Abstract
We have investigated the immunological basis of pregnancy-related Plasmodium berghei recrudescence in immune mice with substantial preexisting immunity. Specifically, we examined the relevance of this experimental model to the study of pregnancy-associated malaria (PAM) caused by P. falciparum in women with substantial preexisting protective immunity. We used mice with immunity induced prior to pregnancy and employed flow cytometry to assess their levels of immunoglobulin G (IgG) recognizing antigens on the surfaces of infected erythrocytes (IEs) in plasma. After immunization, the mice did not possess IgG specific for antigens on IEs obtained during pregnancy-related recrudescence but they acquired recrudescence-specific IgG over the course of several pregnancies and recrudescences. In contrast, levels of antibodies recognizing IEs from nonpregnant mice did not increase with increasing parity. Furthermore, maternal hemoglobin levels increased and pregnancy-related parasitemia decreased with increasing parity. Finally, parasitemic mice produced smaller litters and pups with lower weights than nonparasitemic mice. Taken together, these observations suggest that levels of antibodies specific for recrudescence-type IEs are related to the protection of pregnant mice from maternal anemia, low birth weight, and decreased litter size. We conclude that the model replicates many of the key parasitological and immunological features of PAM, although the P. berghei genome does not encode proteins homologous to the P. falciparum erythrocyte membrane protein 1 adhesins, which are of key importance in P. falciparum malaria. The study of P. berghei malaria in pregnant, immune mice can be used to gain significant new insights regarding malaria pathogenesis and immunity in general and regarding PAM in particular.
Pregnancy-associated malaria (PAM) is a major cause of mother-offspring morbidity and mortality in areas with stable transmission of Plasmodium falciparum parasites, despite protective immunity to P. falciparum malaria acquired by the mother prior to the first pregnancy (7, 29). Susceptibility to PAM declines with increasing parity due to the acquisition of protective immunoglobulin G (IgG) with specificity for parasite-encoded, clonally variant surface antigens (VSA) that are selectively expressed on infected erythrocytes (IEs) that become sequestered in the placenta (14, 34). The PAM-specific VSA (VSAPAM) are functionally and antigenically distinct from the VSA expressed by P. falciparum parasites infecting nonpregnant hosts, and the lack of VSAPAM-specific IgG appears to be the main reason for the high susceptibility to PAM in primigravidae possessing substantial preexisting protective immunity (4, 13, 28). The best-studied VSA are the high-molecular-weight P. falciparum erythrocyte membrane protein 1 (PfEMP1) molecules encoded by the var gene family, with about 60 members per haploid parasite genome (17, 35). VAR2CSA appears to be the only PfEMP1 molecule involved in the pathogenesis of, and protective immunity to, PAM (30, 31). Studies of VSA-specific immunity to P. falciparum malaria have been frustrated by the lack of convenient and relevant animal models. Although rodent malaria parasites lack var gene orthologs, antigenic variation and IE sequestration occur with several Plasmodium species (1, 19, 23, 41), and these species possess multigene families that appear to encode IE surface-expressed VSA (6, 16). This fact notwithstanding, only limited information regarding the roles of the products of these gene families in pathogenesis and immunity is available (21, 22).
In a series of papers published in the '80s, Van Zon and coworkers developed a mouse model to study the impact of pregnancy on immunity to P. berghei infection. Importantly, they used the model to demonstrate pregnancy-related recrudescences accompanied by severe clinical symptoms in mice with preexisting acquired protective immunity (38). Furthermore, they found that susceptibility to recrudescence appeared to decrease with increasing parity (39, 40). In these aspects, their model resembles PAM caused by P. falciparum in areas where malaria is endemic, where women generally develop substantial clinical immunity to malaria before reproductive age. In the present study, we reevaluated the model developed by Van Zon et al. in view of the recent evidence pointing to the clinical importance of VSA-specific antibody responses in PAM. We show that the apparent breakdown of preexisting protective immunity to P. berghei K173 infection during pregnancy is in fact the consequence of the emergence of parasites expressing pregnancy-specific VSA to which the animals do not possess antibodies if they have never been pregnant before. Furthermore, antibodies to these pregnancy-specific VSA are acquired in a parity-dependent manner and appear to be related to protection from pregnancy-related recrudescence, maternal anemia, low birth weight, and reduced litter size.
MATERIALS AND METHODS
Mice.
We used BALB/c mice purchased from Taconic, Lille Skensved, Denmark (http://www.taconic.com). The animals were maintained on a 12-h/12-h dark/light cycle with food and water ad libitum at the Department of Experimental Medicine, University of Copenhagen, Copenhagen, Denmark, in accordance with institutional, Danish, and European guidelines for animal experimentation and welfare. All mice used were specific-pathogen free. The Danish Animal Experiments Inspectorate (Dyreforsøgstilsynet) approved all experiments reported in this article (permission code no. 2006/561-1093), as required under Danish law.
Parasites and infections.
We used P. berghei strain K173 parasites (12) for all the experiments reported herein. The parasites were originally obtained as a kind gift from Wijnand Eling. The parasites were maintained by weekly passage in the blood of nonimmunized mice. Infections were initiated by the intraperitoneal injection of 106 IEs in 200 μl of normal saline, and parasitemias were monitored from the third day of infection by microscopic examination of thin, Giemsa-stained smears of blood obtained from tail nicks. This blood was also used to assess hemoglobin levels by using a HemoCue instrument (http://www.hemocue.com). Mice with fulminant parasitemia or severe clinical symptoms were killed.
Immunization.
We used a modification of the immunization protocol described by Eling and Jerusalem (10). In brief, 6- to 8-week-old mice were infected as described above. The infection was suppressed by adding 15 mg/liter sulfadiazine (http://www.sigmaaldrich.com) to the drinking water on day 4 (D4) to D11 and D18 to D25. On D32, the mice were challenged using the same inoculum and route used for immunization. Mice showing very-low-level or microscopically undetectable parasitemia after 1 week were considered immune.
Mating and pregnancy monitoring.
The weights and peripheral blood parasitemia levels of females to be mated were recorded. On the following day (D0), they were put together with males (two to three females and one male per cage) for 4 days. The animals were not disturbed during this period to minimize stress-induced early pregnancy failure. The females were weighed when the males were removed on D4 and then left undisturbed until D10. We took an increase in body weight (from D4 to D10) as evidence of pregnancy. Subsequent abrupt weight loss was taken as an indicator of pregnancy interruption. The parasitemia levels and body weights of the animals were monitored daily from D10. Although parasite recrudescence often occurred spontaneously in pregnant mice (see Results), we generally reinfected mice on D11 to D12 (with 0.2 × 107 to 1 × 107 IEs from pregnant mice) to increase the frequency of recrudescence in immune mice. To determine litter sizes and pup weights, female mice were kept in separate cages from D19. The cages were examined every morning, and all newborn pups were counted and weighed. The mating protocol was repeated until mice of parities 1 through 4 had been obtained.
Plasma for analysis of VSA-specific IgG.
Blood samples were obtained at various time points from different groups of mice, identified as described below. We used samples obtained from immunized females (IF) before the first pregnancy (IF0 group), around D16 of the first pregnancy (IP1 group), or shortly after the first delivery (IPP1 group). We also used similar samples obtained from IF during or shortly after the second pregnancy (IP2 or IPP2 group) or the third pregnancy (IP3 or IPP3 group) or during the fourth pregnancy (IP4 group). It should be noted that mating did not always result in pregnancy, and therefore, there was no clear-cut relationship between age and the time of exposure to parasites for the different IP and IPP samples. Control samples were collected from immunized, nonpregnant females with parities of 1 to 3 (IF>0), from immunized males (IM), and from age-matched, nonimmunized animals that had never been mated or infected (nonimmunized, noninfected [NI] mice). Finally, we used blood samples collected from primigravid, nonimmunized, and never-infected mice around D16 of gestation (NP1 group).
Measurement of VSA-specific antibodies.
Levels of plasma IgG antibodies reacting with antigens on the surfaces of P. berghei IEs were measured by flow cytometry, using a modification of the protocol we developed previously for P. falciparum IEs (33). In brief, IEs from the peripheral blood of either primigravid mice (at D14 to D16 of pregnancy) or nonpregnant mice with parasitemia levels of >5% were collected in heparinized Eppendorf tubes. After centrifugation and the removal of plasma, 50 μl of packed IEs was resuspended in 10 ml of Krebs-Henseleit medium (Sigma-Aldrich) supplemented with 1% bovine serum albumin and the IEs were matured overnight (under an atmosphere of 3%O2, 6% CO2, and 91% N2 at 37°C). The next day, the cultures were enriched with hemozoin-containing late developmental stages by exposure to a strong magnetic field. The DNA of purified late-stage IEs (3 × 106/ml) was labeled with dihydroethidium bromide (Hydroethidine; 1 μg/ml). Labeled cells were incubated at 37°C for 20 min in 96-well microtiter plates (100 μl/well) with murine plasma samples (5 μl/well), washed, and stained with secondary fluorescein isothiocyanate-conjugated horse anti-mouse IgG(H+L) affinity-purified antibody (FI-2000 [1:100]; http://www.vectorlabs.com) at 4°C for 30 min. The samples were analyzed by flow cytometry, and levels of IgG reacting with antigens on the IE surface were quantified as the mean fluorescein isothiocyanate fluorescence index (MFI) of dihydroethidium bromide-positive cells by using WinMDI software (http://facs.scripps.edu/software.html). Although levels of VSA-specific antibodies measured by flow cytometry depend on a number of variables that make interspecies comparisons difficult, the levels we observed in the present study were comparable to levels observed with placental P. falciparum isolates.
Histology.
Mice were anesthetized by intraperitoneal injection (10 μl/g of body weight) of a 1:1 mixture of Hypnorm (http://www.janssenpharmaceutica.be) and Dormicum (http://www.roche.com), each reconstituted 1:1 in sterile water. After the collection of a blood sample from the retro-orbital plexus, the animals were killed by cervical dislocation. Tissues from the placentas, kidneys, livers, spleens, lungs, and brains were collected and fixed by immersion in Zamboni's fixative solution for 24 h at room temperature. The organs were then transferred into 70% ethanol, dehydrated, embedded in paraffin, and cut in a microtome. For the illustrations, we used 4-μm sections, whereas 2-μm sections were used for the evaluation of parasitemia in solid tissues. All sections were stained by hematoxylin and eosin and examined by light microscopy. Hemozoin pigment crystals were examined under polarized light to increase their visibility.
Statistical analyses.
We used the SigmaStat (http://www.systat.com) and CIA (2) software packages for the statistical analyses. Results are reported as means or medians with corresponding 95% confidence intervals. Student's t test and the Mann-Whitney rank-sum test (T) were used to evaluate intergroup differences. The Spearman rank-order coefficient (rs) was used to evaluate parameter association, while multiple linear regression analysis was used to identify significant predictors of hemoglobin levels and birth weights. Differences with P values of <0.05 were considered statistically significant.
RESULTS
Infection and subcurative treatment result in immunity to virulent P. berghei infection.
The infection of nonimmune BALB/c mice with the K173 strain of P. berghei is uniformly lethal (12). However, the mice can be rendered immune to P. berghei by repeated infection and subcurative treatment (10, 11). Using a modification of this protocol, we obtained immunized animals that were highly resistant to challenge. Thus, all of 40 animals infected with 106 IEs and given two rounds of suppressive sulfadiazine treatment had parasitemias of <0.6% 1 week following challenge on D32 (Fig. 1). In contrast, three of three nonimmunized mice infected on the same day developed parasitemias of >15% over this period (Fig. 1). Hemoglobin levels in immunized animals about 14 days after challenge were slightly lower than those in NI control animals [median difference (95% confidence interval), 1.1 g/dl (0.5 to 1.9 g/dl); P(T) = 0.001], probably because of the episodes of patent parasitemia during the immunization period. These results show that immunization by infection and subcurative treatment induces marked but nonsterile immunity that provides a high degree of resistance to challenge infection.
FIG. 1.
Parasitemias during immunization and after challenge. The temporal development of peripheral blood parasitemia in female mice immunized by infection on D0 and two rounds of intermittent, suppressive sulfadiazine treatment was monitored. The immunized mice and nonimmunized control mice were challenged by infection on D32. Data for the immunized mice are shown as medians and 95% confidence intervals, whereas individual results for the control mice are shown.
Pregnancy causes recrudescence of P. berghei parasitemia in immunized mice.
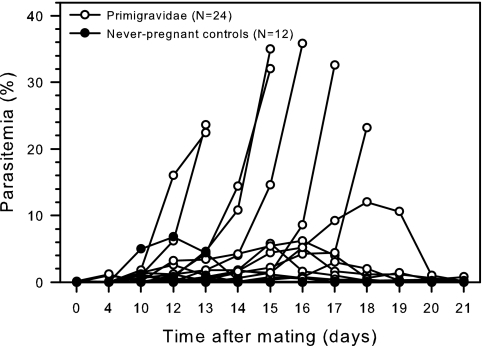
It has long been known that a proportion of mice immune to P. berghei infection develop recrudescent parasitemia during pregnancy (38, 39). This finding was confirmed in the present study, in which we observed recrudescence in 24 of 24 primigravid mice immunized prior to mating (median maximum parasitemia, 2.8%; range, 0.02 to 35.8%), including 7 with fulminant parasitemia (Fig. 2). Although episodes of patent parasitemia in all of 12 immune nonpregnant control females during the same period of time were also observed, the parasitemia levels were much lower (median maximum parasitemia, 0.15%; range, 0.008 to 6.8%) and the difference in the highest observed parasitemia levels between pregnant and nonpregnant animals was significant [median difference, 2.5% (95% confidence interval, 0.73 to 12%); P(T) < 0.01]. These results show that pregnancy can cause recrudescence of parasitemia previously controlled at very low levels by acquired immunity.
FIG. 2.
Pregnancy-related recrudescence of P. berghei in immunized and nonimmunized mice. The development of peripheral blood parasitemia in immunized primigravidae at various time points following conception was evaluated. Data for nonpregnant, immunized control mice monitored in parallel are shown for comparison. Data for individual mice are shown. Mice with parasitemia levels of >15% or signs of severe disease were killed.
Pregnancy-associated recrudescence of P. berghei parasitemia in immunized mice causes maternal anemia.
Pregnancy-associated P. berghei recrudescence in immune mice caused a significant reduction in hemoglobin levels [median difference between IF0 and IP1 mice, 3.5 g/dl (95% confidence interval, 2.4 to 4.8 g/dl); P(T) < 0.001] (Fig. 3A). This difference was not related simply to pregnancy per se. Thus, although hemoglobin levels in NI controls and nonimmunized and never-infected primigravidae (NP1 group) were also significantly different [median difference, 1.7 g/dl (95% confidence interval, 0.8 to 2.8 g/dl); P(T) = 0.001] (Fig. 3A), hemoglobin levels in NP1 mice and IF0 mice were similar [median difference, 0.5 g/dl (95% confidence interval, −0.2 to 1.4 g/dl); P(T) = 0.17] (Fig. 3A). These results show that pregnancy-associated recrudescence can cause maternal anemia.
FIG. 3.
Levels of hemoglobin and parasitemia in different groups of female mice: nonimmunized never-pregnant controls (NI); nonimmunized primigravidae (NP1); immunized, never-pregnant controls (IF0); immunized mice pregnant for the first (IP1), second (IP2), or third (IP3) time; and immunized mice with a recent first (IPP1) or second (IPP2) pregnancy. Data for pregnant mice (NP1, IP1, IP2, and IP3 mice) were obtained around D16 of pregnancy, while postpartum data (for IPP1 and IPP2 groups) were obtained approximately 10 days after delivery. Data are shown as medians (center lines), the central 50% of values (boxes), the central 80% of values (whiskers), and outliers (dots). Parallel horizontal lines indicate statistically significant (P < 0.01) differences. n values indicate the numbers of mice per group. n.d., not determined.
Susceptibility to pregnancy-associated anemia and recrudescence decreases with increasing parity.
It has been shown previously that recrudescence rates are lower during the second pregnancy than during the first pregnancy (38) and that this appears to be due to some kind of pregnancy-dependent immune response (40). Our preliminary data supported these findings and also showed that pregnancy-associated recrudescences during second and third pregnancies were more common if mice were reinfected around D11 of their second or third pregnancies with IEs obtained from primigravid mice with pregnancy-associated recrudescence (data not shown). Taking this approach, we found that hemoglobin levels around D16 of pregnancy correlated with parity (Fig. 3A, groups IP1, IP2, and IP3) [P(rs = 0.40) < 0.001], as did the proportion of animals with anemia (hemoglobin levels of <12 g/dl) (Fig. 4A, groups IP1, IP2, and IP3) [P(χ2 = 14.5) < 0.001]. Corresponding correlations were observed with respect to levels of parasitemia (Fig. 3B, groups IP1, IP2, and IP3) [P(rs = −0.40) < 0.001] and the proportion of mice with patent parasitemia (Fig. 4B) [P(χ2 = 9.3) = 0.009]. Both parasitemia and parity were significant predictors (P < 0.001 for each) of hemoglobin levels in a multiple linear regression model. Taken together, these results show that P. berghei parasitemia adversely affects maternal hemoglobin levels and that acquired immunity reduces recrudescent parasitemias and thereby protects the pregnant mice from anemia.
FIG. 4.
Proportions of animals with anemia (hemoglobin levels of <12 g/dl) and microscopically detectable peripheral blood parasitemia in different groups of IF mice: never-pregnant controls (IF0), mice pregnant for the first (IP1), second (IP2), or third (IP3) time; and mice with a recent first (IPP1) or second (IPP2) pregnancy. Data are presented as described in the legend to Fig. 3.
Immunized mice acquire high levels of IgG with specificity for antigens on the surfaces of IEs.
The immunization protocol used here has been shown previously to result in the acquisition of antibodies with specificity for antigens on the surfaces of P. berghei IEs (32) (Fig. 5). In agreement with these data, we found that levels of surface-reactive IgG with specificity for VSA expressed on the surfaces of P. berghei IEs from nonpregnant mice were significantly different [median difference, 17.6 MFI units (95% confidence interval, 15.3 to 19.9 MFI units); P(T) < 0.001] in NI animals and immunized (IF and IM) mice of comparable ages (Fig. 5A). Also as expected, levels of IgG in the immunized animals did not depend on sex [median difference between IF and IM mice, 0.7 MFI units (95% confidence interval, −2.5 to 3.3 MFI units); P(T) = 0.64] or parity [median difference between IF and IF>0 mice, 2.1 MFI units (95% confidence interval, −0.7 to 5.3 MFI units); P(T) = 0.15] (Fig. 5A). These results show that immunization results in the acquisition of IgG with specificity for antigens on the surfaces of IEs obtained from nonpregnant mice and that this acquisition is independent of sex and parity.
FIG. 5.
VSA-specific IgG levels in different groups of mice. Levels, in plasma, of IgG with specificity for variant antigens on the surfaces of erythrocytes infected with P. berghei obtained either from nonpregnant (A) or from pregnant (B) mice are shown. IgG levels in different groups of mice, NI mice, IM mice, and IF mice with (IF>0) or without (IF0) previous pregnancies, were measured by flow cytometry. Data are presented as described in the legend to Fig. 3.
Pregnancy-related recrudescence is caused by parasites expressing distinct variant antigens on the surfaces of IEs.
Based on the above-described findings, we proceeded to address the hypothesis that the susceptibility to pregnancy-related recrudescence in mice immune to P. berghei infection is due to the expression of pregnancy-specific VSA by the recrudescing parasites, VSA to which the mice do not have antibodies, despite high levels of antibodies to VSA expressed on the surfaces of P. berghei IEs from nonpregnant animals. We found that levels of IgG specific for VSA expressed by pregnancy-associated recrudescence-type IEs in nonimmunized (NI) and immunized (IF and IM) mice were significantly different [median difference, 25.2 MFI units (95% confidence interval, 17.6 to 31.2 MFI units); P(T) < 0.001] (Fig. 5B), similar to the levels of IgG specific for VSA expressed by IEs from nonpregnant mice (Fig. 5A). However, among the immunized animals (IF and IM), the levels of IgG antibody to VSA expressed on the surfaces of IEs obtained from pregnant mice varied with both sex and parity. Thus, antibody levels in IM and IF were different [median difference, 7.4 MFI units (95% confidence interval, −0.2 to 13.2 MFI units); P(T) = 0.06] (Fig. 5B) due to the difference between males (IM) and previously pregnant females [IF > 0; median difference, 9.7 MFI units (95% confidence interval, 4.1 to 14.8 MFI units); P(T) = 0.002] (Fig. 5B). Levels in the males (IM) were similar to levels in never-pregnant females [IF0; median difference, 0.6 MFI units (95% confidence interval, −9.1 to 9.1 MFI units); P(T) = 0.84] (Fig. 5B). Taken together, these results show that the antigens on P. berghei IEs from nonpregnant and pregnant mice are partially different. The simplest explanation for this finding is that there are antigens on the recrudescence-type IEs that are not found on other P. berghei IEs, in addition to antigens that are similar or identical to antigens on IEs from nonpregnant animals.
Levels of antibodies with specificity for the VSA expressed by parasites causing pregnancy-related recrudescences increase with increasing parity.
To further substantiate the hypothesis that the susceptibility to pregnancy-related recrudescence in mice immune to P. berghei infection is due to the expression of pregnancy-specific VSA to which the host does not have specific antibodies, we examined the relationship between IE-specific IgG levels and parity. While there was no apparent association between parity and levels of IgG with specificity for VSA on the surfaces of IEs from nonpregnant animals [P(rs = 0.21) = 0.23] (Fig. 6A), there was a clear correlation between parity and levels of IgG with specificity for recrudescence-type IEs [P(rs = 0.50) = 0.002] (Fig. 6B). These results show that IgG with specificity for recrudescence-type IEs is acquired in a selective and parity-dependent manner, reinforcing the hypothesis of a causal relationship between resistance to pregnancy-related recrudescence and IgG with specificity for antigens on the surfaces of erythrocytes infected with recrudescence-type P. berghei parasites.
FIG. 6.
Relationship between levels of VSA-specific IgG and parity. The parity-dependent acquisition of IgG with specificity for variant antigens on the surfaces of erythrocytes infected with P. berghei obtained from nonpregnant (A) or pregnant (B) mice is depicted. Levels of IgG in immunized mice pregnant for the first (IP1), second (IP2), third (IP3), or fourth (IP4) time were measured by flow cytometry at delivery. Data are presented as described in the legend to Fig. 3.
Erythrocytes infected by mature P. berghei parasites accumulate in the placenta.
Erythrocytes infected by parasites of all maturation stages can be seen in the peripheral blood of P. berghei-infected mice. Nevertheless, erythrocytes infected by mature P. berghei parasites can be sequestered in various tissues (1, 24). Of particular importance here is the recently observed chondroitin sulfate A (CSA)-dependent binding of P. berghei IEs in the placenta (24), which resembles that observed in PAM (13). We found that the placental intervillous spaces in mice with pregnancy-associated recrudescence showed numerous erythrocytes infected with mature, pigment-containing parasites (median, 19.0% [95% confidence interval, 15.1 to 27.3%] of all erythrocytes) but were almost devoid (<1%) of early-developmental-stage parasites (ring forms) (Fig. 7A). In contrast, ring-stage parasitemia (median, 15.0% [95% confidence interval, 8.6 to 18.2%]) dominated over mature-stage parasitemia (median, 7.2% [95% confidence interval, 4.0 to 9.8%]) in the peripheral blood [median difference, 6.8% (95% confidence interval, 3.0 to 11.0%); P(T) < 0.001] (Fig. 7B). These results support earlier evidence that placental accumulation of P. berghei IEs is a feature of pregnancy-related recrudescence (24). We also looked for evidence of the sequestration of IEs in brain, kidney, liver, lung, and spleen tissues. Brains were essentially free of both IEs and hemozoin (the presence of hemozoin is evidence of the phagocytosis of IEs) (data not shown). Hemozoin dominated in spleens, livers, and lungs, consistent with the expected phagocytosis of IEs in these organs (Fig. 7C and data not shown). Low levels of IEs were seen in the kidneys (Fig. 7D). Taken together, these results provide additional support for the hypothesis of preferential sequestration of recrudescence-type IEs in the placenta.
FIG. 7.
P. berghei parasitemia in different tissues. The placenta (A), peripheral blood (B), spleen (C), and kidney (D) of an immunized, pregnant mouse show tissue-specific P. berghei parasitemia.
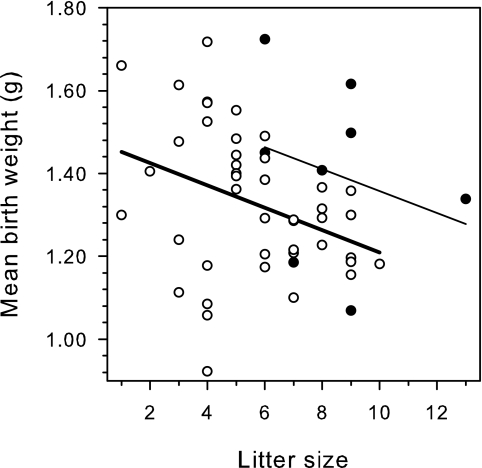
Pregnancy-associated recrudescence is associated with small litters and low pup weights.
Pregnancy-related parasite recrudescence in nonimmune mice has been reported previously to be associated with intrauterine growth retardation and reduced pup weight (24). We found offspring from immunized mice to be smaller (n = 44; mean birth weight, 1.33 g [95% confidence interval, 1.27 to 1.38 g]) than offspring from NI mice (n = 9; mean birth weight, 1.40 g [95% confidence interval, 1.24 to 1.56 g]) (Fig. 8). Furthermore, the average litter size among the control mice (median litter size, 9.0 pups [95% confidence interval, 6 to 9 pups]) was bigger than that among immunized mice (median litter size, 5.5 pups [95% confidence interval, 5.0 to 7.0 pups]). The median difference in litter size was 3 [95% confidence interval, 1 to 4; P(T) = 0.004] (Fig. 8), and litter size was the strongest predictor of birth weight in a linear regression model (P = 0.013). Among immunized mice, those with above-average parasitemia levels between D10 and D18 of pregnancy produced smaller pups (mean birth weight, 1.26 g [95% confidence interval, 1.17 to 1.39 g]) than those with below-average peak parasitemia levels (mean birth weight, 1.39 g [95% confidence interval, 1.32 to 1.47 g]). The mean difference was thus 0.12 g [95% confidence interval, 0.04 to 0.23 g; P(T) = 0.008]. In contrast, the average birth weight of pups from immunized mice with only low-grade parasitemia was not significantly different from that of the pups from nonimmunized mice [mean difference, 0.01 g (95% confidence interval, −0.14 to 0.15 g); P(T) = 0.95].
FIG. 8.
Maternal immune status and pregnancy outcome. The relationship between litter size and birth weight for NI (•) and immunized, infected (○) mice is depicted. Individual datum points and regression lines (light line, nonimmunized mice; heavy line, immunized mice) are shown.
We could not demonstrate that immunized primigravidae produced smaller litters or pups than mice of higher parity, as might have been expected. However, litter size and pup birth weights can be determined only after a successful pregnancy, which renders these markers unreliable for primigravid mice, in which pregnancies are often unsuccessful (resulting in maternal death, fetal resorption, or miscarriage) if high-level parasitemia develops. Our results show that the level of parasitemia in pregnancy-associated P. berghei recrudescence adversely affects litter size and pup weights.
DISCUSSION
In 1915, Clark noted that it “has long been known that it is possible to find an abundance of [malaria] parasites in the placenta” but that it was generally regarded as “a curious feature sometimes encountered” (5). Later, when it was discovered that pregnancy modulates the immune system in order to protect the developing fetus from maternal immune attack (15), many malaria researchers started to see PAM as the inevitable consequence of pregnancy-associated immunosuppression (20). It was known early that primigravidae are particularly susceptible to placental infection (3), but this finding, which is at variance with the immunosuppression hypothesis, was largely ignored—with some notable exceptions (18). Eventually, a coherent understanding of the pathogenesis and immunology of PAM emerged when it was found that P. falciparum IEs being sequestered in the placenta have unique adhesive properties (13) and that susceptibility to PAM is related to levels of antibodies recognizing particular parasite-encoded interclonally variant proteins (the so-called VSAPAM) on the IE surface (14, 34). These insights have spurred the current intensive and worldwide effort to develop vaccines against PAM. However, progress is being hampered by the lack of animal models that exhibit the characteristic features of PAM. Although the chimpanzee parasite P. reichenowi possesses a gene homologous to the P. falciparum var2csa gene implicated in the pathogenesis of PAM (37), higher primates are very impractical experimental model systems for malaria in general, let alone for PAM. The situation is not much better with respect to lower primates, and essentially nothing is known about the relationship between immunity and susceptibility to infection in pregnant monkeys (8). Rodents are the most accessible and therefore best-studied experimental malaria model system, and a number of studies of malaria in pregnant animals are available, including a recent study advocating murine P. berghei infection as a useful model of PAM (24). However, the report by Neres et al. (24) was based exclusively on data from nonimmune animals, and available studies of immunity and susceptibility to infection in pregnant mice either predate the discovery of or ignore the apparent importance of VSA-specific immunity in PAM. In the present study, we show that most, if not all, previously described features of P. berghei infection during pregnancy are consistent with the current, VSA-based understanding of PAM pathogenesis and immunity (29). Thus, in the P. berghei-infected mouse model, pregnancy-associated recrudescences are associated with placental and CSA-dependent sequestration of IEs (9, 24, 36), decrease in frequency and severity with increasing parity (38, 39), have adverse consequences for the pregnant mice and their offspring (24), and occur despite immunity acquired before the first pregnancy (38, 39). Furthermore, we show for the first time that pregnancy-associated recrudescence in P. berghei-infected mice leads to the acquisition of antibodies that are specific for variant antigens expressed only during recrudescence. The acquisition of these antibodies appears to be associated with clinical protection from the consequences of pregnancy-associated recrudescence, such as maternal anemia and low birth weight. However, direct evidence of a causal relationship must await the identification of the antigen(s) involved and its use in vaccination studies.
In common with most other malaria parasites, P. berghei does not possess genes homologous to the var genes encoding the PfEMP1 proteins thought to be the major P. falciparum IE adhesion ligands. Nevertheless, IE sequestration, including placental sequestration during pregnancy, is clearly not restricted to P. falciparum infection (25-27, 36). The capacity for glycosaminoglycan-dependent IE sequestration in the placenta may thus have evolved independently several times. Alternatively, it may be a truly ancient feature, raising the possibility that orthologs of the parasite genes involved in placental IE sequestration in mice exist in P. falciparum. In either case, the identification of the pregnancy recrudescence-related P. berghei genes and the characterization of their products are of considerable interest and a current priority in our laboratories.
In conclusion, we have demonstrated many similarities between PAM in P. falciparum-exposed women and pregnancy-related P. berghei recrudescence in immune mice. This finding opens new opportunities for research on the pathogenesis and immunity of PAM, which remains a major source of poor mother-infant health in large parts of the world.
Acknowledgments
This work was supported primarily by grants to T.S. from Rigshospitalet (9595.91.164) and the Danish Medical Research Council (2112-04-0015). We do not have any conflicting financial interests.
Grethe Gomme is thanked for help with analysis and photography of infected tissues. Jette Pedersen is thanked for help with sectioning and staining of tissues. Lothar Wiese, Kirsten Pihl, and the technical staff at the Department of Experimental Medicine, University of Copenhagen, are thanked for help with the animal work.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Alger, N. E. 1963. Distribution of schizonts of Plasmodium berghei in tissues of rats, mice and hamsters. J. Protozool. 106-10. [DOI] [PubMed] [Google Scholar]
- 2.Altman, D. G., D. Machin, T. N. Bryant, and M. J. Gardner. 2000. Statistics with confidence, 2nd ed. BMJ Books, London, United Kingdom.
- 3.Archibald, H. M.. 1956. The influence of malarial infection of the placenta on the incidence of prematurity. Bull. W. H. O. 15842-845. [PMC free article] [PubMed] [Google Scholar]
- 4.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark, H. C. 1915. The diagnostic value of the placental blood film in æstivo-autumnal malaria. J. Exp. Med. 22427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham, D. A., W. Jarra, S. Koernig, J. Fonager, D. Fernandez-Reyes, J. E. Blythe, C. Waller, P. R. Preiser, and J. Langhorne. 2005. Host immunity modulates transcriptional changes in a multigene family (yir) of rodent malaria. Mol. Microbiol. 58636-647. [DOI] [PubMed] [Google Scholar]
- 7.Desai, M., F. O. Ter Kuile, F. Nosten, R. McGready, K. Asamoa, B. Brabin, and R. D. Newman. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 793-104. [DOI] [PubMed] [Google Scholar]
- 8.Desowitz, R. S. 2001. Animal models of malaria in pregnancy: setting a good example, p. 127-157. In P. E. Duffy and M. Fried (ed.), Malaria in pregnancy. Deadly parasite, susceptible host. Taylor & Francis, London, United Kingdom.
- 9.Desowitz, R. S., K. K. Shida, L. Pang, and G. Buchbinder. 1989. Characterization of a model of malaria in the pregnant host: Plasmodium berghei in the white rat. Am. J. Trop. Med. Hyg. 41630-634. [DOI] [PubMed] [Google Scholar]
- 10.Eling, W., and C. Jerusalem. 1977. Active immunization against the malaria parasite Plasmodium berghei in mice: sulfathiazole treatment of a P. berghei infection and development of immunity. Tropenmed. Parasitol. 28158-174. [PubMed] [Google Scholar]
- 11.Eling, W., and C. Jerusalem. 1977. Active immunization against the malaria parasite Plasmodium berghei in mice. The immunizing inoculum. Tropenmed. Parasitol. 28293-301. [PubMed] [Google Scholar]
- 12.Eling, W., A. Van Zon, and C. Jerusalem. 1977. The course of a Plasmodium berghei infection in six different mouse strains. Z. Parasitenkd. 5429-45. [DOI] [PubMed] [Google Scholar]
- 13.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science 2721502-1504. [DOI] [PubMed] [Google Scholar]
- 14.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395851-852. [DOI] [PubMed] [Google Scholar]
- 15.Hunt, J. S. 1992. Immunobiology of pregnancy. Curr. Opin. Immunol. 4591-596. [DOI] [PubMed] [Google Scholar]
- 16.Kaviratne, M., V. Fernandez, W. Jarra, D. Cunningham, M. R. Galinski, M. Wahlgren, and P. R. Preiser. 2003. Antigenic variation in Plasmodium falciparum and other Plasmodium species, p. 291-318. In A. Craig and A. Scherf (ed.), Antigenic variation. Academic Press, London, United Kingdom.
- 17.Leech, J. H., J. W. Barnwell, L. H. Miller, and R. J. Howard. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 1591567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGregor, I. A. 1984. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 33517-525. [DOI] [PubMed] [Google Scholar]
- 19.McLean, S. A., C. D. Pearson, and R. S. Phillips. 1982. Plasmodium chabaudi: antigenic variation during recrudescent parasitaemias in mice. Exp. Parasitol. 54296-302. [DOI] [PubMed] [Google Scholar]
- 20.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11178-183. [DOI] [PubMed] [Google Scholar]
- 21.Mota, M. M., K. N. Brown, V. E. Do Rosário, A. A. Holder, and W. Jarra. 2001. Antibody recognition of rodent malaria parasite antigens exposed at the infected erythrocyte surface: specificity of immunity generated in hyperimmune mice. Infect. Immun. 692535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mota, M. M., K. N. Brown, A. A. Holder, and W. Jarra. 1998. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surfaces of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infect. Immun. 664080-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mota, M. M., W. Jarra, E. Hirst, P. K. Patnaik, and A. A. Holder. 2000. Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way. Infect. Immun. 684135-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neres, R., C. R. Marinho, L. A. Goncalves, M. B. Catarino, and C. Penha-Goncalves. 2008. Pregnancy outcome and placenta pathology in Plasmodium berghei infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS ONE 3e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oduola, A. M., J. H. Phillips, S. S. Spicer, and R. M. Galbraith. 1986. Plasmodium berghei: histology, immunocytochemistry, and ultrastructure of the placenta in rodent malaria. Exp. Parasitol. 62181-193. [DOI] [PubMed] [Google Scholar]
- 26.Pavia, C. S., and C. J. Niederbuhl. 1991. Immunization and protection against malaria during murine pregnancy. Am. J. Trop. Med. Hyg. 44176-182. [DOI] [PubMed] [Google Scholar]
- 27.Poovassery, J., and J. M. Moore. 2006. Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta. Infect. Immun. 742839-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 1653309-3316. [DOI] [PubMed] [Google Scholar]
- 29.Rogerson, S. J., L. Hviid, P. E. Duffy, R. F. G. Leke, and D. W. Taylor. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7105-117. [DOI] [PubMed] [Google Scholar]
- 30.Salanti, A., M. Dahlbäck, L. Turner, M. A. Nielsen, L. Barfod, P. Magistrado, A. T. R. Jensen, T. Lavstsen, M. F. Ofori, K. Marsh, L. Hviid, and T. G. Theander. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2001197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. R. Jensen, M. P. K. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49179-191. [DOI] [PubMed] [Google Scholar]
- 32.Schetters, T. P., C. C. Hermsen, A. A. Van Zon, and W. M. Eling. 1988. Stage-specific proteins of Plasmodium berghei-infected red blood cells detected by antibodies of immune mouse serum. Parasitol. Res. 7569-72. [DOI] [PubMed] [Google Scholar]
- 33.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35329-336. [DOI] [PubMed] [Google Scholar]
- 34.Staalsoe, T., C. E. Shulman, J. N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against the clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363283-289. [DOI] [PubMed] [Google Scholar]
- 35.Su, X., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 8289-100. [DOI] [PubMed] [Google Scholar]
- 36.Tegoshi, T., R. S. Desowitz, K. G. Pirl, Y. Maeno, and M. Aikawa. 1992. Placental pathology in Plasmodium berghei-infected rats. Am. J. Trop. Med. Hyg. 47643-651. [DOI] [PubMed] [Google Scholar]
- 37.Trimnell, A. R., S. M. Kraemer, S. Mukherjee, D. J. Phippard, J. H. Janes, E. Flamoe, X. Z. Su, P. Awadalla, and J. D. Smith. 2006. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol. Biochem. Parasitol. 148169-180. [DOI] [PubMed] [Google Scholar]
- 38.Van Zon, A. A., and W. M. Eling. 1980. Depressed malarial immunity in pregnant mice. Infect. Immun. 28630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Zon, A. A., and W. M. Eling. 1980. Pregnancy associated recrudescence in murine malaria (Plasmodium berghei). Tropenmed. Parasitol. 31402-408. [PubMed] [Google Scholar]
- 40.Van Zon, A. A., W. M. Eling, and C. C. Hermsen. 1985. Pregnancy-induced recrudescences strengthen malarial immunity in mice infected with Plasmodium berghei. Parasitology 919-17. [DOI] [PubMed] [Google Scholar]
- 41.Yoeli, M., and B. J. Hargreaves. 1974. Brain capillary blockage produced by a virulent strain of rodent malaria. Science 184572-573. [DOI] [PubMed] [Google Scholar]