Abstract
Bacillus anthracis secretes two bipartite toxins, edema toxin (ET) and lethal toxin (LT), which impair immune responses and contribute directly to the pathology associated with the disease anthrax. Edema factor, the catalytic subunit of ET, is an adenylate cyclase that impairs host defenses by raising cellular cyclic AMP (cAMP) levels. Synthetic cAMP analogues and compounds that raise intracellular cAMP levels lead to phenotypic and functional changes in dendritic cells (DCs). Here, we demonstrate that ET induces a maturation state in human monocyte-derived DCs (MDDCs) similar to that induced by lipopolysaccharide (LPS). ET treatment results in downregulation of DC-SIGN, a marker of immature DCs, and upregulation of DC maturation markers CD83 and CD86. Maturation of DCs by ET is accompanied by an increased ability to migrate toward the lymph node-homing chemokine macrophage inflammatory protein 3β, like LPS-matured DCs. Interestingly, cotreating with LT differentially affects the ET-induced maturation of MDDCs while not inhibiting ET-induced migration. These findings reveal a mechanism by which ET impairs normal innate immune function and may explain the reported adjuvant effect of ET.
Bacillus anthracis secretes three proteins that combine to form two distinct exotoxins, edema toxin (ET) and lethal toxin (LT) (13). These two exotoxins share a receptor-binding subunit, protective antigen (PA), but differ in their catalytic moieties, with the combination of PA plus edema factor (EF) forming ET and the combination of PA plus lethal factor (LF) forming LT. Following secretion, PA binds to host cells via one of two identified cell surface receptors, anthrax toxin receptor 1 (ANTXR1) and anthrax toxin receptor 2 (ANTXR2) (9, 54). PA must be proteolytically activated by host proteases such as furin, which allows for oligomerization and subsequent binding of EF and/or LF (25, 40, 42, 51). The toxin complex is then endocytosed and trafficked to an acidic endosomal compartment, where the low pH triggers a conformational change in PA, leading to an insertion in the endosomal membrane and translocation of EF and LF into the cytosol, where they induce their cytotoxic effects (1, 22, 25, 41, 66, 67).
LF is a zinc-dependent metalloproteinase that cleaves and inactivates mitogen-activated protein kinase kinases (MKKs), thereby blocking signaling through the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and Jun N-terminal protein kinase pathways (16, 61, 62). LT induces cell death in macrophages and dendritic cells (DCs) (3, 16, 22, 47, 49, 62). Independent of cell death, LT also impairs cellular responses such as cytokine secretion, actin-based motility in neutrophils, and endothelial cell barrier function (2, 18, 64).
EF is a calcium- and calmodulin-dependent adenylate cyclase that raises cyclic AMP (cAMP) levels (36, 37). Early work demonstrated that ET inhibits the phagocytic process in neutrophils (45). ET has been shown to cooperate with LT to impair monocyte-derived DC (MDDC) cytokine response and T-lymphocyte (T-cell) activation state (46, 60). In addition, it has been hypothesized that ET acts synergistically with LT to promote death of the host (48, 57).
It is becoming clear that a major role for anthrax toxins is to inhibit immune cell function during infection (8). DCs are potentially early targets of anthrax toxins during the initial stages of B. anthracis infection, given their location at the sites of pathogen entry (11, 60). DCs are potent antigen-presenting cells (APCs) that bridge the innate and adaptive immune responses through direct pathogen neutralization, cytokine production, and T-cell activation (7). These cells are present in most tissues in an immature state, with an enhanced ability for antigen capture. Upon antigen capture, DCs undergo a maturation process and migrate to lymph nodes. Maturation is associated with reduced phagocytic and endocytic capacity, increased cytokine secretion, changes in cell surface markers, including increased membrane expression of major histocompatibility complex class II and costimulatory molecules, and increased T-cell stimulatory function (6, 7). Interestingly, DCs were suggested to contribute to the dissemination of spores through phagocytosis, leading to a systemic B. anthracis infection (10, 12).
Following phagocytosis of B. anthracis spores, DCs initiate a maturation process that is counteracted through the activities of de novo-synthesized anthrax toxins (10-12). ET and LT each target distinct DC cytokine pathways, cooperating to inhibit cytokine secretion (2, 11, 60). In addition, ET may alter DC maturation by raising cAMP levels. Indeed, cAMP analogues or agents that raise cAMP levels (i.e., cholera toxin [CT]) lead to an aberrant maturation of DCs in which some functions associated with mature DCs are altered (23, 24, 33). Given the observations that cAMP-elevating agents induce an altered activation state in DCs, we hypothesized that ET might also modulate the function of these cells. In this study we report on ET-induced phenotypic and functional changes in DCs, including migration of DCs toward the lymph node-homing chemokine, macrophage inflammatory protein 3β (MIP-3β). Given that ET is produced together with LT during infection, we explore how the changes induced by ET are affected by the presence of LT.
MATERIALS AND METHODS
Reagents and toxins.
Dibutyryl cAMP (dcAMP), camptothecin, forskolin, lipopolysaccharide (LPS) from Escherichia coli, and polymyxin B were purchased from Sigma-Aldrich (St. Louis, MO). CT was purchased from List Biological Laboratories (Campbell, CA). Purified LF (30) was provided by J. Mogridge (University of Toronto, Toronto, Ontario, Canada). PA expression plasmid PA-pET22b was kindly provided by John Collier (Harvard Medical School, Cambridge, MA) and transformed into E. coli BL21(DE3) cells. EF and LF(H719C) expression plasmids EF-pET15b and LF(H719C)-pET15b were kindly provided by J. Ballard (Oklahoma University Health Sciences Center, Oklahoma City, OK) and transformed into E. coli BL21(DE3) cells. To produce toxin subunits, a fresh colony of the appropriate E. coli transformant was inoculated into a 20-ml starter culture of Luria Bertani (LB) Lennox medium (EMD Biosciences, Inc., Darmstadt, Germany) with 100 μg/ml ampicillin and grown overnight at 37°C. The following day, a 1:50 dilution was made in a 2-liter baffled Erlenmeyer flask of LB Lennox medium with 100 μg/ml ampicillin (EMD Biosciences, Inc.). The culture was allowed to grow at 37°C with shaking at 250 rpm until an optical density of 1.0 was reached. PA-expressing cultures were then induced with a final concentration of 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) (Gold Biotechnology, St. Louis, MO) and allowed to grow at 30°C with shaking at 250 rpm for 4 h. EF- and LF(H719C)-expressing cultures were induced with a final concentration of 0.1 mM IPTG and allowed to grow at 16°C with shaking at 250 rpm for 16 h. PA was isolated from the periplasm and purified over a Macro-Prep high Q column (Bio-Rad Laboratories, Hercules, CA). EF and LF(H719C) were purified using Ni+ affinity chromatography (Amersham Biosciences, Piscataway, NJ) as previously described (63). Endotoxin was removed from PA, EF, and LF(H719C) protein preparations by using the Detoxi-Gel endotoxin removing gel (Pierce, Rockford, IL). Purified proteins were assayed for endotoxin by using the Limulus amebocyte lysate kit (BioWhittaker, Cottonwood, AZ) that has a detection minimum of 0.03 endotoxin units/ml.
Generation of MDDCs.
Monocytes were isolated from peripheral blood obtained from healthy donors through the Virology Core of the UCLA AIDS Institute (institutional review board approval no. 06-06-102). Monocytes were purified using RosetteSep monocyte enrichment cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) according to manufacturer's protocol. Approximately 5.0 × 106 cells were seeded onto a 100-mm dish and differentiated in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and penicillin-streptomycin-glutamine (PSG) cocktail (Invitrogen, Carlsbad, CA), plus 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and 100 ng/ml interleukin-4 (IL-4) (PeproTech, Rocky Hill, NJ). Fresh media and cytokines were added on day 4, and cultures were maintained for an additional 3 days to complete differentiation. On day 7, nonadherent cells were collected and combined with adherent cells released with calcium- and magnesium-free Dulbecco's phosphate-buffered saline (PBS) (Cellgro, Manassas, VA) plus 1 mM EDTA. Approximately 4.0 × 105 cells per well were seeded onto a 12-well plate and treated as indicated in the figure legends in the presence of 50 ng/ml GM-CSF and 100 ng/ml IL-4.
Analysis of cell surface marker expression.
MDDCs were treated as indicated in the figure legends for 48 h. To measure cell surface marker expression, MDDCs were harvested by scraping and washing with Dulbecco's PBS and were then incubated on ice for 1 h with the following fluorophore-conjugated antibodies: anti-DC-SIGN-fluorescein isothiocyanate (FITC) (R&D Systems, Minneapolis, MN), anti-CD14-phycoerythrin (Caltag, Burlingame, CA), anti-CD83-APC (Caltag), anti-HLA-DR-APC (Caltag), anti-CD86-FITC (Caltag), and anti-CCR7-phycoerythrin (R&D Systems) diluted in media per manufacturer's suggestions. Cells were then washed twice with PBS and fixed in 1% paraformaldehyde. MDDC surface marker expression was analyzed using FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed using Cell Quest (BD) and FlowJo (Tree Star, Inc.) software.
Chemotaxis and invasion assays.
MDDCs were treated as indicated in the figure legends for 48 h. Following treatment, cells were collected from wells and counted. For the chemotaxis assay, RPMI 1640 medium containing 3% FBS and PSG, plus 50 ng/ml GM-CSF, 100 ng/ml IL-4, and 200 ng/ml CCL19 (MIP-3β) (PeproTech), was added to the bottom of a 24-well plate. MDDCs (2.5 × 104 per well) under each treatment condition (in triplicate) were added to the top of an uncoated insert with a pore size of 5 μm (Costar, Lowell, MA) in serum-free RPMI 1640 containing PSG, plus 50 ng/ml GM-CSF and 100 ng/ml IL-4. Cells were allowed to migrate for 4 h at 37°C. For the invasion assay, RPMI 1640 medium containing 3% FBS and PSG, plus 50 ng/ml GM-CSF, 100 ng/ml IL-4, and 200 ng/ml CCL19, was added to the bottom of a 24-well plate. MDDCs ( 2.5 × 104 per well) under each treatment condition (in triplicate) were added to the top of a Matrigel-coated insert with a pore size of 8 μm (BD Biosciences) in RPMI 1640 medium containing 3% FBS and PSG, plus 50 ng/ml GM-CSF and 100 ng/ml IL-4. Cells were allowed to migrate for 22 h at 37°C. Inserts were then removed, cells that had not migrated were aspirated off the top, and cells that had migrated and were adherent to the bottom of inserts were removed with trypsin (Invitrogen). Cells that migrated to the bottom well were collected and pooled with the trypsinized cells. Pooled migrated cells were stained with trypan blue (Invitrogen) and counted using a hemocytometer. Statistical analysis was performed as described in the figure legends using GraphPad PRISM 4 software (GraphPad Software, Inc., La Jolla, CA).
WCE and immunoblotting.
Cells were treated with the indicated toxin and/or drug for the indicated times and washed three times with cold PBS. Cell pellets were lysed on ice for 30 min with MCF7 lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8], 0.1% sodium dodecyl sulfate, 1% Triton X-100, 5 mM MgCl2) containing complete mini protease inhibitor cocktail (Roche, Mannheim, Germany). After centrifugation, the supernatants (whole-cell extracts [WCE]) were saved. Protein concentrations were determined using the Bio-Rad Bradford protein assay reagent (Bio-Rad Laboratories). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed using 5 μg of WCE. Antibodies used were reactive against MEK-2 (N-20) (Santa Cruz Biotechnology, Santa Cruz, CA), α-tubulin (Sigma), procaspase-3 (BD Biosciences) or cleaved caspase-3 (Cell Signaling Technology, Danvers, MA) (procaspase-3 and caspase-3 antibodies were both kindly provided by F. Tamanoi [UCLA]). Secondary antibodies for immunoblotting were horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; Zymed, San Francisco, CA) or horseradish peroxidase-conjugated goat anti-mouse IgG(H+L) (AnaSpec Inc.; San Jose, CA).
RESULTS
ET induces maturation of MDDCs.
During DC maturation, cell surface expression of proteins involved in antigen presentation, DC migration, and T-cell stimulation increases, while expression of markers of immature DCs decreases (6, 7, 50). To determine whether ET induces maturation of DCs in a manner similar to that of LPS, MDDCs were incubated with either LPS or ET for 48 h and then assayed for cell surface phenotype by flow cytometry. For ET challenge, cells and media were pretreated with the lipid A-neutralizing agent polymyxin B (43) prior to addition of toxin as previously determined to ensure that any observed effects are specific to ET and not due to potential LPS contamination (39). Treatment with either ET or LPS led to a reduced expression of DC-SIGN, a marker of immature DCs (Fig. 1A). Furthermore, expression of the DC-specific maturation marker CD83 and the costimulatory molecule CD86 was increased upon treatment (Fig. 1A). As predicted, treating with PA or EF alone did not alter expression of the surface markers tested (data not shown). Furthermore, treating with the cAMP analogue dcAMP (Fig. 1A), CT, or forskolin (Fig. 1B) led to a similar maturation as induced by ET. These results suggest that ET, like other cAMP-inducing agents, can promote maturation of DCs.
FIG. 1.
ET induces maturation of MDDCs. MDDCs were pretreated with 10 μg/ml polymyxin B for 45 min and were then treated with either 100 ng/ml LPS (no pretreatment with polymyxin B), 300 μM dcAMP, 100 ng/ml PA plus 100 ng/ml EF (ET), or no treatment (NT) (A), or 50 μM forskolin, 20 ng/ml of CT, or no treatment (B). Cells were harvested 48 h after treatment and incubated with anti-DC-SIGN-FITC, anti-CD83-APC, and anti-CD86-FITC (panel A only) and then subjected to flow cytometric analysis. The data depicted represent the results for one of six independent experiments conducted on the MDDCs generated from six different donors. The histograms depict data for the unstained sample (shaded histogram), data for the stained sample (solid black line), and the geometric mean fluorescence under each treatment condition.
ET induces migration toward MIP-3β.
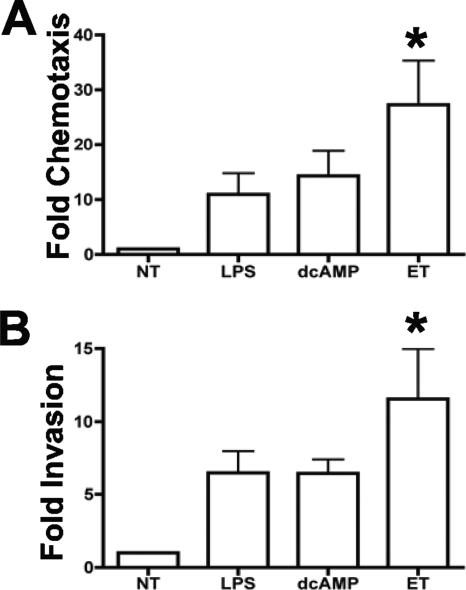
As a consequence of maturation induced upon encountering a pathogen, DCs migrate toward lymph nodes and present processed antigens to T cells in order to prime an adaptive immune response. Migration to secondary lymphoid organs requires chemotaxis toward lymph node chemokines and invasion through extracellular matrix (ECM). Movement through ECM requires the expression of adhesins and metalloproteinases that facilitates migration (34, 58). We, therefore, next sought to determine whether ET-treated DCs chemotax toward the lymph node-homing chemokine, MIP-3β, and whether these ET-treated cells are capable of invading through ECM. MDDCs were treated with LPS, dcAMP, or ET and then assayed for chemotaxis toward MIP-3β in a transwell assay or assayed for their ability to invade through ECM by employing a modified transwell assay in which porous membranes were coated in Matrigel, a biologically active basement membrane matrix. All three treatments induced robust chemotaxis toward MIP-3β and invasion through Matrigel, compared to those induced in untreated DCs (Fig. 2A and B). The low level of migration of untreated DCs is consistent with the lack of responsiveness of immature DCs to MIP-3β. Furthermore, whereas all three treatments induced migration toward MIP-3β, only the migration induced by ET treatment of MDDCs showed statistical significance (P < 0.01) (Fig. 2A and B). Finally, robust migration required the presence of an MIP-3β gradient, as all treatments led to a similar low level of migration as that of untreated cells in the absence of this chemokine (data not shown).
FIG. 2.
ET induces migration toward MIP-3β. MDDCs were pretreated with 10 μg/ml polymyxin B for 45 min and were then treated with either 100 ng/ml LPS (no pretreatment with polymyxin B), 300 μM dcAMP, 100 ng/ml PA plus 100 ng/ml EF (ET), or no treatment (NT). (A) Following 48 h of treatment, the migration of treated MDDCs toward MIP-3β was measured using a transwell assay employing uncoated inserts (5-μm pore). Data are shown as migration compared with that of immature (NT) MDDCs for each donor and are the average increase in migration for six donors ± standard deviation (SD). Statistical significance was calculated using one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test. *, P < 0.01, compared to NT. (B) Following 48 h of treatment, the migration of treated MDDCs for five donors was measured using a transwell assay employing Matrigel-coated inserts (8-μm pore). Data are shown as the increase in invasion compared with that of immature (NT) MDDCs for each donor and are the average values for five donors ± SD. Statistical significance was calculated using one-way ANOVA with Bonferroni's multiple comparison test. *, P < 0.01, compared to NT.
Effects of LT on ET-mediated DC maturation and chemotaxis.
In the context of a B. anthracis infection, DCs are simultaneously exposed to both ET and LT. Because LT inhibits MKK signaling important for DC maturation and migration (2, 49, 60, 65), we next sought to determine whether LT interferes with ET-induced maturation of DCs. First, changes in surface expression markers of treated MDDCs were measured. As expected, LT treatment alone did not induce MDDC maturation (Fig. 3A). Simultaneous treatment with LT did not interfere with the ET-induced decrease in DC-SIGN expression (Fig. 3A). However, the presence of LF did dampen the ET-induced increase in both CD83 and CD86 expression (Fig. 3A). This response was dependent on LF activity since cotreating with a catalytically inactive LF, LF(H719C) (mLT) (32), led to similar CD83 upregulation as that induced by treating with ET alone (Fig. 3B).
FIG. 3.
Effects of LT on ET-mediated DC maturation and chemotaxis. (A and B) MDDCs were pretreated with 10 μg/ml polymyxin B for 45 min and were then treated either with 100 ng/ml PA plus 100 ng/ml EF (ET), 100 ng/ml PA plus 100 ng/ml LF (LT), 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF (ET/LT), or no treatment (NT) (A) or with 100 ng/ml PA plus 100 ng/ml EF (ET), 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF (ET/LT), 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF(H719C) (ET/mLT), or no treatment (NT) (B). Cells were harvested 48 h after treatment and incubated with anti-DC-SIGN-FITC, anti-CD83-APC, and anti-CD86-FITC and then subjected to flow cytometric analysis. The data depicted represent the results for one of five independent experiments conducted on MDDCs generated from five different donors. The histograms depict data for the unstained sample (shaded histogram), data for the stained sample (solid black line), and the geometric mean fluorescence under each treatment condition. (C) MDDCs were pretreated with 10 μg/ml polymyxin B for 45 min and then treated with either 100 ng/ml PA plus 100 ng/ml EF (ET), 100 ng/ml PA plus 100 ng/ml LF (LT), 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF (ET/LT), or 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF(H719C) (ET/mLT). Following 48 h of treatment, the migration of MDDCs toward MIP-3β was measured using a transwell assay employing uncoated inserts (5-μm pore). Data are shown as the increase in migration compared with that of immature (NT) MDDCs for each donor and are the average values for five donors ± SD. Statistical significance was calculated using one-way ANOVA with Bonferroni's multiple comparison test. **, P < 0.05, compared to NT. (D) Cells were pretreated with 10 μg/ml polymyxin B for 45 min and then treated for 2 h with 500 ng/ml PA plus 500 ng/ml EF (ET), 500 ng/ml PA plus 500 ng/ml LF (LT), or 500 ng/ml PA plus 500 ng/ml EF plus 500 ng/ml LF (ET/LT) or were left untreated (NT). WCEs (20 μg/sample) were analyzed by Western blotting using antibodies specific for MEK2 (N terminus) or α-tubulin.
We next sought to determine if MDDCs treated with ET and LT would still migrate toward MIP-3β. LT inhibits Hsp27-dependent neutrophil migration as well as macrophage and T-cell chemotaxis (17, 18, 52). This inhibitory effect is attributed to the ability of LT to inhibit MKK signaling (52, 65) and so could potentially have an inhibitory role in ET-induced MDDC migration. Interestingly, LF did not significantly reduce ET-induced migration (Fig. 3C) even though MEK cleavage is observed (Fig. 3D). Treatment with LT alone did not significantly increase migration to a level above that observed in untreated controls (Fig. 3C), a result that was predicted since LT treatment does not lead to MDDC maturation (Fig. 3A).
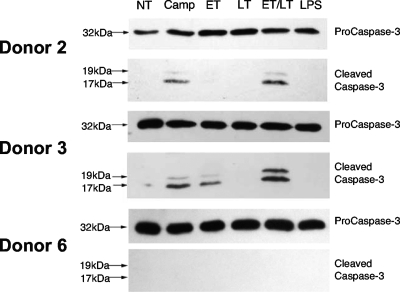
During testing of different donors for the ability of MDDCs to migrate toward MIP-3β, we did not observe a decrease in viability following LT treatment, in contrast to previous reports (3, 49). Interestingly, while ET plus LT (ET+LT) treatment did not decrease viability in the majority of donors tested, one donor (donor 3) reproducibly demonstrated a reduced viability after ET+LT treatment. Upon further inspection, we observed that ET+LT treatment strongly induced caspase-3 activation in donor 3 similar to that induced by the caspase-3-activating compound camptothecin (Fig. 4). Strikingly, the appearance of the 17-kDa and 19-kDa caspase-3 fragments, indicative of activation, varied between donors (Fig. 4). Because decreased MDDC viability would be seen as decreased migration in the assay presented in Fig. 3C, the slight inhibitory effect of LT on ET-mediated migration may be due to either a direct inhibitory effect of LF on migration in all donors or reduced cell viability in a subset of donors. Indeed, when data in Fig. 3C were analyzed so as to exclude donor 3, no difference in migration was seen between ET and ET+LT treatments (data not shown), indicating that LF does not directly influence ET-mediated migration.
FIG. 4.
ET and LT differentially activate caspase-3 in MDDCs. MDDCs were generated from three different healthy donors (donors 2, 3, and 6) and either treated with 100 ng/ml LPS or pretreated with 10 μg/ml polymyxin B for 45 min and then treated with 10 μM camptothecin (Camp) to induce caspase-3-mediated apoptosis, 100 ng/ml PA plus 100 ng/ml EF (ET), 100 ng/ml PA plus 100 ng/ml LF (LT), or 100 ng/ml PA plus 100 ng/ml EF plus 100 ng/ml LF (ET/LT), or no treatment (NT). Following 24 h of treatment, WCEs were generated and analyzed (5 μg/sample) by Western blotting using antibodies specific for procaspase-3 or cleaved caspase-3.
DISCUSSION
DCs are potent APCs with an important role in the development of pathogen-specific immune responses. Phenotypic and functional changes mark the DC maturation response to pathogen and inflammatory stimuli. Here, we demonstrate that in an in vitro system, ET induces maturation of human DCs and migration toward the lymph node-homing chemokine MIP-3β.
Toxins that increase cAMP levels induce expression of DC maturation surface markers, such as CD83, CD86, and HLA-DR (4, 5, 23, 56), a response strictly dependent on cAMP (4, 5). However, this maturation is aberrant, and DCs activated by such toxins are hampered in their ability to produce cytokines, such as IL-12 and tumor necrosis factor alpha. Also, maturation of DCs induced by the combination of LPS and CT or by Bordetella bronchiseptica infection leads to secretion of high levels of the anti-inflammatory cytokine IL-10 (23, 56). In a similar fashion, anthrax ET alters DC cytokine profiles during a B. anthracis infection, with a decrease in tumor necrosis factor alpha and IL-12 secretion and an increase in IL-10 secretion (11, 60).
In this study, we tested the hypothesis that ET promotes DC maturation and determined whether LT interferes with this response. We found that ET induced maturation, as determined by changes in expression of three DC surface markers. Interestingly, while LT did not affect the ET-induced decrease in DC-SIGN expression, it did dampen the increase in both CD83 and CD86 expression. The observed ET-induced DC maturation is accompanied by an increased ability to migrate toward the lymph node-homing chemokine, MIP-3β. MKK signaling plays an important role in chemotaxis (65), and LT inhibits chemotaxis of macrophages, T cells, and neutrophils toward stromal cell-derived factor 1α, MIP-1α, and N-formyl-methionyl-leucyl-phenylalanine, respectively (18, 52). Strikingly, LT did not block ET-induced migration toward MIP-3β even though MEK cleavage is observed. This suggests that ET-induced migration is, in large part, independent of MKK signaling. Further, this differential effect of LT on ET-induced responses suggests that anthrax toxins have evolved to work cooperatively in order to modulate specific immune cell functions.
Although we observed no LT-mediated cell death in MDDCs from the donors tested, we did note that MDDCs from a single donor showed decreased viability following treatment with both ET and LT. Further characterization of cells from this donor demonstrated that treatment with ET activated caspase-3, a key mediator of apoptosis. In addition, caspase-3 activation increased with the addition of LF, suggesting that LT and ET act synergistically to induce DC death. Donor variation in capase-3 activation and cell death implies that additional factors, including host genetic background, influence host response to anthrax toxins. Indeed, genetic background differences have been suggested to influence the manner in which LT mediates DC killing in mice (3).
Anthrax toxins alter immune cell functions, including alteration of the chemotactic response. LT inactivation of MKK signaling leads to inhibition of neutrophil, macrophage, and T-cell chemotaxis (17, 18, 52), while ET inhibits endothelial cell, macrophage, and T-cell migration (29, 52). Interestingly, a recent report demonstrated that ET increases motility of infected macrophages in the presence of a serum gradient (31). The contrasting migratory capacities of ET-treated macrophages observed by the two studies may be due to differences in assay setup, differences in macrophage generation, and/or the chemotactic signal used (31, 52). Indeed, distinct functional subsets of macrophages have been described, and their phenotype may change in response to alterations in their microenvironment (59). It is conceivable that ET may have differential effects on macrophages depending on their phenotypic programming. Our findings are in line with those of Kim et al. (31) and support a role for ET-mediated maturation and induction of migration in monocyte-lineage cell types.
Although cAMP contributes to DC chemotaxis (38, 53), prostaglandin E2, which increases cAMP levels, is not able to significantly promote migration toward lymph node-homing chemokines in the absence of an additional maturation signal (38, 53). In contrast, the ET-induced increase in macrophage motility is dependent only on the ability of EF to raise cAMP levels (31). In the present work, we show that cAMP signaling induced by ET is sufficient to induce DC chemotaxis and invasion through Matrigel. The ability of ET to promote migration toward MIP-3β may be due in part to the potency of the catalytic component of ET (EF) or the manner in which EF induces cAMP signaling (15, 28, 37). Why does ET promote MDDC migration? Given the positioning of DCs at sites of infection, the ability of ET to activate and promote exit of these cells from the periphery may be a mechanism for enhanced dissemination of B. anthracis into sterile tissues or for immune evasion. Recently, it was demonstrated that ET produced by germinating spores promotes migration of macrophages in the presence of a serum gradient (31). It was suggested that ET-induced migration may serve to accelerate the transport of intracellular germinating bacilli to regional lymph nodes, thereby establishing a systemic infection (31). Alveolar macrophages have been implicated in promoting spore dissemination leading to establishment of the systemic infection, although recent studies support a role for lung DCs in this process (10, 12, 26). Thus, in a manner analogous to that proposed by Kim and colleagues (31), spores germinating within DCs may use ET to promote rapid migration from the lung, allowing bacilli to enter the circulatory system. However, it is not clear whether ET is produced before DCs or macrophages exit the lung, and the temporal relationship between germination, toxin production, and phagocyte migration must be tested before conclusions can be drawn regarding the relevance of this model. Alternatively, ET produced during a localized infection, such as is the case for cutaneous anthrax, may serve an immune evasion role by promoting efflux of DCs away from the site of infection.
Regardless of its role in virulence, the ability of ET to promote DC migration toward MIP-3β is important for the potential use of ET as an adjuvant in a similar fashion as that proposed for other cAMP-elevating agents (19, 21). Several bacterial products, including the enterotoxins produced by Vibrio cholerae CT and E. coli heat labile toxin (hLT), function as potent mucosal adjuvants (21, 27). In particular, CT has been shown to promote migration of antigen-loaded DCs to T- and B-cell areas of the Peyer's patches (55). Yet, while CT and hLT are useful adjuvants, their ability to induce watery diarrhea upon oral administration or the link of nasal administration of hLT to effects on the central nervous system may limit their use in humans (35, 44). ET also functions as an adjuvant for mucosal immune responses (19), but unlike hLT, it does not target central-nervous-system tissues, making ET an alternative to the enterotoxin adjuvants (14, 19, 44). Finally, the fact that ET promotes DC migration toward MIP-3β may represent a means to boost the efficacy of vaccines consisting of inactive pathogens, inert particles, or soluble antigens that may not trigger DC migration or maturation (20, 55).
Acknowledgments
This work was funded by National Institutes of Health (NIH) award AI057870 to K.A.B. F.J.M.-A. is supported by the NIH award 5F31AI061837-03. We also acknowledge support of the UCLA Jonsson Comprehensive Cancer Center (JCCC) and the UCLA AIDS Institute and the flow cytometry, virology, mucosal immunology cores, which are supported by the NIH awards CA-16042 and AI-28697 (UCLA-CFAR grant), the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Abrami, L., M. Lindsay, R. G. Parton, S. H. Leppla, and F. G. van der Goot. 2004. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 166645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424329-334. [DOI] [PubMed] [Google Scholar]
- 3.Alileche, A., E. R. Serfass, S. M. Muehlbauer, S. A. Porcelli, and J. Brojatsch. 2005. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS Pathog. 1e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 705533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72962-969. [PubMed] [Google Scholar]
- 6.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18767-811. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 8.Banks, D. J., S. C. Ward, and K. A. Bradley. 2006. New insights into the functions of anthrax toxin. Expert Rev. Mol. Med. 81-18. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414225-229. [DOI] [PubMed] [Google Scholar]
- 10.Brittingham, K. C., G. Ruthel, R. G. Panchal, C. L. Fuller, W. J. Ribot, T. A. Hoover, H. A. Young, A. O. Anderson, and S. Bavari. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 1745545-5552. [DOI] [PubMed] [Google Scholar]
- 11.Cleret, A., A. Quesnel-Hellmann, J. Mathieu, D. Vidal, and J. N. Tournier. 2006. Resident CD11c+ lung cells are impaired by anthrax toxins after spore infection. J. Infect. Dis. 19486-94. [DOI] [PubMed] [Google Scholar]
- 12.Cleret, A., A. Quesnel-Hellmann, A. Vallon-Eberhard, B. Verrier, S. Jung, D. Vidal, J. Mathieu, and J. N. Tournier. 2007. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 1787994-8001. [DOI] [PubMed] [Google Scholar]
- 13.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 1945-70. [DOI] [PubMed] [Google Scholar]
- 14.Couch, R. B. 2004. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 350860-861. [DOI] [PubMed] [Google Scholar]
- 15.Dal Molin, F., F. Tonello, D. Ladant, I. Zornetta, I. Zamparo, G. Di Benedetto, M. Zaccolo, and C. Montecucco. 2006. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 255405-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280734-737. [DOI] [PubMed] [Google Scholar]
- 17.During, R. L., B. G. Gibson, W. Li, E. A. Bishai, G. S. Sidhu, J. Landry, and F. S. Southwick. 2007. Anthrax lethal toxin paralyzes actin-based motility by blocking Hsp27 phosphorylation. EMBO J. 262240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.During, R. L., W. Li, B. Hao, J. M. Koenig, D. S. Stephens, C. P. Quinn, and F. S. Southwick. 2005. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 192837-845. [DOI] [PubMed] [Google Scholar]
- 19.Duverger, A., R. J. Jackson, F. W. van Ginkel, R. Fischer, A. Tafaro, S. H. Leppla, K. Fujihashi, H. Kiyono, J. R. McGhee, and P. N. Boyaka. 2006. Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens. J. Immunol. 1761776-1783. [DOI] [PubMed] [Google Scholar]
- 20.Figdor, C. G., I. J. de Vries, W. J. Lesterhuis, and C. J. Melief. 2004. Dendritic cell immunotherapy: mapping the way. Nat. Med. 10475-480. [DOI] [PubMed] [Google Scholar]
- 21.Freytag, L. C., and J. D. Clements. 2005. Mucosal adjuvants. Vaccine 231804-1813. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander, A. M. 1986. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 2617123-7126. [PubMed] [Google Scholar]
- 23.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 302394-2403. [DOI] [PubMed] [Google Scholar]
- 24.Galgani, M., V. De Rosa, S. De Simone, A. Leonardi, U. D'Oro, G. Napolitani, A. M. Masci, S. Zappacosta, and L. Racioppi. 2004. Cyclic AMP modulates the functional plasticity of immature dendritic cells by inhibiting Src-like kinases through protein kinase A-mediated signaling. J. Biol. Chem. 27932507-32514. [DOI] [PubMed] [Google Scholar]
- 25.Gordon, V. M., K. R. Klimpel, N. Arora, M. A. Henderson, and S. H. Leppla. 1995. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect. Immun. 6382-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidi-Rontani, C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10405-409. [DOI] [PubMed] [Google Scholar]
- 27.Hajishengallis, G., S. Arce, C. M. Gockel, T. D. Connell, and M. W. Russell. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 841104-1116. [DOI] [PubMed] [Google Scholar]
- 28.Hong, J., J. Beeler, N. L. Zhukovskaya, W. He, W. J. Tang, and M. R. Rosner. 2005. Anthrax edema factor potency depends on mode of cell entry. Biochem. Biophys. Res. Commun. 335850-857. [DOI] [PubMed] [Google Scholar]
- 29.Hong, J., R. C. Doebele, M. W. Lingen, L. A. Quilliam, W. J. Tang, and M. R. Rosner. 2007. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J. Biol. Chem. 28219781-19787. [DOI] [PubMed] [Google Scholar]
- 30.Kassam, A., S. D. Der, and J. Mogridge. 2005. Differentiation of human monocytic cell lines confers susceptibility to Bacillus anthracis lethal toxin. Cell Microbiol. 7281-292. [DOI] [PubMed] [Google Scholar]
- 31.Kim, C., S. Wilcox-Adelman, Y. Sano, W. J. Tang, R. J. Collier, and J. M. Park. 2008. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc. Natl. Acad. Sci. USA 1056150-6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 131093-1100. [DOI] [PubMed] [Google Scholar]
- 33.la Sala, A., D. Ferrari, S. Corinti, A. Cavani, F. Di Virgilio, and G. Girolomoni. 2001. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 1661611-1617. [DOI] [PubMed] [Google Scholar]
- 34.Lauffenburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell 84359-369. [DOI] [PubMed] [Google Scholar]
- 35.Lavelle, E. C., A. Jarnicki, E. McNeela, M. E. Armstrong, S. C. Higgins, O. Leavy, and K. H. Mills. 2004. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J. Leukoc. Biol. 75756-763. [DOI] [PubMed] [Google Scholar]
- 36.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 793162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leppla, S. H. 1984. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 17189-198. [PubMed] [Google Scholar]
- 38.Luft, T., M. Jefford, P. Luetjens, T. Toy, H. Hochrein, K. A. Masterman, C. Maliszewski, K. Shortman, J. Cebon, and E. Maraskovsky. 2002. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 1001362-1372. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado-Arocho, F. J., J. A. Fulcher, B. Lee, and K. A. Bradley. 2006. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol. Microbiol. 61324-337. [DOI] [PubMed] [Google Scholar]
- 40.Milne, J. C., S. R. Blanke, P. C. Hanna, and R. J. Collier. 1995. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 15661-666. [DOI] [PubMed] [Google Scholar]
- 41.Milne, J. C., and R. J. Collier. 1993. pH-dependent permeabilization of the plasma membrane of mammalian cells by anthrax protective antigen. Mol. Microbiol. 10647-653. [DOI] [PubMed] [Google Scholar]
- 42.Mogridge, J., K. Cunningham, and R. J. Collier. 2002. Stoichiometry of anthrax toxin complexes. Biochemistry 411079-1082. [DOI] [PubMed] [Google Scholar]
- 43.Morrison, D. C., and D. M. Jacobs. 1976. Inhibition of lipopolysaccharide-initiated activation of serum complement by polymyxin B. Infect. Immun. 13298-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350896-903. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien, J., A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paccani, S. R., F. Tonello, R. Ghittoni, M. Natale, L. Muraro, M. M. D'Elios, W. J. Tang, C. Montecucco, and C. T. Baldari. 2005. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 201325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 2972048-2051. [DOI] [PubMed] [Google Scholar]
- 48.Pezard, C., P. Berche, and M. Mock. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 593472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reig, N., A. Jiang, R. Couture, F. S. Sutterwala, Y. Ogura, R. A. Flavell, I. Mellman, and F. G. van der Goot. 2008. Maturation modulates caspase-1-independent responses of dendritic cells to anthrax lethal toxin. Cell Microbiol. 101190-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Relloso, M., A. Puig-Kroger, O. M. Pello, J. L. Rodriguez-Fernandez, G. de la Rosa, N. Longo, J. Navarro, M. A. Munoz-Fernandez, P. Sanchez-Mateos, and A. L. Corbi. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 1682634-2643. [DOI] [PubMed] [Google Scholar]
- 51.Ren, G., J. Quispe, S. H. Leppla, and A. K. Mitra. 2004. Large-scale structural changes accompany binding of lethal factor to anthrax protective antigen: a cryo-electron microscopic study. Structure 122059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi Paccani, S., F. Tonello, L. Patrussi, N. Capitani, M. Simonato, C. Montecucco, and C. T. Baldari. 2007. Anthrax toxins inhibit immune cell chemotaxis by perturbing chemokine receptor signalling. Cell Microbiol. 9924-929. [DOI] [PubMed] [Google Scholar]
- 53.Scandella, E., Y. Men, S. Gillessen, R. Forster, and M. Groettrup. 2002. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 1001354-1361. [DOI] [PubMed] [Google Scholar]
- 54.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 1005170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shreedhar, V. K., B. L. Kelsall, and M. R. Neutra. 2003. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer's patches. Infect. Immun. 71504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skinner, J. A., A. Reissinger, H. Shen, and M. H. Yuk. 2004. Bordetella type III secretion and adenylate cyclase toxin synergize to drive dendritic cells into a semimature state. J. Immunol. 1731934-1940. [DOI] [PubMed] [Google Scholar]
- 57.Smith, H., and H. B. Stoner. 1967. Anthrax toxic complex. Fed. Proc. 261554-1557. [PubMed] [Google Scholar]
- 58.Sternlicht, M. D., and Z. Werb. 2001. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17463-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stout, R. D., C. Jiang, B. Matta, I. Tietzel, S. K. Watkins, and J. Suttles. 2005. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175342-349. [DOI] [PubMed] [Google Scholar]
- 60.Tournier, J. N., A. Quesnel-Hellmann, J. Mathieu, C. Montecucco, W. J. Tang, M. Mock, D. R. Vidal, and P. L. Goossens. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 1744934-4941. [DOI] [PubMed] [Google Scholar]
- 61.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352(3)739-745. [PMC free article] [PubMed] [Google Scholar]
- 62.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248706-711. [DOI] [PubMed] [Google Scholar]
- 63.Voth, D. E., E. E. Hamm, L. G. Nguyen, A. E. Tucker, I. I. Salles, W. Ortiz-Leduc, and J. D. Ballard. 2005. Bacillus anthracis oedema toxin as a cause of tissue necrosis and cell type-specific cytotoxicity. Cell Microbiol. 71139-1149. [DOI] [PubMed] [Google Scholar]
- 64.Warfel, J. M., A. D. Steele, and F. D'Agnillo. 2005. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 1661871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong, M. M., and E. N. Fish. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 155-14. [DOI] [PubMed] [Google Scholar]
- 66.Zhang, S., A. Finkelstein, and R. J. Collier. 2004. Evidence that translocation of anthrax toxin's lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc. Natl. Acad. Sci. USA 10116756-16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, S., E. Udho, Z. Wu, R. J. Collier, and A. Finkelstein. 2004. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys. J. 873842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]