treated as indicated above or left untreated for 3 days were determined by enzyme-linked immunosorbent assays (ELISA), including assays for tumor necrosis factor alpha (TNF-α) and IL-1β (Pierce Endogen, Rockford, IL), IL-6 (BD Biosciences, San Diego, CA), and IL-12 (R&D Systems, Minneapolis, MN).
Statistical analysis.
Microsoft Excel (Microsoft Corporation, Redmond, WA) was used for statistical analysis. Data were expressed as means ± standard deviations, and statistical significance was determined by Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
CT and LT induce upregulation of surface activation markers.
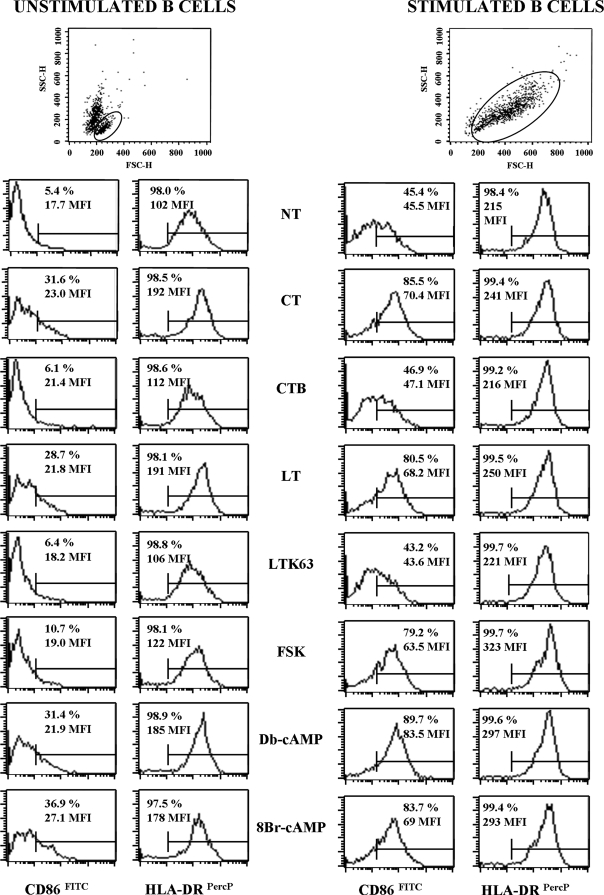
The levels of expression of activation markers CD86, CD80, HLA class I and II molecules, and CD40 on the B-cell surface were examined after treatment with CT, CT-B, LT, LTK63, FSK, or cAMP analogues, such as Db-cAMP and 8Br-cAMP, for 3 and 5 days. As expected, stimulation of B cells cultured in the presence of polyclonal stimuli (stimulated B cells), such as CpG ODN 2006, anti-Ig MAb, and IL-2, induced upregulation of CD86 and HLA-DR markers that was detectable after 3 days of culture compared to the results for unstimulated B cells (Fig. 1). Treatment of both unstimulated and stimulated B cells with CT, LT, FSK, or cAMP analogues induced upregulation of these markers. As shown in Fig. 1, for CD86 expression both the percentage of positive cells and the mean fluorescence intensity (MFI) were strongly increased, whereas for HLA-DR, already expressed on the majority of B cells, there was a marked increase in MFI following treatment with CT, LT, FSK, or cAMP analogues. None of the treatments was able to induce an evident change in CD40 and HLA class I expression, whereas slight downregulation of CD80 expression was observed in stimulated B cells treated with toxin, FSK, or cAMP analogues (data not shown). The presence of CT-B or LTK63 in the culture did not induce modulation of the expression of any of the markers analyzed (Fig. 1). As shown in dot plots in Fig. 1, isolated B cells cultured without polyclonal stimulation showed high mortality. For this reason we decided to perform the next experiments in the presence of polyclonal stimuli, which were also necessary for analysis of B-cell proliferation and cytokine production.
FIG. 1.
Effect of adjuvants on the expression of cell surface activation markers. Enzymatic activity is required for CT and LT to upregulate activation markers on the B-cell surface. Unstimulated B cells (left panel) or B cells stimulated with 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb (right panel) were simultaneously treated with the compounds indicated or left untreated (NT) for 3 days. The expression of cell surface markers was evaluated by FACS analysis of B cells stained with MAbs directed to CD86 and HLA-DR. Dot plots with forward scatter (FSC) and side scatter (SSC) parameters are shown to visualize the amounts of live cells (in the gate) analyzed for surface markers. The percentage of positive cells and the MFI are indicated in each graph. Representative data from five independent experiments are shown. PerCP, peridinin chlorophyll protein.
CT and LT induce an increase in the intracellular cAMP levels.
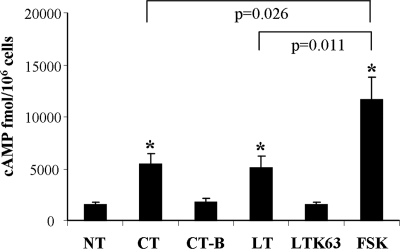
In order to check the enzymatic activity of adjuvants, intracellular cAMP was evaluated in B cells treated with the different compounds. As expected, CT, LT, and FSK, but not CT-B or LTK63, induced increases in intracellular cAMP levels in B cells (Fig. 2). The results showed that there was a statistically significant difference (P < 0.05) between B cells treated with CT, LT, or FSK and untreated control (5,436 ± 1,001, 5,054 ± 1,142, 11,613 ± 2,178, and 1,458 ± 266 fmol/106 cells, respectively). Moreover, FSK-treated B cells contained statistically significant larger amounts of intracellular cAMP than toxin-treated cells (Fig. 2).
FIG. 2.
Effect of adjuvants on intracellular cAMP. The level of intracellular cAMP increases upon treatment with CT, LT, or FSK. Stimulated B cells were treated with the compounds indicated for 24 h in the presence of 3-isobutyl-1-methylxanthine and lysed with HCl. The amount of cAMP was evaluated by an enzyme immunoassay and was expressed in fmol/106 B cells. The asterisks indicate statistically significant differences (P < 0.05) between CT-, LT-, or FSK-treated B cells and untreated B cells (NT). The P values indicate the statistically significant differences between FSK- and CT-treated cells and between FSK- and LT-treated cells. Representative data from three independent experiments are shown.
CT and LT inhibit proliferation of B cells and increase their susceptibility to death.
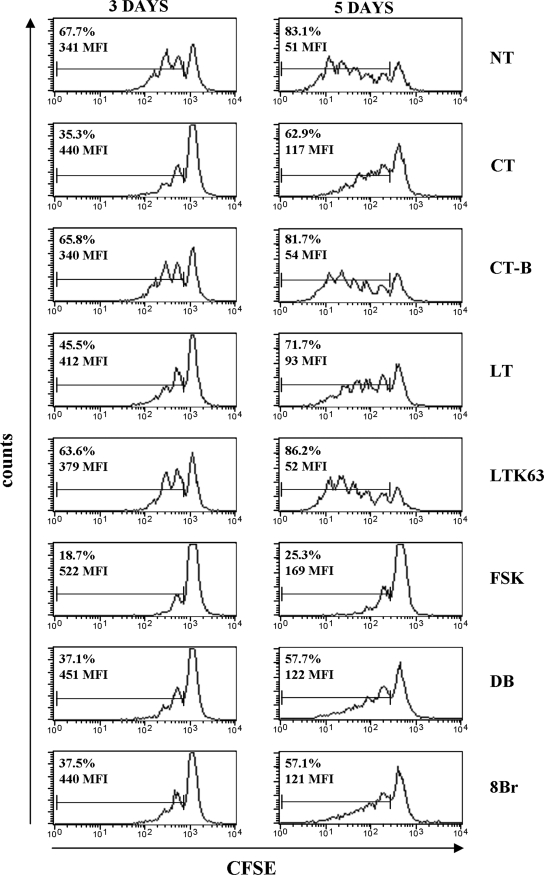
In order to evaluate the effect of adjuvants on cell proliferation, B lymphocytes were stained with CSFE and cultured for 3 and 5 days with polyclonal stimuli and with CT, CT-B, LT, LTK63, FSK, or cAMP analogues or left untreated. Figure 3 shows the results of a representative experiment. Polyclonal stimuli induced proliferation after 3 days of culture (67.7% proliferating B cells), which increased after 5 days (83.1%). Similar percentages of dividing B cells were evident when the cells were treated with CT-B or LTK63 (65.8% and 63.6%, respectively, at 3 days and 81.7% and 86.2%, respectively, at 5 days), suggesting that these compounds did not act on the ability of stimulated B cells to proliferate. Conversely, proliferation of CT-treated B cells, and to a lesser extent LT-treated B cells, was significantly less (P < 0.05) both at day 3 (35.3% and 45.5%, respectively) and at day 5 (62.9% and 71.7%, respectively). Similar results were obtained when B cells were treated with Db-cAMP and 8Br-cAMP (37.1% and 37.5%, respectively, at day 3 and 57.7% and 57.1%, respectively, at day 5). The inhibition of cell proliferation induced by toxins was evident not only based on the percentage of proliferating cells but also based on MFI, which indirectly indicated the rounds of B-cell division under each condition analyzed (Fig. 3). When stimulated B cells were treated with FSK, their ability to proliferate was inhibited even more than it was when they were treated with toxins (18.7% at day 3 and 25.3% at day 5), probably because of the high dose of FSK used (50 μM). The selection of this dose was based on the observation that lower doses were not sufficient to induce activation of human DC (5).
FIG. 3.
Effect of adjuvants on B-cell proliferation. The inhibitory effect of adjuvants on B-cell proliferation is related to the increase in the intracellular cAMP level. CFSE-labeled B cells were stimulated with polyclonal stimuli, including 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb, and simultaneously treated with the compounds indicated or left untreated (NT) for 3 and 5 days. The percentages of dividing cells and the MFI of CFSE proliferating cells are indicated in the graphs. The data shown are data from one representative experiment of five experiments performed. DB, Db-cAMP; 8Br, 8Br-cAMP.
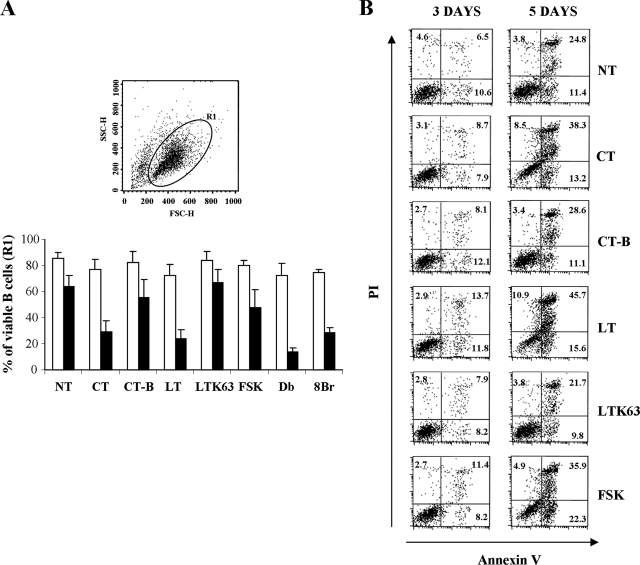
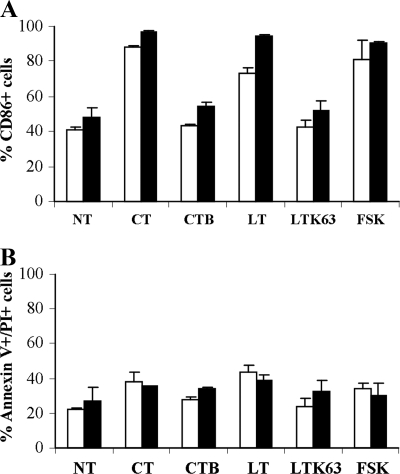
At the same time, in order to understand if the evident inhibition of proliferation observed was related to an increase in B-cell death, the effects of toxins on B-cell viability were evaluated. The percentage of live cells was initially assessed by gating the events (R1) on a dot plot with forward and side scatter parameters (Fig. 4A). On day 3, even if treatment with toxins, FSK, and cAMP analogues resulted in a slight reduction in viability, the viability of B cells was high under all conditions tested, suggesting that at this time cell death was not the cause of the marked inhibition of B-cell proliferation described above. After 5 days of culture the percentage of live B cells decreased to the same level in the control (untreated) or CT-B- or LTK63-treated samples. Treatment with FSK resulted in a further decrease in viability (P < 0.05), whereas CT, LT, and cAMP analogues induced a remarkable reduction in viability (P < 0.05). To confirm these results, we labeled the cells with Annexin V and PI after 3 and 5 days of culture in the presence or absence of adjuvants or FSK. As shown by the results of a representative experiment (Fig. 4B), after 3 days of culture the percentages of labeled cells (PI-positive cells, Annexin V-positive cells, and PI- and Annexin V-positive cells) were similar under all conditions analyzed except for LT-treated B cells, for which there was a slight higher percentage of total labeled cells. After 5 days, treatment with CT, LT, or FSK resulted in evident increases in the percentages of both PI- and Annexin V-positive cells, suggesting that these treatments induced increased susceptibility to death compared to that of B cells that were treated with CT-B or LTK63 or were left untreated.
FIG. 4.
Effect of adjuvants on B-cell viability. The enzymatic activity of CT and LT renders B cells more susceptible to death in long-term culture. B cells were stimulated with polyclonal stimuli, including 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb, and simultaneously treated with the compounds indicated or left untreated (NT) for 3 and 5 days. (A) Percentage of live cells as evaluated by gating the events (R1) on a dot plot with forward scatter (FSC) and side scatter (SSC) parameters. The histogram shows the percentages of gated B cells from five different donors analyzed at day 3 (open bars) and day 5 (filled bars). The error bars indicate standard deviations. Db, Db-cAMP; 8Br, 8Br-cAMP. (B) Stimulated B cells, treated as indicated for 3 and 5 days, were stained with Annexin V-FITC plus PI. The percentages of Annexin V-positive cells, PI-positive cells, and Annexin V-positive PI-positive cells are indicated in the plots. The data shown are data from one representative experiment of five experiments performed.
Toxin-treated B cells are efficient APC.
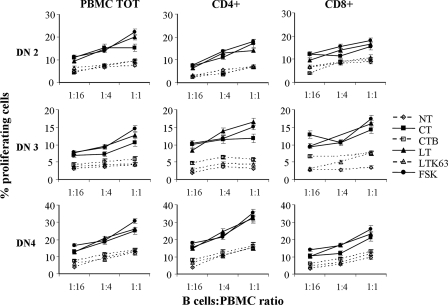
In order to investigate the APC function of stimulated B cells treated with toxins, MLR assays were performed. In this setting, to maintain a good activation state and to avoid cell death, B cells were used as APC after 3 days of stimulation and treatment. Allogeneic T-cell proliferation was analyzed by FACS to determine the content of CFSE after 3 days of coculture with irradiated B cells as stimulators. Figure 5 shows the proliferation of PBMC and CD4+ and CD8+ T cells from three different donors. B cells stimulated with polyclonal stimuli were able to induce low levels of PBMC proliferation. Treatment with toxins or with FSK clearly improved the APC function of stimulated B cells, as shown by the increased proliferation of allogeneic PBMC. In particular, CT-, LT-, or FSK-treated B cells induced both CD4+ and CD8+ T cells to proliferate at any ratio of B cells to PBMC used (1:16 to 1:1) in a dose-dependent manner. The increase in T-cell proliferation ranged from two- to fivefold compared with the T-cell proliferation observed for the untreated control. Similar results were obtained when B cells were treated with Db-cAMP (data not shown). Conversely, treatment of B cells with CT-B and LTK63 did not induce variation in T-cell proliferation compared to the results obtained with untreated B cells at any ratio. These data strongly suggested that CT and LT improved the efficiency of B cells acting as APC and that the mechanism of this effect was related to the increase in the intracellular cAMP level, as indicated by the effect of FSK-treated B cells on allogeneic T-cell proliferation.
FIG. 5.
Proliferation of allogeneic PBMC cocultured with B cells as APC. Activation of B cells by an increased level of intracellular cAMP enhances their ability to present alloantigen in the allogeneic T-cell response. B cells were stimulated with polyclonal stimuli, including 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb, and simultaneously treated with the compounds indicated or left untreated (NT) for 3 days and irradiated. B cells were cocultured with allogeneic PBMC labeled with CFSE at different B cell/PBMC ratios. After 3 days of coculture, cells were collected and stained with anti-human CD4 or anti-human CD8 MAb. The level of PBMC, CD4+, and CD8+ T-cell proliferation was evaluated by FACS analysis. The error bars indicate standard deviations for duplicates. Data from three independent experiments are shown. PBMC TOT, total PBMC.
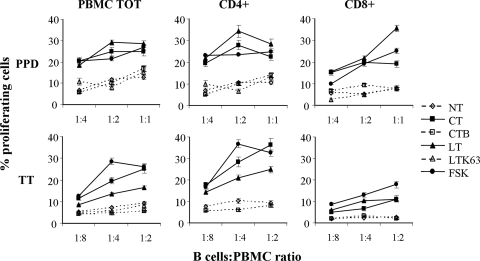
To analyze the antigen-specific T-cell activation induced by B-cell antigen presentation, toxin-treated B cells were used as APC in the autologous system in order to expand the antigen-specific T cells of either PPD or TT responder donors. The isolated B cells were stimulated with CpG, IL-2, and PPD protein or TT, and CT, LT, CT-B, LTK63, or FSK was added on the same day. After 3 days, cells were cocultured with autologous PBMC previously labeled with CFSE. The proliferation of PBMC, CD4+, and CD8+ T cells was evaluated after 5 days of coculture by FACS analysis. As shown in Fig. 6, only treatment with CT, LT, or FSK increased proliferation of autologous PBMC, CD4+, and CD8+ T cells at any ratio of B cells to PBMC used, whereas CT-B or LTK63 did not enhance the antigen-presenting capacity of B lymphocytes.
FIG. 6.
Antigen-specific T-cell proliferation. Activation of B cells by an increased level of intracellular cAMP enhances their ability to present PPD or TT in the autologous T-cell response. B cells derived from a PPD or TT responder donor were stimulated with 2.5 μg/ml of CpG ODN 2006 and 50 U/ml IL-2 in the presence of PPD or TT and treated as indicated or left untreated (NT) for 3 days. Cells were then irradiated and cocultured with autologous PBMC labeled with CFSE at different B cell/PBMC ratios. After 5 days of coculture cells were collected and stained with anti-human CD4 or anti-human CD8 MAb. The levels of PBMC, CD4+, and CD8+ T cell proliferation were evaluated by FACS analysis. The error bars indicate standard deviations for duplicates. PBMC TOT, total PBMC.
In order to check the role of CD86 and HLA-DR in the improvement of the APC function of toxin-treated B cells, MLR experiments were performed in the presence of blocking MAb anti-CD86 and/or MAb anti-HLA-DR. As expected, the presence of both blocking reagents inhibited up to 80% of PBMC proliferation (Table 1) at ratio of B cells to PBMC of 1:4, indicating the evident contribution of these molecules in this system.
TABLE 1.
MLR analysis in the presence of blocking MAbsa
| B-cell treatment | % of proliferating cells (mean ± SD)b
|
|||
|---|---|---|---|---|
| No blocking | Blocking MAbs
|
|||
| Anti-CD86 | Anti-HLA-DR | Anti-CD86 + anti-HLA-DR | ||
| None | 10.17 ± 0.81 | 5.36 ± 0.37 | 3.54 ± 0.08 | 2.07 ± 0.31 |
| CT | 18.00 ± 1.02 | 9.67 ± 0.35 | 4.58 ± 0.29 | 3.61 ± 0.42 |
| CT-B | 11.89 ± 1.17 | 5.90 ± 0.21 | 4.35 ± 0.61 | 2.76 ± 0.40 |
| LT | 20.30 ± 0.28 | 11.42 ± 0.43 | 5.99 ± 0.45 | 3.03 ± 0.74 |
| LTK63 | 9.34 ± 0.62 | 5.52 ± 0.31 | 4.43 ± 0.04 | 3.20 ± 0.35 |
| FSK | 18.86 ± 0.73 | 12.00 ± 1.12 | 6.48 ± 0.50 | 6.05 ± 0.78 |
B cells preincubated with anti-CD86, with anti-HLA-DR, or with both antibodies were not able to induce proliferation of CFSE-labeled PBMC.
The percentage of proliferating cells was determined using a B cell/PBMC ratio of 1:4.
CT and LT inhibit TNF-α production while increasing IL-12, IL-1β, and IL-6 production.
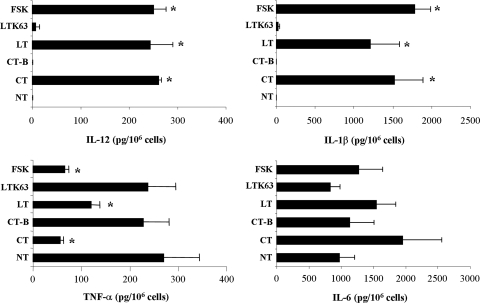
To examine the effect of adjuvants on the production of cytokines, supernatants from B cells cultured for 3 days with polyclonal stimuli and treated with toxins, FSK, or nontoxic counterparts or left untreated were analyzed by ELISA. Figure 7 shows the results obtained for five donors. Treatment with CT, LT, or FSK induced a significant decrease in TNF-α production (56.3, 120.4, and 64.9 pg/106 cells, respectively) compared to the untreated control (270 pg/106 cells). In contrast, treatment with CT-B or LTK63 did not significantly influence the cytokine levels (228 and 237 pg/106 cells). As shown in Fig. 7, toxin- and FSK-treated B cells showed significant production of IL-12 and IL-1β cytokines. In particular, high levels of IL-1β were detected in toxin- and FSK-treated samples (1,520, 1,211, and 1,781 pg/106 B cells treated with CT, LT, and FSK, respectively), whereas low but detectable concentrations of IL-12 were present in B-cell supernatants from CT-, LT-, and FSK-treated samples (260, 243, and 250 pg/106 B cells). Finally, treatment with toxins and FSK induced production of amounts of IL-6 larger than that in untreated B cells (1,947, 1,542, and 1,273 pg/106 cells for B cells treated with CT, LT, and FSK, respectively, versus 970 pg/106 cells for untreated B cells). However, the increase in IL-6 production was not statistically significant, probably due to the high variability among donors. Treatment with CT-B or LTK63 did not result in any significant difference in IL-12, IL-1β, or IL-6 production compared to untreated samples.
FIG. 7.
Cytokine production. CT and LT strongly inhibit the production of TNF-α but increase the production of IL-12, IL-1β, and IL-6. B cells were stimulated with polyclonal stimuli, including 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb, and simultaneously treated with the compounds indicated or left untreated (NT) for 3 days. B-cell culture supernatants from five different donors were collected and analyzed for the presence of the cytokines indicated by ELISA. The error bars indicate standard deviations. The asterisks indicate a statistically significant difference (P < 0.05) between the treatment and control (NT) samples.
Effect of adjuvants on sorted CD27+ and CD27− B-cell subpopulations.
Finally, in order to determine if the different subsets of B cells were targeted differently by adjuvants, CD27+ and CD27− B cells were isolated and treated as described above for unsorted B cells. In particular, CD86 expression and susceptibility to death were analyzed for the two different B-cell populations after 3 days of culture. As shown in Fig. 8A, for both subsets a dramatic increase in the level of CD86 was evident in toxin- or FSK-treated cells compared to the corresponding untreated sample, while the presence of CT-B or LTK63 did not affect CD86 expression. Likewise, for induction of cell death, the results indicated that treatment with toxins had similar effects on the two subsets (Fig. 8B). Indeed, at 3 days there was not a statistically significant difference in the percentages of Annexin V- and PI-labeled cells between the two subpopulations.
FIG. 8.
Effects of CT and LT on CD27+ and CD27− B-cell populations. Toxins act similarly in both subsets in terms of activation and induction of cell death. CD27+ and CD27− B cells were sorted by FACSAria, stimulated with polyclonal stimuli, including 2.5 μg/ml of CpG ODN 2006, 50 U/ml of IL-2, and 2 μg/ml of anti-Ig MAb, and simultaneously treated with the compounds indicated or left untreated (NT) for 3 days. (A) Expression of CD86 was evaluated by FACS analysis of CD27+ and CD27− B-cell subsets. The histograms show the percentages of CD86+ cells for CD27+ (open bars) or CD27− (filled bars) B lymphocytes from two donors. The error bars indicate standard deviations. (B) Stimulated B-cell subsets, treated as indicated for 3 days, were stained with Annexin V-FITC plus PI and analyzed by FACS. The percentages of Annexin V-positive and PI-positive cells are indicated. The error bars indicate standard deviations.
DISCUSSION
The specificity, magnitude, and quality of T-cell-mediated immune responses become conditioned during the early phase of antigen presentation. For this reason, analysis of the effects of adjuvants on APC could help identify the mechanism of action. In the population of APC that includes DC, B cells, and macrophages, DC are the most potent, and for this reason the majority of adjuvant studies are focused on these cells. We and other authors have previously shown that CT and LT were able to mature human DC and inhibit IL-12 and TNF-α production, whereas CT-B and LTK63 (5, 14, 15) did not have these abilities. Several papers described the APC function of B cells, indicating that in vivo B cells provide extra and essential antigen presentation capacity above that provided by DC, optimizing expansion and allowing generation of memory and effector T cells (9, 10, 17, 18, 23). Therefore, we decided to investigate the effects of toxins and their nontoxic counterparts on the antigen-presenting capacity of human B cells. In the present study we performed an in vitro comparative evaluation of the APC function of human B cells after treatment with CT, CT-B, LT, LTK63, or FSK, a direct activator of adenylate cyclase, as a positive control for an increase in intracellular cAMP, or directly with cAMP analogues, such as Db-cAMP and 8Br-cAMP. Our results show that the enzymatic activity of toxins is crucial for in vitro activation of human B cells and improvement of their APC capacity. Indeed, CT and LT, which increase intracellular cAMP levels, induced an evident activation state of human B cells, as judged by changes in surface phenotype, whereas none of the enzymatically inactive derivatives of CT or LT tested in this study were able to modify activation markers. In addition, the functional changes in B cells, including inhibition of proliferation, susceptibility to cell death, cytokine production, and an increase in the antigen-presenting capability induced by CT or LT, can be mimicked consistently by using the pharmacological agonist FSK or cAMP analogues.
To avoid the high rate of mortality of unstimulated B cells and to prolong the in vitro cultures, we decided to perform experiments in the presence of polyclonal stimuli. These stimuli, including antibody to human Ig as an antigen surrogate, CpG as a Toll-like receptor agonist, and IL-2 as a growth factor, are required for activation of both naïve and memory B cells in the absence of CD4+ T cells (7). Treatment with toxins was able to increase the expression of CD86 and HLA class II, confirming results obtained by other workers (2). Conversely, CT-B and LTK63, even if they were used at concentrations higher than those used for the toxins, were unable to upregulate the activation markers on human B cells. Recently, Schnitzler et al. showed that treatment of murine B cells with CT-B induced a sequence of signaling events related to cellular activation and surface molecule expression (37). In our study, treatment of both unstimulated and polyclonal activated human B cells with CT-B did not induce any variations in surface activation markers and antigen presentation, suggesting that there is a difference in behavior between human and murine B cells. The precise mechanism of action of these adjuvants has not been completely elucidated, and there are controversies concerning the requirements for and roles of the A and B subunits of these toxins both in vitro and in vivo (reviewed in reference 16). Factors involved in the dissimilar findings include the route of administration, the characteristics of the vaccine antigen, contamination of the adjuvant with endotoxin or with holotoxin, and the species of animal used. The difference between the human and murine cell responses to nontoxic derivatives of toxins could be another important factor that should be taken into account in the design and development of mucosal adjuvants suitable for human vaccination.
It is known that the second messenger cAMP can have immunosuppressive effects on T and B lymphocytes (28, 32, 38). We confirmed these findings, showing that CT, LT, FSK, and cAMP analogues inhibited the proliferation of B cells induced by polyclonal stimuli. FSK-treated B cells produced larger amounts of intracellular cAMP than toxin-treated cells, suggesting that the difference could represent a possible reason for the more pronounced inhibition of proliferation seen for FSK-treated cells. These results are in agreement with those obtained previously by our group (39) and by Johnson et al. (21), showing that increased levels of cAMP were able to inhibit in a dose-dependent manner anti-CD3- or IL-2-induced T-cell proliferation. In addition, toxins and FSK made B cells more susceptible to death. Conversely, treatment with CT-B or LTK63, which lack enzymatic activity, did not alter either the ability of stimulated B cells to proliferate or the induction of cell death. These results were expected, since it has been shown that cAMP is involved in the regulation of apoptosis in B progenitor and mature B cells by inducing activation of protein kinase A (25, 29), suggesting that physiological ligands that control cellular cAMP levels could play an important role in the regulation of B-cell maturation in vivo. Indeed, compounds acting on the increase in intracellular cAMP content could have pleiotropic effects on the immune cells, inducing both suppressive (inhibition of proliferation) and stimulatory signals (activation) at the same time. The final effect observed in vitro and even more in vivo upon treatment with toxins is probably due to a balance of these signals.
In order to determine if the effects of toxins were directed mostly toward a particular subset of B cells, such as CD27+ or CD27− populations, we first evaluated the expression of GM1 on gated CD27+ and CD27− B cells by using FITC-labeled CT-B. The results indicated that there was a slightly higher level of binding to CD27− B cells (data not shown). However, when CD86 expression and induction of cell death after treatment with toxins were evaluated for the two different B-cell populations, the results indicated that CT and LT acted similarly in both subsets. Taken together, our data indicated that the toxins induced a real increase in CD86 expression in total B cells and did not cause selective depletion of the population with a low level of CD86 expression.
Finally, to investigate the effects of the adjuvants on the antigen-presenting function of human B cells, both allo-MLR and antigen-specific T-cell proliferation tests were performed. The ability of B cells treated with CT, LT, or FSK to induce T-cell proliferation was evident in both the assays. The data could be explained by the fact that toxins and FSK were able to induce an activation state with upregulation of costimulatory molecules and HLA class II, which was responsible for the increased antigen-presenting function observed. Indeed, as expected, the use of blocking MAbs against CD86 and/or HLA-DR in the MLR assay resulted in a high level of inhibition of PBMC proliferation. Again, treatment with CT-B and LTK63 did not improve the APC capacity of stimulated B cells, further confirming the role of cAMP in the adjuvant activity of toxins. We and other authors reported that CT and LT inhibit IL-12 and TNF-α production by human DC, partially explaining the polarization of CD4+ T cells toward a Th2 phenotype observed when CT- or LT-treated DC were used as APC (5, 14, 15). In this study, we detected strong inhibition of TNF-α production upon treatment of B cells with CT, LT, or FSK, further supporting the role of cAMP in the modulation of B-cell functions. Surprisingly, in contrast to the results for DC treated with CT or LT, we observed IL-12 production in toxin-treated B cells and strong production of IL-1β. As reported in other papers (reviewed in reference 16), we confirmed that CT and LT induced an increase in IL-6 production. This peculiar pattern of cytokines induced by treatment with toxins could be important for the generation of an environment able to drive the Th1/Th2 polarization of T cells. Additional studies are required to investigate this issue.
In this work, several observations support the hypothesis that CT and LT directly activate human B cells predominantly by elevating the intracellular cAMP level. We cannot exclude the possibility that there is concomitant involvement of other factors, including the signaling induced by binding with their receptors. Although in our model system the presence of enzymatic activity is required for the adjuvanticity of toxins, suggesting that LTK63 and CT-B do not act directly on these APC, we cannot rule out the possibility that there is an indirect effect on APC that is induced by these compounds in vivo. Our results are in agreement with the results of other studies based on a requirement for the A subunit of CT for the induction of adjuvanticity of B cells. Indeed, it has been demonstrated that CTA1-DD, an adjuvant based on the CT A subunit genetically linked to two Ig-binding domains (DD) of staphylococcal protein A, but not enzymatically inactive mutants, was able to target and activate B cells and act as a good mucosal adjuvant in vivo (1, 12). In this study, our objective was to determine if the enzymatic activity is mandatory for the APC function of B cells, and therefore we focused on reagents with 0% or 100% activity. It would be interesting to check the minimum level of enzymatic activity required for adjuvanticity with human B cells by using recombinant enterotoxins with greatly reduced enzymatic activity.
Together with our previous data (14, 15), results obtained in this study allow us to conclude that the adjuvanticity of toxins, measured as in vitro activation and the antigen-presenting ability of human APC (both DC and B cells), is stringently correlated to the presence of the enzymatic activity involved in the increase in the intracellular cAMP content.
Acknowledgments
We thank Andrea Cara for suggestions and critical reading of the manuscript, Laura Pancotto for purification of LT, and Emanuele Fanales-Belasio and Maria Rosaria Pavone-Cossut for performing the endotoxin analysis.
This study was carried out with financial support from Commission of the European Communities Sixth Framework Programme contract LSHP-CT-2003-503240 (Mucosal Vaccines for Poverty-Related Diseases) (M.T.D.M.) and from grants from the Italian AIDS National Program (contract 45G/C) (M.T.D.M.).
P.R. and G.D.G. are Novartis employees. The other authors declare no conflict of interest.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Agren, L. C., L. Ekman, B. Löwenadler, J. G. Nedrud, and N. Y. Lycke. 1999. Adjuvanticity of the cholera toxin A1-based gene fusion protein, CTA1-DD, is critically dependent on the ADP-ribosyltransferase and Ig-binding activity. J. Immunol. 1622432-2440. [PubMed] [Google Scholar]
- 2.Anastassiou, E. D., H. Yamada, M. L. Francis, J. J. Mond, and G. C. Tsokos. 1990. Effects of cholera toxin on human B cells. Cholera toxin induces B cell surface DR expression while it inhibits anti-mu antibody-induced cell proliferation. J. Immunol. 1452375-2380. [PubMed] [Google Scholar]
- 3.Arce, S., H. Nawar, M. W. Russell, and T. D. Connell. 2005. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation in lymphoid cells. Infect. Immun. 732718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arce, S., H. F. Nawar, G. Muehlinghaus, M. W. Russell, and T. D. Connell. 2007. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect. Immun. 751413-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 705533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudner, B. C., O. Balland, M. M. Giuliani, P. Von Hoegen, R. Rappuoli, D. Betbeder, and G. Del Giudice. 2002. Enhancement of protective efficacy following intranasal immunization with vaccine plus a nontoxic LTK63 mutant delivered with nanoparticles. Infect. Immun. 704785-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B Cells. Science 2982199-2202. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P., and F. E. Johansen. 2005. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 20632-63. [DOI] [PubMed] [Google Scholar]
- 9.Constant, S., N. Schweitzer, J. West, P. Ranney, and K. Bottomly. 1995. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J. Immunol. 1553734-3741. [PubMed] [Google Scholar]
- 10.Crawford, A., M. Macleod, T. Schumacher, L. Corlett, and D. Gray. 2006. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J. Immunol. 1763498-3506. [DOI] [PubMed] [Google Scholar]
- 11.Douce, G., M. Fontana, M. Pizza, R. Rappuoli, and G. Dougan. 1997. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect. Immun. 652821-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson, A., and N. Y. Lycke. 2003. The CTA1-DD vaccine adjuvant binds to human B cells and potentiates their T cell stimulating ability. Vaccine 22185-193. [DOI] [PubMed] [Google Scholar]
- 13.Fontana, M. R., R. Manetti, V. Giannelli, C. Magagnoli, A. Marchini, R. Olivieri, M. Domenighini, R. Rappuoli, and M. Pizza. 1995. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect. Immun. 632356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 302394-2403. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi, M. C., and M. T. De Magistris. 2003. Maturation of human dendritic cells induced by the adjuvant cholera toxin: role of cAMP on chemokine receptor expression. Vaccine 21856-861. [DOI] [PubMed] [Google Scholar]
- 16.Hajishengallis, G., S. Arce, C. M. Gockel, T. D. Connell, and M. W. Russell. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 841104-1116. [DOI] [PubMed] [Google Scholar]
- 17.Heit, A., K. M. Huster, F. Schmitz, M. Schiemann, D. H. Busch, and H. Wagner. 2004. CpG-DNA aided cross-priming by cross-presenting B cells. J. Immunol. 1721501-1507. [DOI] [PubMed] [Google Scholar]
- 18.Hoft, D. F., C. S. Eickhoff, O. K. Giddings, J. R. Vasconcelos, and M. M. Rodrigues. 2007. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J. Immunol. 1796889-6900. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren, J., N. Lycke, and C. Czerkinsky. 1993. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine 111179-1184. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren, J., J. Adamsson, F. Anjuère, J. Clemens, C. Czerkinsky, K. Eriksson, C. F. Flach, A. George-Chandy, A. M. Harandi, M. Lebens, T. Lehner, M. Lindblad, E. Nygren, S. Raghavan, J. Sanchez, M. Stanford, J. B. Sun, A. M. Svennerholm, and S. Tengvall. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97181- 188. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, K. W., B. H. Davis, and K. A. Smith. 1988. cAMP antagonizes interleukin 2-promoted T-cell cycle progression at a discrete point in early G1. Proc. Natl. Acad. Sci. USA 856072-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura, Y. I., R. Kawashima, Y. Shirai, R. Kato, T. Hamabata, M. Yamamoto, K. Furukawa, K. Fujihashi, J. R. McGhee, H. Hayashi, and T. Dohi. 2003. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur. J. Immunol. 333205-3212. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow, D. J., A. I. Sperling, M. P. Cooke, G. Freeman, L. Rhee, D. C. Decker, G. Gray, L. M. Nadler, C. C. Goodnow, and J. A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 1531990-1997. [PubMed] [Google Scholar]
- 24.Liu, Y.-J., C. Barthélémy, O. de Bouteiller, C. Arpin, I. Durand, and J. Banchereau. 1995. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity 2239-248. [DOI] [PubMed] [Google Scholar]
- 25.Lomo, J., H. K. Blomhoff, K. Beiske, T. Stokke, and E. B. Smeland. 1995. TGFβ1 and cyclic AMP promote apoptosis in resting human B lymphocytes. J. Immunol. 1541634-1643. [PubMed] [Google Scholar]
- 26.Lycke, N., A. K. Bromander, L. Ekman, U. Karlsson, and J. Holmgren. 1989. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. J. Immunol. 14220-27. [PubMed] [Google Scholar]
- 27.Lycke, N., E. Severinson, and W. Strober. 1991. Molecular effects of cholera toxin on isotype differentiation. Immunol. Res. 10407-412. [DOI] [PubMed] [Google Scholar]
- 28.Majumdar, S., and B. B. Aggarwal. 2003. Adenosine suppresses activation of nuclear factor-jB selectively induced by tumor necrosis factor in different cell types. Oncogene 221206-1218. [DOI] [PubMed] [Google Scholar]
- 29.Myklebust, J. H., D. Josefsen, H. K. Blomhoff, F. O. Levy, S. Naderi, J. C. Reed, and E. B. Smeland. 1999. Activation of the cAMP signaling pathway increases apoptosis in human B-precursor cells and is associated with downregulation of Mcl-1 expression. J. Cell. Physiol. 18071-80. [DOI] [PubMed] [Google Scholar]
- 30.Nashar, T. O., H. M. Webb, S. Eaglestone, N. A. Williams, and T. R. Hirst. 1996. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. USA 93226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nashar, T. O., Z. E. Betteridge, and R. N. Mitchell. 2001. Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4+ T cells. Int. Immunol. 13541-551. [DOI] [PubMed] [Google Scholar]
- 32.Neumann, M., T. Grieshammer, S. Chuvpilo, B. Kneitz, M. Lohoff, A. Schimpl, B. R. Franza, Jr., and E. Serfling. 1995. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 141991-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plant, A., R. Williams, M. E. Jackson, and N. A. Williams. 2003. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur. J. Immunol. 333186-3195. [DOI] [PubMed] [Google Scholar]
- 34.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20493-500. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Pinto, D., and J. Moreno. 2005. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur. J. Immunol. 351097-1105. [DOI] [PubMed] [Google Scholar]
- 36.Ryan, E. J., E. McNeela, M. Pizza, R. Rappuoli, L. O'Neill, and K. H. G. Mills. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 1655750-5759. [DOI] [PubMed] [Google Scholar]
- 37.Schnitzler, A. C., J. M. Burke, and L. M. Wetzler. 2007. Induction of cell signaling events by the cholera toxin B subunit in antigen-presenting cells. Infect. Immun. 753150-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vendetti, S., M. Patrizio, A. Riccomi, and M. T. De Magistris. 2006. Human CD4+ T lymphocytes with increased intracellular cAMP levels exert regulatory functions by releasing extracellular cAMP. J. Leukoc. Biol. 80880-888. [DOI] [PubMed] [Google Scholar]
- 39.Vendetti, S., A. Riccomi, A. Sacchi, E. Sciaraffia, L. Gatta, C. Pioli, and M. T. De Magistris. 2008. Inhibition of T cell proliferation by cholera toxin involves the modulation of costimulatory molecules CTLA-4 and CD28. Immunol. Lett. 11559-69. [DOI] [PubMed] [Google Scholar]
- 40.Verweij, W. R., L. De Haan, M. Holtrop, E. Agsteribbe, R. Brands, G. J. M. Van Scharrenburg, and J. Wilschut. 1998. Musosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine 162069-2076. [DOI] [PubMed] [Google Scholar]
- 41.Wu, H.-Y., and M. W. Russell. 1998. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine 16286-292. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, M. Yamamoto, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 1851203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]