Abstract
Strains of enteropathogenic Escherichia coli (EPEC) generally employ the adhesins bundle-forming pili (Bfp) and intimin to colonize the intestine. Atypical EPEC strains possess intimin but are negative for Bfp and, yet, are able to cause disease. To identify alternative adhesins to Bfp in atypical EPEC, we constructed a transposon mutant library of atypical EPEC strain E128012 (serotype O114:H2) using TnphoA. Six mutants that had lost the ability to adhere to HEp-2 cells were identified, and in all six mutants TnphoA had inserted into the pstSCAB-phoU (Pst) operon. To determine if the Pst operon is required for adherence, we used site-directed mutagenesis to construct a pstCA mutant of E128012. The resultant mutant showed a reduced ability to adhere to HEp-2 cells and T84 intestinal epithelial cells, which was restored by trans-complementation with intact pstCA. To determine if pst contributes to bacterial colonization in vivo, a pstCA mutation was made in the EPEC-like murine pathogen, Citrobacter rodentium. C57BL/6 mice infected perorally with the pstCA mutant of C. rodentium excreted significantly lower numbers of C. rodentium than those given the wild-type strain. Moreover, colonic hyperplasia and diarrhea, which are features of infections with C. rodentium, were not observed in mice infected with the pstCA mutant but did occur in mice given the trans-complemented mutant. As mutations in pst genes generally lead to constitutive expression of the Pho regulon, our findings suggested that the Pho regulon may contribute to the reduced virulence of the pstCA mutants. To investigate this, we inactivated phoB in the pstCA mutants of EPEC E128012 and C. rodentium and found that the phoB mutation restored the adherent phenotype of both mutant strains. These results demonstrate that Pst contributes to the virulence of atypical EPEC and C. rodentium, probably by causing increased expression of an unidentified, Pho-regulated adhesin.
Enteropathogenic Escherichia coli (EPEC) is a prominent cause of diarrhea worldwide, especially among young children (28, 32, 41). In developing countries, EPEC is responsible for endemic infantile diarrhea and is estimated to cause the deaths of several hundred thousand children each year (32, 41). EPEC employs a large number of determinants to colonize the intestine and produces characteristic attaching and effacing (A/E) lesions in the intestinal mucosa (8, 20). The genetic determinants required for the production of A/E lesions are located on a pathogenicity island called the locus of enterocyte effacement (LEE), which encodes a type III protein secretion system, an outer-membrane protein adhesin (called intimin and encoded by the eae gene), and a translocated intimin receptor (Tir), as well as other type III secreted proteins (8, 14). Many EPEC strains also carry an adherence factor plasmid (pEAF) that encodes bundle-forming pili (Bfp), which promote bacterial adherence to epithelial cells and are essential for virulence (7, 25, 39).
Carriage of the bfpA gene, which encodes the major structural pilin subunit, is used to classify EPEC into two major subgroups, known as typical (Bfp positive) and atypical (Bfp negative) EPEC (19, 41). Typical EPEC bacteria adhere to HEp-2 cells in a localized pattern, whereas atypical EPEC, if they adhere to HEp-2 cells at all, do so in a variety of patterns, termed localized-like adherence, diffuse adherence, and aggregative adherence (33, 41). Despite their lack of Bfp, the results of epidemiological, clinical, and volunteer studies indicate that atypical EPEC are able to cause diarrhea (25, 33, 41).
Given that, as a group, atypical EPEC lack Bfp and display variable patterns of adherence to HEp-2 cells, we hypothesized that atypical EPEC strains carry novel adhesin(s) responsible for these phenotypes. Other than intimin, however, only one adhesin has so far been described in an atypical EPEC strain. This is a novel afimbrial adhesin called the locus for diffuse adherence (LDA), which was present in an atypical EPEC strain (O26:H11) isolated from an infant with diarrhea (36). However, the prevalence of LDA in other atypical EPEC strains is low (36). The aim of this study was to identify the determinants of atypical EPEC strain E128012 (O114:H2) which allow this strain to adhere to HEp-2 cells. Originally isolated from an infant with sporadic diarrhea in Bangladesh, E128012 shows localized-like adherence to HEp-2 cells and, when fed to volunteers, caused diarrhea of severity similar to that caused by a typical EPEC strain, E2348/69 (25). Our results indicated that atypical EPEC strain E128012 requires an intact pst-phoU operon to adhere to HEp-2 cells and, moreover, that Citrobacter rodentium strain ICC169, an A/E pathogen of mice that is used as a model of infections with A/E strains of E. coli, requires the same operon for virulence.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Strains were maintained on Luria-Bertani (LB) medium and grown overnight at 37°C with shaking unless otherwise stated. Where necessary, the following antibiotics were used at the indicated concentrations per milliliter: ampicillin (Amp; 100 μg), kanamycin (Kan; 50 μg), tetracycline (Tet; 12.5 μg), chloramphenicol (Cam; 25 μg), and nalidixic acid (Nal; 50 μg). 5-Bromo-4-chloro-3-indolyl-phosphate (XP) was used at a final concentration of 50 μg/ml, together with 0.2% (wt/vol) glucose, to detect alkaline phosphatase activity. To grow bacteria in known concentrations of phosphate, minimal medium containing 121 salts (40), 0.2% (wt/vol) glucose, 0.01 mM Casamino Acids, and 0.01 mM thiamine was made without added phosphate, after which various amounts of KH2PO4 were added to a final concentration of 6.5 mM for high-phosphate medium or 65 μM for low-phosphate medium, respectively. The final concentration of phosphate was determined by flow injection analysis (11).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli strains | ||
| CY70 | E128012 ΔpstCA::kan Kanr | This study |
| CY88 | E128012 ΔpstCA | This study |
| CY91 | E128012 ΔpstCA phoB | This study |
| CY95 | E128012 ΔphoB::kan Kanr | This study |
| E128012 | Wild-type atypical EPEC O114:H2 | 25 |
| E1 | E128012 TnphoA mutant, pstS::TnphoA Kanr | This study |
| E2 | E128012 TnphoA mutant, pstS::TnphoA Kanr | This study |
| E3 | E128012 TnphoA mutant, Kanr | This study |
| E4 | E128012 TnphoA mutant, pstA::TnphoA Kanr | This study |
| E5 | E128012 TnphoA mutant, pstA::TnphoA Kanr | This study |
| E6 | E128012 TnphoA mutant, pstC::TnphoA Kanr | This study |
| C. rodentium strains | ||
| ICC169 | Derivative of C. rodentium ICC168, Nalr | 27 |
| ICA15 | ICC169 ΔpstCA::kan Kanr Nalr | This study |
| ICA18 | ICC169 ΔpstCA ΔphoB::kan Kanr Nalr | This study |
| Plasmids | ||
| pBlueScript (pBSII) SK− | High-copy-no. cloning vector; Ampr | Agilent Technologies |
| pGEM-T Easy | High-copy-no. cloning vector; Ampr | Promega |
| pACYC184 | Medium-copy-no. cloning vector; Camr Tetr | New England Biolabs |
| pRT733 | Delivery vector of TnphoA, derivative of suicide vector pJM703.1; Ampr Kanr | 37 |
| pKD4 | FRT-flanked Kanr cassette template | 12 |
| pACBSR | I-SceI and λ Red recombinase expression plasmid with arabinose-inducible expression; Camr | 18 |
| pCP20 | FLP helper plasmid, temp-sensitive replication; Ampr Camr | 10 |
| pAC1 | 1.8-kb PCR product containing wild-type E. coli E128012 pstCA cloned into SmaI site of pBSII; Ampr | This study |
| pAC2 | 1.9-kb BamHI/EcoRV fragment from pCA1 containing wild-type E. coli E128012 pstCA cloned into the BamHI/EcoRV sites of pACYC184; Camr | This study |
| pAC4 | 1.9-kb fragment containing wild-type C. rodentium ICC169 pstCA cloned into the EcoRV/SalI sites of pACYC184; Camr | This study |
To compare the growth kinetics of the bacterial strains used in this study in different media, overnight cultures of the test strains, grown in LB, were diluted 1 in 50 and allowed to grow at 37°C with shaking in LB, minimal essential medium, or high- or low-phosphate medium in a Klett flask. Absorbance was measured at regular time intervals by using a Klett-Summerson colorimeter (Klett Manufacturing Co., Inc., Brooklyn, NY).
Recombinant DNA techniques.
Routine DNA manipulations were performed by using standard techniques (1, 35), with the buffers and instructions supplied by the manufacturers of the kits and reagents used. Genomic and plasmid DNA were isolated by using the cetyltrimethylammonium bromide method (1) and a Wizard plus SV DNA purification system (Promega, Madison, WI), respectively. PCR amplifications were performed using Vent proofreading DNA polymerase (New England Biolabs, Ipswich, MA) or high-fidelity Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). Synthetic oligonucleotides for PCR and sequencing (Table 2) were obtained from GeneWorks Pty., Ltd. (Hindmarsh, South Australia, Australia).
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′ to 3′)a |
|---|---|
| T1 | AACGGGAAAGGTTCCGTC |
| T2 | CTTGCACAGATAGCGTGG |
| T3 | TGTAGCGGATGGAGATCG |
| T4 | ATGGAAGTCAGATCCTGG |
| pKD4F | TGTGTAGGCTGGAGCTGCTTC |
| pKD4R | CATATGAATATCCTCCTTAG |
| pstCAF | TTCGTTCAGCGTCTGCC |
| pstCAR | AGTTCGGTGATCAGCTCTTC |
| pstCAKanF | CTAAGGAGGATATTCATATGATTACCCTGTGC |
| pstCAKanR | GAAGCAGCTCCAGCCTACACAAGGCTTGGTTGC |
| pstCAISceIF | TAGGGATAACAGGGTAATTTCGTTCAGCGTCTGCC |
| pstCAISceIR | TAGGGATAACAGGGTAATAGTTCGGTGATCAGCTC |
| pstCAcF | ACTTTGTAAACGCGTTTAAACTG |
| pst4′ | TGTTAACCGTGTTTATTCTTCG |
| phoBF | ACGGTAGTATTGAGGAACG |
| phoBR | AGGTAACCCTGTAACACG |
| phoBKanF | CTAAGGAGGATATTCATATGACGGTCGATGTC |
| phoBKanR | GAAGCAGCTCCAGCCTACACATTCTACGACCAG |
| phoBISceIF | TAGGGATAACAGGGTAATACGGTAGTATTGAGG |
| phoBISceIR | TAGGGATAACAGGGTAATAGGTAACCCTGTAAC |
| CrpstCAcF | TACGTTAGTCTGGAAGCACG |
| CrpstCAcR | TTAGCCGTGTTTCTTCTTAGC |
| ICC169phoB-FRT | TCCTCGACTGGATGTTGCCb |
| ICC169phoB-RRT | TCTGGCGGTAAGCATCACCb |
| phoB-FRT | ATGACAGTGCTGTGAATCAAc |
| phoB-RRT | TCAACATCACCACTGGAATAc |
| rpoD-FRT | TGATCATGAAGCTCTGCGTTGAd |
| rpoD-RRT | TCTCAGACCACGGTTTGTTCATd |
| ler-FRT | ACTACCGTAATGAAGACGGACAe |
| ler-RRT | TGCTTCTTTAAGCCAACGTGe |
| eae-FRT | TATCCTGCATTAGGTGGCAAf |
| eae-RRT | GTCACCAAAGGAATCGGAGTf |
Underlined nucleotide sequences are homologous to the flanking regions of the pstCA and phoB genes, and restriction sites for I-SceI are shown in boldface.
Primers used for RT-PCR of ICC169 phoB.
Primers used for RT-PCR of E128012 phoB.
Primers used for RT-PCR of the housekeeping gene rpoD.
Primers used for RT-PCR of ICC169 ler.
Primers used for RT-PCR of ICC169 eae.
Transposon mutagenesis and Southern hybridization.
TnphoA was introduced into atypical EPEC strain E128012 on the suicide plasmid pRT733 by conjugation, as described previously (38). Forty-eight blue colonies were selected on LB agar containing Kan and XP and tested for loss of adherence to HEp-2 cells. A 2.8-kb BglII fragment from pRT733 that spans the BamHI site of TnphoA was labeled with digoxigenin by the random primer method (Roche Diagnostics, Mannheim, Germany) and used in Southern blotting to determine whether mutants contained one or more transposon insertions. The insertion site of TnphoA in each nonadherent mutant was determined by using inverse PCR to amplify the sequences flanking the transposon (29). Briefly, genomic DNA of each nonadherent mutant was digested with BamHI and EcoRV, the resulting BamHI 5′ overhangs were filled in using the Klenow fragment of DNA polymerase I (New England Biolabs), and the products were recircularized by self-ligation. The unknown region was amplified by PCR using primers T1 and T2 for the region upstream of the transposon and primers T3 and T4 for the region downstream of the transposon (Table 2). DNA sequencing was performed by using an ABI Prism BigDye Terminator cycle sequencing kit, version 3.1 (Applied Biosystems). Reaction mixtures were analyzed at the Australian Genome Research Facility (Parkville, Victoria, Australia), and sequences were edited and assembled in contiguous sequences by using the Sequencher program (Gene Codes, Ann Arbor, MI). BLAST searches and sequence analyses were conducted using databases at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/) and the CLUSTAL W (http://clustalw.genome.jp) websites.
Construction of nonpolar pstCA and phoB mutants.
Knockout mutations were constructed in E. coli E128012 and C. rodentium by using overlapping extension PCR (9) and the “gene gorging” technique described by Herring et al. (18). First, ∼0.6 kb of DNA flanking the target genes was amplified by using primer pairs pstCAF/pstCAKanR and pstCAKanF/pstCAR for pstCA and phoBF/phoBKanR and phoBKanF/phoBR for phoB. The fragment length polymorphism (FLP) recombinase target (FRT)-flanked Kan resistance (Kanr) gene from pKD4 (12) was amplified by using primers pKD4F and pKD4R. This product, together with each pair of amplified flanking regions, was used as the template in a PCR using primer pairs pstCAISceIF/pstCAISceIR (pstCA) and phoBISceIF/phoBISceIR (phoB) (Table 2). The I-SceI-flanked PCR products were cloned into pGEM-T Easy to yield the donor plasmids required for gene gorging. These plasmids and pACBSR, which carries the λ Red recombinase genes and the gene for I-SceI under an arabinose-inducible promoter, were cointroduced into electrocompetent E. coli E128012 or C. rodentium cells. Mutants were selected on LB plates supplemented with Kan. All mutations were confirmed by PCR using primers flanking the targeted region and primers within the Kanr gene. When required, the Kanr gene was excised by using the FRT sites that flank the Kanr gene and FLP helper plasmid pCP20 (10). E128012 and ICC169 pstCA phoB::kan double mutants were achieved by the introduction of ΔphoB::kan by allelic exchange in the pstCA mutant strains. The Kanr gene was excised accordingly.
Construction of trans-complementing plasmids.
Wild-type pstCA was amplified from E128012 genomic DNA by using primers pstCAcF and pst4′. The resultant 1.8-kb, gel-purified, blunt-end PCR product was ligated with SmaI-linearized pBSII. This plasmid, designated pAC1, was digested with BamHI and EcoRV to release the insert, which was then ligated to BamHI- and EcoRV-digested pACYC184 to give pAC2, which carried pstCA behind the Tetr promoter of pACYC184.
The wild-type pstCA gene was amplified from C. rodentium genomic DNA (pstCACR) using primers CrpstCAcF and CrpstCAcR. The resultant purified 1.9-kb PCR product was ligated with pGEM-T Easy vector and then linearized by digestion with NcoI, and the 5′ overhangs were filled in as described above. The resultant fragment was then digested with SalI, gel purified, and cloned into EcoRV- and SalI-digested pACYC184 to yield pAC4, which possessed wild-type pstCACR behind the Tetr promoter of pACYC184.
Quantitative real-time RT-PCR.
Overnight cultures of E. coli and C. rodentium strains were inoculated 1:50 in LB and grown to an optical density at 600 nm of 0.6. Ten milliliters of culture was incubated with 20 ml of RNAprotect solution (Qiagen, Valencia, CA) at room temperature for 10 min, after which cells were pelleted and RNA was purified by using a FastRNA pro blue kit (Qbiogene, Inc., Carlsbad, CA). The samples were treated with DNase I before further purification using an RNeasy MinElute kit (Qiagen). Real-time PCR was performed with an MxPro-Mx3005P multiplex quantitative PCR system (Agilent Technologies, Santa Clara, CA). First-strand cDNA synthesis was performed with 5 μg of total RNA, SuperScript II reverse transcriptase (Invitrogen), and random primers (Invitrogen) according to the manufacturer's recommendations. Each 25-μl reaction mixture contained 10 ng cDNA, 300 nM of each specific primer (Table 2), and 12.5 μl 2× SYBR green master mix (Applied Biosystems, Foster City, CA). All reverse transcription-PCR (RT-PCR) data were normalized with the results for the housekeeping gene rpoD, and the relative expression ratio of the target gene was calculated as described by Pfaffl (30).
Adherence of bacteria to cultured epithelial cells.
HEp-2 cell adherence assays were performed as previously described (33). Cells were examined by using bright-field microscopy for characteristic patterns of adherence and photographed with a Leica DC2000 digital camera (Leica Microsystems AG, Wetzlar, Germany). Quantitative bacterial adherence to HEp-2 cells was expressed as the number of cells with five or more attached bacteria as a percentage of the total number of cells counted. Each assay was performed in triplicate, with at least 100 cells counted for each bacterial strain.
To determine the ability of atypical EPEC strain E128012 and its derivatives to adhere to polarized cells, T84 cells of human intestinal origin were grown in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 medium containing 5% fetal calf serum in 5% CO2 at 37°C. For cell adherence assays, T84 cells were seeded in 24-well plates at a density of 7.5 × 104 cells per well and were used when just confluent (7 to 10 days). Before infection with E. coli, the growth medium was replaced with medium containing 0.5% fetal calf serum and 0.5% d-mannose. Overnight cultures of E. coli were diluted 1 in 33 in LB, grown to early log phase at 37°C, and then added to the T84 cells at a multiplicity of infection of 100:1. Bacteria and monolayers were incubated for 3 h at 37°C in 5% CO2, after which nonadherent bacteria were removed by washing in phosphate-buffered saline (PBS) and the numbers of attached bacteria were determined by lysing the T84 cells in 100 μl of 1.0% Triton X-100 (Sigma Chemical Co., St. Louis, MO) and enumerating the bacteria on LB agar.
Alkaline phosphatase.
Alkaline phosphatase activity was determined as described by Brickman and Beckwith (6). Briefly, the optical densities at 600 nm of cultures grown overnight in high- or low-phosphate medium were recorded and a measured amount of the culture was centrifuged to pellet the bacteria. The bacteria were then resuspended in 1 M Tris HCl, pH 8.0, and permeabilized with 0.1% sodium dodecyl sulfate and chloroform. The alkaline phosphatase activity was determined by using p-nitrophenol phosphate and was expressed in Miller units as the mean and standard deviation (SD) of the results of at least three separate assays.
Infection of mice.
Four- to five-week-old male C57BL/6 mice were bred, housed, and maintained in the Department of Microbiology and Immunology animal facility at the University of Melbourne. Animals in this facility are certified free of infection with C. rodentium and other common bacterial, viral, and parasitic infections of laboratory mice. For single-strain infections of mice, each of nine mice per group was inoculated by oral gavage with approximately 2 × 109 CFU of an overnight culture of a test strain of C. rodentium in 200 μl of PBS. Control animals received 200 μl of sterile PBS. Fecal samples were recovered aseptically for up to 20 days after inoculation, and the number of viable C. rodentium bacteria per gram of stool was determined by plating onto selective medium. The limit of detection was 100 CFU/g feces.
For mixed-strain infections, five mice were inoculated perorally with approximately 109 CFU of a mutant or complemented mutant strain together with an approximately equal number of wild-type C. rodentium cells in 200 μl of PBS. Mice were killed 7 days after infection; their colons excised; and the contents removed, serially diluted, and spread on two LB agar plates containing appropriate antibiotics to determine the proportion of wild-type C. rodentium bacteria to mutant or complemented mutant bacteria. The ability of the mutant or trans-complemented mutant to compete with the wild-type strain was determined for three to five mice and expressed as the competitive index (CI), which was the proportion of mutant or complemented mutant to wild-type bacteria recovered from animals divided by the proportion of the mutant or complemented mutant to wild-type bacteria in the inoculum (17). Mutants with a CI of less than 0.5 were considered to be attenuated.
Colonic hyperplasia.
At days 6, 10, 14, and 20 after infection, one mouse from each single-strain-infected group was killed and 4 cm of the colon, beginning at the anal verge, was excised. The contents were removed, and the colon was weighed and fixed in 10% (wt/vol) neutral buffered formalin or 10% (wt/vol) glutaraldehyde for histological examination. Formalin-fixed sections were stained with hematoxylin and eosin as described previously (17) and photographed using a Leica DC2000 digital camera. The crypt heights of well-oriented sections were measured by micrometry, with at least 10 measurements taken in the distal colon of each mouse. Glutaraldehyde-fixed sections were processed and examined by transmission electron microscopy as described previously (34).
Stool water content.
The water content of feces in the distal colon of mice infected with C. rodentium was determined as described by Guttman et al. (15, 16). Briefly, 7 days after inoculation with PBS or a test strain of C. rodentium, mice were killed, and the distal 3.5 cm of the large intestines were excised. The contents of the excised intestines were removed and weighed immediately and again after drying at 37°C for 48 h. The difference between the wet and the dry weights was used to calculate the percentage of water in the gut contents.
Statistical analyses.
Statistical analyses were performed using the Instat and Prism software packages (GraphPad Software, San Diego, CA). A two-tailed P value of <0.05 was taken to indicate statistical significance.
Nucleotide sequence accession numbers.
The complete sequences of the pstSCAB-phoU operon and the phoB genes of EPEC strain E128012 and C. rodentium strain ICC169 have been deposited in the GenBank database under accession numbers FJ377883, FJ393267, FJ415986, and FJ415987.
RESULTS
Isolation of nonadherent mutants of atypical EPEC strain E128012.
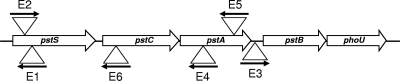
Atypical EPEC strain E128012 was mutagenized using transposon TnphoA. Forty-eight Kanr mutants were isolated that were PhoA positive as evidenced by their blue coloration on XP agar. All 48 mutants were examined for their ability to adhere to HEp-2 cells, and six strains, named E1 to E6, were found to be defective in this regard (Fig. 1). As Southern blot hybridization had shown the presence of single TnphoA insertions in each of strains E1 to E6, the transposon and flanking DNA from each mutant were cloned and sequenced. Homology searches revealed that in all six mutants, TnphoA had inserted into genes belonging to the pstSCAB-phoU operon (Fig. 2). Specifically, mutants E1 and E2 carried insertions in pstS, E6 in pstC, E4 and E5 in pstA, and E3 in the intergenic region between pstA and pstB. Further sequence analysis of E1, E4, E5, and E6 showed that TnphoA had inserted in the wrong orientation, opposite to the direction of transcription, indicating that alkaline phosphatase fusion proteins had not been produced. Therefore, the alkaline phosphatase activity detected in these mutants, as well as in mutant E3, where the transposon was inserted in an intergenic region, most probably resulted from the constitutive overexpression of the endogenous phoA gene of E128012 which had become deregulated as a result of mutations in pst.
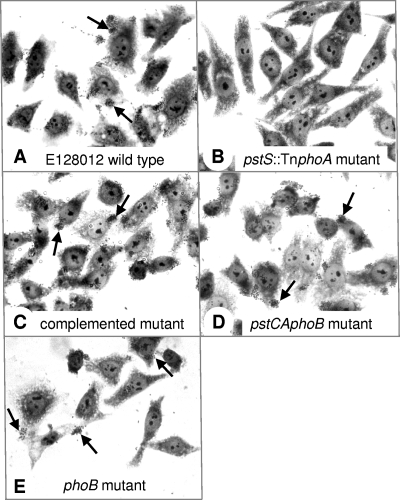
FIG. 1.
Adherence of atypical EPEC strain E128012 and its derivatives to HEp-2 epithelial cells after 3 h. (A) Wild-type atypical EPEC strain E128012 showing localized-like adherence. (B) Strain E2, a representative pstS::TnphoA mutant. Five other TnphoA Pst operon mutants and strain CY88, E128012 ΔpstCA, showed the same phenotype. (C) Strain CY88(pAC2), CY88 trans-complemented with pstCA. (D) Strain CY91, pstCA phoB double mutant of E128012. (E) Strain CY95, phoB mutant of E128012. Arrows point to examples of adherent bacteria. Giemsa stain was used.
FIG. 2.
Diagrammatic representation of the Pst operon of atypical EPEC strain E128012 showing the sites of insertion (triangles) and the orientation (black arrows) of TnphoA in the nonadherent mutants E1 to E6.
Effects of site-directed mutagenesis of pstCA.
To establish whether the Pst operon plays a role in the adherence of E128012 to HEp-2 cells, we constructed a site-directed mutant, disrupted in both pstC and pstA, by excising 1.7 kb of the 1.9 kb that comprises pstCA and replacing it with a 1.6-kb Kanr gene. This mutant was named CY70. To avoid any possible polar effects on downstream genes that may have resulted from the introduction of the Kanr gene, this gene was subsequently deleted from CY70 by using an FLP helper plasmid, pCP20. PCR and sequence analysis revealed that the resultant Kans mutant, named CY88, had lost 1.7 kb of pstCA and the Kanr gene. The adherence of CY88 to HEp-2 and T84 cells was significantly less than that of the wild-type strain E128012 (P < 0.001) (Table 3). Adherence was restored by trans-complementation of CY88 with intact pstCA on pAC2 such that the difference between the complemented mutant and the wild-type strain was no longer significant (P > 0.5). These findings indicated that pstCA is required for E128012 to adhere to HEp-2 and T84 cells.
TABLE 3.
Quantitative assessment of the ability of atypical EPEC strain E128012 and its isogenic derivatives to adhere to cultured epithelial cells
| Strain | Characteristic(s) | Adherence to HEp-2 cells (%)a | Adherence to T84 cells (%)b |
|---|---|---|---|
| E128012 | Wild type | 56.9 ± 8.9 | 109.6 ± 21.7 |
| CY88 | pstCA mutant | 10.4 ± 1.4c | 8.8 ± 6.6c |
| CY88(pAC2) | Trans-complemented pstCA mutant | 42.9 ± 4.0 | 90.8 ± 25.8 |
| CY91 | pstCA phoB double mutant | 54.4 ± 5.4 | 96.9 ± 16.2 |
For HEp-2 cells, bacterial adherence is expressed as the number of cells with at least five attached bacteria as a percentage of the total number of cells counted. At least 100 cells were counted in each assay, which were performed in triplicate. Data are the means ± SD of the results.
For T84 cells, bacterial adherence is expressed as the percentage of the initial inoculum recovered from T84 cells after incubation for 3 h at 37°C and removal of nonadherent bacteria. Data are the means ± SD of the results of five separate determinations.
Significantly lower (P < 0.001) than the results for all other strains tested (two-tailed Student's t test).
To determine if the inability of CY88 to adhere to HEp-2 and T84 cells was in any way due to an impaired ability to grow under the conditions of the adherence assay, we compared the growth kinetics of this strain with those of E128012 under various conditions, including in tissue culture medium and in defined minimal medium supplemented with low or high concentrations of phosphate. In every case, the pstCA mutant grew at the same rate as the wild type (data not shown).
In E. coli, mutations in the pst operon lead to constitutive expression of Pho regulon genes, including phoA and phoB (44). The impact of the pstCA mutation in E128012 on the Pho regulon was assessed by measuring the expression of phoB and phoA. The expression of the phoB gene was determined by using quantitative real-time RT-PCR on strains grown in LB. These studies showed that in CY88 (pstCA mutant strain), transcription of phoB was sixfold greater than in the wild-type strain and in the trans-complemented mutant CY88 (pAC2), which was the same as the wild type in this respect. The levels of expression of alkaline phosphatase by the wild-type strain E128012 (1 Miller unit) and the complemented pstCA mutant strain CY88(pAC2) (15 Miller units) were low when the bacteria were grown in high-phosphate medium and substantially higher [E128012 and CY88(pAC2), 807 and 606 Miller units, respectively] when they were grown in low-phosphate medium. In contrast, CY88 exhibited a constitutive Pho phenotype (>1,000 Miller units) in both high- and low-phosphate media. Together these findings indicate that the pstCA mutation in E128012 resulted in constitutive elevated expression of phoB, phoA, and presumably, other genes in the Pho regulon.
Adherence to epithelial cells by a pstCA phoB double mutant.
Because inactivation of pstCA results in constitutive expression of the PhoR/PhoB regulon (23), we hypothesized that the reduced adherence phenotype of CY88 might be restored if PhoB were also inactivated in CY88. Accordingly, we deleted phoB in CY88 to generate a pstCA phoB double-deletion mutant, named CY91. This strain adhered to HEp-2 and T84 cells to an extent similar to the wild-type EPEC strain (P > 0.5, two-tailed Student's t test) (Table 3), indicating that constitutive expression of one or more genes of the Pho regulon was responsible for the relatively reduced adherence of CY88. Deletion of phoB alone in wild-type E128012 did not affect cell adherence (Fig. 1).
Contribution of pstCA to the virulence of C. rodentium for mice.
As there is no convenient animal model of infection with atypical EPEC, we used Citrobacter rodentium to determine if the Pst operon contributes to the virulence of A/E enterobacteria. C. rodentium strain ICC169 is a natural pathogen of mice that is frequently used as a model of infection with A/E strains of E. coli (5). When fed to mice, C. rodentium colonizes the intestine in large numbers and causes diarrhea accompanied by A/E lesions and colonic hyperplasia, which is used as a quantitative indicator of the severity of infection (5). An insertional deletion was made in the pstCA gene of C. rodentium ICC169 to create the pstCACR mutant, ICA15. This strain and its trans-complemented derivative, ICA15(pAC4), grew at the same rate as each other and as the parent strain under various conditions (data not shown). In mixed-infection experiments, four- to five-week-old C57BL/6 mice were infected with wild-type C. rodentium and ICA15 or ICA15(pAC4) in a 1:1 ratio. Seven days later, the mice were killed and the ability of the C. rodentium strains to compete with each other in vivo was assessed by enumerating the test bacteria in the colon. The results showed that strain ICA15 was out-competed by the wild-type with a CI of 0.11 (P = 0.02, two-tailed Student's t test). When ICA15 was complemented with pAC4, its ability to compete successfully with the wild-type strains was restored (CI = 0.7, P = 0.12).
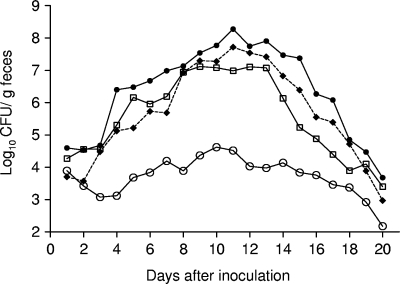
In single-strain infections, the ability of the pstCACR mutant, ICA15, to colonize the mouse intestine was significantly less than that of the wild-type, as evidenced by maximum mean counts of 4.2 × 104 CFU/g feces and 1.9 × 108 CFU/g feces for the mutant and wild-type, respectively (P = 0.002, two-tailed Student's t test) (Fig. 3). In contrast, the pstCA trans-complemented strain, ICA15(pAC4), reached a maximum mean of 5.2 × 107 CFU/g feces, which was comparable to that of the wild-type strain (P > 0.5). These results indicate that C. rodentium requires pstCA to colonize the mouse intestine.
FIG. 3.
Colonization of C57BL/6 mice with derivatives of C. rodentium ICC169. Results are expressed as the mean log10 CFU/g feces from at least five mice at selected time points after inoculation. Mice received approximately 2 × 109 CFU via oral gavage of wild-type C. rodentium strain ICC169 (•); a pstCACR mutant strain, ICA15 (○); a trans-complemented mutant, ICA15(pAC4) (⧫); or a pstCA phoBCR double mutant, ICA18 (□). The limit of detection was 100 CFU/g feces.
Pathological changes in the intestines of mice infected with pstCA derivatives of C. rodentium.
To determine the outcome of infection with the pstCACR mutant ICA15 on colonic pathology, sections of the distal colon were collected 14 days after inoculation of mice with wild-type C. rodentium, ICA15, ICA15(pAC4), or PBS (control); fixed and stained with hematoxylin and eosin; and examined by light microscopy. The colons of mice given PBS and mice infected with mutant ICA15 revealed similar colonic morphology, with no significant difference in crypt height between the two groups of mice (P > 0.3) (Table 4). In contrast, crypt heights in the colons of mice infected with C. rodentium and the complemented mutant ICA15(pAC4) were significantly greater than those in control animals (P = 0.008 and P = 0.001, respectively) (Table 4).
TABLE 4.
Crypt heights and colon weights of mice 14 days after infection with derivatives of C. rodentium
| Strain | Crypt ht (μm)a | Colon wtb (mg/cm) |
|---|---|---|
| ICC169 | 301 ± 63c | 30.6 ± 3.4c |
| ICA15 | 189 ± 11 | 23.4 ± 1.7 |
| ICA15(pAC4) | 250 ± 15c | ND |
| ICA18 | 260 ± 21c | 34.8 ± 4.5c |
| None (PBS control) | 199 ± 18 | 24.1 ± 2.0 |
Only well-oriented crypts taken from the distal colon of each mouse were measured. Data are the means ± SD of the results (n = 10).
Colon weight after removal of contents. Data are the means ± SD of the results (n = 5). ND, not determined.
Significantly different (P < 0.01) from results for control mice inoculated with PBS (Student's t test, two-tailed).
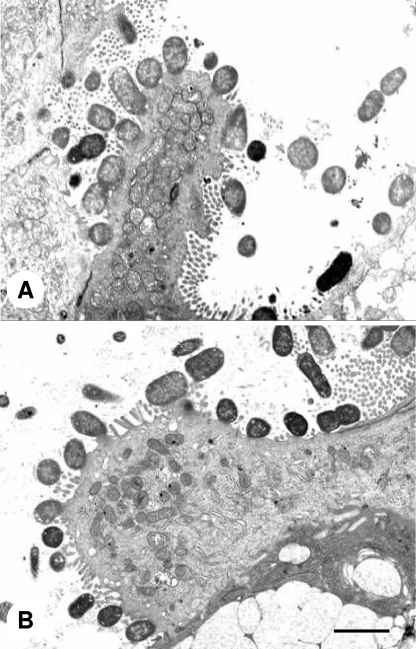
To determine if ICA15, the pstCA mutant of C. rodentium, was affected in terms of its ability to produce A/E lesions, we examined the distal colons of infected mice by using transmission electron microscopy. In mice given either ICC169 or ICA15, large numbers of bacteria were observed intimately associated with the colonic epithelium, in association with characteristic A/E lesions. The observed changes were indistinguishable between the two strains (Fig. 4). The data indicate that the Pst system is not required for the formation of A/E lesions by C. rodentium in mice. These findings were corroborated by quantitative real-time RT-PCR analysis, which showed that the expression of two key LEE-encoded genes, ler and eae, was not significantly different in wild-type C. rodentium and its isogenic pstCA mutant.
FIG. 4.
Transmission electron micrographs of sections of mouse colon 14 days after oral inoculation with wild-type C. rodentium ICC169 (A) or ICA15, an isogenic pstCACR mutant of C. rodentium ICC169 (B), showing extensive A/E lesions. Scale bar, 1 μm. Note that although the numbers of adherent bacteria appear similar in the two panels, C. rodentium adheres to the intestinal epithelium in patches, making electron microscopy an unsuitable method for quantifying adhesion.
Stool water content.
To determine if ICA15, the pstCA mutant of C. rodentium, was affected in terms of its ability to cause diarrhea, we compared the water content of feces in the distal colons of mice infected with wild-type C. rodentium and its derivatives. The results showed that ICA15 was defective in terms of its ability to induce diarrhea, as evidenced by a stool water content that was the same as that in uninfected mice (Table 5). In contrast, feces from mice given wild-type C. rodentium or ICA15(pAC4), the trans-complemented pstCA mutant, contained significantly more water than control mice (Table 5).
TABLE 5.
Water content of feces collected from the distal colons of mice 7 days after infection with derivatives of C. rodentium
| Strain | Characteristic(s) | Water content (%)a |
|---|---|---|
| ICC169 | Wild type | 89.3 ± 4.0b |
| ICA15 | pstCA mutant | 71.6 ± 3.1 |
| ICA15(pAC4) | Trans-complemented pstCA mutant | 84.9 ± 2.9b |
| ICA18 | pstCA phoB double mutant | 90.0 ± 2.8b |
| None (PBS control) | 71.5 ± 5.5 |
Seven days after infection, mice were killed. A 3.5-cm length of distal colon was excised, and the colon contents were weighed immediately and after drying at 37°C for 48 h. The difference in the wet versus dry weights was then used to calculate the percentage of water in the colon contents. Data are the mean values ± SD of the results obtained for four or five mice.
Significantly greater (P < 0.005) than results for control mice inoculated with PBS (two-tailed Student's t test).
Restoration of virulence to ICA15 by mutation of phoB.
Gene expression studies using quantitative real-time RT PCR showed that the expression of phoB in ICA15 was 42-fold greater than in the wild-type strain when both strains were grown in LB. To determine if defective colonization by mutant ICA15 was due to the pstCA mutation directly or caused by gene(s) affected by a deregulated Pho regulon, an ICC169 pstCACR phoB double mutant, known as ICA18, was generated. Single-strain infection experiments with ICA18 showed that the phoB mutation was able to restore the colonizing ability of the pstCACR mutant to a level comparable to that of the wild-type strain (Fig. 3). Moreover, unlike the pstCACR mutant, ICA15, the pstCACR phoB double-deletion mutant, ICA18, was able to induce colonic hyperplasia and diarrhea in mice, as evidenced by significantly increased crypt height, colon weight, and stool water content compared to those in mice given ICA15 or PBS (Tables 4 and 5). These data suggest that one or more genes of the Pho regulon play a key role in the colonic colonization of mice by C. rodentium.
DISCUSSION
The Pho regulon is a global regulatory network that bacteria use to manage phosphate acquisition and metabolism (23). At the core of the regulon is a two-component system which activates or inhibits transcription, comprising PhoR, an inner-membrane histidine kinase sensor protein, and PhoB, a response regulator that is a DNA-binding protein (4, 44). In E. coli, the Pho regulon comprises at least 47 genes (23), although this is likely to be an underestimate given that as many as 400 genes in E. coli respond to environmental concentrations of phosphate (42).
A key component of the Pho regulon is the Pst system, which captures periplasmic inorganic phosphate and transports it into the cytosol. Pst comprises four elements: PstS, a periplasmic protein that binds inorganic phosphate; PstC and PstA, which form an inner membrane channel for phosphate transport; and PstB, a permease that provides the energy needed to transport phosphate (23). The Pst system also regulates the entire Pho regulon by preventing the activation of PhoB in phosphate-rich environments. Thus, in E. coli, mutations in the Pst system lead to constitutive expression of the Pho regulon regardless of phosphate concentration. Although PhoB is normally activated by PhoR, it is subject to cross-regulation by other sensor proteins in response to a variety of environmental signals other than phosphate (23). At least six such histidine kinases, QseC, ArcB, CreC, KpdD, BaeS, and EnvZ, can activate PhoB in the absence of PhoR (45). The Pho regulon is also interrelated with the stress response (23).
Despite extensive research on phosphate uptake and phosphate-related gene regulation in bacteria, evidence of the contribution of the Pho regulon to virulence gene expression has emerged only recently (reviewed in reference 23). For example, mutations in pst genes can interfere with the expression of virulence-associated type III protein secretion systems of Edwardsiella tarda and Salmonella enterica (3, 26, 31) and diminish the virulence of avian pathogenic E. coli for chickens (22). In addition, pstS mutants of porcine EPEC show reduced adherence to piglet ileal explants (2), and a phoB mutant of Vibrio cholerae showed reduced ability to colonize the rabbit small intestine (43). Given that the intestine contains high concentrations of phosphate, these observations suggest that stimuli other than phosphate concentrations are responsible for the reduced virulence of some Pho regulon mutants. Among the Pho-regulated systems that may be relevant in this regard are responses to changes in pH and other environmental stimuli; the expression of surface components, including adhesins; and the capacity to form biofilms (reviewed in reference 23).
In this study, we used TnphoA mutagenesis to identify adhesins of E. coli E128012, an atypical EPEC strain of proven pathogenicity (25) that adheres to HEp-2 cells in a localized-like pattern. In all six PhoA-positive, nonadherent mutants that we identified, TnphoA had inserted into the pst operon. The results of subsequent sequence and deletion analysis and trans-complementation studies confirmed that strain E128012 requires pstCA to adhere to HEp-2 and T84 epithelial cells. In addition, by showing that adhesion could be restored to a pstCA mutant of E128012 by inactivating phoB, we established that the contribution of pstCA to bacterial adherence is exerted via the Pho regulon. Similar results were obtained in mouse infection studies with site-directed pstCA and phoB mutants of C. rodentium, thus establishing the role of the Pho regulon in the virulence of some A/E strains of enterobacteria. In addition, our finding that pst mutants of EPEC and C. rodentium grew equally well in high- and low-phosphate medium indicated that phosphate starvation was not responsible for the attenuation of these strains and suggested that, as with some other enteric pathogens, Pho regulation in these bacteria may be effected via cross-regulation by signals other than phosphate concentration (23).
Recently, Ferreira and Spira reported that the pst operon enhances the adhesion of E. coli LRT9, a typical EPEC strain, to cultured epithelial cells (13). They concluded that the reduced cell adherence of a pst mutant of LTR9 was not mediated via the Pho regulon, because adherence was not restored by mutating phoB. They also found that the pst mutant showed reduced expression of the principal adhesins of typical EPEC, namely, Bfp and intimin, partly as a consequence of reduced expression of the per operon, which positively regulates the expression of bfp and several LEE-encoded genes, including eae. Our findings are in broad agreement with those of Ferreira and Spira regarding the requirement by EPEC for an intact pst operon to adhere to epithelial cells and that signaling through pst is probably unrelated to phosphate concentrations, but there are several important points of difference. First, atypical EPEC and C. rodentium lack the adherence factor plasmid, pEAF, and hence do not express Bfp or Per. Second, attenuation of the pst mutants investigated in this study was clearly mediated through the Pho regulon, because in pst mutants of both EPEC and C. rodentium, the wild-type phenotype was restored to the mutants after inactivation of phoB. Third, the attenuation of a pstCA mutant of C. rodentium for mice was evidently not mediated via reduced expression of LEE-encoded genes, given that the expression of two key LEE genes, namely ler and eae, was normal in the pstCA mutant and that the mutant evoked A/E lesions indistinguishable in extent and severity from those induced by the parent strain in the colons of mice (Fig. 4). The observation that mice infected with a pst mutant of C. rodentium developed A/E lesions but not colonic hyperplasia shows that these two pathological outcomes are not interdependent. This confirms our previous observations that a prerequisite of colonic hyperplasia is extensive colonization of the colon by C. rodentium (21, 24) and suggests that any situation which reduces colonization is likely to affect hyperplasia.
In conclusion, we have shown that pst genes acting through the Pho regulon are required by atypical EPEC to adhere to epithelial cells and by C. rodentium to colonize the mouse intestine and cause diarrhea. Although we did not achieve our original aim, namely, to identify novel adhesins of atypical EPEC, our findings indicate that adherence of atypical EPEC and C. rodentium is mediated by one or more adhesins that are negatively regulated either by PhoB itself or by PhoB-regulated genes. We are currently using microarray analysis to identify downstream genes that EPEC and C. rodentium require for adherence.
Acknowledgments
We are grateful to Danijela Krmek and Kim Huett for assisting with C. rodentium infections, to Lee Sau Fung for assistance with tissue culture, and to Ji Yang and Judyta Praszkier for valuable advice.
This work was supported by grants from the Australian National Health and Medical Research Council.
Editor: A. Camilli
Footnotes
Published ahead of print on 2 March 2009.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2003. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 2.Batisson, I., M. P. Guimond, F. Girard, H. An, C. Zhu, E. Oswald, J. M. Fairbrother, M. Jacques, and J. Harel. 2003. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect. Immun. 714516-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter, M. A., and B. D. Jones. 2005. The fimYZ genes regulate Salmonella enterica serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect. Immun. 731377-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, A. G., M. Sola, F. X. Gomis-Ruth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10701-713. [DOI] [PubMed] [Google Scholar]
- 5.Borenshtein, D., M. E. McBee, and D. B. Schauer. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 2432-37. [DOI] [PubMed] [Google Scholar]
- 6.Brickman, E., and J. Beckwith. 1975. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J. Mol. Biol. 96307-316. [DOI] [PubMed] [Google Scholar]
- 7.Brinkley, C., V. Burland, R. Keller, D. J. Rose, A. T. Boutin, S. A. Klink, F. R. Blattner, and J. B. Kaper. 2006. Nucleotide sequence analysis of the enteropathogenic Escherichia coli adherence factor plasmid pMAR7. Infect. Immun. 745408-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli, J., W. Deng, and B. B. Finlay. 2000. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell. Microbiol. 21-9. [DOI] [PubMed] [Google Scholar]
- 9.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 1831259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1589-14. [DOI] [PubMed] [Google Scholar]
- 11.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton. 1998. Standard methods for the examination of water and wastewater, 20th edition, p.4-149-4-150. American Public Health Association, Washington, DC.
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira, G. M., and B. Spira. 2008. The pst operon of enteropathogenic Escherichia coli enhances bacterial adherence to epithelial cells. Microbiology 1542025-2036. [DOI] [PubMed] [Google Scholar]
- 14.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30911-921. [DOI] [PubMed] [Google Scholar]
- 15.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8634-645. [DOI] [PubMed] [Google Scholar]
- 16.Guttman, J. A., F. N. Samji, Y. Li, W. Deng, A. Lin, and B. B. Finlay. 2007. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell. Microbiol. 9131-141. [DOI] [PubMed] [Google Scholar]
- 17.Hart, E., J. Yang, M. Tauschek, M. Kelly, M. J. Wakefield, G. Frankel, E. L. Hartland, and R. M. Robins-Browne. 2008. RegA, an AraC-like protein, is a global transcriptional regulator that controls virulence gene expression in Citrobacter rodentium. Infect. Immun. 765247-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring, C. D., J. D. Glasner, and F. R. Blattner. 2003. Gene replacement without selection: regulated suppression of amber mutations in Escherichia coli. Gene 331153-163. [DOI] [PubMed] [Google Scholar]
- 19.Kaper, J. B. 1996. Defining EPEC. Rev. Microbiol. 27(Suppl. 1)130-133. [Google Scholar]
- 20.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, M., E. Hart, R. Mundy, O. Marches, S. Wiles, L. Badea, S. Luck, M. Tauschek, G. Frankel, R. M. Robins-Browne, and E. L. Hartland. 2006. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 742328-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamarche, M. G., C. M. Dozois, F. Daigle, M. Caza, R. Curtiss III, J. D. Dubreuil, and J. Harel. 2005. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect. Immun. 734138-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamarche, M. G., B. L. Wanner, S. Crépin, and J. Harel. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32461-473. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. F., M. Kelly, A. McAlister, S. N. Luck, E. L. Garcia, R. A. Hall, R. M. Robins-Browne, G. Frankel, and E. L. Hartland. 2008. A C-terminal class I PDZ binding motif of EspI/NleA modulates the virulence of attaching and effacing Escherichia coli and Citrobacter rodentium. Cell. Microbiol. 10499-513. [DOI] [PubMed] [Google Scholar]
- 25.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152550-559. [DOI] [PubMed] [Google Scholar]
- 26.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1821872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundy, R., D. Pickard, R. K. Wilson, C. P. Simmons, G. Dougan, and G. Frankel. 2003. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol. Microbiol. 48795-809. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao, P. S., Y. Yamada, Y. P. Tan, and K. Y. Leung. 2004. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol. Microbiol. 53573-586. [DOI] [PubMed] [Google Scholar]
- 32.Robins-Browne, R. M. 1987. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev. Infect. Dis. 928-53. [DOI] [PubMed] [Google Scholar]
- 33.Robins-Browne, R. M., A.-M. Bordun, M. Tauschek, V. Bennett-Wood, J. Russell, F. Oppedisano, N. A. Lister, K. A. Bettelheim, C. K. Fairley, M. I. Sinclair, and M. E. Hellard. 2004. Atypical enteropathogenic Escherichia coli: a leading cause of community-acquired gastroenteritis in Melbourne, Australia. Emerg. Infect. Dis. 101797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins-Browne, R. M., A. M. Tokhi, L. M. Adams, and V. Bennett-Wood. 1994. Host-specificity of enteropathogenic Escherichia coli from rabbits: lack of correlation between adherence in vitro and pathogenicity for laboratory animals. Infect. Immun. 623329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Scaletsky, I. C. A., J. Michalski, A. G. Torres, M. V. Dulguer, and J. B. Kaper. 2005. Identification and characterization of the locus for diffuse adherence, which encodes a novel afimbrial adhesin found in atypical enteropathogenic Escherichia coli. Infect. Immun. 734753-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 1711870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 842833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobe, T., T. Hayashi, C. G. Han, G. K. Schoolnik, E. Ohtsubo, and C. Sasakawa. 1999. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect. Immun. 675455-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torriani, A. 1966. Alkaline phosphatase from Escherichia coli. p. 224-234. In G. L. Cantoni and R. Davies (ed.), Procedures in nucleic acid research. Harper & Row, Inc., New York, NY.
- 41.Trabulsi, L. R., R. Keller, and T. A. T. Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanBogelen, R. A., E. R. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 1784344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Krüger, W. M., S. Humphreys, and J. M. Ketley. 1999. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate-limitation response and intestinal colonization. Microbiology 1452463-2475. [DOI] [PubMed] [Google Scholar]
- 44.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381. In F. C. Neidhardt, R. I. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. F. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 45.Zhou, L., G. Grégori, J. M. Blackman, J. P. Robinson, and B. L. Wanner. 2005. Stochastic activation of the response regulator PhoB by noncognate histidine kinases. J. Integr. Bioinform. 211-24. [Google Scholar]