Abstract
In studies of immunity to malaria, the absence of febrile malaria is commonly considered evidence of “protection.” However, apparent “protection” may be due to a lack of exposure to infective mosquito bites or due to immunity. We studied a cohort that was given curative antimalarials before monitoring began and documented newly acquired asymptomatic parasitemia and febrile malaria episodes during 3 months of surveillance. With increasing age, there was a shift away from febrile malaria to acquiring asymptomatic parasitemia, with no change in the overall incidence of infection. Antibodies to the infected red cell surface were associated with acquiring asymptomatic infection rather than febrile malaria or remaining uninfected. Bed net use was associated with remaining uninfected rather than acquiring asymptomatic infection or febrile malaria. These observations suggest that most uninfected children were unexposed rather than “immune.” Had they been immune, we would have expected the proportion of uninfected children to rise with age and that the uninfected children would have been distinguished from children with febrile malaria by the protective antibody response. We show that removing the less exposed children from conventional analyses clarifies the effects of immunity, transmission intensity, bed nets, and age. Observational studies and vaccine trials will have increased power if they differentiate between unexposed and immune children.
Malaria is a pressing global health problem (36). The correlates of immunity in observational field-based studies are often used to guide vaccine design (22), in which the chosen definition of immunity to malaria is usually the absence of febrile malaria. However, the findings obtained with this approach are often inconsistent, and responses to a specific antigen are associated with protection in some studies but not in others (4, 6, 7, 9-12, 23, 29). This may be because of parasite polymorphism (38), because of a confounding association between protective and nonprotective responses, because the endpoint of mild febrile malaria is not specific (26), or because rapidly waning antibody responses are not a stable predictive measure for the follow-up period (15).
In studies in Kilifi, Kenya, associations between specific antibody responses and protection were stronger in children who had asymptomatic parasitemia at the start of monitoring (5, 16, 20, 28, 30, 31). This might imply that there is premunition, where a chronic low-level infection is required to provide immunity against further infection (35), and that antibody responses are more long lived in the presence of asymptomatic parasitemia (1). Alternatively, antibody responses measured in the presence of a challenge with asymptomatic parasitemia may be more informative than antibody responses measured without current exposure. For instance, protection against hepatitis B is predicted by the antibody titer shortly after vaccination, even when antibody titers subsequently become undetectable (32). However, it may simply be that parasitemia reflects greater exposure to malaria and hence a greater power to detect associations.
In this study, we cleared asymptomatic parasitemia with highly effective antimalarials in order to identify newly acquired parasitemia during follow-up. We compared children who acquired asymptomatic parasitemia with children who developed febrile malaria by examining the associations with known markers of exposure and immunity. We then examined what impact excluding “unexposed” children had on conventional survival analyses in order to determine whether such analyses should be more widely used to study outcomes in observational studies or clinical trials.
MATERIALS AND METHODS
Study design.
The data presented here were generated during a randomized controlled trial of a candidate malaria vaccine. The details of the study design are described elsewhere (3). The participants were 1 to 6 years old (inclusive), healthy, and residents of the Junju sublocation in Kilifi District, Kenya. Vaccination had no effect on either the incidence of febrile episodes, the prevalence of asymptomatic parasitemia, the parasite density (3), or the anti-variant surface antigen (VSA) antibodies (P = 0.57) and is not considered further here. Ethical approval was obtained from the Kenyan Medical Research Institute National Ethics Committee, the Central Oxford Research Ethics Committee, and the London School of Hygiene and Tropical Medicine Ethics Committee. Parents of all children were approached for informed consent before the study began. Blood was taken for plasma and cross-sectional assessments of malaria parasitemia before all children were treated with antimalarials at the start of follow-up and again after 3 months.
Drug treatment.
Immediately following the first cross-sectional bleed, curative antimalarial treatment consisting of 7 days of directly observed dihydroartemisinin monotherapy was administered (2 mg per kg on the first day, followed by 1 mg per kg for 6 days). This regimen is highly effective when it is directly observed (18, 39), and parasite clearance was confirmed by examining slides taken 7 days after completion of treatment.
Follow-up.
Children were visited every week by field workers. When the temperature of a child was above 37.5°C, a blood film was made and a rapid near-patient test for malaria was conducted. When the mother reported fever but the temperature was below 37.5°C, blood film examination and rapid testing were not performed. Instead, the field worker returned to visit the child three times in the next 24 h. Rapid testing and blood film examination were performed if the temperature was elevated on any of these visits. Parents could bring their child in for assessment between regular weekly visits if they thought that the child had developed fever. Field workers were residents in the villages in which the study was conducted and were readily accessible to the parents. The treatment for episodes of malaria was the Government of Kenya-recommended first-line treatment, artemether-lumefantrine. Cross-sectional blood film examination was performed for all children before they cleared asymptomatic parasitemia and once after 3 months of follow-up.
Bed net use was assessed by field workers visiting the subject's home at the beginning of the study. They observed whether the bed net was hung over the child's sleeping space, asked about recent treatment, and examined the number of holes that could admit a fingertip. Children were considered net users if an untreated bed net had less than three holes that could admit a fingertip (25). Children without nets or with untreated nets with three or more holes were considered nonusers.
Laboratory studies.
The A4 parasite clone of Plasmodium falciparum was cultured in vitro to the mature trophozoite stage in blood group O erythrocytes. A4 was previously derived from the endothelial binding line ITO4 for CD36 and ICAM-1 binding (33). A4 was chosen because of the results of previous studies showing that the responses measured correlated well with immunity to malaria (20). The major target of immunity to parasite-derived antigen on the red cell surface antigen appears to be to VSA (6, 27), and flow cytometry-based assays to detect this response are widely used (2, 14). Briefly, an infected erythrocyte suspension was adjusted to a 4% hematocrit in phosphate-buffered saline containing 0.5% bovine serum albumin and ethidium bromide (10 μg/ml). Then 11.5 μl of the suspension was incubated with 1 μl of an individual's plasma in 96-well U-bottom plates (Falcon, Becton Dickinson, United States) for 30 min at room temperature. The cells were then washed three times with phosphate-buffered saline containing 0.5% bovine serum albumin by centrifugation at 1,000 rpm for 3 min each time. Fifty microliters of fluorescein isothiocyanate-conjugated sheep anti-human immunoglobulin G (IgG) (Binding Site, United Kingdom) at a 1:50 dilution was then added to the cells and incubated for 30 min at room temperature. Following a further series of washes, 1,000 infected erythrocytes were acquired on an FC500 flow cytometer (Beckman Coulter), and the proportion of erythrocytes binding IgG was determined using CXP analysis software (Beckman Coulter). Nonspecific IgG binding was controlled for by subtracting the proportion of infected erythrocytes binding IgG in plasma from eight nonexposed United Kingdom donors.
Thick and thin blood smears were stained with 10% Giemsa stain and examined at a magnification of ×1,000 for asexual forms of P. falciparum, and the results were expressed in number of parasites per μl using an assumed white or red cell count. Films were read in duplicate and by a third reader if one film was positive and the other film was negative or if the calculated densities differed by more than tenfold. The final result was a geometric mean of the only two readings or the two closest readings.
Categorization of outcome.
Children were assigned to one of the following three categories: febrile malaria (one or more episodes of an axillary temperature greater than 37.5°C, with P. falciparum parasitemia greater than 2,500 parasites per μl [26] during the 3 months of follow-up), acquiring asymptomatic infection (no detected episode of febrile malaria but asymptomatic parasitemia at the second cross-sectional bleed after 3 months), or uninfected (no episode of febrile malaria identified and no parasites in the 3-month cross-sectional sample). Children with episodes of parasitemia with <2,500 parasites per μl and fever during follow-up were excluded, since the episodes may have been either febrile malaria or asymptomatic parasitemia (26).
Analysis.
We used STATA version 10 (StataCorp, Texas) to fit logistic regression models comparing each combination of two of the three outcomes (febrile malaria, asymptomatic malaria, and uninfected). Age groups were classified by quartile for logistic regression studies. Antibody titers were analyzed as continuous variables. Odds ratios were adjusted by village and age (as a categorical variable in quartiles) in all analyses. There was one child or at most two children per household, so it was not practical to adjust by household. We then fitted Cox survival models, where the endpoint was febrile malaria, and restricted the analysis by removing children who were uninfected during follow-up or who had asymptomatic malaria during follow-up. Age group and village were retained as factors in the Cox regression models. For significance testing for interactions we used likelihood ratio tests comparing a model containing an interaction term with a model using the same variables but containing no interaction term.
RESULTS
In the cohort of 381 children, 66 (17%) had an episode of febrile malaria (i.e., fever and a parasitemia of >2,500 parasites/μl) during the 3 months of follow-up. Eighty-five (22%) acquired asymptomatic parasitemia during the 3 months (without a febrile episode), and 208 children (55%) remained uninfected. Twenty-two (6%) had a febrile episode with less than 2,500 parasites/μl and were excluded from further analysis. There were no significant differences in this excluded group for age (P = 0.43), anti-VSA levels (P = 0.61), village (P = 0.61), or parasitemia at baseline (P = 0.83).
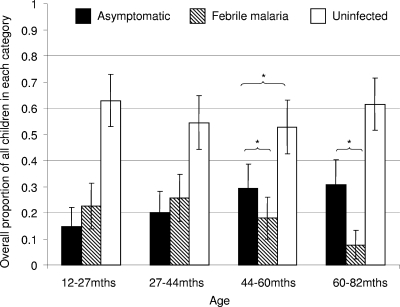
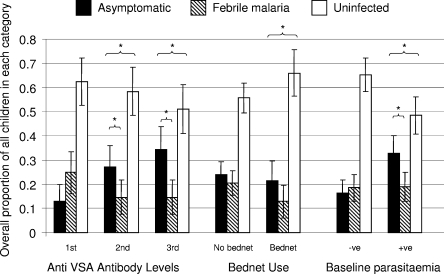
We studied this categorization of outcomes by examining the effects of factors that have known effects against malaria. We used age (known to be associated with immunity to malaria), a specific protective antibody response associated with protection (20), bed nets (17), village, and parasitemia at the first cross-sectional bleed (before treatment with antimalarials). The data obtained are shown in Fig. 1, 2, and 3. Age was associated with a shift away from febrile malaria to acquiring asymptomatic parasitemia, without a change in the overall incidence of infection (Fig. 1). Antibodies to the infected red cell surface were associated with acquiring asymptomatic infection rather than febrile malaria or remaining uninfected (Fig. 2). Bed net use was associated with remaining uninfected rather than acquiring asymptomatic infection or febrile malaria (Fig. 2).
FIG. 1.
Proportions of children having asymptomatic or febrile malaria and of children remaining malaria free over 3 months of observation sorted by age group. Age groups are defined according to quartiles. An asterisk indicates statistical significance (P < 0.05) for logistic regression for a shift in the balance between asymptomatic parasitemia and febrile malaria or between asymptomatic parasitemia and uninfected status (P < 0.05).
FIG. 2.
Proportions of children having asymptomatic or febrile malaria and of children remaining malaria free over 3 months of observation sorted by anti-VSA antibody level, bed net use, and parasitemia. Tertiles of the antibody response to VSA are used, along with bed net use and parasitemia at the first cross-sectional survey (before curative treatment was given). An asterisk indicates statistical significance (P < 0.05) for logistic regression for a shift in the balance between asymptomatic parasitemia and febrile malaria or between asymptomatic parasitemia and uninfected status (P < 0.05).
FIG. 3.
Proportions of children having asymptomatic or febrile malaria and of children remaining malaria free over 3 months of observation sorted by village. An asterisk indicates statistical significance (P < 0.05) for logistic regression for a shift in the balance between asymptomatic parasitemia and febrile malaria or between asymptomatic parasitemia and uninfected status (P < 0.05).
We studied the strength of these associations and their significance by using logistic regression models (Table 1). Increasing age was associated with a significant trend in odds ratios in favor of developing asymptomatic parasitemia rather than febrile malaria. However, age had no clear effect on the risk of febrile malaria compared to uninfected status. The anti-VSA antibodies acted similarly, with increasing antibody levels significantly favoring the development of asymptomatic parasitemia rather than febrile malaria but not affecting febrile malaria compared with uninfected status. Bed net use was not associated with an altered risk of asymptomatic parasitemia compared with febrile malaria but was associated with increased odds of remaining uninfected compared with either developing febrile disease (P = 0.017) or acquiring asymptomatic parasitemia (P = 0.13).
TABLE 1.
Logistic regression models to examine factors associated with malaria infection
| Factora | n | Febrile vs Asymptomatic
|
Asymptomatic vs uninfected
|
Febrile vs uninfected
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | ||
| Age | ||||||||||
| 12-27 mo | 101 | 1 | 1 | 1 | ||||||
| 27-44 mo | 100 | 0.64 | 0.2-1.9 | 0.4 | 1.4 | 0.6-3.4 | 0.39 | 1.3 | 0.6-2.7 | 0.5 |
| 44-60 mo | 99 | 0.19 | 0.06-0.6 | 0.004 | 2.4 | 1.1-5.5 | 0.03 | 1 | 0.5-2.2 | 0.99 |
| 60-82 mo | 105 | 0.09 | 0.02-0.3 | <0.001 | 1.9 | 0.9-4.3 | 0.1 | 0.34 | 0.1-0.9 | 0.03 |
| A4 anti-VSA antibodies | 314 | 0.08 | 0.01-0.9 | 0.036 | 7.8 | 2.03-30 | 0.003 | 1.14 | 0.25-5.3 | 0.86 |
| Bed net | ||||||||||
| No bed net | 281 | 1 | 1 | 1 | ||||||
| Bed net | 102 | 1 | 0.34-3.1 | 0.95 | 0.42 | 0.2-0.9 | 0.017 | 0.52 | 0.2-1.2 | 0.13 |
| Village | ||||||||||
| Gongoni | 82 | 1 | 1 | 1 | ||||||
| Junju | 109 | 0.07 | 0.02-0.3 | <0.001 | 2.9 | 1.3-6.5 | 0.009 | 0.38 | 0.14-1.0 | 0.06 |
| Kolewa | 112 | 0.51 | 0.02-0.3 | 0.28 | 0.99 | 0.4-2.5 | 0.98 | 1 | 0.4-2.4 | 0.98 |
| Mapawa | 71 | 0.50 | 0.1-2.2 | 0.36 | 0.40 | 0.1-1.2 | 0.09 | 0.39 | 0.1-1.0 | 0.06 |
| Mwembe Tsungu | 31 | 3.9 | 0.6-27 | 0.27 | 0.26 | 0.05-1.3 | 0.11 | 0.89 | 0.3-2 | 0.83 |
| Parasite negative | 115 | |||||||||
| Parasite positive | 270 | 0.25 | 0.09-0.7 | 0.01 | 5.4 | 2.4-12 | <0.001 | 1.2 | 0.6-2.1 | 0.7 |
Parasite negative, blood film negative for parasites at cross-sectional bleed at the start of the study; parasite positive, blood film positive for parasites at cross-sectional bleed for the first cross-sectional bleed, before curative antimalarials were given. Analyses are adjusted for village and age.
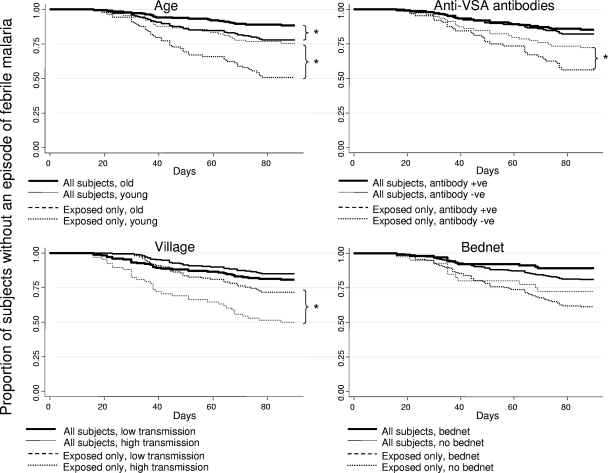
Hence, anti-VSA antibodies and age were negatively associated with developing febrile disease rather than developing asymptomatic parasitemia but were not associated with developing febrile disease rather than remaining uninfected. Bed net use was associated with uninfected status but not with asymptomatic parasitemia compared with febrile malaria. Taken together, these findings suggest that many uninfected children were simply unexposed. We therefore examined the impact that the less exposed group had on standard survival analyses of time to febrile malaria (Fig. 4)
FIG. 4.
Survival plots for time to febrile malaria before and after “unexposed” children were excluded. Statistically significant differences (P < 0.05) as determined by Cox regression are indicated by an asterisk. Children are divided into two categories according to age (old [44 to 82 months] versus young [12 to 44 months]), according to levels of antibody to VSA (high [15 to 85% recognition] versus low [0 to 15% recognition]), according to village (high transmission versus low transmission), and according to bed net use. The plots are survival plots for each categorization of children. Each plot shows the results of the analysis of all subjects and also a repeated analysis after the unexposed children were excluded.
Survival analyses.
The time to febrile disease increased with age (hazard ratio [HR], 0.49; 95% confidence interval [95% CI], 0.3 to 0.8; P = 0.007), but the difference increased once the less exposed children were removed from the analysis (HR, 0.36; 95% CI, 0.2 to 0.6; P < 0.0005). For all children, there was a nonsignificant trend for anti-VSA antibodies to be associated with less febrile disease (HR, 0.82; 95% CI, 0.5 to 1.5; P = 0.52), but the difference was more evident once less exposed children were removed from the analysis (HR, 0.50; 95% CI, 0.3 to 0.9; P = 0.025). Similarly, there was no difference for comparisons of high- and low-transmission villages when all children were analyzed (HR, 1.3; 95% CI, 0.8 to 2; P = 0.34), but when the less exposed children were excluded, there was significantly more febrile malaria in the low-transmission villages (HR, 2.2; 95% CI, 1.4 to 3.7; P = 0.001). Transmission intensity was defined by the overall proportion of children in a village who remained uninfected during follow-up (Fig. 3).
There was a strong interaction between age and village (P < 0.00005); living in low-transmission villages was most strongly associated with increased risk for the oldest quartile (HR, 10.2; 95% CI, 2.5 to 50; P = 0.002) and nonsignificantly protective for the youngest quartile (HR, 0.64; 95% CI, 0.3 to 1.2; P = 0.19). In the intermediate age quartiles, the HRs for the effect of village were 4.6 (95% CI, 1.9 to 11; P = 0.001) and 0.93 (95% CI, 0.51 to 1.7; P = 0.8) for the second- and third-oldest quartiles, respectively.
Removing the less exposed children did not increase the separation of the survival plots by bed net use. Although bed nets were nonsignificantly associated with protection from febrile malaria for all children (HR, 0.59, 95% CI, 0.3 to 1.3; P = 0.18), removing the less exposed children from analysis reduced the difference in the rates of febrile malaria (HR, 0.67, 95% CI, 0.3 to 1.5; P = 0.3).
Previous studies of other cohorts in this population found that specific antibodies were associated with protection from febrile malaria only in subjects who were parasitemic at baseline. In our study, analyzing only children who were parasitemic at baseline did increase the estimate of antibody-mediated protection (HR, 0.7; 95% CI, 0.3 to 1.6; P = 0.4) but not as effectively as excluding less exposed children, as described above.
Interactions of asymptomatic parasitemia with age.
Having asymptomatic parasitemia in the pretreatment cross-sectional survey was associated with an increased chance of acquiring asymptomatic parasitemia during the 3 months after curative antimalarials were administered, both in comparison with febrile malaria and in comparison with remaining uninfected (Table 1). However, the summary odds ratios are misleading since there is significant variation in the effect of parasitemia by age. The association of parasitemia at the start of the study with a reduced risk of febrile malaria compared with asymptomatic parasitemia was evident only in older children. In contrast, parasitemia was associated with an increased risk of febrile malaria in young children, based on comparison with uninfected status (Table 2).
TABLE 2.
Logistic regression models to examine the effect of parasitemia at the start of the study on malaria by age groupa
| Age (mo) | Febrile vs Asymptomatic
|
Asymptomatic vs uninfected
|
Febrile vs uninfected
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| 12-27 | 1.1 | 0.2-6.6 | 0.9 | 2.3 | 0.6-8.5 | 0.2 | 3.0 | 1.0-9.2 | 0.05 |
| 27-44 | 0.50 | 0.1-2.8 | 0.4 | 3.6 | 1.2-11 | 0.03 | 1.5 | 0.5-4.2 | 0.4 |
| 44-60 | 0.47 | 0.09-2.6 | 0.3 | 2.4 | 0.9-6.5 | 0.09 | 1.5 | 0.5-4.9 | 0.5 |
| 60-82 | 0.09 | 0.01-1.6 | 0.1 | 2.1 | 0.7-6.1 | 0.2 | 0.8 | 0.2-4 | 0.8 |
The odds ratios are odds ratios of parasite-positive children to parasite-negative children. Analyses were adjusted for village and age.
We also tested whether the same interaction between parasitemia and age group could be seen in a previously studied cohort (20). The odds ratios for febrile malaria associated with parasitemia were 16.5 (95% CI, 2.2 to 100), 4.0 (95% CI, 1.1 to 15), 1.0 (95% CI, 0.3 to 3.8), and 1.36 (95% CI, 0.3 to 6) for the children who were 5 to 24 months, 24 to 48 months, 48 to 72 months, and 72 to 96 months old, respectively. For 96- to 120-month-old children asymptomatic parasitemia completely predicted protection.
DISCUSSION
Studies that investigate the ability of naturally acquired immunity or experimental vaccinations to protect against malaria often use febrile malaria as an endpoint. However, children who do not have an episode of febrile malaria may be protected in a number of different ways. They may be genuinely immune, or they may simply have avoided exposure to infective mosquito bites. Since curative antimalarials were used at the start of monitoring in the current study, parasitemia at the 3-month cross-sectional bleed was necessarily acquired during the period of monitoring and so reflects exposure during follow-up. The shift from younger children developing febrile malaria to older children acquiring asymptomatic parasitemia suggests that there is acquisition of adaptive immunity with age. The constant proportion of uninfected children with increasing age suggests that children did not acquire immunity to parasitization per se but acquired immunity that prevented parasitization leading to febrile disease. We may infer that the children who acquired asymptomatic parasitemia were immune to febrile malaria and that the children who remained parasite negative were simply not exposed to malaria during the 3 months of follow-up.
One might think that a single blood film following parasite clearance is likely to be a poor indicator of exposure. Despite the careful monitoring during the 3 months following parasite clearance, it is possible that episodes of febrile malaria were missed or treated with antimalarials from another source and that the infection was cleared without detection. An infection might also be missed because of sterilizing immunity (or because of immunity that produces submicroscopic parasitemia). We reasoned that sterilizing immunity was rare in children. In support of this conclusion, the proportion of uninfected children does not steadily rise with increasing age, suggesting that immunity to infection (as opposed to immunity to febrile disease) is not acquired in this age group. However, the significant odds ratio for a lower risk of febrile malaria compared with remaining uninfected in 60- to 82-month-old children suggests that this oldest group may have acquired some immunity that cleared infections. Poor compliance with the 7 days of artemether treatment might have resulted in recrudescent infection (rather than reinfection), although treatment was directly observed and only 3% of febrile episodes occurred in the first 2 weeks of monitoring.
Nevertheless, despite these potential limitations for categorizing exposure in this way, the known associations of anti-VSA antibodies, exposure, and age were consistent with the conclusion that the majority of members of the uninfected group were “unexposed.” Furthermore, exclusion of the “unexposed” group increased the ability of the survival analysis to detect immunity to febrile malaria. Given the limitations of our approach, it is unlikely that this definition of exposure is perfect. It is probably more correct to refer to this group as “less exposed” rather than “unexposed.” Despite this shortcoming, is our classification of exposure useful in clarifying aspects of malaria epidemiology? The results of conventional survival analysis with and without the less exposed children suggest that it is. Including this group in survival analyses obscured completely the true protective effect of anti-VSA antibodies and the transmission zone and partially obscured the effect of age. Excluding the less exposed group made the immunity to febrile disease associated with these variables obvious.
High transmission was protective in older age groups but not in younger age groups, suggesting that this paradoxical effect of higher transmission is explained by more rapidly acquired immunity. It has previously been shown that a higher-transmission setting results in more febrile malaria in early childhood, followed by less malaria in later childhood (37). Our cohort did not include children younger than 1 year old; hence, the predominant effect of low transmission was protective. Higher-transmission villages were associated with a shift toward asymptomatic parasitemia and away from febrile malaria (Fig. 3). This finding supports our conclusion that the protective effect of living in a high-transmission village was due to blood stage immunity.
We measured transmission by determining the rate of infection over 3 months (adding asymptomatic and febrile disease) rather than by entomological methods. This protocol may reflect housing conditions or behavioral differences as much as mosquito populations, but it is the actual exposure to malaria that is relevant to the child. In theory, one could convert the observed infection rates to a force of infection, but heterogeneity in transmission would make a simple arithmetic calculation inaccurate (34). Furthermore, not every infective bite results in infection even in a nonimmune subject (8).
Parasitemia has been associated with protection from malaria (21), but it has also been associated with susceptibility (20). The direction of causality is unclear (13). We found that in young children parasitemia at baseline was associated with a shift from uninfected status to febrile malaria but that was associated with a shift from febrile malaria to subsequent asymptomatic parasitemia in older children. This result can be understood if asymptomatic parasitemia is considered a marker of exposure. In children without immunity (i.e., young children), higher exposure is associated with more frequent febrile malaria. Once immunity has been acquired (i.e., in older children), exposure is instead associated with reduced susceptibility to febrile malaria. Variation between studies could then be explained by the relative ages and immunities of the children studied.
Could exposure and immunity be distinguished without using curative antimalarials at the start of monitoring? Multiple cross-sectional surveys to identify repeated asymptomatic parasitemia define exposure better than a single measurement without prior treatment with antimalarials (24). Household has been used as an effective marker for exposure (19). However, these approaches require a threshold (number of positive slides for the former approach or incident rates for households for the latter approach) to separate individual children into “exposed” and “unexposed” categories. After drug clearance, reinfection with asymptomatic parasitemia is a binary outcome and eliminates the need for an arbitrary cutoff. However, it would be difficult to conduct such analyses using passive case detection, since this would increase the misclassification of the “unexposed” group. The follow-up period was short, and it may be that this increased the accuracy of a single blood film to measure exposure.
In summary, in studies that use protection against febrile malaria as the indicator of immunity to malaria, children who do not have an episode of febrile malaria may be immune or may simply be unexposed. These groups can be distinguished by the analysis that we describe here. This definition now needs to be tested for a wide range of specific antibody responses and should be considered for proof-of-concept vaccine trials.
Acknowledgments
This study was supported by KEMRI and the Wellcome Trust. The data on malaria episodes were from a vaccine study funded by the Gates Malaria Partnership at the London School of Hygiene and Tropical Medicine. K. Marsh is supported by Wellcome Trust grant no. 077092. P. C. Bull and G. Warimwe are funded by Wellcome Trust grant no. 076030. P. Bejon is supported by the Oxford Biomedical Research Centre Programme.
There are no conflicts of interest.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 17 February 2009.
This paper is published with the permission of the director of KEMRI.
REFERENCES
- 1.Akpogheneta, O. J., N. O. Duah, K. K. Tetteh, S. Dunyo, D. E. Lanar, M. Pinder, and D. J. Conway. 2008. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect. Immun. 761748-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeson, J. G., E. J. Mann, T. J. Byrne, A. Caragounis, S. R. Elliott, G. V. Brown, and S. J. Rogerson. 2006. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. J. Infect. Dis. 193721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejon, P., J. Mwacharo, O. Kai, T. Mwangi, P. Milligan, S. Todryk, S. Keating, T. Lang, B. Lowe, C. Gikonyo, C. Molyneux, G. Fegan, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2006. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin. Trials 1e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58211-219. [DOI] [PubMed] [Google Scholar]
- 5.Bull, P. C., B. S. Lowe, N. Kaleli, F. Njuga, M. Kortok, A. Ross, F. Ndungu, R. W. Snow, and K. Marsh. 2002. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J. Infect. Dis. 1851688-1691. [DOI] [PubMed] [Google Scholar]
- 6.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6689-692. [DOI] [PubMed] [Google Scholar]
- 8.Davis, J. R., J. R. Murphy, D. F. Clyde, S. Baqar, A. H. Cochrane, F. Zavala, and R. S. Nussenzweig. 1989. Estimate of Plasmodium falciparum sporozoite content of Anopheles stephensi used to challenge human volunteers. Am. J. Trop. Med. Hyg. 40128-130. [DOI] [PubMed] [Google Scholar]
- 9.Dodoo, D., T. Staalsoe, H. Giha, J. A. Kurtzhals, B. D. Akanmori, K. Koram, S. Dunyo, F. K. Nkrumah, L. Hviid, and T. G. Theander. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect. Immun. 693713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 672131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173765-769. [DOI] [PubMed] [Google Scholar]
- 12.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71117-126. [DOI] [PubMed] [Google Scholar]
- 13.Gosling, R. D. 2008. Asymptomatic malaria associated with protection: not causal. Clin. Infect. Dis. 47147. (Author's reply, 47:147-148.) [DOI] [PubMed] [Google Scholar]
- 14.Kinyanjui, S. M., P. Bull, C. I. Newbold, and K. Marsh. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187667-674. [DOI] [PubMed] [Google Scholar]
- 15.Kinyanjui, S. M., D. J. Conway, D. E. Lanar, and K. Marsh. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinyanjui, S. M., T. Mwangi, P. C. Bull, C. I. Newbold, and K. Marsh. 2004. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J. Infect. Dis. 1901527-1533. [DOI] [PubMed] [Google Scholar]
- 17.Lengeler, C. 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2CD000363. [DOI] [PubMed] [Google Scholar]
- 18.Looareesuwan, S., P. Wilairatana, C. Viravan, S. Vanijanonta, P. Pitisuttithum, and D. E. Kyle. 1997. Open randomized trial of oral artemether alone and a sequential combination with mefloquine for acute uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 56613-617. [DOI] [PubMed] [Google Scholar]
- 19.Mackinnon, M. J., T. W. Mwangi, R. W. Snow, K. Marsh, and T. N. Williams. 2005. Heritability of malaria in Africa. PLoS Med. 2e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackintosh, C. L., T. Mwangi, S. M. Kinyanjui, M. Mosobo, R. Pinches, T. N. Williams, C. I. Newbold, and K. Marsh. 2008. Failure to respond to the surface of Plasmodium falciparum infected erythrocytes predicts susceptibility to clinical malaria amongst African children. Int. J. Parasitol. 381445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Males, S., O. Gaye, and A. Garcia. 2008. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. 46516-522. [DOI] [PubMed] [Google Scholar]
- 22.Marsh, K., and S. Kinyanjui. 2006. Immune effector mechanisms in malaria. Parasite Immunol. 2851-60. [DOI] [PubMed] [Google Scholar]
- 23.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83293-303. [DOI] [PubMed] [Google Scholar]
- 24.Mwangi, T. W., G. Fegan, T. N. Williams, S. M. Kinyanjui, R. W. Snow, and K. Marsh. 2008. Evidence for over-dispersion in the distribution of clinical malaria episodes in children. PLoS ONE. 3e2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwangi, T. W., A. Ross, K. Marsh, and R. W. Snow. 2003. The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast. Trans. R. Soc. Trop. Med. Hyg. 97369-372. [DOI] [PubMed] [Google Scholar]
- 26.Mwangi, T. W., A. Ross, R. W. Snow, and K. Marsh. 2005. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J. Infect. Dis. 1911932-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75281-292. [DOI] [PubMed] [Google Scholar]
- 28.Osier, F. H., G. Fegan, S. D. Polley, L. Murungi, F. Verra, K. K. Tetteh, B. Lowe, T. Mwangi, P. C. Bull, A. W. Thomas, D. R. Cavanagh, J. S. McBride, D. E. Lanar, M. J. Mackinnon, D. J. Conway, and K. Marsh. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 762240-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perraut, R., L. Marrama, B. Diouf, C. Sokhna, A. Tall, P. Nabeth, J. F. Trape, S. Longacre, and O. Mercereau-Puijalon. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191264-271. [DOI] [PubMed] [Google Scholar]
- 30.Polley, S. D., D. J. Conway, D. R. Cavanagh, J. S. McBride, B. S. Lowe, T. N. Williams, T. W. Mwangi, and K. Marsh. 2006. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 244233-4246. [DOI] [PubMed] [Google Scholar]
- 31.Polley, S. D., T. Mwangi, C. H. Kocken, A. W. Thomas, S. Dutta, D. E. Lanar, E. Remarque, A. Ross, T. N. Williams, G. Mwambingu, B. Lowe, D. J. Conway, and K. Marsh. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23718-728. [DOI] [PubMed] [Google Scholar]
- 32.Puro, V., G. De Carli, S. Cicalini, F. Soldani, U. Balslev, J. Begovac, L. Boaventura, M. Campins Marti, M. J. Hernandez Navarrete, R. Kammerlander, C. Larsen, F. Lot, S. Lunding, U. Marcus, L. Payne, A. A. Pereira, T. Thomas, and G. Ippolito. 2005. European recommendations for the management of healthcare workers occupationally exposed to hepatitis B virus and hepatitis C virus. Eur. Surveill. 10260-264. [PubMed] [Google Scholar]
- 33.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, D. L., J. Dushoff, R. W. Snow, and S. I. Hay. 2005. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438492-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, T., I. Felger, M. Tanner, and H. P. Beck. 1999. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans. R. Soc. Trop. Med. Hyg. 93(Suppl. 1)59-64. [DOI] [PubMed] [Google Scholar]
- 36.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 3491650-1654. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe, K., N. Sakihama, I. Rooth, A. Bjorkman, and A. Farnert. 2007. High frequency of recombination-driven allelic diversity and temporal variation of Plasmodium falciparum msp1 in Tanzania. Am. J. Trop. Med. Hyg. 761037-1045. [PubMed] [Google Scholar]
- 39.White, N. J., and P. Olliaro. 1998. Artemisinin and derivatives in the treatment of uncomplicated malaria. Med. Trop. 5854-56. [PubMed] [Google Scholar]