Abstract
The etiology of rheumatic fever and rheumatic heart disease (RF/RHD) is believed to be autoimmune, involving immune responses initiated between streptococcal and host tissue proteins through a molecular mimicry mechanism(s). We sought to investigate the humoral and cellular responses elicited in a Lewis rat model of group A streptococcus M-protein- or peptide-induced experimental valvulitis/carditis, a recently developed animal model which may, in part, represent human rheumatic carditis. Recombinant streptococcal M5 protein elicited opsonic antibodies in Lewis rats, and anti-M5 antisera recognized epitopes within the B- and C-repeat regions of M5. One peptide from the streptococcal M5 protein B-repeat region (M5-B.6, amino acids 161 to 180) induced lymphocytes that responded to both recombinant M5 and cardiac myosin. Rats immunized with streptococcal M5 protein developed valvular lesions, distinguished by infiltration of CD3+, CD4+, and CD68+ cells into valve tissue, consistent with human studies that suggest that RF/RHD are mediated by inflammatory CD4+ T cells and CD68+ macrophages. The current study provides additional information that supports the use of the rat autoimmune valvulitis model for investigating RF/RHD.
There is a wealth of evidence to indicate that the immunopathogenic mechanisms in rheumatic heart disease involve autoimmunity as a result of molecular mimicry between streptococcal and host tissue proteins (7, 16), although the precise mechanisms are not completely understood. Progress has been made in this area of research by analyzing cellular and humoral responses of peripheral blood samples from rheumatic fever and rheumatic heart disease (RF/RHD) patients (11, 15, 17, 19). However, study of T cells and associated cytokines involved in initiating tissue damage is required. Limited access to tissue samples from RF/RHD patients is a major obstacle to these studies, and an acceptable animal model would facilitate further studies. An animal model, in which the immunopathological mechanisms or outcome of disease resembles those that occur in humans, is a logical adjunct to human studies.
For many years, attempts to establish a suitable animal model for RF/RHD had limited success, with none of the proposed models displaying the same pathological changes as those seen in human patients (23). The rat autoimmune valvulitis (RAV) model, developed by Quinn and colleagues (30) whereby Lewis rats immunized with recombinant streptococcal M protein develop hallmark RHD lesions in heart valves, has shown promise as a suitable animal model of rheumatic carditis.
A role for molecular mimicry in RF/RHD immunopathogenesis has also been supported by the study of Quinn (30) and by others using the RAV model (14). Peripheral blood T-cell lines from M-protein-immunized rats proliferated in response to cardiac myosin (30), and T cells from heart lesions of cardiac myosin-immunized rats also responded to peptides from the B-repeat region of M protein (14). The RAV model has also been used in our laboratory to induce valvulitis/carditis by immunizing Lewis rats with C-terminal M-protein peptides (26).
In this study, B- and T-cell responses in Lewis rats immunized with group A streptococcus (GAS) M5 protein or selected M5 peptides were examined to further validate the use of the RAV model as a suitable animal model for RF/RHD. Immunostaining of cellular infiltrates in valvular and myocardial tissue revealed that heart damage observed in streptococcal M-protein-immunized rats is mediated by CD4+ T cells and macrophages, in agreement with human studies (19).
MATERIALS AND METHODS
Animals.
Lewis rats (LEW/SsN; albino: a,h,c: RT1) were originally purchased from Animal Resources Centre (Canning Vale, Western Australia, Australia) and subsequently bred at the Small Animal Breeding Facility at James Cook University, Townsville, Australia. The rats were fed with a pelleted protein-rich commercial diet and water ad libitum. Experimental procedures were conducted with approval from James Cook University Animal Ethics Committee and in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council). Depending on the experimental design, treatment groups included from three to five rats, with appropriate control groups.
Antigens.
M protein is a major virulence factor of group A streptococcus, and serotype 5 was chosen for this study due to its potential as a “rheumatogenic” strain (34). The extracellular domain of GAS M5 protein (amino acid residues 1 to 450) was amplified by PCR from genomic DNA from GAS reference strain M5T5/B/PS PHLS (provided by The Townsville Hospital, Townsville, Australia) by standard methods. The forward primer M5-5-169 (GCGCGGATCCGCCGTGACTAGGGGTACA) and reverse primer M5-5-1330 (GCGCGTCGACTTGACCTTTACCTGGAACAGC) containing restriction sites (underlined) to allow directional cloning were based on the M5 nucleotide sequence reported by Miller et al. (27). The DNA fragment was designed to encode a truncated form of the GAS M5 protein containing the A-, B- and C-repeat regions but lacking the N-terminal signal sequence, the C-terminal cell wall-spanning region and sorting sequence. The M5 DNA was ligated into expression vector pQE-30 (Qiagen) to form the pQE30.m5 construct and subsequently transformed in Escherichia coli BL21(pREP4). Plasmid DNA, extracted from positive clones, was sequenced in both directions, and the recombinant M5 protein (rM5) consensus sequence, constructed from three independent sequence reactions, was analyzed using BLAST search of the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/). Deduced amino acid sequence and multiple-protein alignments were performed using the software provided on the ExPASy Proteomics Server (http://au.expasy.org/tools/dna.html). rM5 protein, comprised of 427 amino acid residues with a calculated molecular mass of 48.5 kDa, has the highest identity (99%) with Streptococcus pyogenes serotype 5 M-protein strain NCTC8193 as published by Whatmore and Kehoe (UniProtKB/TrEMBL accession no. Q54510) (35).
The histidine-tagged rM5 was overexpressed in E. coli and purified by Ni-nitrilotriacetic acid affinity chromatography according to the manufacturer's instructions (QiaExpressionist; Qiagen). The buffer was changed to phosphate-buffered saline (PBS) (pH 7.2) on a PD10 buffer exchange column (Amersham), and the protein was concentrated by ultrafiltration and then filter sterilized (0.22 μm). Protein concentration and purity were assessed by bicinchoninic acid and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, respectively. Potential endotoxin contamination was tested by the Limulus amoebocyte lysate method using an E-Toxate kit (Sigma) before storage at −20°C. No endotoxin was found in the purified rM5 used in our studies.
Six (20-mer) peptides from the B-repeat region and four peptides from the C-repeat region of GAS M5 protein which overlap by 10 amino acid residues (Table 1) were synthesized at the Queensland Institute of Medical Research, Brisbane, Australia, by solid-phase peptide synthesis using Boc (tert-butoxycarbonyl) chemistry, as described previously (20). The M5 peptides contained a free amine on the N terminus and a free amide on the C terminus. Peptides were assessed for purity by high-performance liquid chromatography, lyophilized, and stored at −20°C. Prior to use, the peptides were reconstituted in sterile PBS. Porcine cardiac myosin, which exhibits 97% sequence identity with human and rat cardiac myosin, was purchased from Sigma (Castle Hill, Australia) and used for immunization of rats as a positive control.
TABLE 1.
20-mer peptides with 10-aa overlap from the B- and C-repeat regions of GAS M5
| Peptide | Sequencea | Amino acid positions |
|---|---|---|
| M5-B.1 | TRQELANKQQESKENEKALN | 111-130 |
| M5-B.2 | ESKENEKALNELLEKTVKDK | 121-140 |
| M5-B.3 | ELLEKTVKDKIAKEQENKET | 131-150 |
| M5-B.4 | IAKEQENKETIGTLKKILDE | 141-160 |
| M5-B.5 | IGTLKKILDETVKDKIAKEQ | 151-170 |
| M5-B.6 | TVKDKIAKEQENKETIGTLK | 161-180 |
| M5-C.7 | NKISEASRKGLRRDLDASRE | 285-304 |
| M5-C.8 | LRRDLDASREAKKQVEKALE | 295-314 |
| M5-C.9 | AKKQVEKALEEANSLKAALE | 305-324 |
| M5-C.10 | EANSKLAALEKLNKELEESK | 315-334 |
Amino acid sequences of overlapping peptides from S. pyogenes M5 used in this study. Position numbers are from the mature protein sequence published by Miller et al. (27).
Immunization of rats.
To induce carditis, rats were immunized as described previously (26). Briefly, female Lewis rats, 8 to 12 weeks old, were immunized subcutaneously on day 0 in one rear foot with 0.5 mg of rM5 or M5 peptide emulsified 1:1 with complete Freund's adjuvant in a total volume of 200 μl following intraperitoneal anesthetization with ketamine and xylazine at 10 mg and 0.2 mg per 250 g of body weight, respectively. On days 1 and 3, rats received 1 × 1010 whole killed Bordetella pertussis cells (strain T1F1, sourced from stocks held at James Cook University, Townsville, Australia) given intraperitoneally as a coadjuvant to promote a Th1 immune response and facilitate the development of autoimmunity (33). Seven days after the initial immunization, the rats were boosted subcutaneously in the flank with 0.5 mg of rM5 or M5 peptide emulsified 1:1 with incomplete Freund's adjuvant in a total volume of 200 μl. All rats were euthanized at 21 days following initial immunization. Control rats were immunized with PBS and adjuvant only.
Bactericidal assay.
The ability of sera from rM5-immunized rats to promote the opsonization of GAS serotype 5 was determined in an indirect bactericidal assay (24). Bactericidal activity of immune sera was calculated as the percent reduction in CFU in immune serum compared to CFU grown in control serum after 3 hours of incubation. An opsonization inhibition assay (2) whereby sera were preincubated with rM5 prior to the bactericidal assay was also performed.
ELISA.
Immunoglobulin G (IgG) reactivity in control and immune rat sera toward rM5 or M5 peptides was measured by an indirect enzyme-linked immunosorbent assay (ELISA). Following optimization by checkerboard titration, antigen at 10 μg/ml was coated onto Nunc Maxisorp F96 plates in bicarbonate coating buffer (pH 9.6) (TropBio, Townsville, Australia) at 100 μl per well and incubated overnight at 4°C. The plates were washed five times with wash buffer (PBS [pH 7.2], 0.05% Tween 20) and blocked with 200 μl of postcoating buffer (TropBio) for 1 h at 37°C. Serial twofold dilutions, starting at 1:50, of pooled rat sera from each group were added to duplicate wells at 100 μl per well and incubated for 1 h at 37°C. After the wells were washed as described above, horseradish peroxidase-conjugated goat anti-rat IgG (Jackson ImmunoResearch, West Grove, PA) at a 1:5,000 dilution was added and incubated for 1 h at 37°C. A positive (rM5) and a negative (PBS) serum sample were included on each plate as controls. After a final wash, 2,2′-azinobis(3-ethylbenthiazolinesulfonic acid) (ABTS) substrate solution (KPL) was added for 20 min at room temperature before absorbance was measured at 414 nm with a reference wavelength of 492 nm on a Multiskan microplate reader (Titertek). Data were processed using Genesis V3.00 software (Labsystems) and reported as mean absorbance ± standard error of the mean (SEM) at 1:100 dilution with background subtracted.
Lymphocyte proliferation assays.
The specificity and reactivity of lymphocytes from immunized rats were determined by measuring their proliferative response to antigen stimulation in a tritiated [3H]thymidine incorporation assay. Pooled mononuclear cells (MNC) from rat spleens in each group were cultured in 96-well cell culture plates (Nunc) in triplicate wells at 105 cells per well and stimulated with 10 μg/ml individual M5 peptides, rM5, or cardiac myosin or 5 μg/ml concanavalin A (ConA) as positive control for 96 to 168 h. Negative-control cells were left unstimulated. The culture medium consisted of RPMI 1640 medium (Invitrogen) supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), 2 mM l-glutamine, 10 mM HEPES buffer, and 2.5% heat-inactivated autologous rat serum in a total volume of 200 μl per well. Cells were harvested onto glass fiber mats at 24-h intervals on days 4 to 7 of culture after pulsing for 4 h with 0.25 μCi [3H]thymidine (Amersham). When dry, scintillation fluid was applied to the mats, and the number of CPM was determined in a MicroBeta scintillation counter. Proliferative response of cells is reported as stimulation index (SI), calculated as a ratio between the mean CPM in stimulated cells and CPM in unstimulated cells (CPM of test wells/CPM of control wells). Although SI values as low as 2.0 have been reported as positive for peptide restimulation (21), in this study we chose an SI of ≥3.0 as representing a positive response.
Histology.
The hearts from rM5-immunized rats and PBS controls were fixed in neutral buffered formalin overnight and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) and examined using a light microscope (Olympus BH2) fitted with a QImaging camera, for evidence of myocarditis or valvulitis, including inflammatory mononuclear infiltration, fibrosis, and necrosis. Image manipulation was restricted to adjustments to contrast or brightness using Microsoft Picture Manager software.
Immunohistochemistry.
All procedures were carried out at room temperature unless otherwise stated, and PBS (pH 7.2) or Tris-buffered saline (pH 7.5) was used for rinsing sections between incubation steps. The antibody against rat CD4 was found to be unsuitable for use in formalin-fixed, paraffin-embedded sections; therefore, frozen sections were used. The hearts were fixed in 4% paraformaldehyde for 4 h, rinsed in PBS, then soaked 24 to 48 h in 30% sucrose prior to embedding in Tissue-Tek OCT medium and freezing in liquid nitrogen. Cryostat sections (5 to 7 μm) were mounted on SuperfrostPlus slides (Menzel-Glaser), air dried overnight, acetone fixed at 4°C for 10 min, air dried, and stored at −70°C. Prior to use, frozen sections were warmed to room temperature and rinsed to remove OCT compound. Formalin-fixed, paraffin-embedded sections (4 to 6 μm) were deparaffinized in xylene and rehydrated with graded ethanol. For antibodies requiring microwave antigen retrieval, sections were treated with citric acid (pH 6.0) three times for 5 min each time. Endogenous peroxidase was blocked with 0.3% H2O2 for 30 min. Sections were rinsed in PBS three times for 5 min each time and incubated with 1.5% normal horse serum in PBS for 30 min. Excess serum was removed without rinsing, and sections were incubated overnight at 4°C with primary antibody against pan T-cell marker CD3 (clone G4.18; eBiosciences), T helper cell CD4 or T cytotoxic cell CD8 (W3/25 and OX8; Cedarlane Laboratories), and macrophage lysosomal membrane molecule CD68 (ED1; Chemicon). Biotinylated horse anti-mouse secondary antibody (Vector Laboratories) at 1:50 or 1:100 dilution was applied for 30 min, and sections were incubated for 30 min with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch). Positively stained cells were visualized with fast red (BioGenex). Valvular inflammatory infiltrates were scored as follows: 0, no staining; 1, less than five cells in a valve; 2, more than five cells but no foci; 3, one to two focal lesions; and 4, more than two lesions. Myocardial infiltrates were scored as follows: 0, isolated cells throughout tissue; 1, one or two small foci; 2, more than two small foci; 3, one or more large focal lesions; and 4, Aschoff-type lesion. For each animal, a valve or myocardial score of ≥2 was classified as positive.
Statistical analysis.
Data were analyzed using SPSS version 14.0 software and where necessary, transformed to ensure a Gaussian population. In opsonophagocytosis assays, an unpaired, two-tailed Student's t test was performed on normally distributed data to test for differences between group means with a P value of ≤0.01 regarded as significant.
RESULTS
Lewis rats immunized with rM5 develop opsonic antibodies against GAS.
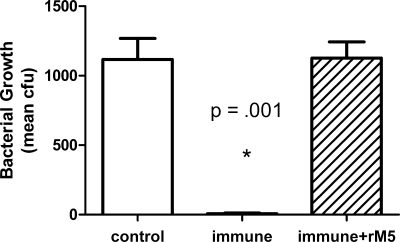
Sera from each group of rats were pooled and tested for functional activity against GAS using an indirect bactericidal assay. As shown in Fig. 1, heat-inactivated sera from rM5-immunized rats had significantly higher (P < 0.001) bactericidal activity (>98%) than sera from control rats, reducing the number of CFU from an inoculum size of 370 ± 40 (mean ± SEM) to 14 ± 9 after 3 h of incubation in human blood. In the presence of sera from control rats, the number of CFU increased to 1,146 ± 89. Preabsorption of the rM5 antisera with rM5 protein completely abrogated this bactericidal activity against GAS (1,168 ± 123 CFU).
FIG. 1.
Bactericidal activity in rM5 antiserum against GAS serotype M5. In an indirect bactericidal assay, rM5 antisera produced a 98% reduction of GAS M5 bacteria in nonimmune human blood. Preabsorption of rM5 antisera with rM5 completely abrogated opsonization. Results are expressed as mean CFU ± SEM from duplicate assays and are representative of two independent experiments. The value for the immune group was significantly different (P < 0.001 by two-tailed Student's t test) from the values for the other two groups as indicated by the asterisk.
Peptides from the B-repeat region and C-repeat region of M5 protein contain B-cell epitopes.
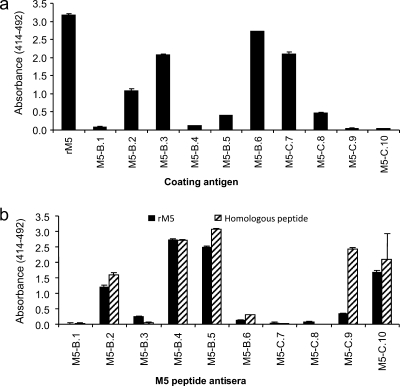
The IgG response in rats immunized with rM5 or M5 peptides was tested by indirect ELISA. Initially, a pooled sera sample from rM5-immunized rats (n = 5) was evaluated against individual peptides to determine whether the peptides contained epitopes that were recognized by antibodies raised against the full-length rM5 protein. High reactivity (defined as titers of ≥6,400) to peptides M5-B.2, M5-B.3, and M5-B.6 from the B-repeat region and M5-C.7 from the C-repeat region and medium reactivity (800 < titer < 3,200) to peptides M5-B.5 and M5-C.8 was observed in rM5 antiserum (Fig. 2a). Next, the ability of individual M5 peptides to elicit a humoral immune response in vivo was assessed. The reactivity of each one of the pooled sera from rats immunized with individual M5 peptides (n = 3 per group) toward the immunizing, homologous M5 peptide or the full-length rM5 protein is shown in Fig. 2b. Five of 10 groups of sera from rats immunized with M5 peptides had strong responses (titers of ≥3,200) to the respective homologous peptide. These were M5-B.2, M5-B.4, M5-B.5, M5-C.9, and M5-C.10; four of these anti-M5 peptide sera (M5-B.2, M5-B.4, M5-B.5, and M5-C.10) reacted strongly to the full-length rM5 protein.
FIG. 2.
Antibody responses to M5 protein and peptides in Lewis rats. (a) IgG antibody reactivity of serum from rM5-immunized Lewis rats to rM5 and individual M5 peptides. (b) IgG antibody reactivity of serum from peptide-immunized Lewis rats to homologous M5 peptide or rM5.
Peptides from the B-repeat region and C-repeat region of M5 protein contain T-cell epitopes.
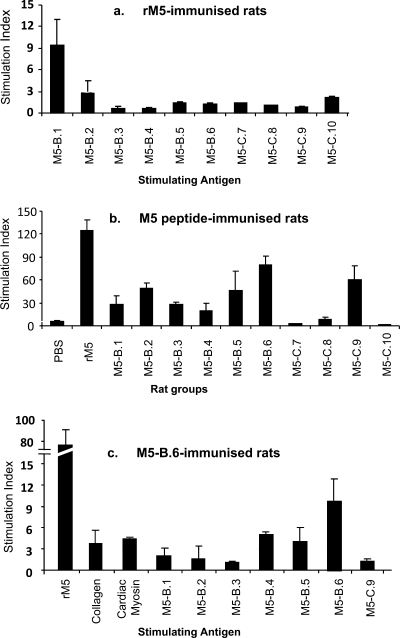
T-cell responses in immunized rats (n = 3 to 5 per group) were determined by measuring the maximal proliferative response of MNC derived from the spleens of immunized rats using a standard tritiated [3H]thymidine incorporation assay. MNC from rM5-immunized rats proliferated in response to stimulation with peptide M5-B.1 (SI = 9.5 ± 3.5), but not to any of the other M5 peptides (Fig. 3a). rM5-sensitized MNC also responded strongly to recall antigen rM5 protein and ConA (SI = 211 ± 41 and 31.9 ± 1.9, respectively). MNC from naïve, nonimmunized rats and control rats immunized with PBS and adjuvant alone responded positively to ConA (SI = 76 ± 27 and 37 ± 3, respectively), but not to any of the other antigens tested. MNC derived from 5 of 10 M5 peptide-immunized rat groups (M5-B.1, M5-B.2, M5-B.5, M5-B.6, and M5-C.9) produced a significant recall response to the homologous immunizing peptide, while all but two groups (M5-C.7 and M5-C.10) reacted significantly to the full-length rM5 protein (Fig. 3b). MNC derived from rats immunized with one B-repeat region peptide, M5-B.6 (amino acids [aa] 161 to 180), also proliferated in response to stimulation with cardiac myosin (SI = 4.4 ± 0.1) (Fig. 3c). In contrast, no positive reactivity with cardiac myosin was demonstrated by any of the other peptide groups, with SI values of 2.1 or less.
FIG. 3.
T-cell proliferative responses in Lewis rats. (a) Responses of splenic lymphocytes from rats immunized with rM5 to individual peptides. (b) Responses of splenic lymphocytes from rats immunized with individual M5 peptides to the full-length rM5 protein. (c) Responses of splenic lymphocytes from rats immunized with peptide M5-B.6. Results are expressed as stimulation index (SI), calculated as mean test CPM/mean medium control CPM. Error bars represent standard errors (SEMs). Broken line indicates significant proliferative response cutoff value (SI = 3).
GAS M5 induces inflammatory infiltrates in the hearts of Lewis rats.
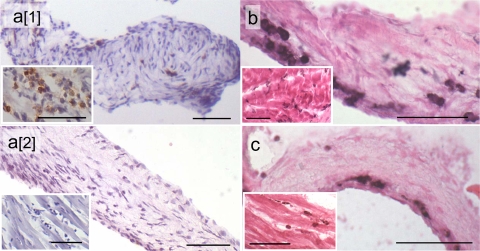
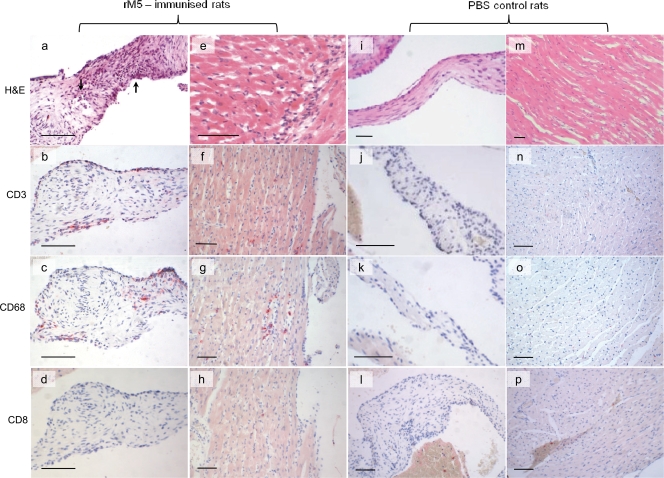
We sought to determine whether rats exposed to GAS M protein developed signs of valvulitis or myocarditis which are similar to the hallmark lesions reported for RF/RHD patients (31, 32). H&E-stained sections of hearts from rats immunized with rM5 or M5 peptides were examined for evidence of cardiac lesions and compared to heart tissue from control rats immunized with PBS and adjuvant only. The extent of pathology in rM5-immunized rats varied in individual animals. Five of seven animals had histological evidence of either valvular or myocardial infiltration by inflammatory cells. Four animals had mild inflammatory infiltration of the valves, and four rats had signs of small foci of infiltrating MNC, neutrophils, and myocyte necrosis in myocardial tissue. Three rats had both valvular and myocardial infiltrates. Immunohistochemical analysis was carried out to phenotype the infiltrating cells. Our anti-rat CD4 antibody was unsuitable for use in formalin-fixed paraffin sections; however, CD4+ T cells were demonstrated in frozen sections of rM5-immunized rats, but not in control rats (Fig. 4a). In paraffin sections (Fig. 5), T cells were distinguished with an antibody against pan T-cell marker CD3. MNC in valvular lesions therefore consisted mainly of CD3+, CD4+, and CD68+ cells, with few CD8+ cells present, supporting the opinion that CD4+ T helper cells and macrophages play a key role in valvular damage. In the myocardium, MNC infiltrates stained positive for CD3, CD4, CD8, and CD68. No lesions were apparent in the valves or myocardium in any of the control rats or rats immunized with individual M5 peptides except for peptide M5-B.6. In one of three animals immunized with peptide M5-B.6, myocardial and valvular lesions were evident by positive staining for CD4+ and CD68+ cells in frozen sections (Fig. 4b and c).
FIG. 4.
CD4+ T cells in valves and myocardium (insets) of rats immunized with rM5 (a[1]) and PBS (a[2]) and CD4+ T cells (b) and CD68+ macrophages (c) in rats immunized with peptide M5-B.6. The frozen sections were immunostained using diaminobenzidine (DAB) as a chromogen and hematoxylin as a counterstain (a) and metal-enhanced DAB counterstained with eosin (b and c). Bars = 50 μm.
FIG. 5.
Lewis rats immunized with rM5 showed evidence of valvulitis/carditis characterized by infiltration of T cells and macrophages. rM5-immunized rats showed evidence of mononuclear cell infiltrates in the valves (a) and myocardium (e) and are distinguished from adjuvant controls which showed no evidence of pathology (i and m). Valvular infiltrates in rM5-immunized rats stained for CD3+ T cells (b) and CD68+ macrophages (c), but not CD8+ T cells (d). Myocardial lesions in rM5-immunized rats were also characterized by CD3+ (f) and CD68+ (g) infiltrating cells with few CD8+ T cells (h). In contrast, no CD3+, CD68+, or CD8+ infiltrates were present in adjuvant control valves (j, k, and l) or myocardium (n, o, and p). Paraffin sections stained with H&E (a, e, i, and m) or immunostained using fast red as chromogen. Bars = 50 μm.
DISCUSSION
To study the pathogenesis of rheumatic heart disease, a number of animal models, including primates, rabbits, mice, and rats, have been used in the past and have contributed, in part, to our understanding of RF/RHD. However, the RAV model first described by Quinn et al. in 2001 (30) has shown the most promise for further unraveling the complex immune responses involved in the development of RF/RHD. An important aspect of any model is to validate its relevance to the human condition, which we have proposed with regard to an animal model for RHD. In this study, Lewis rats were immunized with streptococcal rM5 to induce lesions in the myocardium and heart valves that have similarities to those seen in RF/RHD. The data presented here, therefore, support the use of the RAV model for investigating immune responses in RF/RHD.
Antibody responses toward GAS M protein have been extensively studied in both humans and various animal models (1, 3, 4, 10, 29), and identification of cardiac myosin (10, 22) as an important autoantigen has indicated a role for autoimmunity in the pathogenesis of RHD (6, 8). Opsonophagocytosis, mediated by serum opsonins, such as antibodies or complement, is a key mechanism for host protective immunity against streptococcal infection. M protein has been shown to contribute to virulence, conferring resistance to phagocytosis under nonimmune conditions by interfering with the complement pathway (5). The indirect bactericidal test is a widely used assay for measuring growth or killing of hemolytic streptococci in blood and has been used extensively to demonstrate opsonic anti-M protein antibodies in sera of mice (10) and humans (2, 10, 25, 36). Our data show conclusively that rM5 is capable of eliciting opsonic antibodies in Lewis rats, demonstrated by over a 98% reduction of CFU in nonimmune human blood in the presence of immune rat sera. Antibodies in opsonic sera from rM5-immunized rats recognized peptides from the B-repeat region and C-repeat region of M5 protein. When sera from peptide-immunized rats were tested in ELISAs, 5 of 10 peptides produced a strong antibody response toward both rM5 and the original immunizing peptide (Fig. 2b).
Strong evidence from human (6, 11, 12, 16, 18), murine (9), and rat (14, 30) studies now suggests that while humoral responses may initiate RF/RHD by triggering endothelial inflammation in the heart, the key mediators of heart lesions are autoreactive T cells that, via molecular mimicry, also recognize heart tissue proteins. Faé et al. (12) were the first to demonstrate that heart-infiltrating T-cell clones from RHD patients simultaneously recognized streptococcal M5 protein and heart tissue proteins/peptides, including peptides from the light meromyosin and S2 regions of human cardiac myosin. Another important study using human T-cell clones from an RHD patient determined potential sites of mimicry between streptococcal M protein and several α-helical host proteins, such as cardiac myosin, laminin, or tropomyosin (11). In that study, two M5 protein sequences TIGTLKKILDETVKDKIA (aa 151 to 167) and IGTLKKILDETVKDKLAK (aa 176 to 193) were the dominant peptides recognized by human T-cell clones.
Cunningham et al. (9) immunized mice with peptides spanning the M5 protein (aa 1 to 308) and found that only two peptides (B1A111-129 and B3B202-219) produced heart lesions. When cardiac myosin-sensitized T cells were stimulated with M5 peptides, dominant cardiac myosin cross-reactive epitopes were identified in the NT4/NT5 (aa 40 to 76) and B1B2/B2/B3A regions (aa 137 to 193) as well as the C-terminal C3A region (aa 293 to 308), demonstrated by high proliferation toward these peptides. In our study, only one B-repeat region peptide (M5-B.1, aa 111 to 130) produced a strong T-cell response in rM5-immunized rats. However, it is often found that T cells taken from animals that have been immunized with whole protein show minimal proliferative responses when stimulated with individual peptides from the whole protein (28). This can be attributed to the low frequencies of T cells in the periphery that respond to each individual peptide. For individual peptide immunizations, all T cells from rats immunized with B-region peptides in this study proliferated strongly in response to the full-length rM5 protein, while only one C-region peptide (M5-C.9, aa 305 to 324) produced a T-cell response against rM5. Peptide M5-B.6 was found to contain a strong T-cell epitope as well as a myosin-cross-reactive epitope. We consider this cross-recognition seen in M5-B.6-sensitized T cells toward cardiac myosin as significant: while recognition of “nonself” antigen by peripheral T cells is readily observed ex vivo, cross-recognition of “self” antigen is more difficult to demonstrate. T cells with high affinity for self antigens are normally removed from the repertoire during intrathymic deletion; those that escape to the periphery most likely have low to intermediate affinity for self antigens. These T cells are subject to peripheral tolerance control but may be activated under certain conditions and subsequently contribute to autoimmune disease. Ellis et al. (11) demonstrated significant cardiac myosin cross-reactivity in T-cell clones derived from RHD patients. These clones had a 100-fold-higher sensitivity to streptococcal M protein than to cardiac myosin in dose-response studies. The amino acid sequence for M5-B.6 (aa 161 to 180) lies within the region reported by others to contain a strong myosin cross-reactive epitope recognized by T cells from patients with acute rheumatic fever (15) and from BALB/c mice (9).
Quinn et al. (30) reported the induction of valvulitis and focal myocarditis in Lewis rats which were histologically similar to human RHD lesions following immunization with rM6. It was concluded that sequences from the A-repeat region of M protein, but not from the B- or C-repeat regions, were capable of causing valvulitis. In contrast, our previous report found that when injected into Lewis rats, a pool of 15 overlapping C-repeat region peptides (aa 337 to 492) was capable of causing valvulitis, evidenced by focal infiltration by MNC in the aortic valve (26). In the present study, lesions were observed in some mitral and aortic valves as well as the triscuspid valve of rats immunized with rM5. We further validated the RAV model by phenotyping the cellular infiltrates in these lesions. Infiltrating cells were predominantly comprised of CD4+ T cells and CD68+ macrophages, consistent with analysis of valvular lesions found in patients with RF/RHD. On the basis of immunohistochemical staining of 15 rheumatic valves obtained at surgery, Fraser et al. observed aggregated macrophages during the initial stages of inflammation, followed by lymphocytic infiltration and neovascularization (13). Guilherme et al. (15) observed a predominance of CD4+ T cells in valvular lesions, and this was further confirmed by studies carried out on heart valves from patients by Roberts et al. (31). In our current work, one peptide (M5-B.6), found to contain a strong B-cell epitope and a myosin cross-reactive T-cell epitope, also induced heart lesions. Although animal models cannot completely substitute for human studies, these findings confirm that the Lewis rat is the most appropriate animal model for studying the immunopathological changes that occur in RF/RHD and enables parallels to be drawn with human disease.
Acknowledgments
This work was supported in part by the National Heart Foundation of Australia (GB06B2497) and the National Health and Medical Research Council of Australia (project grant 540419).
We thank Madeleine Cunningham for her critical review of the manuscript.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Beachey, E. H., M. Bronze, J. B. Dale, W. Kraus, T. Poirier, and S. Sargent. 1988. Protective and autoimmune epitopes of streptococcal M proteins. Vaccine 6192-196. [DOI] [PubMed] [Google Scholar]
- 2.Brandt, E. R., W. A. Hayman, B. Currie, S. Pruksakorn, and M. F. Good. 1997. Human antibodies to the conserved region of the M protein: opsonization of heterologous strains of group A streptococci. Vaccine 151805-1812. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, E. R., P. J. Yarwood, D. J. McMillan, H. Vohra, B. Currie, L. Mammo, S. Pruksakorn, J. Saour, and M. F. Good. 2001. Antibody levels to the class I and II epitopes of the M protein and myosin are related to group A streptococcal exposure in endemic populations. Int. Immunol. 131335-1343. [DOI] [PubMed] [Google Scholar]
- 4.Bronze, M. S., E. H. Beachey, and J. B. Dale. 1988. Protective and heart-crossreactive epitopes located within the NH2 terminus of type 19 streptococcal M protein. J. Exp. Med. 1671849-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson, F., C. Sandin, and G. Lindahl. 2005. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway. Mol. Microbiol. 5628-39. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham, M. W. 2003. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front. Biosci. 8s533-s543. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, M. W. 2004. T cell mimicry in inflammatory heart disease. Mol. Immunol. 401121-1127. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham, M. W., S. M. Antone, J. M. Gulizia, B. A. McManus, and C. J. Gauntt. 1993. Alpha-helical coiled-coil molecules: a role in autoimmunity against the heart. Clin. Immunol. Immunopathol. 68118-123. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham, M. W., S. M. Antone, M. Smart, R. Liu, and S. Kosanke. 1997. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect. Immun. 653913-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham, M. W., J. M. McCormack, P. G. Fenderson, M. K. Ho, E. H. Beachey, and J. B. Dale. 1989. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J. Immunol. 1432677-2683. [PubMed] [Google Scholar]
- 11.Ellis, N. M. J., Y. Li, W. Hildebrand, V. A. Fischetti, and M. W. Cunningham. 2005. T cell mimicry and epitope specificity of cross-reactive T cell clones from rheumatic heart disease. J. Immunol. 1755448-5456. [DOI] [PubMed] [Google Scholar]
- 12.Faé, K. C., D. Diefenbach da Silva, S. E. Oshiro, A. C. Tanaka, P. M. Pomerantzeff, C. Douay, D. Charron, A. Toubert, M. Cunningham, J. Kalil, and L. Guilherme. 2006. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J. Autoimmun. 1765662-5770. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, W. J., I. Haffejee, and K. Cooper. 1995. Rheumatic Aschoff nodules revisited: an immunohistological reappraisal of the cellular component. Histopathology 27457-461. [DOI] [PubMed] [Google Scholar]
- 14.Galvin, J. E., M. E. Hemric, S. D. Kosanke, S. M. Factor, A. Quinn, and M. W. Cunningham. 2002. Induction of myocarditis and valvulitis in Lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am. J. Pathol. 160297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilherme, L., E. Cunha-Neto, V. Coelho, R. Snitcowsky, P. M. Pomerantzeff, R. V. Assis, F. Pedra, J. Neumann, A. Goldberg, M. E. Patarroyo, F. Pileggi, and J. Kalil. 1995. Human heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation 92415-420. [DOI] [PubMed] [Google Scholar]
- 16.Guilherme, L., and M. Cunningham. 2006. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity 3931-39. [DOI] [PubMed] [Google Scholar]
- 17.Guilherme, L., N. Dulphy, C. Douay, V. Coelho, E. Cunha-Neto, S. E. Oshiro, R. V. Assis, A. C. Tanaka, P. M. Pomerantzeff, D. Charron, A. Toubert, and J. Kalil. 2000. Molecular evidence for antigen-driven immune responses in cardiac lesions of rheumatic heart disease patients. Int. Immunol. 121063-1074. [DOI] [PubMed] [Google Scholar]
- 18.Guilherme, L., and J. Kalil. 2002. Rheumatic fever: the T cell response leading to autoimmune aggression in the heart. Autoimmun. Rev. 1261-266. [DOI] [PubMed] [Google Scholar]
- 19.Guilherme, L., S. E. Oshiro, K. C. Faé, E. Cunha-Neto, G. Renesto, A. C. Goldberg, A. C. Tanaka, P. M. Pomerantzeff, M. H. Kiss, C. Silva, F. Guzman, M. E. Patarroyo, S. Southwood, A. Sette, and J. Kalil. 2001. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect. Immun. 695345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houghten, R. A. 1985. General method for the rapid solid phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 825131-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwai, L. K., M. Yoshida, A. Sadahiro, W. R. da Silva, M. L. Marin, A. Goldberg, M. A. Juliano, L. Juliano, M. A. Shikanai-Yasuda, J. Kalil, E. Cunha-Neto, and L. R. Travassos. 2007. T-cell recognition of Paracoccidioides brasiliensis gp43-derived peptides in patients with paracoccidioimycosis and healthy individuals. Clin. Vaccine Immunol. 14474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisher, K., and M. W. Cunningham. 1985. Myosin: a link between streptococci and heart. Science 227413-415. [DOI] [PubMed] [Google Scholar]
- 23.Krishna, G., and R. R. Iyer. 1999. Animal models of rheumatic fever and rheumatic carditis, p. 195-208. In J. Narula, R. Virmani, K. S. Reddy, and R. Tandon (ed.), Rheumatic fever. American Registry of Pathology, Washington, DC.
- 24.Lancefield, R. C. 1957. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. 106525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancefield, R. C. 1959. Persistence of type-specific antibodies in man following infection with group A streptococci. J. Exp. Med. 110271-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lymbury, R. S., C. Olive, K. A. Powell, M. F. Good, R. G. Hirst, J. T. LaBrooy, and N. Ketheesan. 2003. Induction of autoimmune valvulitis in Lewis rats following immunization with peptides from the conserved region of group A streptococcal M protein. J. Autoimmun. 20211-217. [DOI] [PubMed] [Google Scholar]
- 27.Miller, L., L. Gray, E. Beachey, and M. Kehoe. 1988. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J. Biol. Chem. 2635668-5673. [PubMed] [Google Scholar]
- 28.Miyakoshi, A., W. K. Yoon, Y. Jee, and Y. Matsumoto. 2003. Characterization of the antigen specificity and TCR repertoire, and TCR-based DNA vaccine therapy in myelin basic protein-induced autoimmune encephalomyelitis in DA rats. J. Immunol. 1706371-6378. [DOI] [PubMed] [Google Scholar]
- 29.Quinn, A., E. E. Adderson, P. G. Shackelford, W. L. Carroll, and M. W. Cunningham. 1995. Autoantibody germ-line gene segment encodes VH and VL regions of a human anti-streptococcal monoclonal antibody recognizing streptococcal M protein and human cardiac myosin epitopes. J. Immunol. 1544203-4212. [PubMed] [Google Scholar]
- 30.Quinn, A., S. Kosanke, V. A. Fischetti, S. M. Factor, and M. W. Cunningham. 2001. Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect. Immun. 694072-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts, S., S. Kosanke, S. T. Dunn, D. Jankelow, C. M. Duran, and M. W. Cunningham. 2001. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J. Infect. Dis. 183507-511. [DOI] [PubMed] [Google Scholar]
- 32.Saffitz, J. E. 2008. The heart, p. 456-459. In R. Rubin (ed.), Rubin's pathology: clinicopathologic foundations of medicine, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
- 33.Silver, P. B., C. C. Chan, B. Wiggert, and R. R. Caspi. 1999. The requirement for pertussis to induce EAU is strain-dependent: B10. RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Investig. Ophthal. Vis. Sci. 402898-2905. [PubMed] [Google Scholar]
- 34.Stollerman, G. H. 1991. Rheumatogenic streptococci and autoimmunity. Clin. Immunol. Immunopathol. 61131-142. [DOI] [PubMed] [Google Scholar]
- 35.Whatmore, A. M., and M. Kehoe. 1994. Horizontal gene transfer in the evolution of group A streptococcal emm-like genes: gene mosaics and variation in Vir regulons. Mol. Microbiol. 11363-374. [DOI] [PubMed] [Google Scholar]
- 36.Yoonim, N., C. Olive, C. Pruksachatkunakorn, and S. Pruksakorn. 2006. Bactericidal activity of M protein conserved region antibodies against group A streptococcal isolates from the Northern Thai population. BMC Microbiol. 671-77. [DOI] [PMC free article] [PubMed] [Google Scholar]