Abstract
Salmonella enterica serotype Typhimurium causes acute inflammatory diarrhea in humans. Flagella contribute to intestinal inflammation, but the mechanism remains unclear since most mutations abrogating pattern recognition of flagellin also prevent motility and reduce bacterial invasion. To determine the contribution of flagellin pattern recognition to the generation of innate immune responses, we compared in two animal models a nonmotile, but flagellin-expressing and -secreting serotype Typhimurium strain (flgK mutant) to a nonmotile, non-flagellin-expressing strain (flgK fliC fljB mutant). In vitro, caspase-1 can be activated by cytosolic delivery of flagellin, resulting in release of the interferon gamma inducing factor interleukin-18 (IL-18). Experiments with streptomycin-pretreated caspase-1-deficient mice suggested that induction of gamma interferon expression in the murine cecum early (12 h) after serotype Typhimurium infection was caspase-1 dependent but independent of flagellin pattern recognition. In addition, mRNA levels of the CXC chemokines macrophage inflammatory protein 2 and keratinocyte-derived chemokine were markedly increased early after serotype Typhimurium infection of streptomycin-pretreated wild-type mice regardless of flagellin expression. In contrast, in bovine ligated ileal loops, flagellin pattern recognition contributed to increased mRNA levels of macrophage inflammatory protein 3α and more fluid accumulation at 2 h after infection. Collectively, our data suggest that pattern recognition of flagellin contributes to early innate host responses in the bovine ileal mucosa but not in the murine cecal mucosa.
Salmonella enterica serotype Typhimurium is a major cause of gastroenteritis in humans, which is characterized by acute intestinal inflammation and diarrhea (11, 36). One of the serotype Typhimurium virulence factors contributing to intestinal inflammation are flagella. Nonflagellated serotype Typhimurium mutants have been shown to cause less inflammation than their isogenic parents do after infection of bovine ligated ileal loops (59), streptomycin-pretreated mice (65, 74), and chickens (24).
Several possible mechanisms by which flagella may contribute to eliciting proinflammatory responses have been proposed. Flagella are surface appendages of serotype Typhimurium that are required for motility and chemotaxis. Motility contributes to serotype Typhimurium invasion of intestinal epithelial cell lines by increasing bacterial contact with host cells (26, 27). The invasion-associated type III secretion system (T3SS-1) is important for inducing intestinal inflammation in animal models (1, 20, 70, 81). Nonmotile serotype Typhimurium mutants may thus cause reduced intestinal inflammation in vivo because the efficiency of T3SS-1-mediated invasion is reduced.
In addition to its role in motility and invasion, the proteinaceous monomer of the flagellar filament, flagellin, has been shown to be a potent activator of the innate immune response in tissue culture models. Flagellin is an agonist of Toll-like receptor 5 (TLR5) (21), a pattern recognition receptor (PRR) of the innate immune system expressed on the basolateral surface of intestinal epithelial cells (15, 16) and on the surface of a subset of intestinal dendritic cells (71). In addition, flagellin is delivered into the cytosol of macrophages by the T3SS-1 of serotype Typhimurium (12, 38, 68), where it activates the cytosolic interleukin-1β (IL-1β) converting enzyme-protease activating factor (IPAF), a nucleotide-binding and oligomerization domain-like receptor (NLR) of the innate immune system. Recognition of flagellin by IPAF leads to activation of the inflammasome (i.e., caspase-1), followed by proteolytic activation of IL-1β and IL-18 (12, 38).
Although the molecular mechanisms by which flagella influence interaction with host cells have been determined using tissue culture models, it remains unclear which of these mechanisms are operational in vivo. The principal reason for this is that most mutations that prevent the biosynthesis of flagella are associated with a pleiotropic phenotype, including an absence of motility, reduced invasion and reduced stimulation of TLR5 and IPAF. For example, inactivation of the two flagellin genes (fliC and fljB) reduces inflammation in vivo (24, 59, 65, 74), but it is not clear whether the lack of motility or the lack of flagellin pattern recognition exhibited by the fliC fljB mutant is responsible for this observation. Because of the pleiotropic phenotypes of the mutants under study, genetic approaches used in previous reports were not able to distinguish between a reduction in pattern recognition and a reduction in invasiveness as possible causes for reduced inflammatory responses elicited by nonmotile serotype Typhimurium mutants. Therefore, conclusive evidence for a contribution of flagellin pattern recognition to inflammation in vivo is still lacking.
Here, we applied a combination of innovative bacterial genetics, mouse genetics, and bovine ligated ileal loop experiments to overcome current limitations and test the hypothesis that flagellin pattern recognition contributes to the initiation of inflammation in vivo.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in the present study are listed in Table 1. Strains were routinely grown with aeration at 37°C in LB broth (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl/liter) or on LB agar plates unless noted otherwise. When appropriate, antibiotics were added at the following concentrations: chloramphenicol (Cm), 0.03 mg/ml; carbenicillin, 0.1 mg/ml; kanamycin (Kan), 0.05 mg/ml; nalidixic acid, 0.05 mg/ml; streptomycin, 0.1 mg/ml; and tetracycline, 0.01 mg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Plasmid or strain | Relevant characteristicsa | Source or reference |
|---|---|---|
| Plasmids | ||
| pGP704 | oriR6K mobRP4 bla | 40 |
| pHP45Ω | ori(pMB1) bla; Strepr | 46 |
| pRDH10 | oriR6K mobRP4 cat sacRB; Tcr | 28 |
| pSPN29 | oriR6K mobRP4 cat sacRB, upstream and downstream regions of fliC | This study |
| pSW127 | oriR6K mobRP4 bla, internal fragment of invA | This study |
| Strains | ||
| E. coli | ||
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 lacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen |
| CC118 λpir | araD139 Δ(ara leu)7697 ΔlacX74 phoA20 galE galK thi rpsE rpoB argEam recA1 λpir | 22 |
| DH5α λpir | F−endA1 hsdR17 (r− m+) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U189 φ80lacZΔM15 λpir | 44 |
| S17-1 λpir | C600::RP4 2-(tet::Mu) (kan::Tn7) λpir recA1 thi pro hsdR (r− m+) | 61 |
| S. enterica serovar Typhimurium | ||
| LT2 | Laboratory strain | ATCC 700720 |
| IR715 | Fully virulent spontaneous nalidixic acid-resistant derivative of serotype Typhimurium strain ATCC 14028 | 66 |
| EHW26 | IR715 fliC::Tn10 fljB5001::MudJ | 48 |
| SPN286 | IR715 fliC5050::MudJ | This study |
| SPN287 | IR715 fljB5001::MudCm | This study |
| SPN303 | IR715 ΔfliC(−25 to +1494) | This study |
| SPN313 | IR715 ΔfliC(−25 to +1494) fljB5001::MudCm | This study |
| SPN315 | IR715 flgK5396::MudJ ΔfliC(−25 to +1494) fljB5001::MudCm | This study |
| SW215 | IR715 flgK5396::MudJ | 76 |
| SW358 | IR715 flgK5396::MudJ ΔfliC(−25 to +1494) fljB5001::MudCm invA::pSW127 | This study |
| SW399 | IR715 invA::pSW127 | This study |
| SW473 | IR715 ΔfliC(−25 to +1494) fljB5001::MudJ | This study |
| TH4881 | LT2 pNK2880(Carbr, Tn10 tnpA[ats-1 ats-2]) fliC5050::MudJ fljB5001::MudCm | 51 |
| TH5507 | LT2 flgK5396::MudJ | 3 |
Carbr, carbenicillin resistance; Tcr, tetracycline resistance; Specr, spectinomycin resistance.
Construction of serotype Typhimurium mutants.
Standard cloning techniques were performed as described previously (55). Deletion of the fliC gene of serotype Typhimurium was achieved by allelic exchange. The primers listed in Table 2 were used to amplify the regions upstream and downstream of fliC of serotype Typhimurium LT2, respectively. The PCR products were purified, digested with XbaI, and ligated. The ligation mixture served as a template for a subsequent PCR to amplify the linked flanking region construct. The PCR product encompassing the two flanking regions of the fliC coding sequence was ligated into pCR2.1 by using the TOPO cloning kit (Invitrogen). This plasmid was digested with BamHI, and the resulting ΔfliC(−25 to +1494) cassette was ligated into BamHI-digested pRDH10, generating pSPN29. pSPN29 was introduced into SPN286 by conjugation, selecting for single-crossover events. Sucrose selection was performed as described previously (30) to delete fliC5050::MudJ by allelic exchange, yielding SPN303. The deletion was confirmed by PCR. To inactivate the invA gene by insertion mutagenesis, an internal fragment of the serotype Typhimurium IR715 invA gene was amplified and cloned into pCR2.1. The resulting plasmid was digested with SmaI and XhoI, the invA fragment ligated into SmaI- and SalI-digested pGP704. The resulting plasmid (pSW127) was then introduced into serotype Typhimurium IR715 by conjugation. Integration in the chromosome at the appropriate location was verified by PCR.
TABLE 2.
Primers used for mutagenesis in this study
| Purpose/target | Primer sequence (5′-3′)a | Source |
|---|---|---|
| Amplification of the upstream and the downstream region of fliC | TTTGGCGGATCCTTCCAGCGGCTCTTTACG | This study |
| TATGGCTCTAGATGATGTTATTGGGCTGTTGC | ||
| TATGCGTCTAGAGATTGATTCACCGACACG | This study | |
| TTTGGCGGATCCTACACCTGTTCCAGTTCG | ||
| Insertional inactivation of invA | CCCGGGTGAAATTATCGCCACG | This study |
| CTCGAGTCATCGCACCGTCAAAG |
Restriction endonuclease cleavage sites are underlined.
Generalized transduction.
Phage P22 HT int-105 was used to generate generalized transducing lysates of serotype Typhimurium as previously described (39). Transductants were struck for single colonies on Evans blue uridine agar (8) and light green colonies were cross-struck against P22 H5 to confirm phage sensitivity. A P22 lysate of TH4881 was used to transduce serotype Typhimurium IR715 to Kanr and Cmr separately, thus generating SPN286 and SPN287. A P22 lysate of SPN287 was used to transduce fljB5001::MudCm into SPN303, generating SPN313. The flgK5396::MudJ mutation was transduced from TH5507 into SPN313, yielding SPN315. To construct SW358, a P22 phage lysate of SW399 was used to transduce invA::pSW127 into SPN315. A phage lysate of EHW26 was used to transduce fljB5001::MudJ into SPN303, creating SW473.
Flagella staining.
Staining of unfixed bacteria was carried out as described previously (35). Briefly, 0.002 ml of bacterial culture was added to 0.005 ml of a mixture of 1 part 12% crystal violet in ethanol and 10 parts mordant [10% tannic acid, 2.5% phenol in saturated aqueous KAl(SO4)2]. Digital images were created by using a Zeiss Axiovert 200M inverted microscope and Zeiss Axiocam MRC5/Zeiss AxioVision 4.5 software.
Motility assays.
For motility assays, plates containing 10 g of tryptone/liter, 5 g of NaCl/liter, and 0.3% agar were inoculated with a single colony from an LB agar plate and incubated at 37°C for 8 h. The experiment was performed in triplicate.
Analysis of extracellular flagellin.
Serotype Typhimurium strains were grown for 16 h at 37°C with aeration, diluted 1:50 in 6 ml of LB broth, and grown for 3 h at 37°C with aeration. Flagella were sheared by treating the bacterial culture with a T25 disperser (IKA) on ice for 1 min. The resulting suspension was centrifuged at 3,220 × g for 10 min at 4°C. To 5 ml of the supernatant, trichloroacetic acid was added to a final concentration of 10% (wt/vol), followed by centrifugation at 12,000 × g for 30 min at 4°C. The pellet was washed two times with ice-cold acetone and resuspended in 0.05 ml of loading buffer. Next, 0.01 ml of this solution was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5), transferred to polyvinylidene fluoride membrane (Millipore) using a semidry transfer system (Bio-Rad Laboratories), and detected with rabbit Salmonella H antiserum i (Difco) and an alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Bio-Rad Laboratories). The experiment was performed three times with identical results. Salmonella H antiserum 1,2 was obtained from the Robert-Koch Institute, Wernigerode, Germany.
RNA extraction, reverse transcription-PCR (RT-PCR), and real-time PCR.
RNA was extracted from tissue culture cells and animal tissue as described previously using Tri-Reagent (Molecular Research Center) according to the instructions of the manufacturer. RNA samples (1,000 ng) were reverse transcribed in a 0.050-ml volume (TaqMan reverse transcription reagents; Applied Biosystems), and 0.004 ml of cDNA was used as a template for each Real time PCR in a 0.025-ml total reaction volume. Real-time PCR was performed using the primers listed in Table 3, as well as SYBR green (Applied Biosystems) and GeneAmp 7900 sequence detection system. The data were analyzed by using the comparative CT method (Applied Biosystems). Target gene transcription of each sample was normalized to the respective levels of GAPDH mRNA.
TABLE 3.
Primers used for real-time qRT-PCR in this study
| Species | Target gene(s) | Sequence (5′-3′) | Source or reference |
|---|---|---|---|
| Homo sapiens | IL-8 (CXCL8) | GCCAACACAGAAATTATTGTAAAGCTT | 67 |
| CCTCTGCACCCAGTTTTCCTT | |||
| MIP3A (CCL20) | CTGCTTTGATGTCAGTGCTGCTAC | 25 | |
| CTGCCGTGTGAAGCCCACAATAAA | |||
| GAPDH | CCAGGAAATGAGCTTGACAAAGT | 48 | |
| CCCACTCCTCCACCTTTGAC | |||
| Mus musculus | Ifng | TCAAGTGGCATAGATGTGGAAGAA | 43 |
| TGGCTCTGCAGGATTTTCATG | |||
| Il6 | GAGGATACCACTCCCAACAGACC | 43 | |
| AAGTGCATCATCGTTGTTCATACA | |||
| Il17a | GCTCCAGAAGGCCCTCAGA | 43 | |
| AGCTTTCCCTCCGCATTGA | |||
| Kc (Cxcl1) | TGCACCCAAACCGAAGTCAT | 23 | |
| TTGTCAGAAGCCAGCGTTCAC | |||
| Mip2 (Cxcl2) | AGTGAACTGCGCTGTCAATGC | 23 | |
| AGGCAAACTTTTTGACCGCC | |||
| Mip3a (Ccl20) | CCAGGCAGAAGCAAGCAACT | 43 | |
| TCGGCCATCTGTCTTGTGAA | |||
| Gapdh | TGTAGACCATGTAGTTGAGGTCA | 43 | |
| AGGTCGGTGTGAACGGATTTG | |||
| Bos taurus | IFNG | TGATGGCATGTCAGACAGCA | 37 |
| GGCACAAGTCATATAGCCTGACAC | |||
| IL17A | CTTGGACTCTCCACCGCAA | This study | |
| GTCCACCTTCCCTTCAGCA | |||
| IL-8 (CXCL8) | CTCTGTGTGAAGCTGCAGTTCTGTC | This study | |
| ATTTGGGGTGGAAAGGTGTGG | |||
| GRO (CXCL1, CXCL2, CXCL3) | GATGCTGTTCCTGCTCCTGG | This study | |
| TGAGGTGAATCCCCTGCAA | |||
| MIP3A (CCL20) | GCTCCTGGCTGCTTTGATGT | This study | |
| TGGCCAGCTGCTGTGTGA | |||
| GAPDH | TTCTGGCAAAGTGGACATCGT | 49 | |
| GCCTTGACTGTGCCGTTGA |
Tissue culture experiments.
The colorectal carcinoma cell line T84 (ATCC CCL-248) was obtained from the American Type Culture Collection. T84 cells were routinely cultured in Dulbecco modified Eagle medium/F-12 medium (Gibco), containing 1.2 g of sodium bicarbonate/liter, 2.5 mM l-glutamine, 15 mM HEPES, and 0.5 mM sodium pyruvate (Gibco) supplemented with 10% fetal calf serum (FCS). For assays, T84 cells were seeded in 24-well plates at a density of ∼105 cells per well, incubated for 24 h with serum-containing medium, and 24 h with serum-free medium.
Gentamicin protection assay.
To determine invasion of T84 cells, Salmonella strains were grown for 16 h at 37°C with aeration, diluted 1:50 in LB broth, and incubated for 3 h at 37°C with aeration. Bacteria were added to epithelial cells at a multiplicity of infection (MOI) of 5 for 1 h. Cells were washed four times with Dulbecco phosphate-buffered saline (DPBS; Gibco), and serum-free Dulbecco modified Eagle medium/F-12 medium containing 0.1 mg of gentamicin (Gibco)/ml was added. After 90 min, cells were washed four times with DPBS, and intracellular bacteria were quantified by spreading serial 10-fold dilutions of T84 cell lysates (1% Triton X-100) on LB agar plates to determine the number of CFU. The experiment was performed three times.
Stimulation of T84 cells.
A portion (5 ml) of LB broth was inoculated with 0.1-ml overnight culture and incubated at 37°C for 3 h. Flagella were sheared by treating the bacterial culture with a T25 disperser (IKA) for 1 min on ice. The resulting suspension was centrifuged at 3,220 × g for 10 min at 4°C, and the supernatant was filter sterilized. Then, 0.005 ml of this solution was added per well to stimulate T84 cells for 60 min. As controls, either purified flagellin from serotype Typhimurium (InvivoGen) at a final concentration of 0.002 mg/ml or 0.01 ml of endotoxin-free PBS per well was added. The experiment was performed three times.
Secretion of IL-1β by bone-marrow-derived macrophages (BMDM).
Primary macrophages from C57BL/6/J and Caspase-1−/− mice were isolated as described previously (75). Briefly, femurs were flushed with complete medium (RPMI, 10% FCS, 2 mM glutamine, and 0.1 mg of penicillin and streptomycin/ml). Cells were cultured in complete medium supplemented with L-cell conditioned medium (53) at 37°C in the presence of 5% CO2. After 72 h, the medium was removed, and fresh complete medium supplemented with L-cell conditioned medium was added. On day 7, adherent cells were harvested by treatment with trypsin-EDTA (Gibco), and the resulting cell suspension was centrifuged at 1,000 rpm for 10 min. Cells were seeded in 24-well plates at a density of 5 × 105 cells per well and incubated for 48 h at 37°C in 5% CO2 in RPMI containing 10% FCS. At 4 h prior to infection, macrophages were treated with purified 100 ng of lipopolysaccharide (LPS; E. coli O55:B5; Sigma)/ml. A 5-ml portion of LB broth was inoculated with a single colony from a plate, followed by incubation at 30°C for 10 h. The medium containing LPS was replaced with fresh medium, and the macrophages were infected at an MOI of 5. To synchronize the infection, the plate was centrifuged at 250 × g for 5 min at 25°C and subsequently incubated at 37°C and 5% CO2 for 25 min. Cells were washed twice with 0.5 ml of DPBS, and subsequently, 0.5 ml of RPMI containing 10% FCS and 0.025 mg of gentamicin/ml were added. Cell culture supernatants were collected 18 h after infection, and the remaining macrophages were lysed with 1% Triton X-100 in DPBS. Bacterial numbers were enumerated by spreading 10-fold serial dilutions on LB agar plates.
The concentration of IL-1β in the culture supernatants was determined using a mouse IL-1β specific enzyme-linked immunosorbent assay (ELISA) kit (e-Bioscience) according to the instructions of the manufacturer. The experiment was performed three times.
Mouse strains and infection.
C57BL/6/J (Jackson Laboratory) and Caspase-1−/− mice in the C57BL/6 background (34) were housed under specific-pathogen-free conditions and were provided with water and food ad libitum. Mice (10 to 12 weeks of age) received 20 mg of streptomycin intragastrically 24 h prior to infection (7). For oral infection, Salmonella strains were grown for 16 h aerobically at 37°C. Each mouse was inoculated either with 0.1 ml of sterile LB broth or with 1 × 109 to 2 × 109 CFU of serotype Typhimurium in 0.1 ml of LB broth. At 12 h after infection, animals were sacrificed. Peyer's patches (five to seven per animal), as well as the center and the distal parts (including the lymphoid patch) of the ceca, were frozen in liquid nitrogen for nucleic acid extraction. To determine the numbers of viable Salmonella, Peyer's patches (three per animal), mesenteric lymph nodes, livers, and spleens were homogenized in PBS, and 10-fold serial dilutions were plated on LB agar plates containing nalidixic acid.
Specifically, to obtain the data depicted in Fig. 4A, B, and C, as well as Fig. 5, eight wild-type animals were inoculated with sterile broth, four wild-type animals were inoculated with IR715, nine wild-type animals were inoculated with SW215, and nine wild-type animals were inoculated with SPN315. In addition, four Caspase-1−/− mice were inoculated with sterile LB broth, six Caspase-1−/− mice were inoculated with SPN315, and five Caspase-1−/− animals were inoculated with SW215. To obtain the data depicted in Fig. 4D and E, groups of five animals were inoculated with either sterile broth, SW215, or SPN315.
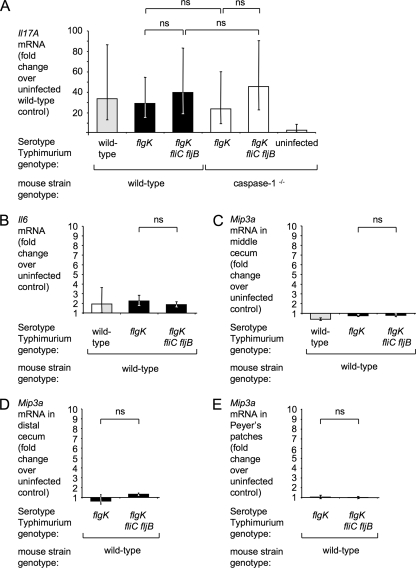
FIG. 4.
Changes in mRNA levels in the murine intestinal mucosa 12 h after serotype Typhimurium infection. (A to C) Relative expression of Il17A (A), Il6 (B), an Mip3a (C) in the middle cecum. Mip3a mRNA levels in the cecal patch containing distal cecum (D) and in ileal Peyer's patches (E). Streptomycin pretreated C57BL/6 wild-type and caspase-1 mice were infected with 109 CFU of IR715 (wild-type), SW215 (flgK), or SPN315 (flgK fliC fljB). Fold changes were standardized to the mean expression level of the respective transcript in uninfected wild-type mice. The genotype of the mice infected with each bacterial strain is indicated below each panel. The data are shown as geometric means (bars) ± the standard error. Strains that differ only in their capacity to induce flagellin pattern recognition (flgK mutant and flgK fliC fljB mutant) are highlighted by black or white bars. ns, not statistically significant.
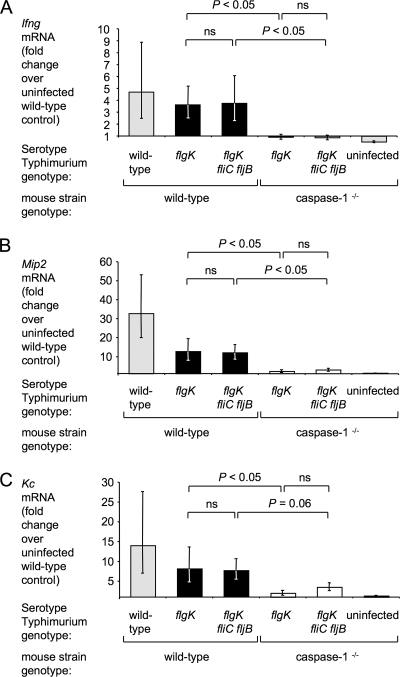
FIG. 5.
Changes in relative expression of the caspase-1-dependent transcripts Ifng (A), Mip2 (B), and Kc (C) in the middle cecum of streptomycin-pretreated mice induced by IR715 (wild-type), SW215 (flgK), or SPN315 (flgK fliC fljB). Relative gene expression was determined by real-time qRT-PCR, and fold changes were standardized to the mean expression level of the respective transcripts in uninfected wild-type mice. The genotype of the mice infected with each bacterial strain is indicated below each panel. The data are shown as geometric means (bars) ± the standard error. Strains that differ only in their capacity to induce flagellin pattern recognition (flgK mutant and flgK fliC fljB mutant) are highlighted by black or white bars. ns, not statistically significant.
Experiments were performed in accordance with Institutional Animal Care and Use Committee Guidelines.
Bovine ligated ileal loop model.
Bovine ligated ileal loop surgery was performed as described previously (56). Salmonella strains were grown for 16 h at 37°C with aeration, diluted 1:50 in LB broth, and incubated for 3 h at 37°C with aeration.
Four milk-fed, 3- to 4-week-old calves, clinically healthy and culture negative for fecal excretion of serotype Typhimurium, were initially anesthetized with propofol and maintained under anesthesia with isoflurane. A laparotomy was performed, leaving the ileum exposed. Loops, ranging in length from 5 to 8 cm, were tied with spacer loops of 1 cm in between experimental loops. Each loop was intraluminally inoculated with either sterile 3 ml of LB broth or a suspension of 2 × 109 CFU serotype Typhimurium in 3 ml of LB broth. After the injection, loops were placed back in the abdominal cavity. At 2 h after infection, the loops were resected, weighed, drained of fluid, reweighed, and opened longitudinally. Biopsies (6 mm) were punched in the mucosa, and the underlying serosa was removed. Biopsies were frozen in liquid nitrogen for nucleic acid extraction. The weight of the luminal fluid was measured by subtracting the weight of the drained loop from the weight of the resected loop, normalized by the length of the loop (in centimeters). The fluid response of each loop was corrected by subtracting the fluid accumulation from the corresponding uninfected loop. For one animal, significant hemorrhage in the uninfected loop was observed, and data for fluid accumulation were therefore excluded from the analysis.
To determine the tissue invasion of serotype Typhimurium, two 6-mm biopsy punches per loop were washed three times with 5 ml of DBPS, incubated at 25°C for 1 h in 0.1 mg of gentamicin/ml in DPBS, and homogenized in 5 ml of DBPS. Serial 10-fold dilutions of the resulting suspensions were plated on LB agar containing nalidixic acid.
Statistical analysis.
For statistical analysis of ratios (i.e., the fold increases in gene expression or invasion data expressed as a percentage of the inoculum), values were transformed logarithmically to calculate geometric means and for further statistical analysis. A parametric test (Student t test) was used to calculate whether differences in fold changes of gene expression or invasion between treatment groups were statistically significant (P < 0.05). For data from tissue culture experiments and bovine ligated ileal loop experiments, paired statistical analysis was used, while for data from mouse experiments unpaired statistical analysis was used.
RESULTS
Genetic strategy to assess the importance of flagellin pattern recognition during intestinal inflammation.
Our understanding of the role of flagella in eliciting inflammation has been hampered by the fact that these surface appendages are important for invasion (26, 27, 32), as well as for pattern recognition (12, 15, 21, 38). Compared to the serotype Typhimurium wild-type strain, flagellin gene fliC (encoding H1 flagellin) and fljB (encoding H2 flagellin) mutants exhibit a reduced ability to elicit inflammation in animal models of serotype Typhimurium-induced gastroenteritis (24, 59, 65, 74). However, it is not clear from these results whether flagella contribute to inflammation because motility is required for invasion or because flagellin is a potent pathogen-associated molecular pattern recognized by PRRs. An in vitro characterization of a fliC fljB mutant was performed to demonstrate this point. As shown in Fig. 1, a fliC fljB mutant was nonflagellated (Fig. 1A), nonmotile (Fig. 1B), lacked expression of the phase H1 flagellin FliC (Fig. 1C), and exhibited reduced invasiveness for human colonic cancer T84 cells (Fig. 1D). Unlike culture supernatants of the serotype Typhimurium wild-type strain, culture supernatants of the fliC fljB mutant did not elicit transcription of macrophage inflammatory protein 3α (MIP-3α; CCL20) and IL-8 (CXCL8), encoded by the MIP3A and the IL-8 genes, respectively (Fig. 2). In T84 cells, this process has been previously shown to depend on pattern recognition of flagellin by TLR5 (15). Furthermore, the fliC fljB mutant was defective in eliciting release of IL-1β from murine BMDM (Fig. 3A), a process previously described to depend on the activation of IPAF and caspase-1 by pattern recognition of flagellin in the host cell cytosol (12, 38, 68). These data confirm that comparison of the serotype Typhimurium wild-type strain to a fliC fljB mutant is not suited for distinguishing between pattern recognition and invasion as possible mechanisms by which flagella contribute to inflammation in vivo.
FIG. 1.
In vitro characterization of serotype Typhimurium flagellar mutants. The genotypes of the serotype Typhimurium strains IR715 (wild-type), SW473 (fliC fljB), SW215 (flgK), SPN315 (flgK fliC fljB), SW399 (invA), and SW358 (flgK fliC fljB invA) are indicated at the bottom of each panel. Strains chosen for in vivo comparison (flgK mutant and flgK fliC fljB mutant) are highlighted by a box (A to C) or black bars (D). (A) Staining for flagella using crystal violet. Bar, 5 μm. (B) Motility of serotype Typhimurium mutants on plates containing 0.3% agar. (C) Determination of flagellin amounts exported by serotype Typhimurium strains regardless of assembly into filaments. Sheared surface-associated structures, as well as bacterial culture supernatant, were precipitated with trichloroacetic acid and analyzed by Western blotting with rabbit anti-Hi serum (αHi). The molecular mass and position of standard protein is indicated on the left. (D) Invasion of T84 epithelial cells by serotype Typhimurium. Cells were infected for 1 h, washed with PBS, and then treated with gentamicin for 90 min before bacteria were counted. The data are shown as geometric means (bars) from three independent experiments ± the standard error. ns, not statistically significant.
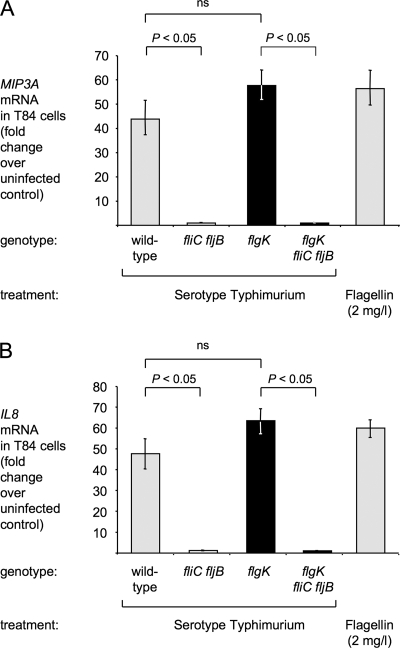
FIG. 2.
MIP3A (A) and IL-8 (B) transcription in T84 cells is induced by bacterial flagellin. Bacterial culture supernatants were prepared by shearing off surface-associated structures, removing bacterial cells by centrifugation, and filter sterilization. T84 cells were stimulated for 1 h with 0.005 ml of sterile bacterial culture supernatant or 2 mg of purified serotype Typhimurium flagellin/liter. RNA was extracted and relative gene expression was determined by real-time qRT-PCR. The genotypes of the serotype Typhimurium strains IR715 (wild-type), SW473 (fliC fljB), SW215 (flgK), and SPN315 (flgK fliC fljB) are indicated at the bottom of each panel. Strains chosen for in vivo comparison (flgK mutant and flgK fliC fljB mutant) are highlighted by black bars. The data are expressed as fold increases of gene expression in infected cells over uninfected cells. The data are shown as geometric means (bars) from three independent experiments ± the standard error. ns, not statistically significant.
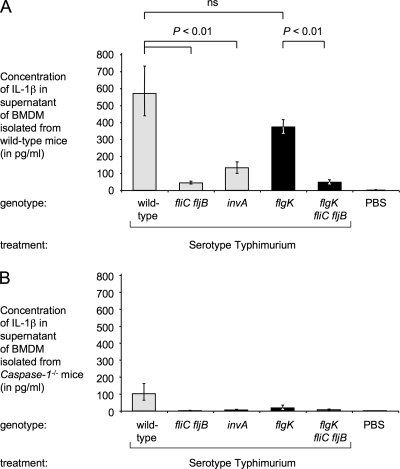
FIG. 3.
Flagellin-dependent IL-1β secretion by BMDM. BMDM from wild-type (A) or caspase-1-deficient (B) mice were treated with 100 ng of LPS/ml for 4 h and infected with serotype Typhimurium strains IR715 (wild-type), SW473 (fliC fljB), SW399 (invA), SW215 (flgK), or SPN315 (flgK fliC fljB) at an MOI of 5 for 18 h. The concentration of IL-1β in the culture supernatant was determined by ELISA. The data are shown as geometric means (bars) from three (A) or four (B) independent experiments ± the standard error. Strains chosen for in vivo comparison (flgK mutant and flgK fliC fljB mutant) are highlighted by black bars. ns, not statistically significant.
To overcome this limitation, we developed a genetic strategy that allowed us to distinguish between traits that depend on the flagellin monomer (pattern recognition) from those that rely on the presence of an intact flagellar filament (motility and invasion). Disruption of the flgK gene, encoding a flagellar hook-associated protein, abolishes the formation of flagellar filaments (69), resulting in a loss of motility; however, the expression of flagellin is not affected (18). We reasoned that a serotype Typhimurium flgK-null mutant and a flgK fliC fljB triple mutant should be equally invasive but differ in their ability to induce recognition of flagellin by the innate immune system. To test this hypothesis, an flgK mutant and an flgK fliC fljB mutant were characterized in vitro. The flgK mutant and the flgK fliC fljB mutant were both nonflagellated (Fig. 1A) and nonmotile (Fig. 1B). TLR5 is thought to be stimulated exclusively by monomeric flagellin because the TLR5-binding site of flagellin is not accessible in polymerized flagellar filaments (62). To determine the amount of secreted and/or assembled flagellin, we sheared off surface structures from all bacterial strains, separated the intact cells from soluble factors, and analyzed the supernatant for the presence of FliC. Western blot analysis showed that the serotype Typhimurium wild-type strain and the flgK mutant secreted comparable amounts of FliC, whereas no FliC secretion was detected in the flgK fliC fljB mutant (Fig. 1C). Importantly, the flgK mutant and the flgK fliC fljB mutant did not differ in their invasiveness for human colonic epithelial cancer cells (Fig. 1D). The invasiveness of the flgK mutant and the flgK fliC fljB mutant was significantly lower (P < 0.05) than that of the serotype Typhimurium wild-type strain but significantly higher (P < 0.05) than that of a flgK fliC fljB invA mutant (Fig. 1D), which does not produce a functional T3SS-1. These data suggested that the flgK and flgK fliC fljB mutants were equally invasive and differed only in the expression of flagellin monomers.
To determine the capacity of the flgK and the flgK fliC fljB mutants to stimulate TLR5-mediated innate immune responses regardless of bacterial invasion, T84 cells were treated with sterile bacterial culture supernatants. Culture supernatants were prepared by shearing surface associated factors and removing insoluble factors by centrifugation. RNA was extracted after 1 h and MIP3A (CCL20) and IL-8 (CXCL8) mRNA levels were determined by real-time quantitative RT-PCR (qRT-PCR). Stimulation of T84 cells with culture supernatants from both the serotype Typhimurium wild-type strain and the flgK mutant resulted in marked induction of MIP3A and IL-8 transcription (Fig. 2). In addition, MIP3A and IL-8 transcription could be induced by stimulation of T84 cells with purified flagellin in accordance with previous observations (4, 15, 78). However, the stimulation with supernatants from the flgK fliC fljB mutant did not result in increased MIP3A and IL-8 mRNA levels in this model (P < 0.05).
The ability of the flgK and the flgK fliC fljB mutants to induce IPAF/caspase-1-dependent secretion of mature IL-1β by BMDM was investigated next. BMDM were infected at an MOI of 5, and the concentration of secreted IL-1β in the supernatant was determined by ELISA 18 h after infection. BMDM infected with the wild-type strain secreted 570 pg of IL-1β/ml (Fig. 3A). Previous studies showed that detection of IL-1β in this model is dependent on a functional T3SS-1, as well as the expression of both flagellins (12, 38). Consistent with these previous reports, we found that a mutation in invA, encoding a structural component of the T3SS-1, significantly reduced IL-1β secretion (Fig. 3A). The amount of IL-1β secreted by BMDM infected with the flgK mutant (370 pg/ml) was not significantly different than that secreted by BMDM infected with the serotype Typhimurium wild-type strain. In contrast, IL-1β secretion was markedly reduced when BMDM were infected with the flgK fliC fljB mutant. Secretion of IL-1β was caspase-1 dependent, since BMDM isolated from caspase-1-deficient mice secreted significantly (P < 0.05) reduced amounts of IL-1β upon infection with the wild-type strain and the flgK mutant (Fig. 3B). In this experiment, no statistically significant difference in numbers of intracellular bacteria was observed between bacterial strains at the end of the experiment (data not shown).
Collectively, these data confirmed that the serotype Typhimurium wild-type strain and the fliC fljB mutant differed in their motility (Fig. 1B) and invasiveness (Fig. 1D) and their ability to induce pattern recognition in vitro by activating TLR5 expressed on epithelial cells (Fig. 2) and IPAF/caspase-1 expressed by BMDM (Fig. 3). In contrast, the flgK mutant and the flgK fliC fljB mutant differed only in their ability to induce TLR5 and IPAF/caspase-1 recognition in vitro (Fig. 2 and 3), while their invasiveness for epithelial cells was comparable (Fig. 1D). These data supported that comparison of the flgK mutant with the flgK fliC fljB mutant would be a discerning genetic strategy for determining the contribution of flagellin pattern recognition to inflammation in vivo.
Early transcription of CXC chemokines and Ifng in the ceca of streptomycin-pretreated mice is caspase-1 dependent but flagellin independent.
The goal of the present study was to assess the role of flagellin pattern recognition in eliciting intestinal inflammation using two animal models: the streptomycin-pretreated mouse (7) and bovine ligated ileal loops (56). The bovine ligated ileal loop model is suited for analyzing responses that develop within the first 12 h after infection. To improve comparison, we chose to use an early time point (12 h after infection) in streptomycin-pretreated mice. Severe pathological changes are restricted to the ceca of streptomycin-pretreated mice, whereas inflammatory changes in the ileum are mild or absent at early time points after infection (7). Therefore, the cecal mucosa was used to study acute intestinal inflammation in mice. To test the hypothesis that proinflammatory gene expression depends on the presence of bacterial flagellin, wild-type mice (C57BL/6) were pretreated with streptomycin and orally infected with the serotype Typhimurium wild-type strain, the flgK mutant, the flgK fliC fljB mutant, or sterile LB (mock infection), respectively. All bacterial strains were carrying plasmid pHP45Ω (46) to confer resistance to streptomycin. Mice were euthanized 12 h after infection, RNA extracted from the cecum, and the relative gene expression was determined by real-time qRT-PCR. No statistically significant differences in bacterial numbers were observed in the colon contents and the Peyer's patches of flgK mutant and flgK fliC fljB mutant-infected mice (data not shown).
Compared to mock-infected mice, animals infected with the serotype Typhimurium wild-type strain led to an increase in cecal mRNA levels of murine IL-17 (encoded by the Il17a gene) (34-fold), IFN-γ (encoded by Ifng) (5-fold), MIP-2 (encoded by Mip2) (33-fold), and keratinocyte-derived chemokine (encoded by Kc) (14-fold) in the cecum (Fig. 4 and 5). Il17a, Ifng, Kc, and Mip2 mRNA levels induced by the flgK mutant and the serotype Typhimurium wild-type strain were not significantly different, indicating that despite the lack of motility, the flgK mutant was still able to elicit profound innate host responses.
In vitro, flagellin can activate cells through TLR5 or IPAF/caspase-1. To distinguish between these two mechanisms in vivo, we sought to identify marker genes, whose expression depends on only one of these pathways using mouse genetics. However, uninfected TLR5-deficient mice display abnormally high cytokine expression in the cecum compared to the wild-type mouse strain (73), rendering this approach unsuitable for TLR5-dependent genes. Therefore, we focused on the IPAF/caspase-1 pathway. To identify cytokines whose expression requires caspase-1 activity, mice deficient in caspase-1 (bred on a C57BL/6 background) were treated with streptomycin and then intragastrically infected with the flgK mutant, the flgK fliC fljB mutant, or sterile media (mock infection). The host responses 12 h after infection were compared to those of mock-infected wild-type animals. No compensatory effects on cytokine or chemokine expression were observed in mock-infected caspase-1-deficient mice (Fig. 4 and 5). Upregulation of Il17a mRNA upon serotype Typhimurium infection was independent of caspase-1 (Fig. 4A). In contrast, induction of Ifng, Mip2, and Kc transcription was caspase-1 dependent (Fig. 5). However, we did not detect any statistically significant differences between animals infected with the flgK mutant or the flgK fliC fljB mutant with regard to cecal mRNA levels of Il6, Il17a, Ifng, Mip2, or Kc (Fig. 4 and 5). In summary, in streptomycin-pretreated mice, caspase-1 was required for increased Ifng, Mip2, and Kc transcription at 12 h after serotype Typhimurium infection. However, our results suggested that the activation of IPAF/caspase-1 and the induction of the cytokine response were flagellin independent in this model.
Flagellin pattern recognition contributes to fluid accumulation in bovine ligated ileal loops.
The bovine ileal loop model has been used extensively to study early events in the host response to serotype Typhimurium (56, 57, 81). To investigate whether pattern recognition of flagellin contributes to eliciting inflammation in the ileal mucosa of calves, we compared host responses 2 h after infection of loops with either the serotype Typhimurium wild-type strain, the flgK mutant, the flgK fliC fljB mutant, or sterile broth (mock infection).
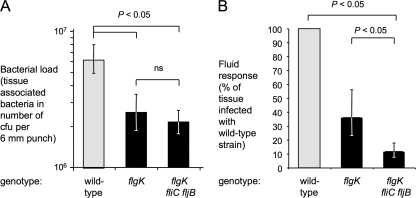
The basic premise of our genetic approach was to ensure that strains used for comparisons are equally invasive and differ only in their ability to elicit pattern recognition signaling. Therefore, we determined the number of bacteria residing inside the tissue by killing extracellular bacteria with gentamicin, homogenizing the treated biopsies, and plating the resulting homogenates on selective agar plates. The motile wild-type strain was found to be more invasive than the nonmotile flgK and flgK fliC fljB mutants (Fig. 6A), indicating that flagella contribute to invasion of the mucosa in the bovine ligated ileal loop model. Furthermore, no significant difference in tissue-associated bacterial numbers was observed between the flgK and the flgK fliC fljB mutant.
FIG. 6.
Contribution of motility-mediated invasion and pattern recognition to fluid accumulation in bovine ligated ileal loops. Loops were infected with IR715 (wild type), SW215 (flgK), or SPN315 (flgK fliC fljB) for 2 h. The genotype of each bacterial strain is indicated below each panel. (A) Recovery of serotype Typhimurium from Peyer's patch-associated mucosa. Punches (6 mm) were washed three times with PBS and treated for 1 h with 0.1 mg of gentamicin/ml before determination of bacterial numbers. The data are shown as geometric means (bars) from four animals ± the standard error. (B) Fluid accumulation elicited by serotype Typhimurium. The data are shown as geometric means (bars) from three animals ± the standard error. Strains that differ only in their capacity to induce flagellin pattern recognition (flgK mutant and flgK fliC fljB mutant) are highlighted by black bars. ns, not statistically significant.
Fluid secretion into the ileal lumen, a surrogate of diarrhea (56), was measured at 2 h after infection (Fig. 6B). The flgK mutant elicited less fluid secretion (36%) than the wild-type strain, indicating that motility-mediated invasion is a prerequisite for the full induction of host responses. The flgK fliC fljB mutant elicited significantly less fluid accumulation (P < 0.05, 12% of response in loops infected with the wild-type strain) than the flgK mutant, suggesting that flagellin pattern recognition contributed to host responses at 2 h after infection.
Flagellin pattern recognition contributes to the induction of MIP3A expression in bovine ileal loops.
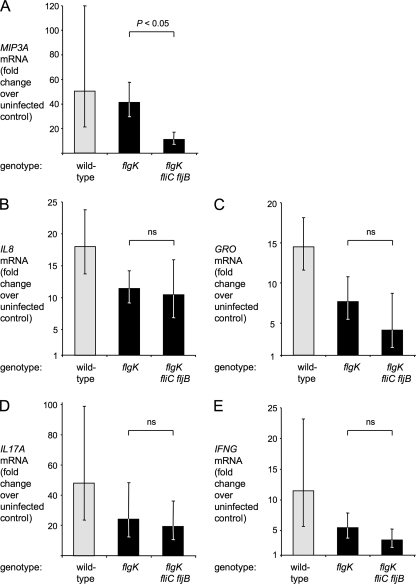
We then measured the relative mRNA levels of the genes encoding MIP-3α, IL-8, GRO, IL-17, and IFN-γ (the bovine genes encoding these cytokines are termed MIP3A, IL-8, GRO, IL17A and IFNG, respectively). Due to the high sequence similarity between the bovine GRO genes, encoding for GROα (CXCL1), GROβ (CXCL2), and GROγ (CXCL3), primers used for the real-time qRT-PCR were predicted to amplify all three transcripts; hence, we refer to the observed transcript as GRO genes.
The serotype Typhimurium wild-type strain elicited a robust host response 2 h after infection, with markedly increased mRNA levels of MIP3A (50-fold), IL-8 (18-fold), GRO (14-fold), IL17A (48-fold), and IFNG (11-fold) genes compared to mock-infected controls (Fig. 7). The mRNA levels in loops infected with the flgK mutant were lower than those in loops infected with the serotype Typhimurium wild-type strain, but these differences were not statistically significant for any assayed gene. The flgK fliC fljB mutant induced transcription of IL-8, GRO genes, IL17A, and IFNG at levels comparable to the flgK mutant, indicating that upregulation of these genes was independent of flagellin pattern recognition. However, MIP3A mRNA levels were found to be significantly (P < 0.05) higher in loops infected with the flagellin-expressing flgK mutant than in loops infected with the nonflagellated flgK fliC fljB mutant (Fig. 7A). In contrast, Mip3a was not induced in the middle cecum, in the distal cecum containing the cecal patch, or in the small intestinal Peyer's patches of streptomycin-pretreated mice infected with flgK mutant or flgK fliC fljB mutant 12 h after infection (Fig. 4).
FIG. 7.
Changes in the relative expression of MIP3A (A), IL-8 (B), GRO (C), IL17A (D), and IFNG (E) in the mucosa of bovine ligated ileal loops 2 h after serotype Typhimurium infection. Loops were infected with IR715 (wild type), SW215 (flgK), or SPN315 (flgK fliC fljB). The genotype of each bacterial strain is indicated below each panel. The data are shown as geometric means (bars) from four animals ± the standard error. Strains that differ only in their capacity to induce flagellin pattern recognition (flgK mutant and flgK fliC fljB mutant) are highlighted by black bars. ns, not statistically significant.
Collectively, our data show that invasion-independent flagellin pattern recognition contributed significantly to fluid accumulation and MIP3A expression during the early phase of serotype Typhimurium infection in calves.
DISCUSSION
The role of caspase-1 in the pathogenesis of serotype Typhimurium infection has been an active field of study in recent years. Mice lacking caspase-1 were initially found to be more resistant to oral serotype Typhimurium infection (41); however, increased susceptibility was reported for an independently generated caspase-1-deficient mouse strain (29). Consistent with a defect in controlling bacterial infection, pathological changes in the cecum of streptomycin-pretreated mice are more profound in caspase-1-deficient mice than in wild-type mice (29). Caspase-1 is required for proteolytic activation of IL-1β and IL-18 (17). Mice deficient for producing either IL-1β or IL-18 have enhanced susceptibility to serotype Typhimurium infection (52). IL-18, formerly known as IGIF (for IFN-γ inducing factor) is a potent inducer of IFN-γ production by T cells (42), which involves an innate mechanism that is antigen independent (63). Both IL-1β and IL-18 stimulate macrophages to produce CXC chemokines in vitro (47, 77). Here, we report that increased expression of murine Ifng, Kc, and Mip2 mRNA in the cecum at 12 h after serotype Typhimurium infection was strictly dependent on caspase-1, indicating that activation of the inflammasome plays an important role during the early phase of intestinal infection in mice. The inflammasome is activated upon assembly, a process that requires an NLR (IPAF, NALP1, or NALP3), as well as the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (33). Interestingly, while flagellin is able to activate caspase-1 in BMDM in vitro (12, 38), we found flagellin to be dispensable for caspase-1-dependent Ifng transcription in the cecal mucosae of mice. An alternative mechanism for activating caspase-1 involves TLR4 and the ATP-sensitive purinergic P2X7 receptor; however, serotype Typhimurium does not activate the inflammasome through this pathway in vitro (10, 13). Additional work is needed to identify the bacterial pathogen-associated molecular pattern and its cognate host NLR responsible for caspase-1-dependent IFN-γ production in the intestine of streptomycin-pretreated mice early after serotype Typhimurium infection.
The IL-18/IFN-γ axis has been implicated in amplifying inflammatory responses early after serotype Typhimurium infection in tissue (63). Interaction of serotype Typhimurium with phagocytes induces additional amplification mechanisms contributing to intestinal inflammation, including the IL-23/IL-17 axis (19, 50). Thus, analysis of RNA isolated from the inflamed intestine is expected to reveal responses that have been amplified and may differ from the gene expression profile observed in tissue culture. Our results indicate that flagellin pattern recognition was not required for IFNG transcription early after infection of bovine ligated ileal loops, a result that did not provide support for the idea that flagellin contributes to caspase-1 activation in the bovine ileal mucosa. However, flagellin pattern recognition was required for MIP3A transcription induced in the bovine ileal mucosa and for induction of MIP3A transcription in human epithelial T84 cells. Flagellin-dependent changes in gene expression in T84 cells are mediated through TLR5 (15). These data are consistent with the idea that stimulation of TLR5 by serotype Typhimurium flagellin may be involved in activating a subset of inflammatory changes early after infection of the bovine ileal mucosa, including expression of MIP3A.
MIP-3α (CCL20) is expressed by the follicle-associated epithelium overlying organized lymphoid structures such as the Peyer's patches (14) and is the sole known chemokine ligand for CCR6, a receptor protein expressed by a subset of dendritic cells, B cells, and memory T cells (6, 31, 45). MIP-3α and CCR6 are thought to be involved in lymphoid tissue homeostasis (9, 72), but expression of MIP-3α is strongly increased upon stimulation with proinflammatory signals (14). Model epithelia treated with serotype Typhimurium flagellin upregulate human MIP3A expression, which promotes transepithelial migration of dendritic cells (60). In vivo, recruitment of CCR6-positive dendritic cells to the follicle-associated epithelium has been observed during oral serotype Typhimurium infection of mice and is a prerequisite for activation of T-cell responses (54). It is interesting in this context that ca. 50% of Salmonella-specific CD4+ T cells generated during serotype Typhimurium infection recognize flagellin (2). Collectively, these previous studies provide a model suggesting that flagellin-dependent induction of MIP-3α expression may contribute to orchestrating host responses during serotype Typhimurium infection.
Previous studies have shown that flagella contribute to early cecal inflammation in streptomycin-pretreated mice predominantly by providing motility (64, 65). In accordance with these findings, our results suggest that motility-mediated invasion contributes to eliciting a full inflammatory response in the bovine ligated loop model. However, our data suggest that, in contrast to the cecal mucosa of mice, flagellin pattern recognition contributes to initiating inflammatory responses in the bovine ileal mucosa. Given the differences in disease manifestation between mice and calves (58, 79, 80), identification of such differences may be expected. Calves develop a localized infection with diarrhea, while streptomycin-pretreated mice develop bacteremia. Oral infection of calves results in acute exudative inflammation in the terminal ileum and colon (70). In contrast, neutrophil influx is induced artificially in mice during serotype Typhimurium infection by streptomycin pretreatment, and severe lesions remain restricted to the cecum (7). The finding that flagellin contributed to intestinal inflammation in the calf but not in mice may be due to differences in the expression of PRRs in intestinal tissue. However, additional studies are needed to understand why serotype Typhimurium infection causes different disease manifestations in mice and calves.
Acknowledgments
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant number C06 RR12088-01 from the National Center for Research Resources, National Institutes of Health. Work in A.J.B.'s and L.G.A.'s laboratory was supported by Public Health Service grants AI040124, AI044170, AI079173, and AI076246. P.T. was supported by Chiang Mai University, Thailand. T.H. was supported by Kitasato University, Japan. S.-P.N. was supported by the Floyd and Mary Schwall Fellowship, University of California, Davis, CA.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Ahmer, B. M., J. van Reeuwijk, P. R. Watson, T. S. Wallis, and F. Heffron. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol. Microbiol. 31971-982. [DOI] [PubMed] [Google Scholar]
- 2.Alaniz, R. C., L. A. Cummings, M. A. Bergman, S. L. Rassoulian-Barrett, and B. T. Cookson. 2006. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J. Immunol. 1773983-3993. [DOI] [PubMed] [Google Scholar]
- 3.Aldridge, P., J. Karlinsey, and K. T. Hughes. 2003. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol. Microbiol. 491333-1345. [DOI] [PubMed] [Google Scholar]
- 4.Anderle, P., M. Rumbo, F. Sierro, R. Mansourian, P. Michetti, M. A. Roberts, and J. P. Kraehenbuhl. 2005. Novel markers of the human follicle-associated epithelium identified by genomic profiling and microdissection. Gastroenterology 129321-327. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. J. Wiley & Sons, Inc., New York, NY.
- 6.Baba, M., T. Imai, M. Nishimura, M. Kakizaki, S. Takagi, K. Hieshima, H. Nomiyama, and O. Yoshie. 1997. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 27214893-14898. [DOI] [PubMed] [Google Scholar]
- 7.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochner, B. R. 1984. Curing bacterial cells of lysogenic viruses by using UCB indicator plates. BioTechniques 2234-240. [Google Scholar]
- 9.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12495-503. [DOI] [PubMed] [Google Scholar]
- 10.Cook, P., S. Totemeyer, C. Stevenson, K. A. Fitzgerald, M. Yamamoto, S. Akira, D. J. Maskell, and C. E. Bryant. 2007. Salmonella-induced SipB-independent cell death requires Toll-like receptor-4 signalling via the adapter proteins Tram and Trif. Immunology 122222-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day, D. W., B. K. Mandal, and B. C. Morson. 1978. The rectal biopsy appearances in Salmonella colitis. Histopathology 2117-131. [DOI] [PubMed] [Google Scholar]
- 12.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7576-582. [DOI] [PubMed] [Google Scholar]
- 13.Franchi, L., T. D. Kanneganti, G. R. Dubyak, and G. Nunez. 2007. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 28218810-18818. [DOI] [PubMed] [Google Scholar]
- 14.Fujiie, S., K. Hieshima, D. Izawa, T. Nakayama, R. Fujisawa, H. Ohyanagi, and O. Yoshie. 2001. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3α/CCL20 in mucosal epithelial cells through NF-κB. Int. Immunol. 131255-1263. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 10799-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386619-623. [DOI] [PubMed] [Google Scholar]
- 18.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 1732301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godinez, I., T. Haneda, M. Raffatellu, M. D. George, T. A. Paixao, H. G. Rolan, R. L. Santos, S. Dandekar, R. M. Tsolis, and A. J. Baumler. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 762008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hapfelmeier, S., K. Ehrbar, B. Stecher, M. Barthel, M. Kremer, and W. D. Hardt. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72795-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 22.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, L., V. D. Dixit, V. de Mello-Coelho, and D. D. Taub. 2004. Age-associated alterations in CXCL1 chemokine expression by murine B cells. BMC Immunol. 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal, M., V. J. Philbin, G. S. Withanage, P. Wigley, R. K. Beal, M. J. Goodchild, P. Barrow, I. McConnell, D. J. Maskell, J. Young, N. Bumstead, Y. Boyd, and A. L. Smith. 2005. Identification and functional characterization of chicken Toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect. Immun. 732344-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johanesen, P. A., and M. B. Dwinell. 2006. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect. Immun. 743437-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, B. D., C. A. Lee, and S. Falkow. 1992. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect. Immun. 602475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, G. W., L. A. Richardson, and D. Uhlman. 1981. The invasion of HeLa cells by Salmonella typhimurium: reversible and irreversible bacterial attachment and the role of bacterial motility. J. Gen. Microbiol. 127351-360. [DOI] [PubMed] [Google Scholar]
- 28.Kingsley, R. A., R. Reissbrodt, W. Rabsch, J. M. Ketley, R. M. Tsolis, P. Everest, G. Dougan, A. J. Baumler, M. Roberts, and P. H. Williams. 1999. Ferrioxamine-mediated iron(III) utilization by Salmonella enterica. Appl. Environ. Microbiol. 651610-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lara-Tejero, M., F. S. Sutterwala, Y. Ogura, E. P. Grant, J. Bertin, A. J. Coyle, R. A. Flavell, and J. E. Galan. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 2031407-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawes, M., and S. Maloy. 1995. MudSacI, a transposon with strong selectable and counterselectable markers: use for rapid mapping of chromosomal mutations in Salmonella typhimurium. J. Bacteriol. 1771383-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao, F., R. Alderson, J. Su, S. J. Ullrich, B. L. Kreider, and J. M. Farber. 1997. STRL22 is a receptor for the CC chemokine MIP-3α. Biochem. Biophys. Res. Commun. 236212-217. [DOI] [PubMed] [Google Scholar]
- 32.Liu, S. L., T. Ezaki, H. Miura, K. Matsui, and E. Yabuuchi. 1988. Intact motility as a Salmonella typhi invasion-related factor. Infect. Immun. 561967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariathasan, S., K. Newton, D. M. Monack, D. Vucic, D. M. French, W. P. Lee, M. Roose-Girma, S. Erickson, and V. M. Dixit. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430213-218. [DOI] [PubMed] [Google Scholar]
- 34.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2021043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayfield, C. I., and W. E. Inniss. 1977. A rapid, simple method for staining bacterial flagella. Can. J. Microbiol. 231311-1313. [DOI] [PubMed] [Google Scholar]
- 36.McGovern, V. J., and L. J. Slavutin. 1979. Pathology of salmonella colitis. Am. J. Surg. Pathol. 3483-490. [DOI] [PubMed] [Google Scholar]
- 37.Meade, K. G., E. Gormley, M. B. Doyle, T. Fitzsimons, C. O'Farrelly, E. Costello, J. Keane, Y. Zhao, and D. E. MacHugh. 2007. Innate gene repression associated with Mycobacterium bovis infection in cattle: toward a gene signature of disease. BMC Genomics 8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7569-575. [DOI] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, New York, NY.
- 40.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 37888-91. [DOI] [PubMed] [Google Scholar]
- 43.Overbergh, L., A. Giulietti, D. Valckx, R. Decallonne, R. Bouillon, and C. Mathieu. 2003. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Technol. 1433-43. [PMC free article] [PubMed] [Google Scholar]
- 44.Pal, D., T. Venkova-Canova, P. Srivastava, and D. K. Chattoraj. 2005. Multipartite regulation of rctB, the replication initiator gene of Vibrio cholerae chromosome II. J. Bacteriol. 1877167-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power, C. A., D. J. Church, A. Meyer, S. Alouani, A. E. Proudfoot, I. Clark-Lewis, S. Sozzani, A. Mantovani, and T. N. Wells. 1997. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3α from lung dendritic cells. J. Exp. Med. 186825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29303-313. [DOI] [PubMed] [Google Scholar]
- 47.Puren, A. J., G. Fantuzzi, Y. Gu, M. S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 101711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Baumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 733367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. E. Winter, C. A. Rossetti, S. D. Lawhon, H. Chu, T. Lau, C. L. Bevins, L. G. Adams, and A. J. Baumler. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 754342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raffatellu, M., R. L. Santos, D. E. Verhoeven, M. D. George, R. P. Wilson, S. E. Winter, I. Godinez, S. Sankaran, T. A. Paixao, M. A. Gordon, J. K. Kolls, S. Dandekar, and A. J. Baumler. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rappleye, C. A., and J. R. Roth. 1997. A Tn10 derivative (T-POP) for isolation of insertions with conditional (tetracycline-dependent) phenotypes. J. Bacteriol. 1795827-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raupach, B., S. K. Peuschel, D. M. Monack, and A. Zychlinsky. 2006. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 744922-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolan, H. G., and R. M. Tsolis. 2007. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect. Immun. 752965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salazar-Gonzalez, R. M., J. H. Niess, D. J. Zammit, R. Ravindran, A. Srinivasan, J. R. Maxwell, T. Stoklasek, R. Yadav, I. R. Williams, X. Gu, B. A. McCormick, M. A. Pazos, A. T. Vella, L. Lefrancois, H. C. Reinecker, and S. J. McSorley. 2006. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity 24623-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, NY.
- 56.Santos, R. L., R. M. Tsolis, S. Zhang, T. A. Ficht, A. J. Baumler, and L. G. Adams. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 694610-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santos, R. L., S. Zhang, R. M. Tsolis, A. J. Baumler, and L. G. Adams. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39200-215. [DOI] [PubMed] [Google Scholar]
- 58.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Baumler. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 31335-1344. [DOI] [PubMed] [Google Scholar]
- 59.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 695619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sierro, F., B. Dubois, A. Coste, D. Kaiserlian, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 9813722-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Nat. Biotechnol. 1784-791. [Google Scholar]
- 62.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 41247-1253. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan, A., R. M. Salazar-Gonzalez, M. Jarcho, M. M. Sandau, L. Lefrancois, and S. J. McSorley. 2007. Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J. Immunol. 1786342-6349. [DOI] [PubMed] [Google Scholar]
- 64.Stecher, B., M. Barthel, M. C. Schlumberger, L. Haberli, W. Rabsch, M. Kremer, and W. D. Hardt. 2008. Motility allows serovar Typhimurium to benefit from the mucosal defense. Cell Microbiol. 101166-1180. [DOI] [PubMed] [Google Scholar]
- 65.Stecher, B., S. Hapfelmeier, C. Muller, M. Kremer, T. Stallmach, and W. D. Hardt. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 24138-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stojiljkovic, I., A. J. Baumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 1771357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stylianou, E., A. Yndestad, L. I. Sikkeland, V. Bjerkeli, J. K. Damas, T. Haug, H. G. Eiken, P. Aukrust, and S. S. Froland. 2002. Effects of interferon-alpha on gene expression of chemokines and members of the tumour necrosis factor superfamily in HIV-infected patients. Clin. Exp. Immunol. 130279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun, Y. H., H. G. Rolan, and R. M. Tsolis. 2007. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 28233897-33901. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki, T., T. Iino, T. Horiguchi, and S. Yamaguchi. 1978. Incomplete flagellar structures in nonflagellate mutants of Salmonella typhimurium. J. Bacteriol. 133904-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Baumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 674879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uematsu, S., M. H. Jang, N. Chevrier, Z. Guo, Y. Kumagai, M. Yamamoto, H. Kato, N. Sougawa, H. Matsui, H. Kuwata, H. Hemmi, C. Coban, T. Kawai, K. J. Ishii, O. Takeuchi, M. Miyasaka, K. Takeda, and S. Akira. 2006. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7868-874. [DOI] [PubMed] [Google Scholar]
- 72.Varona, R., R. Villares, L. Carramolino, I. Goya, A. Zaballos, J. Gutierrez, M. Torres, A. C. Martinez, and G. Marquez. 2001. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Investig. 107R37-R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vijay-Kumar, M., J. D. Aitken, A. Kumar, A. S. Neish, S. Uematsu, S. Akira, and A. T. Gewirtz. 2008. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect. Immun. 761276-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vijay-Kumar, M., H. Wu, R. Jones, G. Grant, B. Babbin, T. P. King, D. Kelly, A. T. Gewirtz, and A. S. Neish. 2006. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 1691686-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson, R. P., M. Raffatellu, D. Chessa, S. E. Winter, C. Tukel, and A. J. Baumler. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 10876-890. [DOI] [PubMed] [Google Scholar]
- 76.Winter, S. E., M. Raffatellu, R. P. Wilson, H. Russmann, and A. J. Baumler. 2008. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol. 10247-261. [DOI] [PubMed] [Google Scholar]
- 77.Yoshimura, T., K. Matsushima, J. J. Oppenheim, and E. J. Leonard. 1987. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL-1). J. Immunol. 139788-793. [PubMed] [Google Scholar]
- 78.Zeng, H., A. Q. Carlson, Y. Guo, Y. Yu, L. S. Collier-Hyams, J. L. Madara, A. T. Gewirtz, and A. S. Neish. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 1713668-3674. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, S., L. G. Adams, J. Nunes, S. Khare, R. M. Tsolis, and A. J. Baumler. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 714795-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Baumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 711-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W. D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 703843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]