Abstract
We have previously shown that a recombinant baculovirus that displays Plasmodium berghei circumsporozoite protein (PbCSP), a homolog of the leading human malaria vaccine candidate, on the viral envelope protected 60% of mice against P. berghei infection. Here, we describe a second-generation baculovirus vaccine based on the “baculovirus dual expression system,” which drives PbCSP expression by a dual promoter that consists of tandemly arranged baculovirus-derived polyhedrin and mammal-derived cytomegalovirus promoters. The baculovirus-based PbCSP vaccine not only displayed PbCSP on the viral envelope but also expressed PbCSP upon transduction of mammalian cells. Immunization with the baculovirus-based PbCSP vaccine elicited high PbCSP-specific antibody titers (predominantly immunoglobulin G1 [IgG1] and IgG2a) and PbCSP-specific CD8+ T-cell responses without extraneous immunological adjuvants in mice, indicating that there was induction of both Th1 and Th2 responses (a mixed Th1/Th2 response). Importantly, upon intramuscular inoculation, the baculovirus-based PbCSP vaccine conferred complete protection against sporozoite challenge. Thus, the baculovirus-based PbCSP vaccine induced strong protective immune responses against preerythrocytic parasites. These results introduce a novel concept for the baculovirus dual expression system that functions as both a subunit vaccine and a DNA vaccine and offer a promising new alternative to current human vaccine delivery platforms.
Malaria, which is transmitted by anopheline mosquitoes, is an enormous public health problem worldwide and every year kills 1 to 2 million people, mostly children residing in Africa. Moreover, global warming and deterioration of public hygiene caused by natural disasters (e.g., cyclones, earthquakes, and tsunami) are major concerns for increased risk of malaria outbreaks in malaria-free areas where residents have no natural immunity against the parasite. The few tools presently available for control of malaria are largely limited to insecticide-treated bed nets and treatment of clinical episodes with antimalarial drugs. Clearly, an effective vaccine for the control of malaria is urgently needed. Many malaria vaccine candidate antigens, adjuvants, and virus-vectored systems have been developed or have been in development over the past 20 years, and only RTS,S antigen formulated with either the AS02A or AS01E adjuvant system consistently confers partial protective immunity against infection by Plasmodium falciparum in populations in areas where malaria is endemic (4, 8). Further improvement of vaccine efficacy may be achieved by developing novel adjuvants and/or vector systems.
The baculovirus Autographa californica nucleopolyhedrosis virus (AcNPV) is an enveloped, double-stranded DNA virus that naturally infects insects. A typical AcNPV particle consists of a rod-shaped nucleocapsid that is 40 to 50 nm in diameter and 200 to 400 nm in long. AcNPV has long been used as a biopesticide and as a tool for efficient production of recombinant complex animal, human, and viral proteins that require folding, subunit assembly, and extensive posttranslational modification in insect cells (33, 34). From a biological safety perspective, AcNPV has emerged as a new vaccine vector with several attractive attributes, including (i) low cytotoxicity, (ii) an inability to replicate in mammalian cells, and (iii) an absence of preexisting antibodies. In recent years, AcNPV has been engineered for application in the following two expression systems.
One system is a baculovirus display system for expression of complex eukaryotic proteins on the surface of the viral envelope in a manner that is analogous to the manner of expression in the bacterial phage display system. This baculovirus display system (known as “baculophage”) involves fusion of the target protein to the N terminus of gp64, the major envelope protein of AcNPV, and provides a tool for analysis of protein-protein interactions and cell-specific targeting in gene transfer (17). Using this baculophage system, vaccine candidate antigens, such as human immunodeficiency virus gp41 (20), rubella virus spike protein (36), and porcine circovirus type 2 capsid protein (19), have been expressed successfully with near-native forms, and some of them induced high titers of antigen-specific antibodies. In terms of vaccine efficacy in vivo, we have shown that the first generation of the AcNPV-based vaccine, which displays Plasmodium berghei circumsporozoite protein (CSP) on the viral envelope, protected 60% of the mice tested against malaria infection, indicating that the baculophage system is a powerful vaccine delivery system (59).
The other expression system is a gene delivery system for mammalian cells. In 1995, Hofmann et al. (25) showed for the first time that recombinant AcNPV that contains the cytomegalovirus (CMV) promoter is able to transduce human hepatocytes and drive expression of a reporter gene under the control of this promoter. For the past decade, AcNPVs that harbor appropriate eukaryotic promoters (known as “BacMam” virus) have been studied widely for DNA transfection in several types of mammalian cells in vitro (3, 7, 10, 16, 25, 39, 44, 50, 54). In contrast to these studies of gene delivery into cultured mammalian cells, a few studies have shown the effectiveness of BacMam as a vaccine vector in vivo. Abe et al. have reported that, although BacMam that expresses the influenza virus hemagglutinin gene confers protection against influenza in mice, similar protection is also provided by wild-type AcNPV (2). In the case of influenza virus, activation of innate immunity by AcNPV may be sufficient to protect against virus infection. In fact, AcNPV itself has strong adjuvant properties, including promotion of humoral and cellular immune responses against coadministered antigens, dendritic cell (DC) maturation, and production of inflammatory cytokines (23).
To improve the partial malaria vaccine efficacy obtained in our previous study using the baculophage system (59), we have developed the “baculovirus dual expression system,” which combines the immunological advances of the baculophage and BacMam systems. Here, we describe the development of a second-generation AcNPV vaccine based on the baculovirus dual expression system, which is capable of displaying P. berghei CSP (PbCSP) on the viral envelope and expressing it upon transduction of mammalian cells. We showed that the AcNPV-based PbCSP vaccine elicited PbCSP-specific humoral and cellular immune responses and consistently conferred complete protection against sporozoite challenge in mice. These results demonstrate that the baculovirus dual expression system provides an avenue for developing a novel vaccine delivery platform not only for malaria but also for other infectious diseases.
MATERIALS AND METHODS
Cell lines.
Sf9 cells were maintained at 27°C in SF900-II medium (Invitrogen, San Diego, CA) supplemented with antibiotics. COS7 and HepG2 cells were maintained at 37°C in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 2 mM l-glutamine, and antibiotics (complete medium).
Recombinant protein.
For expression and purification of recombinant PbCSP (rPbCSP), a gene that encoded amino acids 21 to 305 of PbCSP, which lacked the N-terminal signal peptide and C-terminal glycosylphosphatidylinositol signal sequence, was excised from pcDNA-CS87 (58) by digestion with BamHI and NotI, inserted into the BamHI/NotI sites of pET32-b(+) (Novagen, Madison, WI), and expressed as a thioredoxin fusion protein. rPbCSP was produced and solubilized under denaturing conditions using 6 M guanidine-HCl and then purified by affinity chromatography on a Ni-nitrilotriacetic acid column (Qiagen, Hiden, Germany), followed by dialysis against phosphate-buffered saline (PBS) as described previously (60). The purity of the protein was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the protein yield was estimated by comparison with bovine serum albumin standards. The highly purified rPbCSP was used as an enzyme-linked immunosorbent assay (ELISA) antigen.
Recombinant baculoviruses.
PCR was performed with pBACsurf-1 (Novagen) as the template by using primers pPolh-F2 (5′-CACCCGGACCGGATAATTAAAATGATAACCATCTCGCAAATAAATAAG-3′, with a newly created RsrII site indicated by underlining) and pgp64-R2 (5′-GGTACCATATTGTCTATTACGGTTTCTAATCATAC-3′, with a newly created KpnI site indicated by underlining). A 1.7-kb fragment of the gp64 gene was inserted in pENTR/D-TOPO (Invitrogen) to construct the plasmid pENTR-gp64. A 2.4-kb fragment of the PbCSP-gp64 fusion gene was excised from pBACsurf-CSP (59) by digestion with PstI and KpnI and inserted into the PstI/KpnI sites of pENTR-gp64 to construct the plasmid pENTR-PbCSP-gp64. A 2.6 kbp fragment of the polyhedrin promoter-PbCSP-gp64 fusion gene cassette was excised from pENTR-PbCSP-gp64 by digestion with RsrII and KpnI and inserted into the RsrII/KpnI sites of pTriEX-3 (Novagen) to construct the transfer plasmid pTriEx-PbCSP-gp64. To construct a baculovirus transfer vector, pBAC-CMV-PbCSP, a 1.8-kb fragment of the CMV promoter-PbCSP gene cassette was excised from pcDNA-CS87 (58) by digestion with BglII and XhoI and inserted into the BglII/XhoI sites of pBACgus-1 (Novagen). The recombinant baculoviruses AcNPV-Dual-PbCSP and AcNPV-CMV-PbCSP were generated in Sf9 cells by cotransfection of the recombinant transfer plasmids pTriEX-PbCSP-gp64 and pBAC-CMV-PbCSP, respectively, with BacVector-2000 DNA (Novagen) used according to the manufacturer's protocol. The recombinant baculovirus AcNPV-PbCSPsurf has been described previously as AcNPV-CSPsurf (59). Purification of baculovirus particles was performed as described previously (59). The purified baculovirus particles were free of endotoxin (<0.01 endotoxin unit/109 PFU), as determined by use of an Endospecy endotoxin measurement kit (Seikagaku Co., Tokyo, Japan).
Immunoblotting.
The protein samples were separated on a 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to an Immobilon transfer membrane (Millipore, Bedford, MA), and probed with anti-PbCSP monoclonal antibody (MAb) 3D11 specific for the repeats of PbCSP (MR4, Manassas, VA) or anti-gp64 MAb (BD Biosciences, Bedford, MA). Bound antibodies were subsequently detected as described previously (59).
Immunoelectron microscopy.
Purified virus samples were absorbed onto carbon-coated copper grids and incubated with anti-PbCSP MAb 3D11 for 1 h. The grids were incubated with goat anti-mouse immunoglobulin G (IgG) labeled with 5-nm gold particles (British BioCell International, Cardiff, United Kingdom). Unbound gold conjugates were removed by five sequential 2-min washes with PBS. The virus-immunogold complexes were then negatively stained by incubation of the grids for 4 min in 2% phosphotungstic acid (pH 7.2). Excess stain was removed, and the grids were air dried. Images of viruses were obtained with a JEM 1200EX transmission electron microscope (JEOL, Tokyo, Japan).
Indirect fluorescence assay (IFA).
COS7 cells were seeded at a density of 5 × 104 cells/well in a collagen type I-coated eight-well chamber slide (BD Biosciences) and transduced with purified baculovirus particles at a multiplicity of infection of 10. After 48 h of incubation, cells were fixed for 15 min in cold acetone-methanol (6:4) and incubated with anti-PbCSP MAb 3D11 and then with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Biosource International, Camarillo, CA). For nuclear staining, the slides were covered with a drop of Vectashield with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA). Bound antibodies were detected by fluorescence microscopy.
Animals and immunization.
Female BALB/c mice were obtained from Nippon Clea (Saitama, Japan) and used at 7 to 8 weeks of age in all experiments. Mice were immunized intramuscularly three times at 3-week intervals with 1 × 108 PFU of AcNPV-Dual-PbCSP, AcNPV-PbCSPsurf, or wild-type AcNPV (AcNPV-WT). All methods used for care and handling of the animals were in accordance with the guidelines for animal care and use prepared by Jichi Medical University and Otsuka Pharmaceutical.
Antibody assay.
Sera from immunized mice were collected by tail bleeding 2 weeks after the final immunization, prior to sporozoite challenge. PbCSP-specific antibodies present in prechallenge sera were quantified by ELISA. Precoated ELISA plates with 100 ng/well rPbCSP were incubated with serial dilutions of sera from immunized and control mice. Specific IgG was detected by using horseradish peroxidase-conjugated goat anti-mouse IgG(H+L) (Bio-Rad, Hercules, CA). For determination of the IgG subclasses of anti-PbCSP antibodies, horseradish peroxidase-conjugated rabbit anti-mouse IgG1, IgG2a, IgG2b, and IgG3 antibodies (Zymed Laboratories, San Francisco, CA) were used. The plates were developed with a peroxidase substrate solution [H2O2 and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)]. The optical density at 414 nm of each well was determined using a plate reader. Endpoint titers were expressed as the reciprocal of the highest sample dilution for which the optical density was equal to or greater than the mean optical density of nonimmune control sera.
For the P. berghei sporozoite-specific antibody assay, the serum samples were tested by the IFA using air-dried, methanol-fixed sporozoites as described previously (14).
Sporozoite neutralization assay.
HepG2 cells were seeded at a density of 5 × 104 cells/well in a collagen type I-coated eight-well chamber slide (BD Biosciences) 48 h prior to the addition of sporozoites. The sporozoite neutralization assay was performed using previously described methods (31). Sporozoites (5 × 103 cells) isolated from the salivary glands of mosquitoes were incubated with mouse immune sera and then added to HepG2 cultures. After 72 h of incubation, total RNA was extracted using a QIAamp RNA blood mini kit (Qiagen, Hiden, Germany), and quantification of P. berghei 18S rRNA was performed by real-time reverse transcription-PCR as described previously (11).
ELISPOT assay.
Splenocytes were obtained from mice immunized intramuscularly with AcNPV-based vaccines 2 weeks after the third immunization. PbCSP-specific gamma interferon (IFN-γ)-producing cells in splenocytes were determined by an enzyme-linked immunospot (ELISPOT) assay using the H2-Kd restricted peptide Pb9 (SYIPSAEKI) of PbCSP for in vitro stimulation according to previously described methods (12). Spot-forming cells were enumerated using the KS ELISPOT system (Carl Zeiss, Hallbergmoos, Germany).
P. berghei challenge.
Anopheles stephensi mosquitoes (SDA 500 strain) were infected with P. berghei ANKA by allowing them to feed on P. berghei-infected mice. The mosquitoes used for challenge had salivary gland sporozoite infection rates of 60 to 90% at 18 to 20 days after the infectious blood meal. Two weeks after the third immunization, mice were challenged by using feeding sporozoite-infected A. stephensi mosquitoes or by intravenous inoculation of viable sporozoites into the tail vein.
For the mosquito bite challenge, mosquitoes were allowed to bite each mouse until blood was observed in the gut of five mosquitoes. After feeding, the mosquito salivary glands were dissected, and the presence of sporozoites was confirmed. For the sporozoite inoculation challenge, sporozoites were obtained by dissection of infected mosquito salivary glands and injected through the tail vein at a level of 50 to 1,000 parasites/mouse. For each challenge route, mice were checked for P. berghei blood-stage infection by microscopic examination of Giemsa-stained thin smears of tail blood prepared at least once a week after challenge. A minimum of 50 fields (magnification, ×400) were examined before a mouse was determined to be negative for infection. Protection was defined as the complete absence of blood-stage parasitemia for more than 3 weeks after challenge.
Statistical analysis.
The Mann-Whitney U test was used to compare antibody levels for two groups, Tukey's multiple comparison test was used to compare numbers for different groups in the sporozoite-neutralizing assay and ELISPOT assay, and the two-tailed Fisher's extract probability test was used to compare the numbers of surviving mice in different groups. Statistical analyses were performed using SAS Japan, release 8.1. A P value of <0.05 was considered statistically significant in these analyses.
RESULTS
Construction of baculovirus dual expression system.
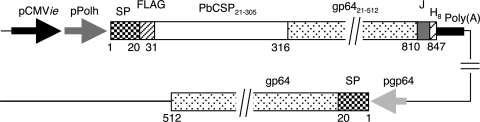
Construction of AcNPV-Dual-PbCSP is shown in Fig. 1. AcNPV-Dual-PbCSP harbored a gene cassette that consisted of the gp64 signal sequence, the PbCSP gene fused to the N terminus of the AcNPV major envelope protein gp64, and the rabbit β-globulin poly(A) signal. Expression of the gene cassette was driven by the dual promoter that consisted of the CMV immediate early enhancer-promoter and the polyhedrin promoter. AcNPV-Dual-PbCSP also had the endogenous gp64 gene in its genome. Thus, a PbCSP-gp64 fusion protein was designed to express both proteins on the viral envelope and in mammalian cells. Construction of AcNPV-PbCSPsurf has been described previously (59).
FIG. 1.
Schematic diagram of AcNPV-Dual-PbCSP genome structure. A gene cassette that consisted of the gp64 signal sequence (SP), the PbCSP gene (PbCSP21-305) fused to the N terminus of the AcNPV major envelope protein gp64 gene (gp6421-512), and the rabbit β-globlin poly(A) signal [Poly(A)] was used. Expression of the gene cassette was driven by the dual promoter, which consisted of the CMV immediate early enhancer-promoter (pCMVie) and the polyhedrin promoter (pPolh). AcNPV-Dual-PbCSP also had the endogenous gp64 gene in its genome. The numbers indicate the amino acid positions of the PbCSP-gp64 fusion protein and endogenous gp64. FLAG, FLAG epitope tag; PbCSP21-305, PbCSP region corresponding to amino acids 21 to 305; gp6421-512, gp64 region corresponding to amino acids 21 to 512; J, junction consisting of 29 unrelated amino acid residues; H8, His tag; pgp64, gp64 promoter.
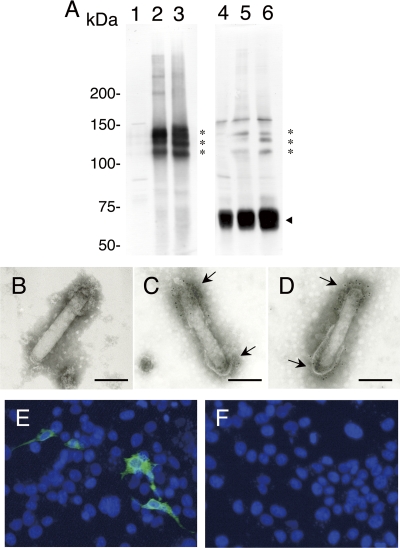
Budded baculovirus particles were purified from culture supernatants and analyzed by Western blotting using anti-PbCSP MAb 3D11, which was specific for the repeats of PbCSP, or anti-gp64 MAb. Anti-PbCSP MAb recognized PbCSP expressed by AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP as a triplet with relative molecular masses of 120, 130, and 140 kDa (Fig. 2, lanes 2 and 3), which corresponded to the predicted relative molecular mass (125 kDa) of the PbCSP-gp64 fusion protein. The triplet bands may have resulted from posttranslational modification or degradation in the insect cells. No specific band reactive with anti-PbCSP MAb was seen in AcNPV-WT (Fig. 2, lane 1). Anti-gp64 MAb recognized both the PbCSP-gp64 fusion protein and endogenous gp64 in AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP particles (Fig. 2, lanes 5 and 6), whereas only endogenous gp64 was detected in AcNPV-WT particles (Fig. 2, lane 4). Thus, PbCSP was incorporated into viral particles of AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP as a PbCSP-gp64 fusion protein.
FIG. 2.
Expression of PbCSP-gp64 fusion protein. (A) Western blot analysis of recombinant AcNPV particles. AcNPV-WT (lanes 1 and 4), AcNPV-PbCSPsurf (lanes 2 and 5), and AcNPV-Dual-PbCSP (lanes 3 and 6) particles were treated with loading buffer containing 1% 2-mercaptoethanol and boiled for 5 min. Expression of the PbCSP-gp64 fusion protein was examined using anti-PbCSP MAb (lanes 1 to 3) and anti-gp64 MAb (lanes 4 and 5). The asterisks and the arrowhead indicate the positions of the PbCSP-gp64 fusion protein and endogenous gp64, respectively. (B to D) Electron micrographs of recombinant AcNPVs displaying PbCSP on the viral envelope. AcNPV-WT (B), AcNPV-PbCSPsurf (C), and AcNPV-Dual-PbCSP (D) were treated with anti-PbCSP MAb, followed by labeling with anti-mouse IgG-gold conjugate. Both ends of the viral envelope were strongly labeled with gold particles (arrows). Bars, 100 nm. (E and F) In vitro expression analysis of PbCSP by transducing recombinant AcNPV particles in mammalian cells. COS7 cells were transduced with AcNPV-Dual-PbCSP (E) and AcNPV-PbCSPsurf (F) at a multiplicity of infection of 10. Forty-eight hours later, cells were fixed with 5% paraformaldehyde, which was followed by permeabilization with 0.1% Triton X-100 in PBS and incubation with anti-PbCSP MAb. Bound antibodies were detected with fluorescein isothiocyanate-labeled anti-mouse IgG by fluorescence microscopy (green). Cell nuclei were visualized by DAPI staining (blue). Original magnification, ×400.
To verify that PbCSP was displayed on the viral envelope, purified viral particles were analyzed by immunoelectron microscopy. As shown in Fig. 2B to D, specific immunogold staining of PbCSP using anti-PbCSP MAb and anti-mouse IgG-gold conjugate was detected on both ends of the viral envelopes of AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP (Fig. 2C and D), whereas no gold staining was seen in AcNPV-WT (Fig. 2B).
Next, we examined the ability of AcNPV-Dual-PbCSP to drive PbCSP expression in mammalian cells by IFA. COS7 cells were exposed to purified particles of AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP. Strong immunofluorescence signals were detected in cells exposed to AcNPV-Dual-PbCSP 48 h after incubation (Fig. 2E), whereas no signal was seen in cells exposed to AcNPV-PbCSPsurf (Fig. 2F). Thus, AcNPV-Dual-PbCSP not only displayed PbCSP on the viral envelope but also expressed it in mammalian cells.
Antibody responses in mice immunized with AcNPV-based vaccines.
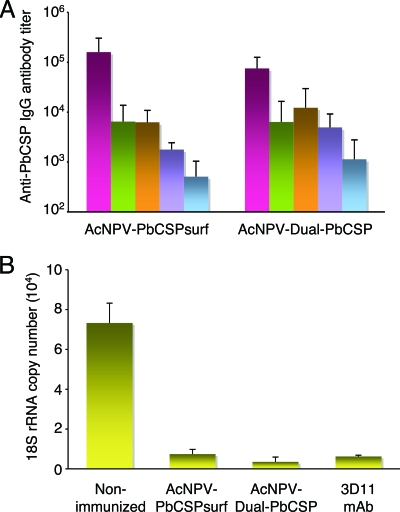
The quantities and isotype profiles of PbCSP-specific antibodies induced by AcNPV-based vaccines were determined by ELISA. Immunization with both AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP induced high levels of PbCSP-specific total IgG (Fig. 3A). Analysis of PbCSP-specific IgG subclasses revealed that AcNPV-PbCSPsurf and AcNPV-PbCSPsurf immune sera contained predominantly IgG1 and IgG2a (IgG1/IgG2a ratios, ∼1.03 and ∼0.52, respectively), indicating that there was activation of both Th1- and Th2-type cells. There was no statistically significant difference in the total IgG titers or the titers of any subclass of IgG between the two groups. We confirmed by IFA that these antibodies strongly bound to P. berghei sporozoites (see Fig. S5 in the supplemental material).
FIG. 3.
Anti-PbCSP antibody response and antibody-mediated sporozoite-neutralizing activity. Cohorts of mice were immunized with AcNPV-Dual-PbCSP or AcNPV-PbCSPsurf. Sera were collected from individual mice 3 weeks after the final immunization. (A) Antibody isotype responses to PbCSP. Individual sera from mice immunized with AcNPV-Dual-PbCSP (n = 13) or AcNPV-PbCSPsurf (n = 33) were tested using ELISA for total IgG, IgG1, IgG2a, IgG2b, and IgG3 antibodies specific for PbCSP. The bars and error bars indicate means and standard deviations. There was no statistically significant difference between the two groups. (B) Sporozoite neutralization assay. Viable P. berghei sporozoites were incubated with sera from mice immunized with AcNPV-Dual-PbCSP or AcNPV-PbCSPsurf. Sporozoites (5 × 103 cells) incubated with sera were added to cultured HepG2 cells, and the levels of P. berghei 18S rRNA were determined by reverse transcription-PCR after 72 h of incubation. The bars and error bars indicate means and standard deviations for 5 of 10 immune serum samples that had a positive ELISA titer for PbCSP (>1:10,000). The controls included P. berghei sporozoites incubated with 25 μg/ml anti-PbCSP MAb 3D11 or nonimmune serum. Compared with the results for the nonimmune serum, all three samples significantly reduced the number of the 18S rRNA copies (P < 0.01).
To determine the role of antibodies, sera from immunized mice were tested for biological activities using an in vitro sporozoite-neutralizing assay. The sera from mice immunized with AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP significantly blocked sporozoite invasion of HepG2 cells (P < 0.01) (Fig. 3B). The mean parasite rRNA levels in the sera from mice immunized with AcNPV-PbCSPsurf and AcNPV-Dual-PbCSP were markedly reduced to 10.2% (7,454 ± 2,331 copies) and 4.8% (3,529 ± 2,411 copies) of the mean parasite rRNA levels in the nonimmune sera (73,245 ± 10,001 copies), respectively. These levels of inhibition were equivalent to that observed when sporozoites were incubated with protective MAb 3D11 specific for PbCSP repeats (8.5%: 6,213 ± 656 copies).
Cellular immune responses in mice immunized with AcNPV-based vaccines.
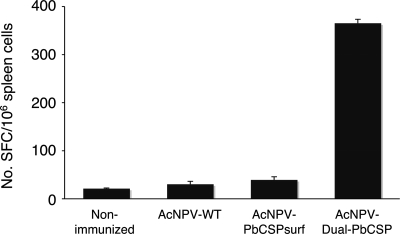
To examine whether the baculovirus dual expression system induced antigen-specific T-cell responses, we performed an ELISPOT assay with splenocytes from immunized mice to visualize IFN-γ secretion. Compared with the results for immunization with AcNPV-PbCSPsurf, immunization with AcNPV-Dual-PbCSP induced significantly higher (P < 0.05) levels of PbCSP-specific IFN-γ-secreting splenocytes in response to stimulation with the Pb9 synthetic peptide, the H2-Kd CD8+ T-cell epitope of PbCSP (Fig. 4). In some experiments, we observed relatively high levels of nonspecific IFN-γ secretion in splenocytes from mice immunized with AcNPV-WT (data not shown), which is consistent with the findings of a previous study (19).
FIG. 4.
ELISPOT responses in mice immunized with AcNPV-based vaccines. The numbers of IFN-γ-secreting cells per 106 splenocytes that reacted with the Pb9 peptide were counted using an ELISPOT assay. The bars and error bars indicate means and standard deviations for the numbers of spot-forming cells (SFC) from triplicate cultures for each group. The group immunized with AcNPV-Dual-PbCSP had significantly higher (P < 0.05) ELISPOT counts than the group immunized with AcNPV-PbCSPsurf.
Protection against natural infection by feeding P. berghei-infected mosquitoes.
To address whether the enhanced CD8+ T-cell responses in mice immunized with AcNPV-Dual-PbCSP improved protective efficacy, we first compared the abilities of AcNPV-Dual-PbCSP and AcNPV-PbCSPsurf to protect mice against challenge with P. berghei. Mice immunized three times were challenged by using feeding P. berghei-infected mosquitoes, and the blood-stage parasites were monitored for a 3-week period. Cumulative data from independent experiments are shown in Table 1. Significant protection was observed in mice immunized with AcNPV-PbCSPsurf (P < 0.01). Notably, all 33 mice immunized with AcNPV-Dual-PbCSP showed complete protection. Not all mosquitoes with infectious sporozoites in their salivary glands successfully initiated malaria infections when they fed on control groups of mice (26% uninfected). Similar infection rates for nonimmunized mice after they were bitten by mosquitoes have been reported by other workers (35, 41).
TABLE 1.
Protection of mice immunized with AcNPV-based vaccines against challenge by feeding P. berghei-infected mosquitoes
| Vaccine | Delayed parasitemia: no. of uninfected mice/total no. (%)a
|
Complete protection: no. of protected mice/total no. (%)b | |
|---|---|---|---|
| 6 days | 10 days | ||
| None | 26/53 (49) | 14/53 (26) | 14/53 (26)c |
| AcNPV-WT | 6/20 (30) | 5/20 (25) | 5/20 (25) |
| AcNPV-CMV-PbCSP | 5/10 (50) | 5/10 (50) | 5/10 (50) |
| AcNPV-PbCSPsurf | 18/20 (90) | 16/20 (80) | 16/20 (80)c,d |
| AcNPV-Dual-PbCSP | 33/33 (100) | 33/33 (100) | 33/33 (100)c,d |
Mice were checked for P. berghei blood-stage infection by microscopic examination of Giemsa-stained thin smears of tail blood prepared at least once a week after challenge. Once parasites appeared in the blood, all mice died.
Complete protection was defined as the complete absence of blood-stage parasitemia for over 3 weeks after challenge. All infected mice died within 3 weeks after challenge.
Cumulative data from two to four independent experiments.
Statistically significantly (P < 0.01) different compared to the nonimmunized group in individual experiments, as calculated by Fisher's exact probability test.
Protection against challenge by intravenous inoculation of P. berghei sporozoites.
For experimental infection, intravenous inoculation of sporozoites dissected from the salivary glands of mosquitoes has been used extensively to evaluate CSP-based vaccines against liver-stage parasites. Immunized mice were challenged by intravenous inoculation of 50, 200, or 1,000 viable sporozoites per mouse. The infection rates in immunized mice were compared with those in naïve control mice (Table 2). When immunized mice were challenged with 200 sporozoites, 70% of the mice did not develop parasitemia within 6 days after challenge (delayed onset of parasitemia), and 60% were completely protected (P < 0.05). Even in the group challenged with 1,000 sporozoites, 40% of the immunized mice showed delayed onset of parasitemia, and 10% were completely protected. Although a higher number of sporozoites resulted in a reduction in the protective efficacy of AcNPV-Dual-PbCSP, the delayed onset of parasitemia at days 6 and 10 after challenge indicated that there was a reduction in the liver-stage parasite burden (>80% reduction for 1-day delayed onset compared with controls [45]). All groups of mice immunized with AcNPV-Dual-PbCSP had consistently higher protection or delayed onset of parasitemia compared with nonimmunized control groups. In the P. berghei-BALB/c model, 1,000 sporozoites delivered by intravenous inoculation were required to establish infection in 100% of the nonimmunized mice, which corresponds to the number described previously (26).
TABLE 2.
Protection of mice immunized with AcNPV-Dual-PbCSP against challenge by intravenous injection of P. berghei sporozoites
| Vaccine | No. of sporozoites injected | Delayed parasitemia: no. of uninfected mice/total no. (%)a
|
Complete protection: no. of protected mice/total no. (%)b | |
|---|---|---|---|---|
| 6 days | 10 days | |||
| None | 50 | 4/15 (27) | 3/15 (20) | 3/15 (20)c |
| AcNPV-Dual-PbCSP | 50 | 14/15 (93) | 10/15 (67) | 10/15 (67)c,d |
| None | 200 | 5/20 (25) | 3/20 (15) | 3/20 (15)c |
| AcNPV-Dual-PbCSP | 200 | 14/20 (70) | 12/20 (60) | 12/20 (60)c,d |
| None | 1,000 | 0/10 (0) | 0/10 (0) | 0/10 (0) |
| AcNPV-Dual-PbCSP | 1,000 | 4/10 (40) | 1/10 (10) | 1/10 (10) |
Mice were checked for P. berghei blood-stage infection by microscopic examination of Giemsa-stained thin smears of tail blood prepared at least once a week after challenge. Once parasites appeared in the blood, all mice died.
Complete protection was defined as the complete absence of blood-stage parasitemia for over 3 weeks after challenge. All infected mice died within 3 weeks after challenge.
Cumulative data from two independent experiments.
Statistically significantly (P < 0.05) different compared to the corresponding nonimmunized group in individual experiments, as calculated by Fisher's exact probability test.
DISCUSSION
We have developed the baculovirus dual expression system, which is designed to facilitate the development of multifunctional vaccines capable of inducing strong humoral and cellular immune responses. In the present study, we applied this system to generation of a novel malaria vaccine (AcNPV-Dual-PbCSP), which possesses a single gene cassette that consists of the PbCSP-gp64 fusion gene under control of the CMV-polyhedrin dual promoter. AcNPV-Dual-PbCSP not only displayed PbCSP on the viral envelope but also expressed it upon transduction of mammalian cells. Immunization with AcNPV-Dual-PbCSP induced high anti-PbCSP antibody titers and PbCSP-specific IFN-γ-secreting CD8+ T cells. Recently, Strauss et al. (53) have developed a P. falciparum CSP-based baculovirus vaccine that possesses two gene cassettes consisting of the PfCSP gene under control of the CMV promoter and the PfCSP-gp64 fusion gene under control of the polyhedrin promoter. Similar to our system, their baculovirus-based vaccine can induce CSP-specific antibody and T-cell responses in mice, suggesting its potential for human malaria vaccine development. Currently available vaccine adjuvants are generally effective at eliciting antibody responses, but few nonreplicating vaccine adjuvants are able to generate strong CD8+ T-cell responses against protein antigens (38). Thus, the baculovirus dual expression system is a novel adjuvant-free and nonreplicating vaccine vector system that is capable of inducing strong humoral and cellular immune responses.
AcNPV-Dual-PbCSP formed “sporozoite-like” particles with an oligomeric PbCSP-gp64 complex (data not shown) (59), and AcNPV itself also possesses strong adjuvant properties for activating DC-mediated innate immunity through MyD88/Toll-like receptor 9-dependent and -independent pathways (1). Thus, the baculovirus dual expression system constitutes an innate immunostimulating complex and functions as a subunit and DNA vaccine for generating strong humoral and cellular immune responses, including CD8+ T-cell responses. The immune mechanism that underlies the protection conferred by the baculovirus dual expression system may result mainly from the humoral and cellular immunity induced by combination of the baculophage- and BacMam-type vaccines. However, further studies are needed to elucidate the exact mechanism, including the involvement of innate immunity.
We evaluated the vaccine efficacy of AcNPV-Dual-PbCSP by using two challenge routes. One of these routes was bites of P. berghei-infected mosquitoes. This route was chosen because it closely mimics the natural route of malaria transmission (27, 56). During blood feeding, mosquitoes probe the skin to search for blood vessels and inject sporozoites along with saliva into the host skin. Most sporozoites injected are deposited in the skin for about 0.5 h, until their motility enables them to invade the dermal blood vessels (5, 55). At this “skin stage” (51, 52), anti-CSP antibodies inhibit sporozoite motility and attachment to target cells and prevent the sporozoites from entering the circulation. Therefore, this challenge route is suitable for evaluating the efficacy of sporozoite-neutralizing antibodies induced by CSP-based vaccines before liver invasion. After mosquito bite challenge, AcNPV-Dual-PbCSP provided a level of protection of 100%, compared with the 80% protection observed with AcNPV-PbCSPsurf. Both immunization with AcNPV-PbCSPsurf and immunization with AcNPV-Dual-PbCSP induced high anti-PbCSP antibody titers, and both immune sera strongly inhibited ∼90% of sporozoite invasion into HepG2 cells in vitro. This suggests that CSP-specific antibodies make a major contribution to protection against natural infection. Clinical trials have reported that the protection mediated by RTS,S/AS02A is linked to antibodies to the CSP repeat region in malaria-naïve (28-30) and malaria-experienced adults (9) and infants (6), suggesting that the anti-CSP antibody titer is an important criterion for CSP-based vaccines.
The other challenge route was intravenous inoculation of viable sporozoites. This route has been used extensively to evaluate CSP-based vaccines against preerythrocytic parasites, because sporozoites can reach the liver within a few minutes when mosquitoes take blood directly from the circulation (49). Although after intravenous challenge with 1,000 sporozoites only 10% protection was obtained with AcNPV-Dual-PbCSP, delayed onset of parasitemia was observed in 40% of the immunized mice. Studies with rodent malaria models have provided definitive evidence that, at the preerythrocytic stage, CD8+ T cells play an important role in killing the parasites in the liver (21, 22, 47, 48). It has been reported that sterile protection against intravenous challenge with 1,000 sporozoites requires induction of 0.1 to 0.2% of the CSP-specific CD8+ spleen T cells (46, 47). Presumably, AcNPV-Dual-PbCSP did not induce this number of PbCSP-specific CD8+ spleen T cells. Consequently, AcNPV-Dual-PbCSP could reduce the number of liver-stage parasites and affect the appearance of the parasite in the blood, but it could not eliminate all infected hepatocytes. The mechanism by which such a response mediates protection is, however, poorly understood. Since passive transfer of MAb against sporozoites can also protect against intravenous sporozoite challenge (40), further studies using knockout mice are required to define the precise protective mechanism of AcNPV-Dual-PbCSP.
Most studies of the development of CSP-based vaccines in animal models have evaluated vaccine efficacy by using intravenous sporozoite challenge (24, 42, 43, 47). In contrast, human clinical trials invariably utilize mosquito bite challenge (15, 18, 37, 57). An ideal CSP-based vaccine should elicit strong CSP-specific humoral and cellular immune responses and confer sterile protection with both challenge routes. Therefore, rational optimization of the baculovirus dual expression system (e.g., combination and route of administration and increase in protein expression levels) is needed to improve vaccine efficacy. Alternatively, the system may serve as a good priming agent in heterologous prime-boost immunization regimens using other vaccine candidates that can elicit CD8+ T-cell responses against CSP, because it can induce both CSP-specific Th1 responses and CSP-specific Th2 responses.
More recently, it has been found that CD8+ T cells that protect against liver-stage parasites are primed in the skin lymph nodes by DC that cross-present CSP of skin-stage parasites, and this CD8+ T-cell activation appears to be dependent on Toll-like receptor 9 signaling (13). The results of this previous study suggest an additional strategy for developing more effective vaccines based on the baculovirus dual expression system. For example, intradermal inoculation of AcNPV-Dual-PbCSP may induce CD8+ T cells active against sporozoites more effectively than intramuscular inoculation does. Other applications of the vaccine platform include mucosal immunization, for which we have found that the baculovirus dual expression system is effective against the erythrocytic stage of the parasite (S. Yoshida, unpublished data).
For clinical application, the baculovirus dual expression system has great potential to improve the safety and manufacture of current vaccines, compared with other virus-vectored malaria vaccine candidates (e.g., vaccinia virus, adenovirus, yellow fever virus, and vesicular stomatitis virus) described in a workshop organized by the PATH Malaria Vaccine Initiative (32). For example, AcNPV virions that are incapable of DNA replication in mammalian cells may provide the highest level of safety. A lot of biological knowledge about baculoviruses has been accumulated from the long-term experience of the silk industry. A fermentation method used in the manufacturing process has already been developed, as baculoviruses are now being utilized for production of clinically useful proteins. The simplicity of production of AcNPV may translate into economical large-scale manufacture of AcNPV-based vaccines. We are now constructing a baculovirus dual expression system for expressing P. falciparum CSP. After evaluating the efficacy and safety of this system, we intend to advance it to the clinical stage.
Supplementary Material
Acknowledgments
We thank Y. Shimada, H. Watanabe, C. Seki, and H. Araki for excellent assistance with the ELISA and with handling of the mosquitoes and mice. We also thank Y. Goto for help with the sporozoite neutralization assay.
This work was supported by grants from the Ministry of Education, Culture, Sports and Science of Japan (grant 16590345) and Otsuka Pharmaceutical Co. Ltd.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 17 February 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abe, T., H. Hemmi, H. Miyamoto, K. Moriishi, S. Tamura, H. Takaku, S. Akira, and Y. Matsuura. 2005. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 792847-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 1711133-1139. [DOI] [PubMed] [Google Scholar]
- 3.Airenne, K. J., M. O. Hiltunen, M. P. Turunen, A. M. Turunen, O. H. Laitinen, M. S. Kulomaa, and S. Yla-Herttuala. 2000. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 71499-1504. [DOI] [PubMed] [Google Scholar]
- 4.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 3641411-1420. [DOI] [PubMed] [Google Scholar]
- 5.Amino, R., S. Thiberge, B. Martin, S. Celli, S. Shorte, F. Frischknecht, and R. Menard. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12220-224. [DOI] [PubMed] [Google Scholar]
- 6.Aponte, J. J., P. Aide, M. Renom, I. Mandomando, Q. Bassat, J. Sacarlal, M. N. Manaca, S. Lafuente, A. Barbosa, A. Leach, M. Lievens, J. Vekemans, B. Sigauque, M. C. Dubois, M. A. Demoitie, M. Sillman, B. Savarese, J. G. McNeil, E. Macete, W. R. Ballou, J. Cohen, and P. L. Alonso. 2007. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet 3701543-1551. [DOI] [PubMed] [Google Scholar]
- 7.Barsoum, J., R. Brown, M. McKee, and F. M. Boyce. 1997. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum. Gene Ther. 82011-2018. [DOI] [PubMed] [Google Scholar]
- 8.Bejon, P., J. Lusingu, A. Olotu, A. Leach, M. Lievens, J. Vekemans, S. Mshamu, T. Lang, J. Gould, M. C. Dubois, M. A. Demoitie, J. F. Stallaert, P. Vansadia, T. Carter, P. Njuguna, K. O. Awuondo, A. Malabeja, O. Abdul, S. Gesase, N. Mturi, C. J. Drakeley, B. Savarese, T. Villafana, W. R. Ballou, J. Cohen, E. M. Riley, M. M. Lemnge, K. Marsh, and L. von Seidlein. 2008. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 3592521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 3581927-1934. [DOI] [PubMed] [Google Scholar]
- 10.Boyce, F. M., and N. L. Bucher. 1996. Baculovirus-mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA 932348-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 311499-1502. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho, L. H., J. C. Hafalla, and F. Zavala. 2001. ELISPOT assay to measure antigen-specific murine CD8+ T cell responses. J. Immunol. Methods 252207-218. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarty, S., I. A. Cockburn, S. Kuk, M. G. Overstreet, J. B. Sacci, and F. Zavala. 2007. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 131035-1041. [DOI] [PubMed] [Google Scholar]
- 14.Charoenvit, Y., M. F. Leef, L. F. Yuan, M. Sedegah, and R. L. Beaudoin. 1987. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect. Immun. 55604-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church, L. W., T. P. Le, J. P. Bryan, D. M. Gordon, R. Edelman, L. Fries, J. R. Davis, D. A. Herrington, D. F. Clyde, M. J. Shmuklarsky, I. Schneider, T. W. McGovern, J. D. Chulay, W. R. Ballou, and S. L. Hoffman. 1997. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J. Infect. Dis. 175915-920. [DOI] [PubMed] [Google Scholar]
- 16.Condreay, J. P., S. M. Witherspoon, W. C. Clay, and T. A. Kost. 1999. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA 96127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies, A. H. 1995. “Baculophage”: a new tool for protein display. Bio/Technology 131046. [DOI] [PubMed] [Google Scholar]
- 18.Dunachie, S. J., M. Walther, J. E. Epstein, S. Keating, T. Berthoud, L. Andrews, R. F. Andersen, P. Bejon, N. Goonetilleke, I. Poulton, D. P. Webster, G. Butcher, K. Watkins, R. E. Sinden, G. L. Levine, T. L. Richie, J. Schneider, D. Kaslow, S. C. Gilbert, D. J. Carucci, and A. V. Hill. 2006. A DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 745933-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan, H., Y. Pan, L. Fang, D. Wang, S. Wang, Y. Jiang, H. Chen, and S. Xiao. 2008. Construction and immunogenicity of recombinant pseudotype baculovirus expressing the capsid protein of porcine circovirus type 2 in mice. J. Virol. Methods 15021-26. [DOI] [PubMed] [Google Scholar]
- 20.Grabherr, R., W. Ernst, O. Doblhoff-Dier, M. Sara, and H. Katinger. 1997. Expression of foreign proteins on the surface of Autographa californica nuclear polyhedrosis virus. BioTechniques 22730-735. [DOI] [PubMed] [Google Scholar]
- 21.Hafalla, J. C., A. Morrot, G. Sano, G. Milon, J. J. Lafaille, and F. Zavala. 2003. Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J. Immunol. 171964-970. [DOI] [PubMed] [Google Scholar]
- 22.Hafalla, J. C., G. Sano, L. H. Carvalho, A. Morrot, and F. Zavala. 2002. Short-term antigen presentation and single clonal burst limit the magnitude of the CD8+ T cell responses to malaria liver stages. Proc. Natl. Acad. Sci. USA 9911819-11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hervas-Stubbs, S., P. Rueda, L. Lopez, and C. Leclerc. 2007. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J. Immunol. 1782361-2369. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman, S. L., M. Sedegah, and R. C. Hedstrom. 1994. Protection against malaria by immunization with a Plasmodium yoelii circumsporozoite protein nucleic acid vaccine. Vaccine 121529-1533. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann, C., V. Sandig, G. Jennings, M. Rudolph, P. Schlag, and M. Strauss. 1995. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA 9210099-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe, R. I., G. H. Lowell, and D. M. Gordon. 1990. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am. J. Trop. Med. Hyg. 42309-313. [DOI] [PubMed] [Google Scholar]
- 27.Jin, Y., C. Kebaier, and J. Vanderberg. 2007. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 755532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kester, K. E., J. F. Cummings, C. F. Ockenhouse, R. Nielsen, B. T. Hall, D. M. Gordon, R. J. Schwenk, U. Krzych, C. A. Holland, G. Richmond, M. G. Dowler, J. Williams, R. A. Wirtz, N. Tornieporth, L. Vigneron, M. Delchambre, M. A. Demoitie, W. R. Ballou, J. Cohen, and D. G. Heppner, Jr. 2008. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 262191-2202. [DOI] [PubMed] [Google Scholar]
- 29.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183640-647. [DOI] [PubMed] [Google Scholar]
- 30.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, Jr., T. Hall, B. T. Wellde, K. White, P. Sun, R. Schwenk, U. Krzych, M. Delchambre, G. Voss, M. C. Dubois, R. A. Gasser, Jr., M. G. Dowler, M. O'Brien, J. Wittes, R. Wirtz, J. Cohen, and W. R. Ballou. 2007. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine 255359-5366. [DOI] [PubMed] [Google Scholar]
- 31.Kumar, K. A., G. A. Oliveira, R. Edelman, E. Nardin, and V. Nussenzweig. 2004. Quantitative Plasmodium sporozoite neutralization assay (TSNA). J. Immunol. Methods 292157-164. [DOI] [PubMed] [Google Scholar]
- 32.Li, S., E. Locke, J. Bruder, D. Clarke, D. L. Doolan, M. J. Havenga, A. V. Hill, P. Liljestrom, T. P. Monath, H. Y. Naim, C. Ockenhouse, D. C. Tang, K. R. Van Kampen, J. F. Viret, F. Zavala, and F. Dubovsky. 2007. Viral vectors for malaria vaccine development. Vaccine 252567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luckow, V. A., and M. D. Summers. 1988. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology 16756-71. [DOI] [PubMed] [Google Scholar]
- 34.Matsuura, Y., R. D. Possee, H. A. Overton, and D. H. Bishop. 1987. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 681233-1250. [DOI] [PubMed] [Google Scholar]
- 35.Medica, D. L., and P. Sinnis. 2005. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 734363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mottershead, D., I. van der Linden, C. H. von Bonsdorff, K. Keinanen, and C. Oker-Blom. 1997. Baculoviral display of the green fluorescent protein and rubella virus envelope proteins. Biochem. Biophys. Res. Commun. 238717-722. [DOI] [PubMed] [Google Scholar]
- 37.Ockenhouse, C. F., P. F. Sun, D. E. Lanar, B. T. Wellde, B. T. Hall, K. Kester, J. A. Stoute, A. Magill, U. Krzych, L. Farley, R. A. Wirtz, J. C. Sadoff, D. C. Kaslow, S. Kumar, L. W. Church, J. M. Crutcher, B. Wizel, S. Hoffman, A. Lalvani, A. V. Hill, J. A. Tine, K. P. Guito, C. de Taisne, R. Anders, W. R. Ballou, et al. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 1771664-1673. [DOI] [PubMed] [Google Scholar]
- 38.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11S63-68. [DOI] [PubMed] [Google Scholar]
- 39.Pieroni, L., D. Maione, and N. La Monica. 2001. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum. Gene Ther. 12871-881. [DOI] [PubMed] [Google Scholar]
- 40.Potocnjak, P., N. Yoshida, R. S. Nussenzweig, and V. Nussenzweig. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 1511504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pumpuni, C. B., C. Mendis, and J. C. Beier. 1997. Plasmodium yoelii sporozoite infectivity varies as a function of sporozoite loads in Anopheles stephensi mosquitoes. J. Parasitol. 83652-655. [PubMed] [Google Scholar]
- 42.Rodrigues, E. G., F. Zavala, R. S. Nussenzweig, J. M. Wilson, and M. Tsuji. 1998. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 161812-1817. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J. R. Rodriguez, I. Bacik, J. R. Bennink, J. W. Yewdell, A. Garcia-Sastre, R. S. Nussenzweig, et al. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 1534636-4648. [PubMed] [Google Scholar]
- 44.Sandig, V., C. Hofmann, S. Steinert, G. Jennings, P. Schlag, and M. Strauss. 1996. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum. Gene Ther. 71937-1945. [DOI] [PubMed] [Google Scholar]
- 45.Sauzet, J. P., B. L. Perlaza, K. Brahimi, P. Daubersies, and P. Druilhe. 2001. DNA immunization by Plasmodium falciparum liver-stage antigen 3 induces protection against Plasmodium yoelii sporozoite challenge. Infect. Immun. 691202-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, N. W., R. L. Podyminogin, N. S. Butler, V. P. Badovinac, B. J. Tucker, K. S. Bahjat, P. Lauer, A. Reyes-Sandoval, C. L. Hutchings, A. C. Moore, S. C. Gilbert, A. V. Hill, L. C. Bartholomay, and J. T. Harty. 2008. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. USA 10514017-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4397-402. [DOI] [PubMed] [Google Scholar]
- 48.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 957648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin, S. C., J. P. Vanderberg, and J. A. Terzakis. 1982. Direct infection of hepatocytes by sporozoites of Plasmodium berghei. J. Protozool. 29448-454. [DOI] [PubMed] [Google Scholar]
- 50.Shoji, I., H. Aizaki, H. Tani, K. Ishii, T. Chiba, I. Saito, T. Miyamura, and Y. Matsuura. 1997. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J. Gen. Virol. 782657-2664. [DOI] [PubMed] [Google Scholar]
- 51.Sidjanski, S., and J. P. Vanderberg. 1997. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am. J. Trop. Med. Hyg. 57426-429. [DOI] [PubMed] [Google Scholar]
- 52.Sinnis, P., and F. Zavala. 2008. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 3275-278. [DOI] [PubMed] [Google Scholar]
- 53.Strauss, R., A. Huser, S. Ni, S. Tuve, N. Kiviat, P. S. Sow, C. Hofmann, and A. Lieber. 2007. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol. Ther. 15193-202. [DOI] [PubMed] [Google Scholar]
- 54.Tani, H., C. K. Limn, C. C. Yap, M. Onishi, M. Nozaki, Y. Nishimune, N. Okahashi, Y. Kitagawa, R. Watanabe, R. Mochizuki, K. Moriishi, and Y. Matsuura. 2003. In vitro and in vivo gene delivery by recombinant baculoviruses. J. Virol. 779799-9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanderberg, J. P., and U. Frevert. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34991-996. [DOI] [PubMed] [Google Scholar]
- 56.Vaughan, J. A., L. F. Scheller, R. A. Wirtz, and A. F. Azad. 1999. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect. Immun. 674285-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webster, D. P., S. Dunachie, J. M. Vuola, T. Berthoud, S. Keating, S. M. Laidlaw, S. J. McConkey, I. Poulton, L. Andrews, R. F. Andersen, P. Bejon, G. Butcher, R. Sinden, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA 1024836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshida, S., S. Kashiwamura, Y. Hosoya, E. Luo, H. Matsuoka, A. Ishii, A. Fujimura, and E. Kobayashi. 2000. Direct immunization of malaria DNA vaccine into the liver by gene gun protects against lethal challenge of Plasmodium berghei sporozoite. Biochem. Biophys. Res. Commun. 271107-115. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida, S., D. Kondoh, E. Arai, H. Matsuoka, C. Seki, T. Tanaka, M. Okada, and A. Ishii. 2003. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology 316161-170. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida, S., H. Matsuoka, E. Luo, K. Iwai, M. Arai, R. E. Sinden, and A. Ishii. 1999. A single-chain antibody fragment specific for the Plasmodium berghei ookinete protein Pbs21 confers transmission blockade in the mosquito midgut. Mol. Biochem. Parasitol. 104195-204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.