Abstract
Considerable effort is being made to understand the acute and memory antibody responses in natural cholera infection, while rather less is known about the roles of cellular immune responses involving T and B lymphocytes. We studied responses in adult patients hospitalized with cholera caused by Vibrio cholerae O1. Peripheral blood mononuclear cells from patients (n = 15) were analyzed by flow cytometry after stimulation with V. cholerae O1 membrane protein (MP) or toxin-coregulated pilus antigen (TcpA). The gamma interferon (IFN-γ) and interleukin-13 (IL-13) responses in stimulated-lymphocyte supernatants were studied. The responses were compared with those of healthy controls (n = 10). Patients responded with increased frequencies of gut-homing CD4+ T cells (CD4+ β7+), gut-homing CD8+ T cells (CD8+ β7+), and gut-homing B cells (CD19+ β7+) at the early and/or late convalescent stages compared to the acute stage. After stimulation with MP or TcpA, proliferation of CD4+ and CD8+ T cells was increased at the acute stage and/or early convalescent stage compared to healthy controls. Increased IL-13 and IFN-γ responses were observed after antigenic stimulation at the acute and convalescent stages compared to healthy controls. Thus, increases in the levels of gut-homing T and B cells, as well as involvement of CD8 and CD4 Th1-mediated (IFN-γ) and CD4 Th2-mediated (IL-13) cytokine responses, take place in acute dehydrating disease caused by V. cholerae O1. Further studies are needed to determine if such responses are also stimulated after immunization with oral cholera vaccines and if these responses play a role in protection following exposure to cholera.
Vibrio cholerae O1 is a common causative agent of acute watery diarrhea in children and adults in the developing world (1, 3, 10, 19). After colonizing the proximal small intestine, this bacterium produces cholera toxin, which induces a profuse secretory diarrhea. Cholera remains a key public health problem that results in epidemics in resource-poor settings.
It is believed that the immune response to cholera is initiated by antigen presentation in the Peyer's patches of the gastrointestinal mucosa, followed by migration of the stimulated antigen-specific B cells to regional lymph nodes and differentiation of these cells into specific antibody-secreting cells (28). Stimulation of the common mucosal immune system leads to production of both local and systemic antibodies (2, 15, 27) to virulence antigens of V. cholerae (25, 28).
Natural cholera infection is believed to give rise to long-term protection against subsequent disease. Robust systemic and mucosal antibodies are produced to the V. cholerae lipopolysaccharide, to cholera toxin, and to colonization factors, including the major subunit of the toxin-coregulated pilus, TcpA (2, 24, 25, 28). We have recently shown that there is induction of memory B-cell responses following infection, which may play a role in longer-lasting protection (14). In addition, recent evidence suggests that an innate component of the immune system may also play a role in the host response to cholera (9, 22, 26). Studies with experimental animals have shown that the mucosal immune response to cholera toxin is T cell dependent and that CD4 T helper cells have an important role (7, 12, 13). However, not much is known about the role of the adaptive cellular immune responses in patients with cholera. The aim of the present study was to decipher the role of T- and B-cell-mediated immune responses in natural cholera infection in adults hospitalized with dehydrating illness, who were followed from the acute stage to convalescence.
MATERIALS AND METHODS
Study group and recruitment.
V. cholerae O1-infected adult male patients (n = 15) who were between 18 and 49 years old were recruited into this study from the hospital of the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh (ICDDR, B), and the degree of dehydration and clinical severity of the disease in the patients were assessed by a physician (30, 31). Ten apparently healthy adult males in the same age group with the same socioeconomic status as the patients and with no history of diarrhea during the previous 3 months were also studied as healthy controls, as in previous studies (26). The study was approved by the Ethical Review Committee of the ICDDR, B.
Sample collection and processing.
Venous blood was collected from patients at the acute stage on the second day of hospitalization (day 2) and on days 7 and 21 after the onset of disease. Single blood samples were collected from the healthy controls. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by density gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden) (27).
Bacteriological examination of patient stools.
Stools from patients with the characteristic rice watery appearance were plated directly on taurocholate-tellurite-gelatin agar and MacConkey agar for culturing V. cholerae (30, 31). V. cholerae O1 was identified by agglutination with specific monoclonal antibodies, and strains were serotyped as Ogawa or Inaba strains (23). Stools were also examined to detect other enteric pathogens, including other Vibrio species, enterotoxigenic Escherichia coli, Salmonella, Shigella, and Campylobacter spp., and were examined directly by microscopy for detection of cyst and vegetative forms of protozoa and ova of helminths (31). The stools of the patients were also tested on subsequent study days. Before recruitment of healthy controls, stools were screened for the enteric bacteria and parasites mentioned above; an individual was not enrolled if he was positive for bacterial pathogens or if he had moderate to high numbers of ova of helminths or protozoa, such as Entamoeba histolytica or giardia.
Antigens used in this study.
Membrane preparations (MP) of V. cholerae O1 Inaba strain T19479 (5 μg/ml) and Ogawa strain X25049 (5 μg/ml) and of E. coli K-12 (5 μg/ml) containing a mixture of bacterial antigens were used (11). Recombinant toxin-coregulated pilus (TcpA) (5 μg/ml) (2) was prepared as previously described (21). Phytohemagglutinin (PHA) (1 μg/ml; Murex, England) was used as a positive control for proliferation of cells and stimulation of cytokine production. All the antigens described above were tested with the study subjects at each of the follow-up points.
Stimulation of PBMCs and analyses by flow cytometry.
To detect the distribution of different phenotypes of lymphocytes, PBMCs (105 cells/sample) were stimulated and then stained for analysis of various cell surface markers with combinations of the following antibodies: anti-CD3-fluorescein isothiocyanate (FITC) (clone SK7), anti-CD4-peridinin-chlorophyll-protein complex (SK3), anti-CD8-FITC (SK1), anti-CD19-FITC (4G7), anti-CD25-allophycocyanin (APC) (2A3), and integrin β7-phycoerythrin (all from BD Pharmingen, San Diego, CA), as well as anti-CD8-APC (UCHT-4; Diatec, Oslo, Norway). Cells were fixed in formaldehyde before flow cytometry was performed with a FACSCalibur instrument equipped with blue and red lasers (BD, San Jose, CA). Mononuclear cells were first gated in forward and side scatter, and then different subpopulations were gated according to different staining patterns; the results were expressed as frequency of expression.
To assess T-cell activation and proliferation in response to cholera antigens, PBMCs were stained with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Vybrant CFDA SE cell tracer kit; Molecular Probes Europe BV, Leiden, The Netherlands) for 15 min, following which cells were stimulated with MP antigens (V. cholerae T19479 and X25049 or E. coli K-12), TcpA, or PHA (at the antigen and mitogen concentrations indicated above) using 2 × 105 cells/100 μl in U-bottom tissue culture plates (Nunc, Denmark). Supernatants were collected after 6 days of stimulation, and cells were stained with anti-β7-phycoerythrin, anti-CD4-peridinin-chlorophyll-protein complex, and anti-CD8-APC. The proliferative response was detected as an incremental loss of CFSE staining intensity (20), and data were analyzed using FlowJo software (TreeStar Inc.). Gamma interferon (IFN-γ) (MABTECH, Sweden) and interleukin-13 (IL-13) (BD Biosciences, Sweden) levels in culture supernatants were measured using enzyme-linked immunosorbent assay kits.
In additional experiments, PBMCs from a subset of cholera patients (n = 3) were further analyzed to determine the T-cell subset responsible for cytokine production. Briefly, PBMCs (107 cells/ml) and anti-CD4 or anti-CD8 Dynabeads were mixed at a 1:1 ratio in glass tubes, incubated for 20 min at 4°C, and separated with a DYNAL MPC-6 magnet (Dynabeads; Dynal AS, Norway). Both the positive and negative populations were stimulated with antigens as described above and analyzed by flow cytometry, and cell culture supernatants were tested for IFN-γ and IL-13.
Humoral immune responses in cholera patients.
The vibriocidal antibody response and cholera toxin-specific immunoglobulin A (IgA) and IgG antibody responses in study participants were studied using previously described procedures (28). An individual with a fourfold increase in the vibriocidal antibody titer at day 7 or day 30 compared to the titer at baseline was considered a responder. For the cholera toxin-specific responses, a twofold increase compared with the baseline was considered a positive response. Vibriocidal responses in healthy controls were also studied.
Statistical analyses.
The Wilcoxon signed-rank test was used to compare immunological responses of cholera patients on different study days, and the Mann-Whitney U test was used to evaluate comparisons of immune responses for patients and controls. A two-tailed P value of ≤0.05 was considered the cutoff for a significant difference. The GraphPad Prism 4 statistical software (GraphPad Software, Inc.) was used for statistical analyses and for preparing figures.
RESULTS
Clinical history of the study group.
All of the cholera patients enrolled suffered from severe dehydration at the time of arrival at the hospital. V. cholerae O1 Inaba serotype (n = 4) and Ogawa serotype (n = 11) were isolated from stools of these patients. Stool microscopy showed a low frequency of different enteric parasites at the acute stage; these parasites included Ascaris lumbricoides (n = 2), Giardia lamblia (n = 1), E. histolytica (n = 1), and Trichuris trichuria (n = 1). At convalescence (day 7 or day 21 following the onset of illness), no enteric bacterial pathogens were detected, while the frequencies of enteric parasites were similar to the original frequencies. No bacterial pathogens were isolated from stools of the healthy controls, although G. lamblia and A. lumbricoides were detected in one control each.
Distribution of T-lymphocyte subsets after the onset of cholera.
The frequency of total CD3+, CD4+, and CD8+ T cells was unchanged over the course of the disease. However, the frequency of activated T cells (CD25low) was increased during the course of disease compared to the frequency in healthy controls (P = 0.003 to P= 0.005), and peak levels occurred on day 2 (P = 0.04 for a comparison with day 7).
Expression of gut-homing lymphocyte subsets after the onset of cholera.
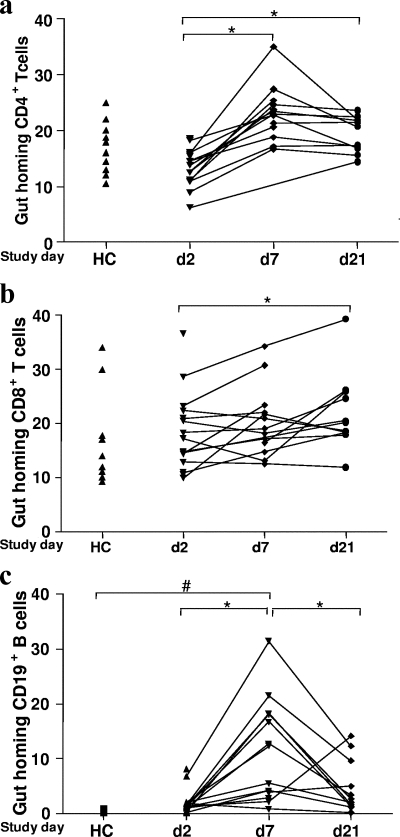
The percentage of gut-homing CD4+ T cells was greater on days 7 and 21 than on day 2 (P = 0.004 to P = 0.0004) (Fig. 1); the percentage of β7+ CD8+ T cells peaked at day 21 (P = 0.03). We also carried out statistical analyses by removing a single subject with a very high level of β7+ CD8+ T cells on day 2, and the difference remained statistically significant (P = 0.03) (Fig. 1b).
FIG. 1.
Expression of gut-homing T (a and b) and B (c) cells in blood after cholera infection and in healthy controls. Flow cytometric analyses were carried out using separated PBMCs for quantification of β7-expressing T and B cells. Lymphocytes were first gated in forward and side scatter, and then T and B cells, respectively, were gated according to the staining pattern. Levels are shown for patients during the acute stage on day 2 (d2) and during convalescence on day 7 (d7) and day 21 (d21) after the onset of diarrhea, as well as for healthy controls (HC). The symbols indicate the percentages of different cells. *, P < 0.05 for a comparison of patients on different days following infection; #, P < 0.05 for a comparison of patients in different phases of the disease and healthy controls. The paired t test and Mann-Whitney U test were used for statistical analysis where necessary.
There was a sevenfold increase in the frequency of gut-homing B cells on day 7 (P = 0.03 to P = 0.001). The frequency declined by day 21 (P = 0.03) but remained higher than the frequency at the acute stage of disease (P = 0.05) or in healthy controls (P = 0.02) (Fig. 1).
Proliferation of T cells in response to V. cholerae antigens.
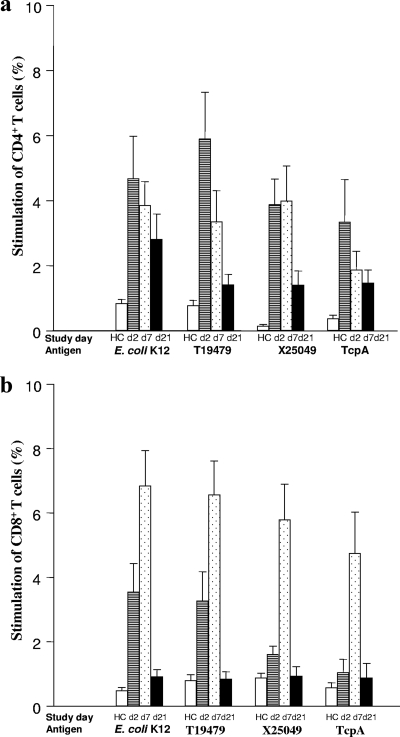
Stimulation with V. cholerae antigens (MP and TcpA) resulted in a >4-fold increase in proliferation of CD4+ T cells on day 2 of disease compared to the results obtained for healthy controls (Fig. 2). A stronger response was seen on day 2 than on day 21 (P = 0.02 to P = 0.03). Stimulation with MP of E. coli also resulted in proliferative responses.
FIG. 2.
Antigen-induced stimulation of PBMCs with membrane protein antigens of V. cholerae (T19479 and X25049) and E. coli K-12, as well as TcpA: responses of CD4 (a) and CD8 (b) T cells after stimulation at the acute stage on day 2 (d2) and at convalescence on day 7 (d7) and day 21 (d21) after the onset of diarrhea. Lymphocytes were first gated in forward and side scatter, and then T and B cells, respectively, were gated according to the staining pattern. Data for healthy controls (HC) are also shown. The proportions of cells that underwent cell division after stimulation with antigens based on incorporation of CFSE are indicated. Each bar indicates the frequency for different cells for patients or healthy controls (geometric means and standard errors of the means are shown). The paired t test and Mann-Whitney U test were used for statistical analysis where necessary.
The CD8+ T cells showed a different pattern of stimulation. Similar to the results for CD4+ T cells, both V. cholerae and E. coli antigens resulted in increased proliferation on day 2 compared to healthy controls, but unlike the results for CD4+ T cells, the peak of the response for CD8+ T cells occurred by day 7 (7- to 10-fold increase on day 7 compared to day 21) (Fig. 2). The proliferative response to the polyclonal activator PHA did not change over the course of disease, and the stimulation index was between 20- and 25-fold (data not shown) at each time point in the study. Similar levels of stimulation with PHA were seen in patients and healthy controls.
Cytokine responses in stimulated PBMCs.
The highest levels of IFN-γ were produced by lymphocytes on days 7 and 21 following stimulation with V. cholerae as well as E. coli antigens (Fig. 3). Higher levels of IFN-γ were produced in PBMCs obtained from cholera patients than in PBMCs obtained from healthy controls (P = 0.02). On the other hand, IL-13 levels were generally higher in lymphocytes recovered from day 2 of disease following antigenic stimulation (Fig. 3).
FIG. 3.
Levels of IFN-γ (a) and IL-13 (b) secretion by stimulated PBMCs obtained from cholera patients and healthy controls. The bars indicate geometric means, and the error bars indicate standard errors of the means. *, P < 0.05 for a comparison of patients on day 2 and day 7; ♣, P < 0.05 for a comparison of patients on day 2 and day 21; ⧫, P < 0.05 for a comparison of patients on day 7 and day 21; #, P < 0.05 for a comparison of patients at different phases of the disease and healthy controls (HC). The paired t test and Mann-Whitney U test were used for statistical analysis where necessary.
The level of production of IL-13 in CD4+-depleted PBMCs was low and was only 25% of the level seen in nondepleted cells (P = 0.04). There was no difference in IL-13 production between nondepleted and CD8-depleted cells (Fig. 4). Thus, the CD4+ T cells were largely responsible for the production of IL-13 (Fig. 4).
FIG. 4.
Determination of IL-13 levels in culture supernatants of fractionated CD4+ and CD8+ T cells after stimulation with TcpA at the acute stage of disease. The bars indicate geometric means, and the error bars indicate standard errors of the means. *, P < 0.05 for a comparison of different cell subsets.
Antibody responses in sera.
Over 93% patients responded with vibriocidal antibodies by day 7 (13/15) or day 30 (14/15) following disease onset. Sera obtained from healthy controls contained no vibriocidal antibodies (the titer was 5 in sera from all 10 volunteers). Similarly, over 90% of the patients responded to cholera toxin by day 7 after the onset of disease in both IgA and IgG isotypes.
DISCUSSION
We have previously analyzed the effects of dehydrating cholera infection on the humoral and innate immune systems in both the systemic and mucosal compartments (22, 27, 28). To our knowledge, this study is the first study to determine the kinetics of cellular responses in cholera patients.
Adaptive B-cell and innate immune responses are elevated both in the mucosal compartment and in the systemic circulation following cholera (19, 22, 26, 28), and the responses in the circulation can be used as a proxy for gut responses, based on the concept of the common mucosal immune system (15, 27, 28). We therefore followed the distribution of different T- and B-cell subsets in the circulation following cholera.
Most striking were the differences in β7-expressing gut-homing lymphocytes during the course of the disease. The levels of the β7-expressing CD19+ B cells and CD4+ T cells increased dramatically in the blood of patients with cholera, peaking at day 7. The level of the gut-homing CD8+ T cells peaked at day 21. This suggests that B cells are activated in the secondary lymphoid organs of the intestinal mucosa and then detected in the blood within 1 week after initiation of infection, with subsequent homing to mucosal effector sites. These findings fit well with our previous observation of a peak of cholera-specific B cells in peripheral blood at the early convalescent stage of disease (27). There was no difference between healthy controls and patients at the acute stage of disease in the levels of gut-homing CD4+ T cells. The reason why the levels of CD4+ T cells are lower at the acute stage than in healthy controls may be because of the initial shift of CD4+ T cells to the mucosal compartment in response to the infecting pathogen. Compared with the gut-homing CD19+ B cells, an increase was seen at day 7, and then there was a decrease at day 21, possibly because of the cells homing back to the mucosa. We observed different patterns for these two different cell types. Moreover, we also observed different levels of gut-homing T and B cells in the healthy controls.
There were increased proliferative responses of both CD4+ and CD8+ T cells to stimulation by cholera antigens at the acute stage of infection. There were responses to both V. cholerae MP, which are heterogeneous mixtures of antigens, including lipopolysaccharide, as well as to a specific cholera antigen, TcpA. CD4+ T-cell activation was observed early following the onset of cholera in the present study; in addition, increased proliferation of CD8+ T cells to cholera antigens was observed at day 7. Involvement of intestinal CD8+ T cells in response to cholera has been described previously (8), and the present results support these findings. The reason for the differences in the kinetics of the CD8+ and CD4+ T-cell responses is unclear. However, it is possible that these differences may be due to the requirement for CD4+ T cells to help activate CD8+ T cells.
Stimulation was also seen with E. coli MP, suggesting that there is a general responsiveness to bacterial antigens following severe dehydrating cholera. However, the proliferative responses to the positive and negative controls remained unchanged over the course of disease, suggesting that the increased stimulation observed was not due to nonspecific activation of T cells in the patients.
The induction of both IFN-γ and IL-13 cytokine responses indicates that there was activation of both Th1 and Th2 CD4+ effector cells. Interestingly, at the acute stage of disease, there was greater production of the Th2-cytokine IL-13 in the cholera antigen-stimulated cell cultures, and the production decreased at convalescence. Activation of an IL-13-dependent Th2 response in cholera patients has not been demonstrated previously. However, cholera toxin has been shown to induce a Th2-mediated immune response in mice (29). Our results demonstrate that CD4+ T cells from subjects suffering from acute cholera produce IL-13 in response to V. cholerae antigens. Previously, we have observed increases in the numbers of mast cells in the intestinal mucosa and high eosinophil levels, as well as IgE anti-V. cholerae antibodies in the systemic circulation after cholera (22). These processes are also known to be dependent on Th2 cells or Th2 cytokines (29).
Taken together, our data show that there is a rapid Th2 response by CD4+ T cells in cholera. Since the patients used live in an area where V. cholerae is endemic and where there are recurring outbreaks of cholera, these individuals may have been exposed and primed to V. cholerae O1 previously. Thus, the T-cell responses detected were likely memory responses. This conclusion is supported by the rapid onset of proliferation of CD4+ T cells to cholera antigens and the production of IL-13.
In addition to the Th2-response seen, there was also a marked increase in IFN-γ secretion by stimulated T cells. Given the kinetics of this response and the ability of both CD4+ and CD8+ T cells to produce IFN-γ after bacterial stimulation (16), there was most likely a contribution to this response from both CD4+ and CD8+ T cells.
In conclusion, we have shown that there is induction of gut-homing B- and T-cell responses, as well as involvement of both Th1 (IFN-γ) and Th2 cytokines (IL-13), in cholera. The increase in the number of gut-homing T and B cells may be needed for directing the immunocytes back to the gut for protection against further infection. Given that natural cholera provides substantial protection against subsequent infection, the responses seen here may need to be stimulated by a cholera vaccine if it is to provide protection from cholera similar to that seen following infection.
Acknowledgments
This study was conducted at the ICDDR, B Centre for Health and Population Research with support from grants from the Swedish Agency for Research Cooperation with Developing Countries (Sida-SAREC grant INT-ICDDR, B-HN-01-AV) and the National Institutes of Health (grant U01 AI058935).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Agtini, M. D., R. Soeharno, M. Lesmana, N. H. Punjabi, C. Simanjuntak, F. Wangsasaputra, D. Nurdin, S. P. Pulungsih, A. Rofiq, H. Santoso, H. Pujarwoto, A. Sjahrurachman, P. Sudarmono, L. von Seidlein, J. L. Deen, M. Ali, H. Lee, D. R. Kim, O. Han, J. K. Park, A. Suwandono, Ingerani, B. A. Oyofo, J. R. Campbell, H. J. Beecham, A. L. Corwin, and J. D. Clemens. 2005. The burden of diarrhoea, shigellosis, and cholera in North Jakarta, Indonesia: findings from 24 months surveillance. BMC Infect. Dis. 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 724448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhury, F., F. Qadri, A. I. Khan, J. B. Harris, R. LaRocque, M. Chowdhury, E. T. Ryan, A. S. Faruque, and S. B. Calderwood. 2008. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr. Infect. Dis. J. 27986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Reference deleted.
- 6.Reference deleted.
- 7.Elson, C. O., and W. Ealding. 1987. Ir gene control of the murine secretory IgA response to cholera toxin. Eur. J. Immunol. 17425-428. [DOI] [PubMed] [Google Scholar]
- 8.Flach, C. F., F. Qadri, T. R. Bhuiyan, N. H. Alam, E. Jennische, J. Holmgren, and I. Lonnroth. 2007. Differential expression of intestinal membrane transporters in cholera patients. FEBS Lett. 5813183-3188. [DOI] [PubMed] [Google Scholar]
- 9.Flach, C. F., F. Qadri, T. R. Bhuiyan, N. H. Alam, E. Jennische, I. Lonnroth, and J. Holmgren. 2007. Broad up-regulation of innate defense factors during acute cholera. Infect. Immun. 752343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass, R. I., S. Becker, M. I. Huq, B. J. Stoll, M. U. Khan, M. H. Merson, J. V. Lee, and R. E. Black. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116959-970. [DOI] [PubMed] [Google Scholar]
- 11.Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kirn, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1008508-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirabayashi, Y., S. I. Tamura, Y. Suzuki, T. Nagamine, C. Aizawa, K. Shimada, and T. Kurata. 1991. H-2-unrestricted adjuvant effect of cholera toxin B subunit on murine antibody responses to influenza virus haemagglutinin. Immunology 72329-335. [PMC free article] [PubMed] [Google Scholar]
- 13.Holmgren, J., A. M. Svennerholm, J. Clemens, D. Sack, R. Black, and M. Levine. 1987. An oral B subunit-whole cell vaccine against cholera: from concept to successful field trial. Adv. Exp. Med. Biol. 216B1649-1660. [PubMed] [Google Scholar]
- 14.Jayasekera, C. R., J. B. Harris, M. S. Bhuiyan, F. Chowdhury, A. I. Khan, A. S. Faruque, G. John, R. LaRocque, E. T. Ryan, R. Ahmad, F. Qadri, and S. B. Calderwood. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 1981055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilhamn, J., S. B. Lundin, H. Brevinge, A. M. Svennerholm, and M. Jertborn. 2003. T- and B-cell immune responses of patients who had undergone colectomies to oral administration of Salmonella enterica serovar Typhi Ty21a vaccine. Clin. Diagn. Lab. Immunol. 10426-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Reference deleted.
- 19.Lopez, A. L., J. D. Clemens, J. Deen, and L. Jodar. 2008. Cholera vaccines for the developing world. Hum. Vaccines 4165-169. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 711755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson, A., A. Tinnert, A. Hamlet, H. Lonroth, I. Bolin, and A. M. Svennerholm. 1998. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin. Diagn. Lab. Immunol. 5288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri, F., T. R. Bhuiyan, K. K. Dutta, R. Raqib, M. S. Alam, N. H. Alam, A. M. Svennerholm, and M. M. Mathan. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 5362-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri, F., A. Chowdhury, J. Hossain, K. Chowdhury, T. Azim, T. Shimada, K. M. Islam, R. B. Sack, and M. J. Albert. 1994. Development and evaluation of rapid monoclonal antibody-based coagglutination test for direct detection of Vibrio cholerae O139 synonym Bengal in stool samples. J. Clin. Microbiol. 321589-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin. Diagn. Lab. Immunol. 4429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qadri, F., R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 714808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 653571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su, S. B., P. B. Silver, P. Wang, C. C. Chan, and R. R. Caspi. 2004. Cholera toxin prevents Th1-mediated autoimmune disease by inducing immune deviation. J. Immunol. 173755-761. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 1994. 25 years of ORS. Joint WHO/ICDDR, B Consultative Meeting on ORS Formulation, Dhaka, Bangladesh. Division of Diarrhoeal and Acute Respiratory Disease Control, World Health Organization, Geneva, Switzerland.
- 31.World Health Organization. 1987. Manual for laboratory investigations of acute enteric infections, p. 9-20. World Health Organization, Geneva, Switzerland.