Abstract
Src family tyrosine kinases (SFKs) phosphorylate immunotyrosine activation motifs in the cytoplasmic tail of multiple immunoreceptors, leading to the initiation of cellular effector functions, such as phagocytosis, reactive oxygen species production, and cytokine production. SFKs also play important roles in regulating these responses through the activation of immunotyrosine inhibitory motif-containing inhibitory receptors. As myeloid cells preferentially express the SFKs Hck, Fgr, and Lyn, we questioned the role of these kinases in innate immune responses to Pneumocystis murina. Increased phosphorylation of Hck was readily detectable in alveolar macrophages after stimulation with P. murina. We further observed decreased phosphorylation of Lyn on its C-terminal inhibitory tyrosine in P. murina-stimulated alveolar macrophages, indicating that SFKs were activated in alveolar macrophages in response to P. murina. Mice deficient in Hck, Fgr, and Lyn exhibited augmented clearance 3 and 7 days after intratracheal administration of P. murina, which correlated with elevated levels of interleukin 1β (IL-1β), IL-6, CXCL1/KC, CCL2/monocyte chemoattractant protein 1, and granulocyte colony-stimulating factor in lung homogenates and a dramatic increase in macrophage and neutrophil recruitment. Augmented P. murina clearance was also observed in Lyn−/− mice 3 days postchallenge, although the level was less than that observed in Hck−/− Fgr−/− Lyn−/− mice. A correlate to augmented clearance of P. murina in Hck−/− Fgr−/− Lyn−/− mice was a greater ability of alveolar macrophages from these mice to kill P. murina in vitro, suggesting that SFKs regulate the alveolar macrophage effector function against P. murina. Mice deficient in paired immunoglobulin receptor B (PIR-B), an inhibitory receptor activated by SFKs, did not exhibit enhanced inflammatory responsiveness to or clearance of P. murina. Our results suggest that SFKs regulate innate lung responses to P. murina in a PIR-B-independent manner.
Although the advent of prophylactic therapy against Pneumocystis jirovecii alone or in combination with highly active antiretroviral therapy has reduced the incidence of pneumonia caused by this fungal pathogen, this infection remains the most prevalent opportunistic infection in individuals with AIDS (25). Furthermore, individuals receiving immunosuppressive therapies, such as individuals undergoing solid organ or hematopoietic cell transplantation, are a growing population susceptible to Pneumocystis pneumonia (34) (33). These observations warrant a more thorough examination of interactions between Pneumocystis and the host in order to define and develop new vaccine and immunotherapeutic approaches to treat Pneumocystis pneumonia.
An intense area of research over the last decade is investigating how lung immune cells recognize and respond to inhaled pathogens, such as P. murina. After inhalation of P. murina into the lungs, one of the first interactions with the host is recognition by the alveolar macrophage. Our laboratory has previously reported that alveolar macrophages recognize P. murina via the beta-glucan receptor dectin-1 (37). Recognition by dectin-1 leads to internalization of P. murina and subsequent killing of the organism, as well as elaboration of the neutrophil-attracting chemokine CXCL2/MIP-2 (37). Toll-like receptor 2 (TLR2) is an additional receptor expressed by alveolar macrophages that mediates CXCL2/MIP-2 production (54) and, in humans, interleukin-8 (IL-8) production in cooperation with the macrophage mannose receptor (39). P. murina infection in TLR2−/− mice is prolonged and associated with a lack of inflammatory responsiveness (51). Other studies have implicated TLR4 in alveolar macrophage recognition of P. murina (6).
Immunoreceptors expressed by cells of the innate immune system are abundant and fall into many different categories, such as scavenger receptors, integrins, immunoglobulin (Ig) superfamily receptors, C-type lectin receptors, and TLRs (41). As expected based on this diversity, immunoreceptor signaling in innate cells is a complex process that differs greatly for different types of receptors. For example, receptors in the C-type lectin family often utilize an immunotyrosine activation motif (ITAM) in the cytoplasmic tail for signaling (2). Receptors in the Ig superfamily, such as SIRPα and Siglec3, contain immunotyrosine inhibitory motifs (ITIMs) that initiate regulatory signals, whereas members of the TLR family signal through multiple intermediate proteins, such as TIRAP, TRIF, and MyD88 (28). One commonality in innate immunoreceptor signaling is the role of Src family tyrosine kinases (SFKs). Our current understanding of how SFKs function is due in large part to studies that have characterized ITAM-associated Fc receptor (FcγR) signaling (32). In FcγR signaling, SFKs phosphorylate two tyrosine residues in the ITAM domain, which leads to the recruitment of Syk and subsequent activation of cellular responses, such as phagocytosis and cytokine and chemokine production (32).
An equally important function of SFKs is to phosphorylate ITIMs, which leads to the recruitment of SHP-1 or SHIP-1 phosphatases and subsequent regulation or inhibition of responses (21). The phosphorylation of ITIMs by SFKs is often responsible for the regulation of responses initiated by many types of immunoreceptors. Mice deficient in the SFKs Hck and Fgr have enhanced chemokine receptor signaling as a result of lower phosphorylation of the ITIM in the inhibitory receptor paired Ig receptor B (PIR-B) (55). Mice deficient in PIR-B were also found to be hyperresponsive to chemokine signaling (55). PIR-B ITIM activation has also been shown to regulate TLR-mediated macrophage responses to some gram-positive and gram-negative bacteria (26). Other studies have shown that the SFK Lyn phosphorylates an ITIM of platelet endothelial cell adhesion molecule 1 in mast cells, leading to regulated FcɛRI responses (45).
Macrophage-mediated recognition of P. murina involves a variety of receptors that may be dependent on SFKs for induction as well as the regulation of responses; therefore, we sought to investigate the role of SFKs in innate immune responses to P. murina. In this study, we made the surprising observation that deficiency of Hck, Fgr, and Lyn resulted in paradoxically augmented lung clearance of P. murina and enhanced cytokine and chemokine production. This response was not due to impaired PIR-B inhibitory responses as PIR-B−/− mice were not capable of enhanced P. murina lung clearance. We propose that the SFKs Hck, Fgr, and Lyn regulate innate immune responses to P. murina and that, therefore, novel therapeutics to control the activity of SFKs may be beneficial in treating pulmonary infections.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice that were 6 to 8 weeks old were purchased from the National Cancer Institute, National Institutes of Health (Bethesda, MD). Hck−/− Fgr−/− Lyn−/− mice and Lyn−/− mice, originally developed by Clifford Lowell, University of California—San Francisco (23), were graciously provided by Shaoguang Li, The Jackson Laboratory, Bar Harbor, ME. PIR-B−/− mice, originally developed by Toshiyuki Takai, Tohoku University (46), were graciously provided by Hiromi Kubagawa, University of Alabama at Birmingham. All animals were housed in a specific-pathogen-free facility and handled according to institutionally recommended guidelines.
Alveolar macrophage isolation.
Mice were anesthetized by intraperitoneal injection of ketamine-xylazine and sacrificed by exsanguination. After this, lungs were lavaged through an intratracheal catheter with prewarmed (37°C) calcium- and magnesium-free phosphate-buffered saline (PBS) supplemented with 0.6 mM EDTA. A total of 10 ml in 0.5-ml increments was used for each mouse, with a 30-s dwell time. The lavage fluids were pooled and centrifuged at 300 × g for 10 min, and the cells were collected for the coculture assay. To ensure that each cell preparation was enriched for macrophages, 25,000 cells were cytospun onto slides and stained with hematoxylin and eosin. Cell preparations were generally >98% enriched for alveolar macrophages.
P. murina isolate and inoculation.
A preparation of P. murina was obtained as previously described (18). Briefly, C.B-17 SCID mice previously inoculated with P. murina were inoculated with a lethal dose of pentobarbital, and the lungs were aseptically removed and frozen at −80°C in 1 ml PBS. Frozen lungs were homogenized through a 70-μm filter and pelleted by centrifugation at 500 × g for 10 min at 4°C. The pellet was resuspended in 1 ml of PBS, and a 1/10 dilution was stained with modified Giemsa stain (Diff-Quik). The number of P. murina cysts was quantified microscopically, and the concentration was adjusted to 2 × 106 cysts/ml. For in vivo challenge, mice were anesthetized with isofluorane and inoculated with 2 × 105 cysts in a 0.1-ml suspension via the intratracheal route. The concentration of some preparations was adjusted to 2 × 106 cysts/ml, and 50-μl aliquots were placed into tubes containing 200 μl of 90% fetal bovine serum supplemented with 10% dimethyl sulfoxide and stored at −80°C. Employing this storage method, stable P. murina viability, as determined by quantitative real-time PCR, can be maintained for more than 1 year (37).
P. murina viability assay.
Macrophages (1 × 105 macrophages in 100 μl) were cocultured with P. murina (1 × 103 cysts in 100 μl) for 24 h at 37°C in the presence of 5% CO2. The controls included P. murina incubated with medium alone. The contents of each well were collected and pelleted by centrifugation at 800 × g for 5 min. The supernatants were discarded, and total RNA was isolated from the cell pellets using TRIZOL reagent (Invitrogen, Carlsbad, CA). The viability of P. murina was analyzed by real-time PCR measurement of the rRNA copy number and was quantified by employing a standard curve for known copy numbers of P. murina rRNA as previously described (38, 56). This methodology detects viable P. murina organisms, as evidenced by the absence of detectable P. murina rRNA in samples subjected to heat inactivation or exposure to trimethoprim-suflamethoxazole. The level of killing was defined as previously described (37).
Real-time PCR analysis of P. murina rRNA in lung tissue.
Total RNA was isolated from the right lung of infected mice by a single-step method using TRIZOL reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. After this, RNA was transcribed to cDNA, and real-time PCR for P. murina rRNA was performed as described previously (38, 56). This assay has a correlation coefficient of 0.98 with P. murina rRNA copy number, and the results correlate with microscopic organism counts (56). The results were expressed as the P. murina copy number.
Analysis of phosphorylated Hck and Lyn.
Alveolar macrophages were isolated as described above and stimulated with P. murina for 10 to 90 min. After this, cell lysates were extracted using PhosphoSafe extraction buffer (Novagen, San Diego, CA), the lysates were clarified by centrifugation, and the total protein concentration of each lysate was determined using a bicinchoninic acid protein assay kit according to the manufacturer's instructions (Pierce, Rockford, IL). Ten micrograms of each lysate was separated on a 4 to 12% bis-Tris sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Invitrogen, Carlsbad, CA), transferred to a polyvinylidene difluoride (PVDF) membrane, and blocked with 5% skim milk overnight at 4°C. After this, the PVDF membrane was incubated with goat polyclonal anti-pHck 411 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-pLyn 507 IgG (Cell Signaling Technologies, Danvers, MA), or chicken anti-beta actin IgY (Novus Biologics) in 2% skim milk (all primary antibodies were used at a dilution of 1:1,000 for 2 h at room temperature), followed by horseradish peroxidase-conjugated donkey anti-goat IgG, horseradish peroxidase-conjugated goat anti-rabbit IgG, or horseradish peroxidase-conjugated rabbit anti-chicken IgY (all at a dilution of 1:2,000 for 2 h at room temperature). Positive bands were identified using an ECL Western blot detection kit (Amersham Biosciences, Piscataway, NJ). Image J software (National Institutes of Health, Bethesda, MD) was used to generate densitometry data for p-Lyn and p-Hck and their beta-actin controls for unstimulated and P. murina-stimulated alveolar macrophages.
Lung cytokine, cell recruitment, and histological analyses.
The left lungs were homogenized in PBS supplemented with Complete Mini protease inhibitor tablets (Roche), and the homogenates were clarified by centrifugation and stored at −80°C. Samples were analyzed to determine the protein levels of 23 cytokines and chemokines using a Bio-Plex multiplex suspension cytokine array (Bio-Rad Laboratories) according to the manufacturer's instructions. The data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories). For cell recruitment, mice were inoculated with 2 × 105 P. murina cysts via the intratracheal route. Three days postinoculation, bronchoalveolar lavage was performed, and cells were stained for F4/80 (macrophages), Gr-1 (neutrophils), and CD3 (T cells) and assessed by flow cytometry. For lung histology, the left lungs were collected and fixed in 4% formalin. The fixed lungs were embedded in paraffin and then processed and stained by the Comparative Pathology Laboratory at the University of Alabama at Birmingham. Imaging was performed using a Nikon Eclipse 90i microscope and Nikon NIS-Elements imaging analysis software.
Statistical analysis.
Data were analyzed using GraphPad Prism statistical software (GraphPad Software, San Diego, CA). Comparisons between groups were made by using the two-tailed unpaired Student t test when data were normally distributed and by using the two-tailed Mann-Whitney U test when the data were not normally distributed. In in vitro experiments comparing wild-type and deficient samples, the two-tailed paired Student t test was employed. A P value of <0.05 was considered significant.
RESULTS
P. murina activates SFKs in alveolar macrophages.
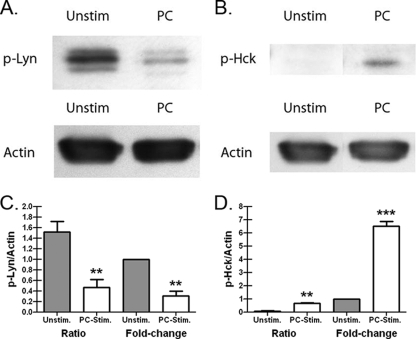
Eight members of the SFK group are present in different immune cell populations and have been widely studied in receptor signaling events using deficiency and overexpression model systems (27). In a monocyte/macrophage cell lineage, Hck, Fgr, and Lyn are the predominantly expressed SFKs (9, 16, 21), although expression of the SFKs Fyn (16, 19) and Yes (36) has also been demonstrated. Perhaps one of the best-described functions of Hck, Fgr, and Lyn in macrophages is the phosphorylation of the ITAM domain in the Fcγ receptor, which leads to the recruitment of Syk (5, 32). In addition, pathogen-associated molecular patterns, such as lipopolysaccharide (LPS) and beta-glucan/zymosan, induce phosphorylation of the SFKs Hck and Lyn in macrophages (17, 53). To determine whether P. murina could activate SFKs in innate cells, we isolated alveolar macrophages from naïve mice and stimulated them with live P. murina. Figure 1 shows representative Western blots of Lyn (Fig. 1A) and Hck (Fig. 1B) activation in alveolar macrophages stimulated with P. murina. Quantitative analysis indicated that P. murina induced a >2-fold decrease in phosphorylation of Lyn at Tyr507 (Fig. 1C), the inhibitory tyrosine that suppresses Lyn catalytic activity (43), parallel with a >6-fold increase in the phosphorylation of Hck (Fig. 1D). Thus, alveolar macrophage interactions with P. murina lead to phosphorylation of the SFK Hck, while they reduce the level of tonically inactivated Lyn.
FIG. 1.
P. murina activates SFKs in alveolar macrophages. Alveolar macrophages were isolated from 6- to 8-week-old, male C57BL/6 mice and stimulated for 10 to 90 min with P. murina (PC) at a ratio of macrophages to total P. murina cells of 1:100. The controls included alveolar macrophages cultured in medium alone (Unstim). After this, cell lysates were extracted, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to PVDF membranes, and immunoblotted with anti-pHck 411 or rabbit anti-pLyn 507 IgG. Positive bands were identified using an ECL Western blot detection kit and were subsequently analyzed using Image J software (NIH). The blots are representative blots for (A) p-Lyn and (B) p-Hck levels in unstimulated and P. murina-stimulated alveolar macrophages. Cumulative data from three independent studies employing Image J software were used to determine the area under the curve values for (C) p-Lyn and (D) p-Hck and their beta-actin controls for unstimulated and P. murina-stimulated alveolar macrophages. The data are expressed both as the ratio of p-Lyn or p-Hck to actin and as the change after unstimulated samples were normalized to a value of 1. Data are the means ± standard errors of the means. ** and ***, P < 0.01 and P < 0.001, respectively (paired two-tailed Student's t test).
Enhanced lung clearance of P. murina in Hck−/− Fgr−/− Lyn−/− and Lyn−/− mice.
Our results indicate that SFKs are activated in response to P. murina. To determine whether the Hck, Fgr, and Lyn tyrosine kinases played a role in innate lung defense against P. murina, wild-type C57BL/6 and Hck−/− Fgr−/− Lyn−/− (Src triple knockout [TKO]) mice were challenged intratracheally with P. murina, and the fungal burden was determined by real-time PCR. Figure 2A shows that 3 days after challenge, Src TKO mice surprisingly had significantly lower levels of P. murina in their lungs, levels that were approximately 30% of those observed in wild-type C57BL/6 mice. We further assessed the lung burden 7 days after challenge and observed that Src TKO mice maintained a higher level of clearance than wild-type controls (Fig. 2B). To further assess the augmented-clearance phenotype observed in Src TKO mice, we also challenged Lyn-deficient mice and observed similar, but not as robust, clearance of P. murina 3 days postchallenge (Fig. 2C). Thus, Src TKO and Lyn−/− mice have enhanced innate immune mechanisms that clear P. murina from the lungs.
FIG. 2.
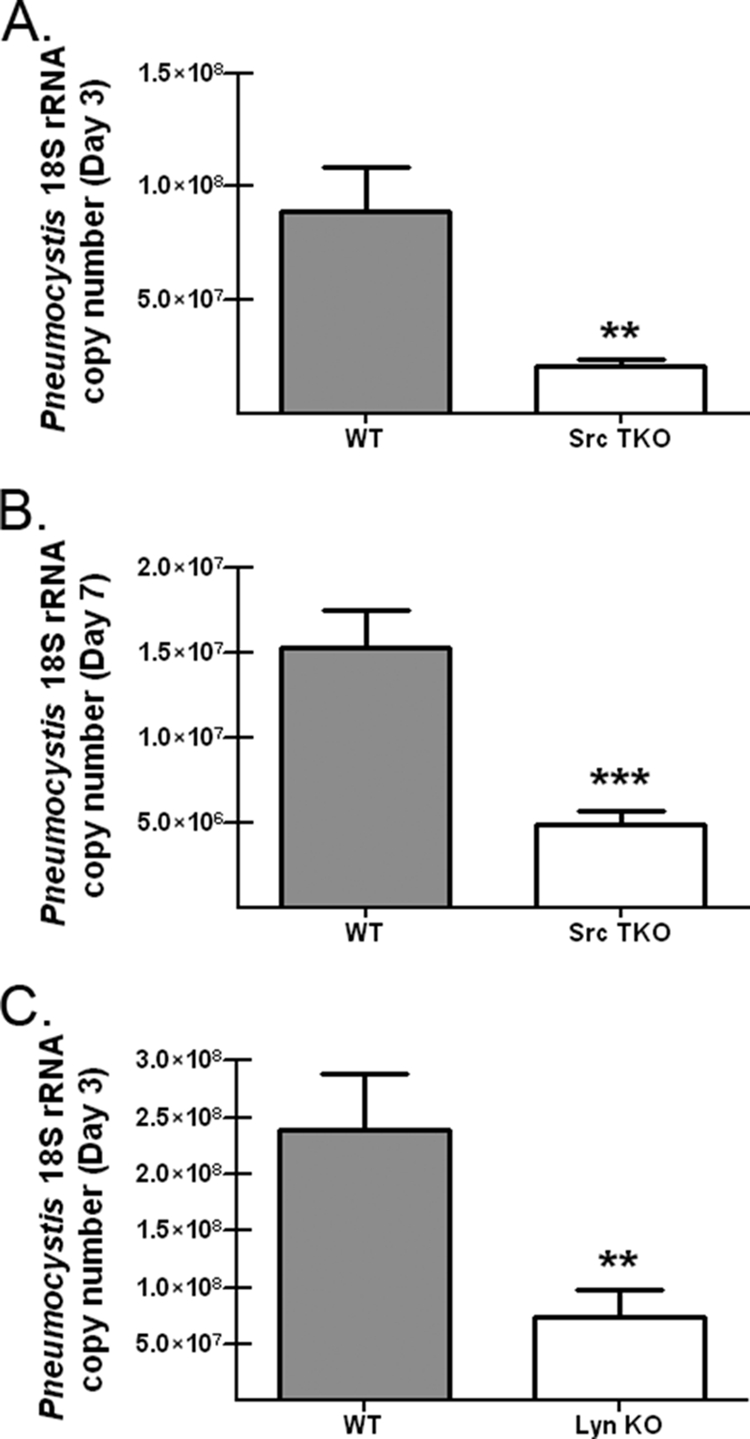
Enhanced lung clearance of P. murina in Hck−/− Fgr−/− Lyn−/− and Lyn−/− mice. C57BL/6 (WT) and Hck−/− Fgr−/− Lyn−/− (Src TKO) mice were inoculated with 2 × 105 P. murina cysts via the intratracheal route. (A and B) At 3 days (A) and 7 days (B) postinoculation, lungs were collected, and the P. murina burden was determined by using real-time PCR to determine the P. murina rRNA copy number. The data are cumulative data from three independent studies with five mice per group. The bars and error bars indicate the mean P. murina rRNA copy numbers and the standard errors of the means. ** and ***, P < 0.01 and P < 0.001, respectively (unpaired two-tailed Student's t test). (C) C57BL/6 and Lyn−/− mice were inoculated with 2 × 105 P. murina cysts via the intratracheal route, and 3 days postinoculation lungs were collected and the P. murina burden was determined by using real-time PCR to determine the P. murina rRNA copy number. The data are cumulative data from three independent studies with five mice per group. The bars and error bars indicate the means and standard errors of the means. **, P < 0.01 (unpaired two-tailed Student's t test).
Increased lung proinflammatory response in P. murina-exposed Hck−/− Fgr−/− Lyn−/− mice.
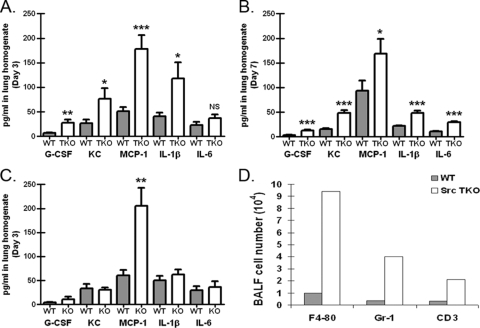
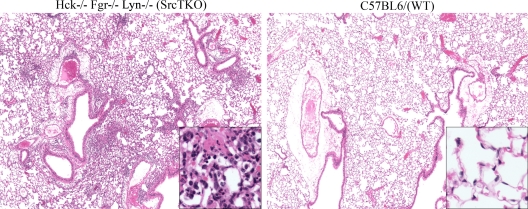
Since P. murina challenge of Src TKO mice resulted in augmented clearance, we asked whether cytokine and chemokine profiling of P. murina-exposed lungs could provide insight into the phenotype observed for these mice. To examine this possibility, wild-type C57BL/6 and Src TKO mice were intratracheally challenged with P. murina, and cytokine and chemokine protein levels in lung homogenates were determined by using a multiplex suspension array. Figure 3A shows that 3 days after challenge, despite augmented clearance of P. murina and thus a lower fungal burden (Fig. 2), in Src TKO mice there were significant increases in the levels of the proinflammatory cytokines IL-1β and IL-6, the hematopoietic factor granulocyte colony-stimulating factor (G-CSF), and the chemokines CXCL1/KC and CCL2/monocyte chemoattractant protein 1 (MCP-1). The elevated inflammatory responsiveness of Src TKO mice was also observed at 7 days postchallenge (Fig. 3B). In contrast, the level of the inflammatory response in P. murina-exposed Lyn−/− mice was not near the level observed in Src TKO mice, as MCP-1 was the lone mediator whose level was observed to be significantly higher (Fig. 3C). Analysis of bronchoalveolar lavage fluid cell populations for Src TKO mice by flow cytometry revealed a substantial increase in macrophages, neutrophils, and T cells 3 days postchallenge (Fig. 3D). Furthermore, hematoxylin and eosin staining of lung tissue sections revealed profound inflammatory cell recruitment in Src TKO mice (Fig. 4, left panel). In contrast, there was significantly less inflammatory cell recruitment in wild-type mice (Fig. 4, right panel). Thus, augmented innate clearance of P. murina in Src TKO mice is associated with an enhanced lung inflammatory response.
FIG. 3.
Increased lung proinflammatory response in P. murina-exposed Hck−/− Fgr−/− Lyn−/− mice. C57BL/6 (WT) and Hck−/− Fgr−/− Lyn−/− (TKO) mice were inoculated with 2 × 105 P. murina cysts via the intratracheal route. (A and B) At 3 days (A) and 7 days (B) postinoculation, lungs were collected, and clarified supernatants from lung homogenates were analyzed for G-CSF, CXCL1/KC, CCL2/MCP-1, IL-1β, and IL-6 levels by Bio-Plex. The data are cumulative data from three independent studies with five mice per group. The bars and error bars indicate the means and standard errors of the means. * and ***, P < 0.05 and P <0.001, respectively (unpaired two-tailed Student's t test). (C) Three days postinoculation, lungs were collected from Lyn−/− mice, and clarified supernatants from lung homogenates were analyzed for G-CSF, CXCL1/KC, CCL2/MCP-1, IL-1β, and IL-6 levels by Bio-Plex. The data are cumulative data from three independent studies with five mice per group. The bars and error bars indicate the means and standard errors of the means. **, P < 0.01 (unpaired two-tailed Student's t test). (D) C57BL/6 and Hck−/− Fgr−/− Lyn−/− (Src TKO) mice were inoculated with 2 × 105 P. murina cysts via the intratracheal route. Three days postinoculation, bronchoalveolar lavage was performed, and cells were stained for F4/80 (macrophages), Gr-1 (neutrophils), and CD3 (T cells) and assessed by flow cytometry. The data are representative data from one of two independent studies. BALF, bronchoalveolar lavage fluid.
FIG. 4.
Histological evidence for inflammatory differences in the lungs of P. murina-exposed Hck−/− Fgr−/− Lyn−/− mice: representative hematoxylin- and eosin-stained lung sections from (A) Hck−/− Fgr−/− Lyn−/− (Src TKO) mice and (B) wild-type (WT) mice challenged intratracheally with 2 × 105 P. murina cysts for 3 days. Original magnification, ×40. (Insets) Magnification, ×200.
Hck−/− Fgr−/− Lyn−/− alveolar macrophages are more efficient at killing P. murina.
Previous studies employing macrophages from Hck−/− Fgr−/− Lyn−/− (Src TKO) mice have shown intact responses to LPS (23), as well as an attenuated, but still present, ability to mediate phagocytosis via Fc receptors (8). To assess the involvement of the SFKs Hck, Fgr, and Lyn in alveolar macrophage-mediated effector functions against P. murina, alveolar macrophages were isolated from wild-type C57BL/6 and Src TKO mice and analyzed to determine their abilities to kill P. murina. Figure 5 shows that not only did alveolar macrophages from Src TKO mice exhibit intact killing of P. murina, but the level of killing activity was approximately 50% higher than the level observed for wild-type mice. Thus, augmented innate clearance of P. murina in Src TKO mice is associated with enhanced alveolar macrophage-mediated killing of P. murina in vitro.
FIG. 5.
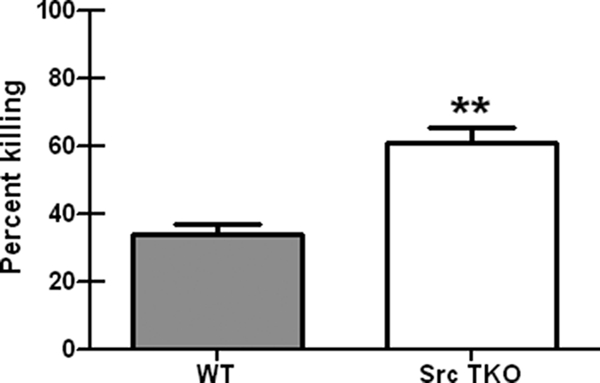
Hck−/− Fgr−/− Lyn−/− alveolar macrophages are more efficient at killing P. murina. Alveolar macrophages were isolated from 6- to 8-week-old, male C57BL/6 (WT) or Hck−/− Fgr−/− Lyn−/− (Src TKO) mice and cocultured overnight with P. murina at a ratio of macrophages to P. murina cysts of 100:1. The controls included P. murina cultured in the absence of macrophages. After this, RNA was isolated from the contents of each well, and quantitative real-time PCR to determine the P. murina rRNA copy number was performed. The data are the cumulative results from three independent studies. The bars and error bars indicate the means and standard errors of the means. **, P <0.01 (unpaired two-tailed Student's t test).
Enhanced responses to P. murina in Hck−/− Fgr−/− Lyn−/− mice are independent of PIR-B.
PIR-B is an ITIM-containing receptor expressed predominantly by macrophages and B cells that is constitutively phosphorylated by SFKs (14). PIR-B is inactive in both Hck−/− Fgr−/− mice (55) and Lyn−/− mice (30) due to a reduction in or the absence of tonic phosphorylation (40). In turn, the responses of neutrophils and dendritic cells from PIR-B−/− mice are similar to the responses of Hck−/− Fgr−/− neutrophils and dendritic cells (55). Moreover, mice deficient in PIR-B have exacerbated cytokine responses to Staphylococcus aureus (26) and Salmonella enterica serovar Typhimurium (44). To determine if the enhanced inflammatory phenotype observed in Src TKO mice was due to a lack of PIR-B inhibitory signaling, we challenged PIR-B-deficient mice with P. murina and assessed the lung burden and cytokine and chemokine levels 3 days after challenge. Figure 6A shows that in contrast to Src TKO and Lyn−/− mice, PIR-B−/− mice did not display enhanced clearance of P. murina. Furthermore, there were not enhanced cytokine and chemokine levels in the lung homogenates of PIR-B−/− mice 3 days postchallenge (Fig. 6B). Thus, these results suggest that PIR-B is not involved in the regulation of lung innate immune responses to P. murina.
FIG. 6.
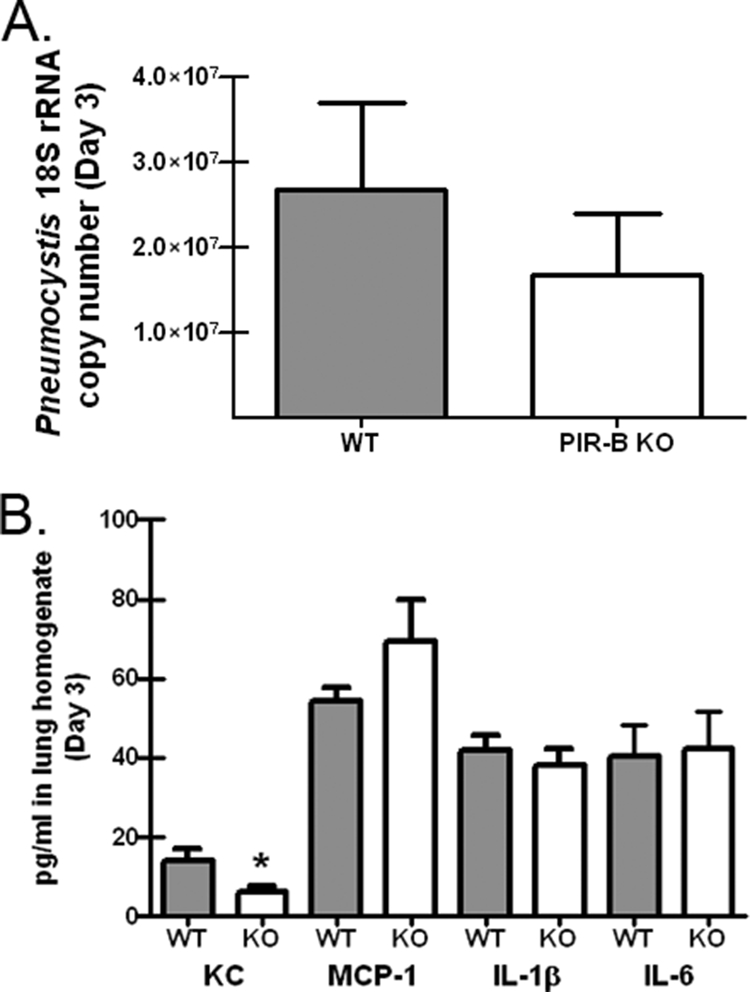
Enhanced responses to P. murina in Hck−/− Fgr−/− Lyn−/− mice are independent of PIR-B. (A) C57BL/6 (WT) and PIR-B−/− mice (PIR-B KO) were inoculated with 2 × 105 P. murina cysts via the intratracheal route, and 3 days postinoculation lungs were collected and the P. murina burden was determined by using real-time PCR to determine the P. murina rRNA copy number. The data are cumulative data from two independent studies with five mice per group. The bars and error bars indicate the means and standard errors of the means. (B) Three days postinoculation, lungs were collected, and clarified supernatants from lung homogenates were analyzed for CXCL1/KC, CCL2/MCP-1, IL-1β, and IL-6 levels by Bio-Plex. The data are cumulative data from two independent studies with five mice per group. The bars and error bars indicate the means and standard errors of the means. *, P < 0.05 (unpaired two-tailed Student's t test).
DISCUSSION
Cells of a myeloid lineage utilize the SFKs Hck, Fgr, and Lyn to mediate classical ITAM signaling, such as FcγR signaling (21). However, these SFKs are now recognized as enzymes that are critical for regulating signaling events associated with a variety of receptor classes, including chemokine receptors, adhesion molecules, and lectins (1). In this study, we show that SFKs were activated in response to P. murina and have a regulatory effect on anti-P. murina immune responses in vivo.
Our original prediction was that Hck−/− Fgr−/− Lyn−/− mice would be susceptible to P. murina infection, based on the recognized role of SFKs in ITAM-mediated signaling to receptors, such as Fcγ (8) and dectin-1 (47), which are known to play a role in eliminating P. murina from the lungs (22, 35, 37). However, the first evidence for SFK-mediated regulation of immune responses against P. murina was lung clearance in Hck−/− Fgr−/− Lyn−/− mice. We assessed the P. murina burden 3 days after intratracheal challenge, a time point at which we hypothesized innate immune responses were actively engaged. At this time point, Hck−/− Fgr−/− Lyn−/− mice exhibited a greater ability to eliminate P. murina from the lungs than wild-type mice. This was not an artifact of normal early clearance of the P. murina inoculum, as TLR2−/− mice assessed in parallel did not display the augmented clearance observed in Hck−/− Fgr−/− Lyn−/− mice (unpublished data). More efficient clearance in Hck−/− Fgr−/− Lyn−/− mice was not restricted to a single time point, as enhanced clearance was also observed 7 days after P. murina challenge. We further observed that the augmented clearance in Lyn−/− mice, while not as robust as that observed in Hck−/− Fgr−/− Lyn−/− mice, nevertheless provides strong evidence that SFKs regulate the mechanism(s) behind innate clearance of P. murina. One concern may be that other Src family members compensate for the loss of Hck, Fgr, and Lyn. However, the initial report describing Hck−/− Fgr−/− Lyn−/− mice demonstrated that no additional Src family members were present in Hck−/− Fgr−/− Lyn−/− macrophages (23).
Lung infection with P. murina is almost exclusively localized within alveolar spaces; therefore, not surprisingly, alveolar macrophages constitute the first line of defense in protection of the lung from P. murina infection. It is widely reported that phagocytosis by alveolar macrophages is the predominant mechanism of P. murina clearance from the lungs (20). SFKs have been implicated in many signaling pathways in macrophages, and their activation through a diverse set of immunoreceptors results in overlapping and complementary functions for family members. In turn, SFKs have been termed “rheostats” because they influence the magnitude of macrophage responses (21). To this end, we demonstrated that alveolar macrophages from Hck−/− Fgr−/− Lyn−/− mice had a greater capacity to kill P. murina in vitro, which provides a mechanism associated with the enhanced P. murina lung clearance observed in these mice in vivo and further supports a role for Hck, Fgr, and Lyn in regulating the killing capacity of alveolar macrophages for P. murina. We also investigated whether the alveolar macrophage inflammatory response to P. murina was also increased and found that this was case for some mediators, such as MCP-1, although the results were not consistent (data not shown).
Although the levels of P. murina were lower in the lungs of Hck−/− Fgr−/− Lyn−/− mice, the levels of proinflammatory mediators, such as IL-1β, IL-6, G-CSF, CCL2/MCP-1, and CXCL1/KC, were significantly elevated. Previous studies investigating these mediators have provided insight into their roles in lung defense against P. murina. Antibody blockage of the IL-1 receptor in P. murina-infected splenocyte-reconstituted SCID mice leads to defective clearance of the organism (4). P. jirovecii induces IL-1 production by rodent splenocytes (42) and human macrophages (15), and IL-1 is additionally detected in the bronchoalveolar lavage fluid of human immunodeficiency virus-positive individuals with Pneumocystis pneumonia (31), suggesting that IL-1 production is part of the natural inflammatory response to P. murina. The roles of G-CSF and CXCL1/KC (GROα in humans) in defense against P. murina have not been studied, although both of these molecules are present in bronchoalveolar lavage fluid from human immunodeficiency virus-positive individuals with Pneumocystis pneumonia (10, 49). CCL2/MCP-1 is produced by P. murina-stimulated alveolar epithelial cells (50) and is observed in the lungs of P. murina-infected splenocyte-reconstituted SCID mice (52), although studies have yet to determine the consequences of MCP-1 deficiency. While alveolar macrophages may be the source of these mediators in vivo, it is possible that other cell types in the lungs of Hck−/− Fgr−/− Lyn−/− mice are involved in the increased inflammatory cytokine and chemokines levels. Future studies will assess the response to P. murina by additional lung cell populations isolated from Hck−/− Fgr−/− Lyn−/− mice, such as alveolar epithelial cells, which are important sources of inflammatory cytokines and chemokines during P. murina infection (7, 11). Collectively, our results lead us to hypothesize that the lower P. murina levels in the lungs of Hck−/− Fgr−/− Lyn−/− mice, in addition to the augmented killing ability of alveolar macrophages, were also the result of an augmented innate inflammatory reaction. Although many studies have indicated that there are hyperresponsive phenotypes in Hck−/− Fgr−/− Lyn−/−, Hck−/− Fgr−/−, and Lyn−/− mice, Hck−/− Fgr−/− Lyn−/− mice have been shown to be susceptible to pneumococcal meningitis as a result of blunted innate cell recruitment and killing (29), indicating that in some instances (in the nervous system, for example), lack of SFKs results in impaired responses. Nevertheless, increased inflammatory reactivity of Hck−/− Fgr−/− Lyn−/− mice to P. murina may also extend to the level of adaptive immune responses, which will be pursued in future studies in a longer-time-course P. murina infection model.
Our data further lead us to hypothesize that activation of Hck, Fgr, and Lyn promotes a regulatory pathway that controls the magnitude of the innate inflammatory response to P. murina. Evidence that supports this hypothesis comes from data showing that LPS stimulation of Hck−/− Fgr−/− Lyn−/− peritoneal macrophages led to augmented IL-1 and IL-6 production (23). Moreover, Hck−/− Fgr−/− Lyn−/− mice given a low dose of G-CSF exhibit a dramatic increase in blood neutrophil levels (24), which supports our observation (Fig. 3) showing that there are higher G-CSF levels in the lungs of Hck−/− Fgr−/− Lyn−/− mice in the presence of higher numbers of neutrophils. Insight into a regulatory role for Hck, Fgr, and Lyn was initially provided by the phenotype of Lyn−/− mice, which have hyperresponsive B cells, elevated immunoglobulin levels, and immune complex nephritis (13). In an ovalbumin sensitization-challenge model, Lyn−/− mice develop severe, persistent asthma due to strongly Th2-polarized dendritic cells (3). Gene profiling has further shown that FcɛR1-cross-linked Lyn−/− mast cells exhibit increased production of Th2-associated cytokines and chemokines and downregulation of FcγRIIB, a negative regulator of FcɛR1 signaling (12). Hck and Fgr are also reported to have negative regulatory capabilities. Opsonic phagocytosis is elevated in Fgr−/− macrophages as a result of increased association of the phosphatase SHP-1 with an ITIM in the signal regulatory phosphatase binding protein (SIRPα) receptor (48). Studies also point to a regulatory role for Hck and Fgr in neutrophil and dendritic cell chemokine signaling (55). Chemokine stimulation of Hck−/− Fgr−/− cells leads to enhanced ERK1/2 activation, actin polymerization, and chemotaxis, which results from dephosphorylation of the paired Ig-like receptor (PIR-B), an ITIM-containing inhibitory receptor expressed by myeloid cells (55). However, PIR-B deficiency was not associated with an enhanced ability to clear P. murina from the lungs, suggesting that SFK-mediated regulation of innate lung responses to P. murina is independent of PIR-B. Future studies will actively pursue ITIM-containing receptors that are phosphorylated in response to P. murina. Finally, it could be argued that the response to P. murina in the absence of SFK signaling may be more harmful than helpful, based on the significant increase in inflammatory mediator production and inflammatory cell recruitment. However, lung histology did not demonstrate a significant level of lung injury associated with the hyperinflammatory response in Hck−/− Fgr−/− Lyn−/− mice, supporting the hypothesis that there is a more beneficial effect resulting in augmented P. murina clearance. This hypothesis is further supported by the observation that alveolar macrophages from Hck−/− Fgr−/− Lyn−/− mice killed a higher level of P. murina in the virtual absence of a hyperinflammatory response by this cell type.
In summary, we provide evidence that innate immune responses to P. murina are regulated by SFKs. Determining how this regulation occurs could lead to the development of therapeutic strategies that “overcome” this regulation in the immunodeficiency setting in order to augment innate immune mechanisms in susceptible individuals that lack robust adaptive immune responses.
Acknowledgments
This work was supported by grants from the Parker B. Francis Foundation and the American Lung Association and by NIH grant HL080317.
Editor: A. Casadevall
Footnotes
Published ahead of print on 2 March 2009.
REFERENCES
- 1.Abram, C. L., and C. A. Lowell. 2007. The expanding role for ITAM-based signaling pathways in immune cells. Sci. STKE 377re2. [DOI] [PubMed] [Google Scholar]
- 2.Ariizumi, K., G. L. Shen, S. Shikano, S. Xu, R. Ritter, T. Kumamoto, D. Edelbaum, A. Morita, P. R. Bergstresser, and A. Takashima. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 27520157-20167. [DOI] [PubMed] [Google Scholar]
- 3.Beavitt, S. E., K. W. Harder, J. M. Kemp, J. Jones, C. Quilici, F. Casagranda, E. Lam, D. Turner, S. Brennan, P. D. Sly, D. M. Tarlinton, G. P. Anderson, and M. L. Hibbs. 2005. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J. Immunol. 1751867-1875. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., E. A. Havell, L. L. Moldawer, K. W. McIntyre, R. A. Chizzonite, and A. G. Harmsen. 1992. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J. Exp. Med. 176713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley, M. T., P. S. Costello, C. J. Fitzer-Attas, M. Turner, F. Meng, C. Lowell, V. L. Tybulewicz, and A. L. DeFranco. 1997. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J. Exp. Med. 1861027-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding, K., A. Shibui, Y. Wang, M. Takamoto, T. Matsuguchi, and K. Sugane. 2005. Impaired recognition by Toll-like receptor 4 is responsible for exacerbated murine Pneumocystis pneumonia. Microbes Infect. 7195-203. [DOI] [PubMed] [Google Scholar]
- 7.Evans, S. E., P. Y. Hahn, F. McCann, T. J. Kottom, Z. V. Pavlovic, and A. H. Limper. 2005. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 32490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzer-Attas, C. J., M. Lowry, M. T. Crowley, A. J. Finn, F. Meng, A. L. DeFranco, and C. A. Lowell. 2000. Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr and Lyn. J. Exp. Med. 191669-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghazizadeh, S., J. B. Bolen, and H. B. Fleit. 1994. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J. Biol. Chem. 2698878-8884. [PubMed] [Google Scholar]
- 10.Grunewald, T., W. Schuler-Maue, and B. Ruf. 1993. Interleukin-8 and granulocyte colony-stimulating factor in bronchoalveolar lavage fluid and plasma of human immunodeficiency virus-infected patients with Pneumocystis carinii pneumonia, bacterial pneumonia, or tuberculosis. J. Infect. Dis. 1681077-1078. [DOI] [PubMed] [Google Scholar]
- 11.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2782043-2050. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Hansen, V., J. D. J. Bard, C. A. Tarleton, J. A. Wilder, C. A. Lowell, B. S. Wilson, and B. S. Oliver. 2005. Increased expression of gene linked to FcɛRI signaling and cytokine and chemokine production in Lyn-deficient mast cells. J. Immunol. 1757880-7888. [DOI] [PubMed] [Google Scholar]
- 13.Hibbs, M. L., D. M. Tarlinton, J. Armes, D. Grail, G. Hodgson, R. Maglitto, S. A. Stacker, and A. R. Dunn. 1995. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83301-311. [DOI] [PubMed] [Google Scholar]
- 14.Ho, L. H., T. Uehara, C. C. Chen, H. Kubagawa, and M. D. Cooper. 1999. Constitutive tyrosine phosphorylation of the inhibitory paired Ig-like receptor PIR-B. Proc. Natl. Acad. Sci. USA 9615086-15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandil, O., J. A. Fishman, H. Koziel, P. Pinkston, R. M. Rose, and H. G. Remold. 1994. Human immunodeficiency virus type 1 infection of human macrophages modulates the cytokine response to Pneumocystis carinii. Infect. Immun. 62644-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katagiri, K., T. Katagiri, Y. Koyama, M. Morikawa, T. Yamamoto, and T. Yoshida. 1991. Expression of src family genes during monocytic differentiation of HL-60 cells. J. Immunol. 146701-707. [PubMed] [Google Scholar]
- 17.Khadaroo, R. G., A. Kapus, K. A. Powers, M. I. Cybulsky, J. C. Marshall, and O. D. Rotstein. 2003. Oxidative stress reprograms lipopolysaccharide signaling via Src kinase-dependent pathway in RAW 264.7 macrophage cell line. J. Biol. Chem. 27847834-47841. [DOI] [PubMed] [Google Scholar]
- 18.Kolls, J. K., S. Habetz, M. K. Shean, C. Vazquez, J. A. Brown, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. R. Summer, and J. E. Shellito. 1999. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J. Immunol. 1622890-2894. [PubMed] [Google Scholar]
- 19.Li, Y., and B. Chen. 1995. Differential regulation of fyn-associated protein tyrosine kinase activity by macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). J. Leukoc. Biol. 57484-490. [DOI] [PubMed] [Google Scholar]
- 20.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 992110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowell, C. A. 2004. Src-family kinases: rheostats of immune cell signaling. Mol. Immunol. 41631-643. [DOI] [PubMed] [Google Scholar]
- 22.Lund, F. E., K. Schuer, M. Hollifield, T. D. Randall, and B. A. Garvy. 2003. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P. carinii-specific antibody. J. Immunol. 1711423-1430. [DOI] [PubMed] [Google Scholar]
- 23.Meng, F., and C. A. Lowell. 199. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr and Lyn. J. Exp. Med. 91661-1670. [DOI] [PMC free article] [PubMed]
- 24.Mermel, C. H., M. L. McLemore, F. Liu, S. Pereira, J. Woloszynek, C. A. Lowell, and D. C. Link. 2006. Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood 1082562-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris, A., R. M. Wachter, J. Luce, J. Turner, and L. Huang. 2003. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS 1773-80. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama, M., D. M. Underhill, T. W. Petersen, B. Li, T. Kitamura, T. Takai, and A. Aderem. 2007. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J. Immunol. 1784250-4259. [DOI] [PubMed] [Google Scholar]
- 27.Okutani, D., M. Lodyga, B. Han, and M. Liu. 2006. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 291L129-L141. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, L. A. 2006. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 183-9. [DOI] [PubMed] [Google Scholar]
- 29.Paul, R., B. Obermaier, J. Van Ziffle, B. Angele, H. W. Pfister, C. A. Lowell, and U. Koedel. 2008. Myeloid Src kinases regulate phagocytosis and oxidative burst in pneumococcal meningitis by activating NADPH oxidase. J. Leukoc. Biol. 841141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira, S., and C. A. Lowell. 2003. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J. Immunol. 1711319-1327. [DOI] [PubMed] [Google Scholar]
- 31.Perenboom, R. M., R. W. Sauerwein, P. Beckers, A. C. van Schijndel, R. P. van Steenwijk, J. C. Borleffs, R. van Leusen, and J. W. van der Meer. 1997. Cytokine profiles in bronchoalveolar lavage fluid and blood in HIV-seropositive patients with Pneumocystis carinii pneumonia. Eur. J. Clin. Investig. 27333-339. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19275-290. [DOI] [PubMed] [Google Scholar]
- 33.Roblot, F., C. Godet, G. Le Moal, B. Garo, M. Faouzi Souala, M. Dary, L. de Gentile, J. Gandji, Y. Guimard, C. Lacroix, P. Roblot, and B. Becq-Giraudon. 2002. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur. J. Clin. Microbiol. Infect. Dis. 21523-531. [DOI] [PubMed] [Google Scholar]
- 34.Roblot, F., G. Le Moal, C. Godet, P. Hutin, M. Texereau, E. Boyer, T. Prazuck, C. Lacroix, M. Faouzi Souala, F. Raffi, P. Weinbreck, J. M. Besnier, B. Garo, L. de Gentile, and B. Becq-Giraudon. 2003. Pneumocystis carinii pneumonia in patients with hematologic malignancies: a descriptive study. J. Infect. 4719-27. [DOI] [PubMed] [Google Scholar]
- 35.Saijo, S., N. Fujikado, T. Furuta, S. H. Chung, H. Kotaki, K. Seki, K. Sudo, S. Akira, Y. Adachi, N. Ohno, T. Kinjo, K. Nakamura, K. Kawakami, and Y. Iwakura. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 839-46. [DOI] [PubMed] [Google Scholar]
- 36.Shahan, T. A., W. G. Sorenson, J. Simpson, N. A. Kefalides, and D. M. Lewis. 2000. Tyrosine kinase activation in response to fungal spores is primarily dependent on endogenous reactive oxygen production in macrophages. J. Biol. Chem. 27510175-10181. [DOI] [PubMed] [Google Scholar]
- 37.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J. Exp. Med. 1981677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele, C., M. Zheng, E. Young, L. Marrero, J. E. Shellito, and J. K. Kolls. 2002. Increased host resistance against Pneumocystis carinii pneumonia in γδ T-cell-deficient mice: protective role of gamma interferon and CD8+ T cells. Infect. Immun. 705208-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tachado, S. D., J. Zhang, J. Zhu, N. Patel, M. Cushion, and H. Koziel. 2007. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J. Leukoc. Biol. 81205-211. [DOI] [PubMed] [Google Scholar]
- 40.Takai, T. 2005. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology 115433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, P. R., L. Martinez-Pomares, M. Stacey, H. H. Lin, G. D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23901-944. [DOI] [PubMed] [Google Scholar]
- 42.Theus, S. A., M. J. Linke, R. P. Andrews, and P. D. Walzer. 1993. Proliferative and cytokine responses to a major surface glycoprotein of Pneumocystis carinii. Infect. Immun. 614703-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13513-609. [DOI] [PubMed] [Google Scholar]
- 44.Torii, I., S. Oka, M. Hotomi, W. H. Benjamin, T. Takai, J. F. Kearney, D. E. Briles, and H. Kubagawa. 2008. PIR-B-deficient mice are susceptible to Salmonella infection. J. Immunol. 1814229-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Udell, C. M., L. A. Samayawardhena, Y. Kawakami, T. Kawakami, and A. W. Craig. 2006. Fer and Fps/Fes participate in a Lyn-dependent pathway from FcepsilonRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation. J. Biol. Chem. 28120949-20957. [DOI] [PubMed] [Google Scholar]
- 46.Ujike, A., K. Takeda, A. Nakamura, S. Ebihara, K. Akiyama, and T. Takai. 2002. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B−/− mice. Nat. Immunol. 3542-548. [DOI] [PubMed] [Google Scholar]
- 47.Underhill, D. M., E. Rossnagle, C. A. Lowell, and R. M. Simmons. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 1062543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicentini, L., P. Mazzi, E. Caveggion, S. Continolo, L. Fumagalli, J. A. Lapinet-Vera, C. A. Lowell, and G. Berton. 2002. Fgr deficiency results in defective eosinophil recruitment to the lung during allergic airway inflammation. J. Immunol. 1686446-6454. [DOI] [PubMed] [Google Scholar]
- 49.Villard, J., F. Dayer-Pastore, J. Hamacher, J. D. Aubert, S. Schlegel-Haueter, and L. P. Nicod. 1995. GRO alpha and interleukin-8 in Pneumocystis carinii or bacterial pneumonia and adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1521549-1554. [DOI] [PubMed] [Google Scholar]
- 50.Wang, J., F. Gigliotti, S. P. Bhagwat, S. B. Maggirwar, and T. W. Wright. 2007. Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. [DOI] [PubMed]
- 51.Wang, S. H., C. Zhang, M. E. Lasbury, C. P. Liao, P. J. Durant, D. Tschang, and C. H. Lee. 2008. Decreased inflammatory response in Toll-like receptor 2 knockout mice is associated with exacerbated Pneumocystis pneumonia. Microbes Infect. 10334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1999. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect. Immun. 673452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaffran, Y., J. C. Escallier, S. Ruta, C. Capo, and J. L. Mege. 1995. Zymosan-triggered association of tyrosine phosphoproteins and Lyn kinase with cytoskeleton in human monocytes. J. Immunol. 1543488-3497. [PubMed] [Google Scholar]
- 54.Zhang, C., S. H. Wang, M. E. Lasbury, D. Tschang, C. P. Liao, P. J. Durant, and C. H. Lee. 2006. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect. Immun. 741857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, H., F. Meng, C. L. Chu, T. Takai, and C. A. Lowell. 2005. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity 22235-246. [DOI] [PubMed] [Google Scholar]
- 56.Zheng, M., J. E. Shellito, L. Marrero, Q. Zhong, S. Julian, P. Ye, V. Wallace, P. Schwarzenberger, and J. K. Kolls. 2001. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J. Clin. Investig. 1081469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]