Abstract
There is ample evidence that Staphylococcus aureus capsular polysaccharide (CP) promotes virulence. Loss of capsule expression, however, may lead to S. aureus persistence in a chronically infected host. This study was conducted to determine the relative prevalence of nonencapsulated S. aureus in patients with chronic and acute osteomyelitis. Only 76/118 (64%) S. aureus isolates from patients with osteomyelitis expressed CP, whereas all 50 isolates from blood cultures of patients with infections other than osteoarticular infections expressed CP (P = 0.0001). A significantly higher prevalence of nonencapsulated S. aureus was found in patients with chronic osteomyelitis (53%) than in those with acute osteomyelitis (21%) (P = 0.0046). S. aureus isolates obtained from multiple specimens from five of six patients with chronic osteomyelitis exhibited phenotypic (expression of CP, α-hemolysin, β-hemolysin, slime, and the small-colony variant phenotype) and/or genotypic (pulsed-field gel electrophoresis and spa typing) differences. Nonencapsulated S. aureus was recovered from at least one specimen from each chronic osteomyelitis patient. Fourteen isolates obtained from two patients with acute osteomyelitis were indistinguishable from each other within each group, and all produced CP5. In conclusion, we demonstrated that nonencapsulated S. aureus is more frequently isolated from patients with chronic osteomyelitis than from those with acute osteomyelitis, suggesting that loss of CP expression may be advantageous to S. aureus during chronic infection. Our findings on multiple S. aureus isolates from individual patients allow us to suggest that selection of nonencapsulated S. aureus is likely to have occurred in the patient during long-term bone infection.
The development of prosthetic joints has been one of the biomedical success stories of the 20th century. However, despite improvements in surgical techniques, implantation of medical devices is associated with a definite risk of bacterial infection. Among the different etiological agents causing prosthetic device-associated infections, Staphylococcus aureus emerges as one of the most prevalent and difficult-to-eradicate pathogens (25). This pathogen has acquired resistance to virtually all of the antimicrobial agents available, and in recent years, the worldwide emergence of multiresistant clones in hospitals and communities has spurred significant concern (32). S. aureus pathogenicity is due to a vast array of virulence factors, many of which are of broad functionality with considerable redundancy, allowing staphylococci to readily adapt to various environmental niches. There is still no clear knowledge, however, about the individual factors associated with the chronicity of S. aureus infections. Experimental studies conducted in our laboratory with a mouse model of staphylococcal mastitis demonstrated that lack of capsular polysaccharide (CP) expression results in an increased capacity for persistence in the host (35). This capacity was attributed to more effective interaction between unmasked staphylococcal surface ligands and cell receptors, leading to internalization into epithelial cells (8, 35). Studies performed with clinical S. aureus isolates from bovines with subclinical mastitis revealed a high prevalence of strains that express neither CP serotype 5 (CP5) nor CP8 (33). The potential medical importance of S. aureus strains that do not express CP5 or CP8 (nontypeable, NT) has been underscored (10). The major mechanisms responsible for lack of S. aureus CP expression have been thoroughly described and include point mutations in the cap5(8) promoter or in 1 of the 11 genes essential for capsule production (10). Other strains have mutations in genes that regulate capsule expression, such as agr or arlRS (10). In one particular region, many NT strains of S. aureus from bovine mastitis fail to produce CP because the cap5(8) locus suffered an IScap-mediated deletion (36). We hypothesize that loss of CP5 (CP8) expression provides S. aureus a selective advantage for persistence in certain infected hosts. If this is so, other hosts with chronic S. aureus infections would also exhibit a high prevalence of NT variants.
The present study was conducted to determine the relative prevalence of NT S. aureus in 118 patients with chronic versus acute osteomyelitis. In addition, we investigated multiple S. aureus isolates from eight different patients suffering from chronic or acute S. aureus osteomyelitis, which resulted in the recovery of different S. aureus CP phenotypes from the same patients.
MATERIALS AND METHODS
Bacterial isolates.
Individual S. aureus isolates were obtained from 118 patients with osteomyelitis at seven hospitals in Argentina (four in Buenos Aires City, two in Buenos Aires Province, and one in the City of Santa Fe) to assess the prevalence of encapsulated (CP5 and CP8) and NT S. aureus. Sixty-six of these patients suffered from chronic osteomyelitis, 33 suffered from acute osteomyelitis, and the type of osteomyelitis (acute or chronic) remained undefined in 19 patients. The criteria for the diagnosis of chronic osteomyelitis described by Cierny and Mader (9) were used. According to these criteria, the hallmarks of chronic osteomyelitis include a nidus of infected dead bone or scar tissue, an ischemic soft tissue envelope, and a refractory clinical course. In the present study, patients with chronic osteomyelitis were infected for periods exceeding 6 months (range, 6 months to 33 years). Patients with acute infections were infected for <6 months. A total of 50 S. aureus isolates were obtained by blood culture from patients at two hospitals in Buenos Aires City with a diagnosis of an acute disease other than osteoarticular infection. In addition, multiple clinical specimens were obtained from seven patients at the Hospital de Clínicas José de San Martín, Universidad de Buenos Aires, Buenos Aires, Argentina, and one patient at the Policlínico Bancario, Buenos Aires, Argentina, in 2006 and 2007. These eight patients suffered from osteomyelitis, and samples of bone and adjacent tissues were obtained for bacteriological evaluation during surgical procedures.
Phenotypic evaluation.
Isolation and identification of S. aureus were performed according to routine procedures used in the clinical bacteriology laboratory (4). Subcultures of single colonies of homogeneous size and pigmentation from primary isolation agar plates were performed with Trypticase soy agar. Homogeneous colonies from subculture plates were frozen in brain heart infusion-glycerol (20%) until further use. The species was confirmed as S. aureus by PCR amplification of species-specific sequences according to Martineau et al. (23) and/or the S. aureus-specific nuc gene (6). CP5 and CP8 production was assessed by colony immunoblotting and confirmed by immunodiffusion as described elsewhere (20). Isolates yielding no positive precipitation with anti-CP5 or anti-CP8 antibodies in the immunodiffusion test were classified as NT (10). Production of α- and β-hemolysin was assessed by evaluating hemolysis in rabbit and goat blood agar plates, respectively. S. aureus RN6390 was used as a positive standard. Slime production was assessed by production of black colonies on Congo red agar (1). Antibiotic susceptibility was tested according to CLSI recommendations. Auxotrophism of a small-colony variant (SCV) isolate was assayed by evaluation of supplemented growth on chemically defined medium agar around disks impregnated with thymidine, hemin, and menadione as described previously (16).
Genotypic analysis.
Genomic DNA was extracted from S. aureus and purified by a standard procedure (27). The clonality of S. aureus isolates was determined by SmaI pulsed-field gel electrophoresis (PFGE) (34) with a CHEF-DR II apparatus (Bio-Rad, Hercules, CA) as previously described (31). The similarity between PFGE types was evaluated by the Dice coefficient. The resultant matrix was analyzed by the unweighted-pair group method using average linkages, and data were analyzed with the TREECON software for Windows. Sequencing of the polymorphic X region of the protein A gene (spa typing) was performed by a standard procedure as described previously (19). The presence of the cap genes that define the CP5 and CP8 serotypes (cap5H to -J and cap8H to -K, respectively) was ascertained by PCR amplification with primers described elsewhere (36). S. aureus Reynolds (CP5) and Becker (CP8) were used as positive standards.
Statistical analysis.
The distribution of strains in the different experimental groups was analyzed by the Fisher exact test. P values of <0.05 were considered significant. Prism 4.0 software (GraphPad) was used for all calculations.
RESULTS
Higher prevalence of NT S. aureus in patients with chronic osteomyelitis.
Assessment of CP expression on 118 S. aureus isolates from patients with osteomyelitis revealed that only 76 (64%) of the isolates expressed CP (57 CP5 and 19 CP8) whereas all 50 isolates from blood cultures of patients with infections other than osteoarticular infections expressed CP (33 CP5 and 17 CP8) (Table 1). The proportion of CP5+/CP8+ S. aureus isolates from patients with osteomyelitis did not differ significantly from that of isolates recovered by blood culture. Evaluation of 99 isolates from patients with precise diagnosis (chronic or acute osteomyelitis) revealed a significantly higher prevalence of NT S. aureus in patients with chronic osteomyelitis (53%) than in those with acute osteomyelitis (21%) (Table 2). Amplification analysis of the cap genes that determine the CP serotype specificity revealed that 24 (57%) NT S. aureus isolates carried the cap5 genes whereas 18 (43%) of the isolates carried the cap8 genes. The higher proportion of NT isolates in patients with chronic infections was still significant when the analysis was performed within groups of isolates bearing the cap5 or the cap8 genes (Table 2).
TABLE 1.
Encapsulated and NT S. aureus isolates recovered from patients with osteomyelitis or isolated from the blood of patients with a focus of infection other than osteoarticular infection
| Infection | No. (%) of encapsulated isolates, no. CP5+/no. CP8+a | No. (%) NT | Total no. | P valueb |
|---|---|---|---|---|
| Osteoarticular | 76 (64), 57/19c | 42 (36) | 118 | |
| Other (blood culture) | 50 (100), 33/17c | 0 (0) | 50 | 0.0001 |
The values in parentheses represent isolates that express CP as ascertained by colony immunoblotting and immunodiffusion.
Statistical significance of difference, Fisher exact test.
The difference in the proportion of CP5+/CP8+ S. aureus isolates between the two groups (osteoarticular infection versus other) was not statistically significant.
TABLE 2.
Encapsulated and NT S. aureus isolates from patients with chronic or acute osteomyelitisa
| Osteomyelitis | No. (%) of encapsulated isolatesb | No. (%) of NT isolates | Total no. | P valuec |
|---|---|---|---|---|
| Chronic | 31 CP+ (47) | 35 (53) | 66 | 0.0046 |
| Acute | 26 CP+ (79) | 7 (21) | 33 | |
| Chronic | 25 CP5+ (54) | 21 (46)d | 46 | 0.027 |
| Acute | 16 CP5+ (84) | 3 (16)d | 19 | |
| Chronic | 6 CP8+ (30) | 14 (70)e | 20 | 0.035 |
| Acute | 10 CP8+ (71) | 4 (29)e | 14 |
The proportion of capsulated (CP+) and NT S. aureus isolates between groups of patients with chronic and acute disease was also compared within each CP serotype. A total of 99 patients were included.
The values represent isolates that express CP as ascertained by colony immunoblotting and immunodiffusion.
Statistical significance of differences between groups of isolates from patients with acute versus chronic osteomyelitis, Fisher exact test.
These NT isolates exhibited positive PCR amplification for cap5-specific genes.
These NT isolates exhibited positive PCR amplification for cap8-specific genes.
Multiple S. aureus isolates from selected chronically infected patients.
A number of S. aureus isolates were obtained from multiple samples of patients with chronic or acute osteomyelitis. Results from each patient are described separately below and in Table 3. The order in which patients are described in the text follows the importance of the findings in each group of S. aureus strains under scrutiny. The first six patients (identified as no. 85, 92, 94, 95, 107, and 110) suffered from chronic osteomyelitis, whereas the remaining two (no. 100 and 111) suffered from acute osteomyelitis.
TABLE 3.
Forty-one S. aureus isolates from multiple specimens from selected patients with chronic or acute osteomyelitisa
| Osteomyelitis and strain | Sample | α-Hemolysin | β-Hemolysin | CP serotype | cap allele | Slime productione | Susceptibility to methicillinf | Pulsotypeb | spa type |
|---|---|---|---|---|---|---|---|---|---|
| Chronic | |||||||||
| 85a | Bone (purulent secretion) | + | + | CP8 | cap8 | + | S | D | t189 |
| 85b | Bone (tibia, distal) | + | + | CP5 | cap5 | + | R | A9 | t149 |
| 85c | Fibrous soft tissue | − | − | NT | cap8 | + | S | D | t189 |
| 85d | Bone (tibia, proximal) | + | + | NT | cap5 | + | R | A9 | t149 |
| 85e | Bone (transplant) | − | − | NT | cap8 | + | S | D | t189 |
| 85f | Bone (tibia) | − | − | NT | cap8 | + | S | D | t189 |
| Chronic | |||||||||
| 92a | Soft tissue adjacent to bone | − | − | CP8 | cap8 | + | S | C | t065 |
| 92b | Cranial bone | + | + | CP8 | cap8 | + | S | C | t065 |
| 92c | Cranial plate | − | − | CP8 | cap8 | ++ | S | C | t2271 |
| Chronic | |||||||||
| 94a | Hip bone | + | + | CP5 | cap5 | +++ | R | A7 | t149 |
| 94b | Bone cement | + | + | CP5 | cap5 | +++ | R | A7 | t149 |
| 94c | Soft tissue adjacent to bone | + | − | CP5 | cap5 | +++ | R | A7 | t149 |
| 94d | Fistulous purulent secretion | − | − | NT | cap5 | +++ | R | A7 | t149 |
| Chronic | |||||||||
| 95a | Articular fluid | + | + | NT | cap8 | + | S | E1 | t2271 |
| 95b | Bone (astragalus) | + | + | NT | cap8 | + | S | E1 | t2271 |
| 95c | Bone (tibia) | + | + | NT | cap8 | + | S | E1 | t2271 |
| Chronic | |||||||||
| 107a | Bone cement | − | − | CP5 | cap5 | − | R | A2 | t3946 |
| 107a1c | Bone cement | − | − | CP5 | cap5 | +++ | R | A3 | t149 |
| 107b | Bone (femur) | + | + | CP5 | cap5 | − | R | A8 | t149 |
| 107c | Soft tissue adjacent to bone | − | − | NT | cap5 | +++ | R | A5 | Undefinedd |
| 107d | Hip purulent secretion | − | − | CP5 | cap5 | +++ | R | A4 | t149 |
| 107e | Cotyloid cement | + | + | CP5 | cap5 | + | R | A4 | t149 |
| 107f | Cotyloid membrane | − | − | CP5 | cap5 | +++ | R | A6 | t149 |
| Chronic | |||||||||
| 110a | Bone membrane (tibia) | − | − | NT | cap8 | +++ | S | E2 | t3796 |
| 110b | Bone membrane (femur) | − | − | NT | cap8 | +++ | S | E3 | t3796 |
| 110c | Bone cement | − | − | NT | cap8 | +++ | S | E3 | t3796 |
| 110d | Bone (femur) | − | − | NT | cap8 | +++ | S | E3 | t3796 |
| Acute | |||||||||
| 100a | Hip purulent aspirate | + | + | CP5 | cap5 | + | S | B | t002 |
| 100b | Cotyloid membrane | + | + | CP5 | cap5 | + | S | B | NDg |
| 100c | Bone cement | + | + | CP5 | cap5 | + | S | B | ND |
| 100d | Soft tissue adjacent to bone | + | + | CP5 | cap5 | + | S | B | ND |
| Acute | |||||||||
| 111a | Superficial secretion | + | + | CP5 | cap5 | + | R | A1 | t311 |
| 111b | Superficial secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111c | Superficial secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111d | Superficial secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111e | Superficial secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111f | Foot secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111g | Deep secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111h | Deep secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111i | Bone (tibia) secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
| 111j | Bone (fibula) secretion | + | + | CP5 | cap5 | + | R | A1 | ND |
All of the strains produced positive PCR amplification for sequences specific for S. aureus and positive amplification for nuc. These 41 isolates are different from the 99 described in Table 2.
Pulsotype denomination arbitrarily defined for this paper.
Isolate 107a-1 exhibited the SCV phenotype.
DNA extracted from strain 107c did not yield any PCR amplification product with the primers routinely used for spa. It did, however, yield PCR amplification of specific S. aureus sequences (17).
Levels of slime production: −, absent; +, light; ++, moderate; +++, heavy.
S, susceptible; R, resistant.
ND, not done.
(i) Isolates from patient 85.
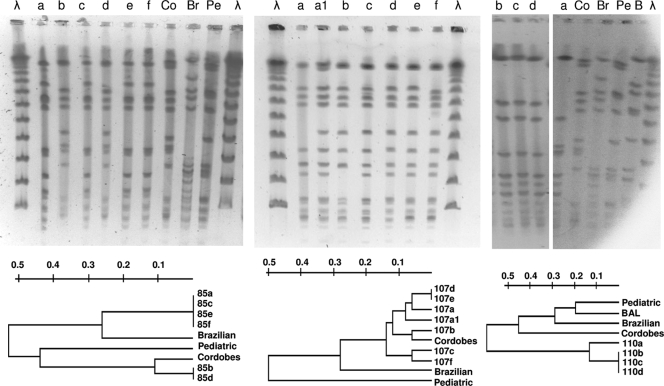
Specimens were obtained from a 20-year-old man with chronic osteomyelitis of the right tibia (Table 3). S. aureus 85a was isolated from a fistulous purulent secretion. Thirteen months later, four samples obtained during a right tibial surgical debridement yielded a culture positive for S. aureus (isolates 85b through 85e). Finally, 4 months afterward, one last S. aureus isolate (85f) was recovered from a specimen from the infected tibia. Strains 85a, 85c, 85e, and 85f belonged to spa type t189; exhibited the same pulsotype, D (Fig. 1, left panel); were susceptible to methicillin; and produced small amounts of slime (Table 3). Strain 85a was CP8+ and produced α- and β-hemolysin, whereas strains 85c, 85e, and 85f were NT and did not express α- and β-hemolysin. These three NT strains carried the cap8 genes, and CP8+ strain 85a had been isolated 13 months before strains 85c, 85e, and 85f. These results indicate that these three NT strains may have derived from CP8+ strain 85a during infection due to a mutation in a regulatory gene (10). On the same day that strains 85c and 85e were recovered, strains 85b and 85d were isolated from two other separate specimens (Table 3). Strains 85b and 85d exhibited the same spa type (t149) and pulsotype (A9), were resistant to methicillin (Fig. 1, left panel), and were mild slime producers. Strain 85b was CP5+, whereas strain 85d was NT. These results plus the facts that both 85b and 85d produced α- and β-hemolysin and that NT strain 85d carried the cap5 genes indicate that NT strain 85d may have derived from strain 85b due to a mutation not involving a regulatory gene. Other conclusions from patient 85 strains are that (i) the selection of NT S. aureus was irrespective of the CP serotype involved, (ii) strains of distinct phenotypes and genotypes can be isolated from the same patient, and (iii) concomitant infection of the patient with different clones of S. aureus (methicillin-resistant S. aureus [MRSA] and methicillin-susceptible S. aureus) can occur during chronic infection.
FIG. 1.
SmaI PFGE band patterns of S. aureus strains isolated from patients 85 (left panel), 107 (center panel), and 110 (right panel). Lanes λ, lambda ladder. Dendrograms show the similarity among isolates from each patient. Representatives of the prevalent Brazilian (Br), Pediatric (Pe), and Cordobes (Co) MRSA clones in Argentina were included in the analysis. BAL is a representative strain of a new emerging MRSA clone in Buenos Aires, obtained from a respiratory specimen of a child with cystic fibrosis (Liliana Jordá Vargas, personal communication).
(ii) Isolates from patient 107.
Six S. aureus isolates identified as strains 107a through 107f were obtained from six samples taken from a 74-year old woman with prosthesis-associated chronic fistulous osteomyelitis of the femur (Table 3). All of these strains displayed the same typical colony morphology and pigmentation following incubation for 24 h at 37°C. All of the S. aureus strains from patient 107 expressed CP5, except one strain (107c), which was NT. Strain 107c carried the cap5 genes and exhibited a pulsotype (A5) of high homology (96%) to those of isolates 107a, 107d, 107e, and 107f (Fig. 1, center panel). There was no correlation between CP5 and hemolysin production. Indeed, CP5+ non-SCV phenotype strains 107b and 107e produced both α- and β-hemolysin whereas CP5+ strains 107a, 107d, and 107f did not express hemolysin activity. These results suggest that the genetic lesion responsible for the 107c strain's lack of CP5 expression may have not been a mutation in a main regulatory gene and that 107c may have evolved from 107a, 107d, or 107f or that strains 107a, 107c, 107d, and 107f may have evolved from a close CP5+ common ancestor. After primary culture of the bone cement sample for 72 h, small, nonhemolytic, nonpigmented colonies were detected in addition to a normal phenotype strain (namely, 107a). These tiny colonies (named strain 107a-1) failed to grow on mannitol-salt agar and yielded a negative coagulase reaction at 4 h but a positive reaction at 18 h. Positive amplification with S. aureus-specific sequences (23) suggested that these colonies represent SCVs (29). The isolate was resistant to gentamicin (MIC = 6 μg/ml), as the remaining six S. aureus isolates obtained from this patient (MICs of 6 to 10 μg/ml). The SCVs retained this phenotype by the seventh passage on blood agar and later started to revert to the normal phenotype. Further characterization of the strain 107a-1 SCV phenotype revealed auxotrophism to hemin (39). Although strains 107a and 107a-1 (SCV) were isolated from the same primary culture plate, they exhibited different PFGE band patterns (ca. 96% similarity). The data also suggest that strain 107c may have not resulted from superinfection with an exogenous strain. The PFGE band patterns and the genetic relatedness of these isolates of the seven strains from patient 107 are depicted in Fig. 1 (center panel). Only two of the seven strains were not discriminated by PFGE analysis (107d and 107e) but were differentiated by the production of α- and β-hemolysin (Table 3). All seven strains exhibited a similarity of greater than 88% and therefore were considered subtypes of the same pulsotype (Table 3). All of the strains from patient 107 were resistant to methicillin and were spa type t149, except strain 107c, which yielded negative PCR amplification with spa and therefore could not be spa typed by the routine procedure. The seven S. aureus strains showed different levels of slime production.
(iii) Isolates from patient 110.
Four S. aureus isolates were obtained from four specimens taken during the surgical debridement of a 79-year-old woman with prosthesis-associated chronic osteomyelitis of the left knee (Table 3). The isolates (110a through 110d) were NT, bore the cap8-specific allele, did not express hemolysins, were susceptible to methicillin, and were heavy slime producers. Strains 110b, 110c, and 110d presented the same pulsotype (E3) (Table 2 and Fig. 1, right panel). Strain 110a (pulsotype E2) exhibited only 78% homology to strains 110b, 110c, and 110d. All four strains from patient 110 belonged to the t3796 spa type group.
(iv) Isolates from patient 94.
Four S. aureus isolates were obtained from a 69-year-old woman with prosthesis-associated chronic osteomyelitis of the left hip (Table 3). Strains 94a, 94b, and 94c expressed CP5, whereas strain 94d was NT. The NT strain bore the cap5-specific allele. The four strains from patient 94 were genealogically indistinguishable from each other, as ascertained by their pulsotype (PFGE gel not shown) and spa type (t149); were resistant to methicillin; and were heavy slime producers. Two isolates expressed hemolysins (94a and 94b), whereas another isolate (94d) did not. Strain 94c expressed α-hemolysin but did not express β-hemolysin (Table 3). These results suggest that NT strain 94d may have emerged as a result of mutation in a regulatory gene (9).
(v) Isolates from patient 92.
Three S. aureus isolates were obtained during the surgical debridement of a 45-year-old man with prosthesis-associated chronic cranial osteomyelitis (Table 3). These strains expressed CP8, but only strain 92b expressed hemolysins. The three strains exhibited the same pulsotype (C), but strain 92c belonged in a spa type group (t2271) different from that of strains 92a and 92b (t065). The three isolates were susceptible to methicillin and exhibited little or moderate slime production.
(vi) Isolates from patient 95.
Three S. aureus isolates were obtained from three specimens taken during the surgical debridement of a 71-year-old man with prosthesis-associated chronic osteomyelitis of the ankle bones (Table 3). There were no phenotypic differences among isolates 95a through 95c. The three strains from patient 95 were NT, carried the cap8 allele, exhibited the same pulsotype (E1) and spa type (t2271), produced a small amount of slime, and were susceptible to methicillin.
Multiple S. aureus isolates from selected acutely infected patients. (i) Isolates from patient 100.
Four S. aureus isolates were obtained from four specimens taken during surgery from a 72-year-old woman (patient 100) with prosthesis-associated acute osteomyelitis of the hip bone (Table 3). These S. aureus strains (100a through 100d) showed no phenotypic differences from each other, and all expressed CP5. These four strains exhibited the same pulsotype (B). Only strain 100a was spa typed (t0002). No differences in the level of slime production were found, and all four isolates were susceptible to methicillin.
(ii) Isolates from patient 111.
Ten S. aureus isolates were obtained from 10 specimens taken during surgery from a 14-year-old boy with acute osteomyelitis of the ankle bones involving the fibula and tibia (Table 3). There were no phenotypic differences among the 10 isolates (111a through 111j). The 10 S. aureus strains from patient 111 expressed CP5 and exhibited the same pulsotype (A1). The spa type of strain 111a was determined (t0002). No differences in the level of slime production were found, and all 10 isolates were resistant to methicillin.
DISCUSSION
S. aureus CP5 or CP8 is produced by ∼80% of human isolates (15). Evidence that CP5 and CP8 promote staphylococcal virulence has come from in vitro experiments, as well as from studies performed with diverse animal models of S. aureus infection and colonization. The experimental studies performed and the major results obtained are described in recent reviews by O'Riordan and Lee (26) and Lee and Lee (22). Loss of capsule expression, however, may play a role in S. aureus persistence in the chronically infected host (8, 35, 36). It has also been shown that NT S. aureus is noticeably prevalent in bovines with subclinical mastitis (long-term infection) and could be as high as 86% (33). Here we demonstrate that NT S. aureus is more frequently isolated from patients with chronic osteomyelitis than from those with acute osteomyelitis. The role of CP in acute invasive infection was corroborated by the finding that all of the S. aureus isolates obtained by blood culture from patients with diseases other than osteomyelitis expressed either CP5 or CP8.
Studies performed with an animal model of mammary infection and with cultures of a bovine epithelial cell line led to the conclusion that loss of capsule expression may give S. aureus an advantage to persist intracellularly (8, 35, 37). The analysis of S. aureus isolates from different specimens obtained from the six patients with chronic osteomyelitis described in this report revealed that, except for one (patient 92), all of them had at least one specimen culture positive for NT S. aureus. The pulsotypes of the NT isolates were identical or exhibited high homology to those of encapsulated (CP5 or CP8) S. aureus isolated from the same patient, suggesting a common ancestor. In one patient (no. 85), an encapsulated S. aureus strain was isolated from an infected bone and in another specimen from the same patient obtained 13 months later, an S. aureus isolate of the same pulsotype and spa type was obtained, but this time it exhibited an NT phenotype. Conversely, two patients with acute osteomyelitis harbored only encapsulated S. aureus of homogeneous pulsotypes (4 and 10 isolates each, pulsotypes B and A1, respectively). Furthermore, S. aureus isolates obtained from the blood of 50 patients with acute disease were all encapsulated. Taken together, our results permit two concurrent hypotheses, one from the evolutionary standpoint and another from the pathogenesis standpoint, to explain the mechanism leading to the high prevalence of NT S. aureus in patients with chronic osteomyelitis.
From the evolutionary standpoint, it can be hypothesized that long-term infection is required for selection of NT S. aureus. This hypothesis involves the concept that NT strains are the consequence of long-term chronic osteomyelitis. From our results, it became apparent that multiple S. aureus isolates from patients suffering from chronic osteomyelitis over long periods of time displayed, in most cases, phenotypic and genotypic diversity. In contrast, isolates from two acutely infected bone and adjacent tissue samples were totally homogeneous in phenotype and genotype. These results support the hypothesis that S. aureus adapts to continuously changing microenvironments in bone and adjacent soft tissue, resulting in the selection of strains with distinct phenotypic features in the chronically infected host. Similar diversity in the S. aureus population was found by Goerke et al. in sputum samples from cystic fibrosis patients who are chronically infected with S. aureus (12). Goerke et al. (12) explained their results, as we can explain ours, by the “insurance hypothesis,” which predicts that a more diverse bacterial community will be better able to resist external stress (5). Therefore, the ability of S. aureus to adapt to changing conditions in the invaded tissue microenvironments during chronic infection would be the key mechanism that permits persistence in the infected host. Many factors present in infected tissues may promote the selection of a bacterial phenotype better adapted for persistence and may be responsible for the diversity of the S. aureus strains isolated from different niches of the same chronically infected patient at the same time. These factors include varying concentrations of CO2 and iron, redox potential, nutrient availability (26), and antimicrobial agents (17), among others. Only two factors, however, have been clearly demonstrated to pose selective pressure for an S. aureus persistent phenotype. These are antibodies to CP (37), which select for NT variants and SCVs, and gentamicin, which selects for SCVs (3, 7). S. aureus SCVs represents a subpopulation that exhibits, among other phenotypic features, a characteristic slow-growth phenotype (30). The clinical importance of S. aureus SCVs as causative agents facilitating persistent and recurrent infections which are refractory to antibiotic treatment has been recognized (29, 30). Osteoarticular tissues (38), as well as the lungs of cystic fibrosis patients (17), have been shown to provide excellent niches with microenvironmental conditions adequate for the selection of S. aureus variants (NT strains and SCVs) which infect and persist over extremely long periods.
From the pathogenesis standpoint, it can also be hypothesized that the lack of capsule or loss of CP expression promotes the establishment of chronic, long-term osteomyelitis. This hypothesis involves the speculation that the emergence of NT strains is the cause of chronic osteomyelitis, not the consequence. It is known that successful adaptation of S. aureus to the host is achieved by regulatory mechanisms in the short term and by inheritable shifts in the population over the long term (12). At the early stages of infection, CP may be required by S. aureus to invade healthy tissue. Replication of S. aureus would provoke changes in the infected tissue, thus creating microenvironments responsible for distinct signals, which would be recognized by the bacteria. The S. aureus cap5/8 locus is under the control of an extremely complex regulatory network (22, 26). Whether these changing signals downregulate the production of CP in vivo in infected bone remains unknown and requires further research. Several studies have demonstrated the expression of CP in vivo (13, 18, 21, 28). Minimal expression of CP5 was observed, however, in tissues obtained from cystic fibrosis patients chronically infected with S. aureus (14, 24). Downregulation of CP expression may generate bacteria with unstable NT phenotypes that are better able to reach the intracellular milieu through a surface adhesin-receptor interaction than are encapsulated bacteria. These regulatory NT variants plus other newly generated stable NT mutants may be the key to persistence. In the presence of antibodies to CP and over a long period of time, stable NT S. aureus would start to emerge at the infection site (37). Beyond the mechanisms responsible for loss of CP expression (inheritable or regulatory), NT S. aureus bacteria can more easily reach the intracellular milieu (8, 35, 37), where they would avoid further removal by antibodies and persist. Our results support both hypotheses (from the evolutionary and pathogenesis standpoints) since it is equally true that the NT phenotype helps S. aureus to become intracellular and persist and that simultaneously long-term infection promotes the selection of NT S. aureus.
Finally, our results also raise the issue of the clinical impact of phenotypic differences in multiple S. aureus isolates from a single infected patient. Whereas criteria for the bacteriological diagnosis of bone infection are generally accepted (2, 11), the phenotypic differences found in multiple S. aureus isolates from a patient with chronic osteomyelitis may have relevance for antimicrobial susceptibility testing. Indeed, in this study, MRSA and methicillin-susceptible S. aureus were isolated from different specimens of the same chronically infected patient. Therefore, in order to ensure selection of the adequate antimicrobial agent for a patient with a chronic bone infection, susceptibility testing should be performed on isolates representative of each specimen with an S. aureus-positive culture.
In conclusion, we demonstrated that NT S. aureus is more frequently isolated from patients with chronic osteomyelitis than from those with acute osteomyelitis, suggesting that loss of CP expression may be advantageous to S. aureus during chronic infection. Our study of multiple S. aureus isolates from individual patients allowed us to suggest that loss of CP expression is likely to have occurred in the patient during long-term bone infection, although one case of reinfection with a different clone of S. aureus was found. An additional observation was that representatives of a homogeneous population of S. aureus can be isolated from multiple clinical samples from patients with acute osteomyelitis. Conversely, a mostly heterogeneous S. aureus population composed of strains with dissimilar phenotypic features and potential different diagnostic relevance was found in multiple specimens from the same patient with chronic osteomyelitis, suggesting the need for adequate guidelines for the clinical bacteriological diagnosis of these patients.
Acknowledgments
This study was partially supported by grants from the Agencia Nacional de Promoción de la Ciencia y la Tecnología, Argentina (ANPCYT PICT 05-32577); the Secretaría de Ciencia y Técnica, Universidad de Buenos Aires, Argentina (UBACyT M-070); and the German Federal Ministry for Education and Science (BMBF) in the context of the Network SkINStaph.
We thank Silvia C. Predari (Instituto de Investigaciones Médicas A. Lanari, UBA, Buenos Aires, Argentina), Adriana N. Procopio (Hospital de Niños R. Gutiérrez, Buenos Aires, Argentina), Emilce Méndez (Hospital J. M. Cullen, Santa Fé, Argentina), and Patricia Vidal (Hospital Ramón Carrillo, Ciudadela, and Santorio Quilmes, Provincia de Buenos Aires, Argentina) for providing some of the S. aureus isolates described in Table 1 of the present study.
We thank Lorena Medina for her dedicated technical assistance.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 9 March 2009.
REFERENCES
- 1.Arciola, C. R., D. Campoccia, S. Gamberini, M. Cervellati, E. Donati, and L. Montanaro. 2002. Detection of slime production by means of and optimised Congo red agar plate test based on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials 234233-4239. [DOI] [PubMed] [Google Scholar]
- 2.Atkins, B. L., N. Athanasou, J. J. Deeks, D. W. M. Crook, H. Simpson, T. E. A. Peto, P. McLardy-Smith, A. R. Berendt, and the OSIRIS Collaborative Study Group. 1998. Prospective evaluation criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J. Clin. Microbiol. 362932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 1701033-1037. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman, T. L. 2003. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically, p. 384-404. In P. R. Murray (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 5.Boles, B. R., M. Thoendel, and P. K. Singh. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. USA 10116630-16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brakstad, O. G., K. Aasbakk, and J. A. Maeland. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 301654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouillette, E., A. Martínez, B. J. Boyll, N. E. Allen, and F. Malouin. 2004. Persistence of a Staphylococcus small colony variant under antibiotic pressure inn vivo. FEMS Immunol. Med. Microbiol. 4135-41. [DOI] [PubMed] [Google Scholar]
- 8.Buzzola, F. R., L. P. Alvarez, L. P. N. Tuchscherr, M. S. Barbagelata, S. M. Lattar, L. Calvinho, and D. O. Sordelli. 2007. Differential abilities of capsulated and noncapsulated Staphylococcus aureus isolates from diverse agr groups to invade mammary epithelial cells. Infect. Immun. 75886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cierny, G., and J. T. Mader. 1984. Adult chronic osteomyelitis. Orthopedics 71557-1564. [DOI] [PubMed] [Google Scholar]
- 10.Cocchiaro, J. L., M. I. Gómez, A. Risley, R. Solinga, D. O. Sordelli, and J. C. Lee. 2006. Molecular characterization of the capsule locus from nontypeable Staphylococcus aureus. Mol. Microbiol. 59948-960. [DOI] [PubMed] [Google Scholar]
- 11.Garvin, K. L., and A. D. Hanssen. 1995. Current concepts review: infection after total hip arthroplasty. J. Bone Jt. Surg. Br. Vol. 77A1576-1588. [DOI] [PubMed] [Google Scholar]
- 12.Goerke, C., M. Gressinger, K. Endler, C. Breitkopf, K. Wardecki, M. Stern, C. Wolz, and B. C. Kahl. 2007. High phenotypic diversity in infecting but not in colonizing Staphylococcus aureus populations. Environ. Microbiol. 93134-3142. [DOI] [PubMed] [Google Scholar]
- 13.Hensen, S. M., M. J. Pavicic, J. A. Lohuis, J. A. de Hoog, and B. Poutrel. 2000. Location of Staphylococcus aureus within the experimentally infected bovine udder and the expression of capsular polysaccharide type 5 in situ. J. Dairy Sci. 831966-1975. [DOI] [PubMed] [Google Scholar]
- 14.Herbert, S., D. Worlitzsch, B. Dassy, A. Boutonnier, J.-M. Fournier, G. Bellon, A. Dalhoff, and G. Döring. 1997. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J. Infect. Dis. 176431-438. [DOI] [PubMed] [Google Scholar]
- 15.Hochkeppel, H. K., D. G. Braun, W. Vischer. A. Imm, S. Sutter, U. Staeubli, R. Guggenheim, E. L. Kaplan, A. Boutonnier, and J. M. Fournier. 1987. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J. Clin. Microbiol. 25526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 1771023-1029. [DOI] [PubMed] [Google Scholar]
- 17.Kahl, B. C., A. Duebbers, G. Lubritz, J. Haeberle, H. G. Koch, B. Ritzerfeld, M. Reilly, E. Harms, R. A. Proctor, M. Herrmann, and G. Peters. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 414424-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiser, K. B., J. M. Cantey-Kiser, and J. C. Lee. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 675001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koreen, L., S. V. Ramaswamy, E. A. Graviss, S. Naidich, J. M. Musser, and B. N. Kreiswirth. 2004. Spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, J. C., M.-J. Liu, J. Parsonnet, and R. D. Arbeit. 1990. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 282612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 611853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, C. Y., and J. C. Lee. 2006. Staphylococcal capsule, p. 456-463. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 23.Martineau, F., F. J. Picard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 1998. Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 36618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Döring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 2841523-1527. [DOI] [PubMed] [Google Scholar]
- 25.Moreillon, P., Y. I. Que, and M. P. Glauser. 2005. Staphylococcus aureus, p. 2321-2351. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Elsevier-Churchill Livingstone, Philadelphia, PA.
- 26.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8151-156. [Google Scholar]
- 28.Poutrel, B., P. Rainard, and P. Sarradin. 1997. Heterogeneity of cell-associated CP5 expression on Staphylococcus aureus strains demonstrated by flow cytometry. Clin. Diagn. Lab. Immunol. 4275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proctor, R. A., P. van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 2095-102. [DOI] [PubMed] [Google Scholar]
- 30.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4295-305. [DOI] [PubMed] [Google Scholar]
- 31.Quelle, L. S., A. Corso, M. Galas, and D. O. Sordelli. 2003. STAR gene restriction profile analysis in epidemiological typing of methicillin-resistant Staphylococcus aureus: description of the new method and comparison with other polymerase chain reaction (PCR)-based methods. Diagn. Microbiol. Infect. Dis. 47455-464. [DOI] [PubMed] [Google Scholar]
- 32.Shams, W. E., and R. P. Rapp. 2004. Methicillin-resistant staphylococcal infections: an important consideration for orthopedic surgeons. Orthopedics 27565-568. [DOI] [PubMed] [Google Scholar]
- 33.Sordelli, D. O., F. R. Buzzola, M. I. Gómez, L. Steele-Moore, D. Berg, E. Gentilini, M. Catalano, A. J. Reitz, T. Tollersrud, G. Denamiel, P. Jeric, and J. C. Lee. 2000. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: genetic and epidemiologic analysis. J. Clin. Microbiol. 38846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover, F. C., R. D. Arbeit, G. Archer, J. Biddle, S. Byrne, R. Goering, G. Hancock, G. A. Hébert, B. Hill, R. Hollis, W. R. Jarvis, B. Kreiswirth, W. Eisner, J. Maslow, L. K. McDougal, J. M. Miller, M. Mulligan, and M. A. Pfaller. 1994. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J. Clin. Microbiol. 32407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuchscherr, L. P. N., F. R. Buzzola, L. P. Alvarez, R. L. Caccuri, J. C. Lee, and D. O. Sordelli. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 737932-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuchscherr, L. P. N., M. I. Gómez, F. R. Buzzola, L. F. Calvinho, J. C. Lee, and D. O. Sordelli. 2007. Characterization of a new variant of IS257 prevalent among bovine isolates of Staphylococcus aureus in Argentina. Infect. Immun. 755483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuchscherr, L. P. N., F. R. Buzzola, L. P. Alvarez, J. C. Lee, and D. O. Sordelli. 2008. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small colony variants of Staphylococcus aureus in mice. Infect. Immun. 765738-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 251250-1251. [DOI] [PubMed] [Google Scholar]
- 39.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Götz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 1794706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]