Abstract
Expression of the gene for the extracellular alkaline protease (aprE) of Bacillus subtilis is subject to regulation by many positive and negative regulators. We have found that aprE expression was increased by disruption of the glutamine synthetase gene glnA. The increase in aprE expression was attributed to a decreased in expression of scoC, which encodes a negative regulator of aprE expression. The glnA effect on scoC expression was abolished by further disruption of tnrA, indicating that aprE expression is under global regulation through TnrA. In the scoC background, however, aprE expression was decreased by glnA deletion, and it was shown that the decrease was due to a defect in positive regulation by DegU. Among the genes that affect aprE expression through DegU, the expression of degR, encoding a protein that stabilizes phosphorylated DegU, was inhibited by glnA deletion. It was further shown that the decrease in degR expression by glnA deletion was caused by inhibition of the expression of sigD, encoding the σD factor, which is required for degR expression. In accordance with these findings, the expression levels of aprE-lacZ in glnA scoC degR and scoC degR strains were identical. These results led us to conclude that glnA deletion brings about two effects on aprE expression, i.e., a positive effect through inhibition of scoC expression and a negative effect through inhibition of degR expression, with the former predominating over the latter.
Bacillus subtilis produces a wide variety of extracellular degradative enzymes such as proteases, α-amylase, levansucrase, and others (1, 19, 27). The extracellular proteases are produced after the end of the exponential growth phase, and among those enzymes, the neutral and alkaline proteases encoded by nprE and aprE, respectively, are the major ones (27). The mechanism of aprE expression has attracted interest in terms of gene expression, since it is temporally controlled and subject to regulation by a large number of positive and negative regulators, apparently for timely and effective use of the enzyme in the habitat (18, 19).
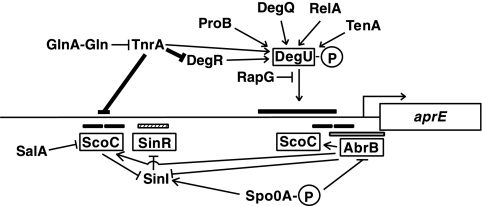
The primary regulators that directly affect aprE expression include the four DNA-binding proteins ScoC, SinR, AbrB, and DegU. ScoC, SinR, and AbrB are negative transcriptional regulators, while DegU constitutes a two-component regulatory system with DegS and exerts a positive effect on aprE transcription (Fig. 1). These regulators play their roles by binding to either upstream regions (ScoC, SinR, and DegU) of the transcriptional initiation point or the transcriptional initiation region (AbrB) of aprE (8, 13, 30, 33). The scoC, sinR, and abrB genes are under the control of the spo0A gene product, and it has been shown that only the cells containing threshold levels of the phosphorylated form of both DegU and Spo0A exhibit aprE expression (35). In addition to these four factors, there are many positive and negative regulators that affect aprE expression indirectly (Fig. 1). The regulators DegQ, DegR, TenA, ProB, RapG, and RelA affect aprE expression through the DegS-DegU route; SenS and SalA do so by affecting transcription of scoC; and SinI does so by inhibiting the SinR function (2, 9, 14, 15, 20, 21, 22, 23, 26). In addition, another negative factor, PaiA, is known, but its mode of action on aprE expression has not been studied since its discovery (12).
FIG. 1.
Regulatory network in aprE expression. The four regulators, which bind upstream regions of aprE, are enclosed by rectangles. The binding sites of ScoC (positions −324 to −295, −292 to −267, −79 to −59, and −35 to −14 relative to the transcription initiation site of aprE), SinR (positions −268 to −220), and AbrB (positions −59 to +25) are shown by the solid, hatched, and open bars, respectively (8, 13, 33). DegU exerts positive effects on the region between positions −164 and −113 (11), and a binding site of DegU was demonstrated within a region spanning positions −146 to +86 (30). GlnA with an asterisk indicates the feedback-inhibited form of GlnA (31, 39). For simplicity, only the phosphorylated form of DegU is shown. The arrows and T-bars show stimulation and inhibition by the regulators, respectively, and the thick lines show the results obtained in this study. The bent arrow depicts the transcription from the aprE promoter. The map is not drawn to scale.
The large amounts of the secreted proteases (the gene products of aprE and nprE) and the large number of regulatory factors controlling aprE expression suggest the importance of these exocellular proteases for the host B. subtilis cells to survive the harsh natural environments. One possible explanation for such high production of the proteases is that they are used to degrade insoluble proteins that happen to be present around the cells in the natural habitats. This may result in the supply of oligopeptides and/or amino acids, from which nitrogen-containing compounds may be derived. However, since the production of the enzymes in large amounts may be a burden to the cell, strict control in response to the nutritional status of the cell must be necessary. One possible candidate for such a regulator is TnrA, which receives information for nitrogen availability in the cell through interaction with feedback-inhibited glutamine synthetase, the glnA gene product (38). On the assumption that the role of the alkaline protease is to degrade high-molecular-weight proteins to supply nitrogen sources, it may be possible that aprE is also under nitrogen regulation through the GlnA-TnrA pathway. In this sense, a nitrogen-replete status in the cell may be a situation where TnrA is inhibited by complex formation with feedback-inhibited GlnA. Conversely, disruption of glnA leading to the release of TnrA from the feedback-inhibited GlnA may mimic a situation where the nitrogen source is scarce.
We have previously shown that glnA deletion results in overexpression of degU and that this was caused by induction of the P2 promoter present in a 3′ region of the degS gene, with which the degU gene constitutes an operon (42). In an attempt to examine whether the signal transduction through GlnA and TnrA is involved in aprE expression, we found that disruption of the glnA gene resulted in an increase in aprE expression, suggesting a link between aprE expression and the GlnA-TnrA system. We show here that a decrease in scoC expression by glnA deletion is the basis for the increase in aprE expression. We also show that an increase in degU expression by the glnA mutation does not contribute to stimulation of aprE expression, because glnA deletion inhibits the expression of sigD, encoding the σD factor, which in turn inhibits σD-dependent expression of degR, whose gene product stabilizes the phosphorylated form of DegU (20).
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Bacillus subtilis strain AY741TN was constructed by replacing chloramphenicol resistance (Cmr) in strain AY741T with neomycin resistance (Nmr) by transformation of a switching plasmid, pCm::Nm (32), provided by the Bacillus Genetic Stock Center.
TABLE 1.
Bacterial strains and plasmids used in this study
| Species and strain or plasmid | Description | Source or reference |
|---|---|---|
| B. subtilis | ||
| CU741 | trpC2 leuC7 | Laboratory stock |
| AY741G | trpC2 leuC7 glnA::Bsr | 42 |
| AY741T | trpC2 leuC7 tnrA::Cmr | 42 |
| AY741TN | trpC2 leuC7 tnrA::Nmr | pCm::Nm × AY741T |
| TT7151 | trpC2 leuC7 degU::NmraprE::pSKD1 | 20 |
| AY741U | trpC2 leuC7 degU::Nmr | TT7151 × CU741 |
| AY741L | trpC2 leuC7 amyE::lacZ (Cmr) (no promoter) | pIS284 × CU741 |
| OAM145 | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) | 22 |
| AY145G | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) glnA::Bsr | AY741G × OAM145 |
| AY145T | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) tnrA::Nmr | AY741TN × OAM145 |
| AY145U | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) degU::Nmr | AY741U × OAM145 |
| AY145GT | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) glnA::BsrtnrA::Nmr | AY741TN × AY145G |
| OAM147 | trpC2 leuC7 amyE::aprE-lacZ(−299, Cmr) | 22 |
| AY147G | trpC2 leuC7 amyE::aprE-lacZ(−299, Cmr) glnA::Bsr | AY741G × OAM147 |
| AY241 | trpC2 leuC7 amyE::aprE-lacZ(−113, Cmr) | BG4200 × CU741 |
| AY241G | trpC2 leuC7 amyE::aprE-lacZ(−113, Cmr) glnA::Bsr | AY741G × AY241 |
| TSU2 | trpC2 leuC7 amyE::scoC-lacZ(−422, Cmr) | 14 |
| TSU2G | trpC2 leuC7 amyE::scoC-lacZ(−422, Cmr) glnA::Bsr | AY741G × TSU2 |
| TSU2T | trpC2 leuC7 amyE::scoC-lacZ(−422, Cmr) tnrA::Nmr | AY741TN × TSU2 |
| TSU2GT | trpC2 leuC7 amyE::scoC-lacZ(−422, Cmr) glnA::BsrtnrA::Nmr | AY741TN × TSU2G |
| TSU31 | trpC2 leuC7 amyE::scoC-lacZ(−167, Cmr) | pSCO256 × CU741 |
| TSU31G | trpC2 leuC7 amyE::scoC-lacZ(−167, Cmr) glnA::Bsr | AY741G × TSU31 |
| TSU32 | trpC2 leuC7 amyE::scoC-lacZ(−144, Cmr) | pSCO279 × CU741 |
| TSU32G | trpC2 leuC7 amyE::scoC-lacZ(−144, Cmr) glnA::Bsr | AY741G × TSU32 |
| TSU33 | trpC2 leuC7 amyE::scoC-lacZ(−122, Cmr) | pSCO301 × CU741 |
| TSU33G | trpC2 leuC7 amyE::scoC-lacZ(−122, Cmr) glnA::Bsr | AY741G × TSU33 |
| TSU34 | trpC2 leuC7 amyE::scoC-lacZ(−73, Cmr) | pSCO350 × CU741 |
| TSU34G | trpC2 leuC7 amyE::scoC-lacZ(−73, Cmr) glnA::Bsr | AY741G × TSU34 |
| TSU2M | TSU2 carrying sequence alterations at the putative TnrA target site | pSCO256 M × CU741 |
| TSU2MG | Same as TSU2 M except for carrying glnA::Bsr | AY741G × TSU2 M |
| TSU2MT | Same as TSU2 M except for carrying tnrA::Nmr | AY741TN × TSU2 M |
| TSU2MGT | Same as TSU2 M except for carrying glnA::Bsr and tnrA::Nmr | AY741TN × TSU2MG |
| OAM157 | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr Tcr | 22 |
| AY157G | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr TcrglnA::Bsr | AY741G × OAM157 |
| AY157U | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr TcrdegU::Kmr | AY741U × OAM157 |
| AY157GU | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr TcrglnA::BsrdegU::Kmr | AY741U × AY157G |
| ODM50 | trpC2 leuC7 amyE::degR1-lacZ(−352, Cmr) | 24 |
| AY50G | trpC2 leuC7 amyE::degR1-lacZ(−352, Cmr) glnA::Bsr | AY741G × ODM50 |
| AY50T | trpC2 leuC7 amyE::degR1-lacZ(−352, Cmr) tnrA::Nmr | AY741TN × ODM50 |
| AY50GT | trpC2 leuC7 amyE::degR1-lacZ(−352, Cmr) glnA::BsrtnrA::Nmr | AY741TN × AY50G |
| ODM612 | trpC2 leuC7 sigD amyE::degRm5-lacZ(Cmr) | 24 |
| AYM612G | trpC2 leuC7 sigD amyE::degRm5-lacZ(Cmr) glnA::Bsr | AY741G × ODM612 |
| ODS200 | trpC2 leuC7 sigD-lacZ(Cmr) | 24 |
| AYS200G | trpC2 leuC7 sigD-lacZ(Cmr) glnA::Bsr | AY741G × ODS200 |
| AYS200T | trpC2 leuC7 sigD-lacZ(Cmr) tnrA::Nmr | AY741TN × ODS200 |
| AYS200GT | trpC2 leuC7 sigD-lacZ(Cmr) glnA::BsrtnrA::Nmr | AY741TN × AYS200G |
| ODF200 | trpC2 leuC7 hag-lacZ(Cmr) | 24 |
| AYF200G | trpC2 leuC7 hag-lacZ(Cmr) glnA::Bsr | AY741G × ODF200 |
| BG4136 | trpC2 hisA1 thr-5 degR::Emr | 40 |
| AY145R | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) degR::Emr | BG4136 × OAM145 |
| AY157R | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr TcrdegR::Emr | OAM157 × AY145R |
| AY157GR | trpC2 leuC7 amyE::aprE-lacZ(−412, Cmr) scoC::Emr TcrdegR::EmrglnA::Bsr | AY741G × AY157R |
| E. coli JM103 | Δ(lac-pro) thi rpsL supE sbcB hsdR4 F′ [traD36 proAB+lacIqlacZΔM15] | 41 |
| Plasmids | ||
| pIS284 | Cmr, E. coli plasmid for insertion of lacZ fusions into B. subtilis amyE locus | I. Smith |
| pCm::Nm | E. coli plasmid to change Cmr to Nmr | M. Steinmetz |
| pSCO256 | pIS284 carrying positions −167 to +139 of the scoC promoter | This study |
| pSCO279 | pIS284 carrying positions −144 to +139 of the scoC promoter | This study |
| pSCO301 | pIS284 carrying positions −122 to +139 of the scoC promoter | This study |
| pSCO350 | pIS284 carrying positions −73 to +139 of the scoC promoter | This study |
| pSCO256 M | pSCO256 carrying altered nucleotides upstream of scoC | This study |
Plasmids and plasmid construction.
The plasmids used in this study are listed in Table 1. Plasmids carrying various upstream regions of scoC were constructed by PCR amplification of the regions studied, followed by cleavage of the PCR products with both EcoRI and BamHI and subsequent cloning into pIS284 that had been treated with the same restriction enzymes. The plasmids thus constructed and the PCR primers used (Table 2) were as follows: for pISCO256, ScoC256 and ScoCR; for pISCO279, ScoC 279 and ScoCR; for pISCO301, ScoC301 and ScoCR; and for pISCO350, ScoC350 and ScoCR. Plasmid pSCO256M was created by a two-step procedure. First, two PCR fragments were prepared with YhaHF plus ScoCMR and ScoCMF plus ScoCR. Second, the resultant PCR fragments were fused by a second PCR in the presence of primers YhaH and ScoCR, followed by cleavage with EcoRI and BamHI and cloning into pIS284 treated with the same restriction enzymes.
TABLE 2.
DNA primers used in this study
| Primer | Nucleotide sequence (5′→3′)a |
|---|---|
| YhaHF | AGTTGAATTCGCACCTTCCTCAGGAAAGC |
| ScoC256 | AGTTGAATTCAGCTGGAGAAAACCTTAC |
| ScoC279 | AGTTGAATTCTAATATCCTATTCAAAAGAAA |
| ScoC301 | AGTTGAATTCAAAATGCGGGCCAAAATTGG |
| ScoC350 | AGTTGAATTCTTCGTCGCAATGGTTTGTGA |
| ScoCMF | GAGAAAATCAGACAGCTGGCGTCGCCTGTTACAAACTAATATCCTATTCAAAAGAAAAA |
| ScoCMR | TTTTTCTTTTGAATAGGATATTAGTTTGTAACAGGCGACGCCAGCTGTCTGATTTTCTC |
| ScoCR | AGTTGGATCCTTCTCGATCGATTTCC |
| SCBio47 | XGCTGAGCCATTTTCTGGGTG |
X, biotin attached to the nucleotide at the 5′ end.
Media.
LB (Lennox) was purchased from Difco Co. Ltd. Schaeffer's sporulation medium (SSM) was prepared as described previously (28). LBG and SSMG were LB and SSM supplemented with 0.2% glutamine, respectively. The concentrations of antibiotics added to media were 5 μg/ml, 15 μg/ml, and 500 μg/ml for Cm, Nm, and blasticidin S (Bs), respectively. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was added at a concentration of 100 μg/ml.
Determination of β-galactosidase activities.
Cells from stock culture were spread on LBG plates containing appropriate antibiotics and X-Gal and grown overnight. The colonies formed were transferred to LBG medium and incubated overnight, and the cultures were then inoculated into SSMG at a concentration of 1%. The levels of β-galactosidase activity (in Miller units) were determined for the samples taken from T−1 (1 hour before the end of exponential growth phase) to T5 as described previously (22). In the experiments for Fig. 6A, determination started from T−1.5. Along with this quantitative assay, expression of lacZ fusions was also confirmed qualitatively by the blue color developed by colonies on X-Gal-containing plates.
FIG. 6.
Inhibition of sigD-lacZ (A) and hag-lacZ (B) expression by glnA deletion. (A) Open circles, ODS200 (wild type); solid circles, AYS200G (glnA); open squares, AYS200T (tnrA); solid squares, AYS200GT (glnA tnrA); diamonds, AY741L (no lacZ promoter). The data are from one of three experiments, in which the variations of the enzyme levels were within 10%. (B) Open circles, ODF200 (wild type); solid circles, AYF200G (glnA). Results from a typical experiment are shown. β-Galactosidase activities were determined as described in Materials and Methods.
Primer extension analysis.
RNA was isolated from strains CU741 and AY741G grown in SMMG as described previously (43). Determination of the transcriptional start site was performed according to the procedure described previously (42), except that the biotinylated primer was SCBio47 and the sequencing ladders were prepared by using a PCR product made with primers YhaHF and ScoCR as a template.
RESULTS
Stimulation of aprE expression by glnA disruption.
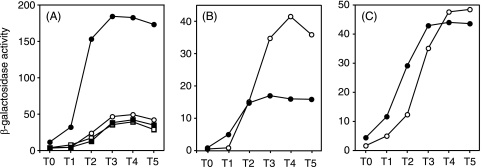
In order to investigate whether expression of the extracellular protease gene aprE is influenced by nitrogen regulation, we estimated aprE expression in strains with and without the intact glnA gene. Figure 2A shows that aprE expression increased about fourfold by deletion of the glnA gene (AY145G) compared to that in the wild-type strain (AY145). Since GlnA regulates the activity of TnrA (39), we also examined the effect of tnrA deletion on aprE expression in those strains. Stimulation of aprE expression was no longer seen in a glnA tnrA double mutant, AY145GT, whereas there was no effect of tnrA disruption alone on aprE expression (AY145T). The glnA gene constitutes an operon with its upstream gene glnR, which also encodes a regulator that controls the expression of the genes for nitrogen regulation (6). It was shown that an in-phase deletion of the glnR gene (glnR57 mutation [29]) did not affect the expression of aprE-lacZ (data not shown). These results show that deletion of the glnA gene resulted in a positive effect on aprE expression through TnrA.
FIG. 2.
Expression of aprE-lacZ fusions containing upstream regions of aprE up to positions −412 (A), −299 (B), and −113 (C) relative to the transcription start site of aprE. (A) Open circles, strain OAM145 (wild type); solid circles, AY145G (glnA); open squares, AY145T (tnrA); solid squares, AY145GT (glnA tnrA). (B) Open circles, OAM147 (wild type); solid squares, AY147G (glnA). (C) Open circles, AY241 (wild type); solid circles, AY241G (glnA). Cell growth and β-galactosidase activities were determined as described in Materials and Methods. Each data set is from one of two experiments, in which the variations of the enzyme levels were within 15%.
Effect of glnA deletion on expression of the four genes encoding primary regulators of aprE expression.
Expression of aprE is known to be controlled directly by the four regulators ScoC, SinR, DegU, and AbrB, which bind to various sequences in the control region of the aprE gene (Fig. 1). To identify the regulator(s) through which the glnA disruption mutation exerts its effect, we examined the influence of the glnA mutation on aprE-lacZ expression in strains carrying deletions upstream of the transcription initiation site of aprE. The strains we used for the experiments for Fig. 2A carried the control region up to position −412 relative to the transcription initiation site of aprE. When this region was deleted to position −299, the glnA mutation caused a 60% reduction of aprE expression (Fig. 2B), in contrast to the stimulation seen in AY157G (Fig. 2A). It was demonstrated previously that the negative effect of ScoC on aprE expression is lost when the sequence upstream of position −299 is removed (22), suggesting that ScoC is involved in the stimulation of aprE expression in the glnA mutant, although the 60% reduction cannot be explained on this basis. On the other hand, there was no effect of glnA deletion in strain AY241G, in which the upstream region of aprE is deleted to position −113 (Fig. 2C). It was shown previously that the target of AbrB resides at positions −59 to +25 upstream of aprE (33), suggesting that abrB is not the target of glnA disruption. This notion was further substantiated by the observation that the enhancing effect of glnA deletion on aprE expression was not affected by mutations in abrB or spo0A, which encodes the repressor of abrB (data not shown).
It is possible from these results that scoC is a candidate for the target of glnA deletion. To test this notion, we examined scoC-lacZ expression in the glnA background. The results showed that glnA deletion caused a decrease in scoC-lacZ expression in the glnA mutant TSU2G compared to the wild-type strain TSU2 (Fig. 3). The reduced expression of scoC-lacZ in strain TSU2G was restored to the wild-type level in strain TSU2GT carrying both glnA and tnrA mutations, whereas the tnrA mutation alone did not affect scoC-lacZ expression (TSU2T). Since ScoC is a negative regulator of aprE expression, these findings with the glnA and tnrA mutants are in parallel with those for aprE-lacZ expression shown in Fig. 2A and confirm that scoC is a target of glnA deletion.
FIG. 3.
Inhibition of scoC-lacZ expression by glnA deletion and recovery by a tnrA null mutation. Open circles, strain TSU2 (wild type); solid circles, TSU2G (glnA); open squares, TSU2T (tnrA); solid squares, TSU2GT (glnA tnrA); diamonds, AY741L (no lacZ promoter). The data set of β-galactosidase activities was from one of two experiments, in which the variations of the enzyme levels were within 15%.
It was also shown that there was no effect of glnA deletion on sinR-lacZ or sinI-lacZ expression (data not shown), indicating that the glnA deletion effect is not via the SinI-SinR route.
We have already shown that degU expression is under positive regulation of TnrA in the glnA background (42). All of these results show that scoC and degU are the targets of glnA deletion among the four primary regulators.
Analysis of the upstream region of scoC.
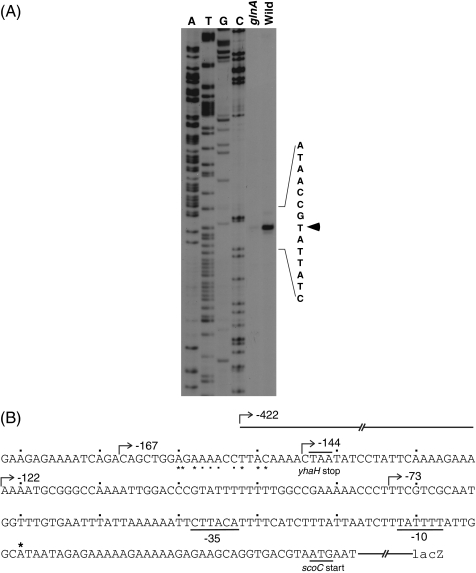
We next investigated the target of glnA deletion in the upstream region of scoC. First, we determined the transcriptional initiation site of the scoC gene. By referring to the profiles of scoC expression in Fig. 3, we isolated RNA samples at T2 from both the wild-type and glnA strains and used them for primer extension analysis. As shown in Fig. 4A, the reverse transcriptase product of the RNA prepared from the wild-type strain CU741 was more intense than that from the glnA mutant CU741G and migrated with a sequence ending in T that corresponds to A in the sense strand located 37 bases upstream of the scoC start codon (Fig. 4B). The transcriptional start site is preceded by putative −35 and −10 regions recognized by σA-type RNA polymerase (10) (Fig. 4B).
FIG. 4.
Determination of the 5′ end of scoC mRNA by primer extension analysis (A) and the sequence upstream of the scoC gene (B). (A) RNAs were isolated from strains CU741 (wild type) and CU741G (glnA) at T2 and used for primer extension analysis as described in Materials and Methods. The arrowhead indicates the transcriptional start site. (B) The asterisk and bent arrows above the sequence indicate the transcriptional start site and the 5′ ends of deletion mutations, respectively. The asterisks and dots under the sequence show the nucleotides that show similarity to the consensus sequence of the TnrA recognition site. The dots above the sequence show the positions introduced at every 10 nucleotides from position −1 relative to the transcriptional initiation site.
Second, we searched for the DNA region upstream of scoC where the glnA mutation exerts its effect. We constructed at the amyE locus lacZ fusions carrying various deletions upstream of the transcriptional initiation site of scoC and examined β-galactosidase levels in these strains. In strains with the 5′ end points of −422 and −167 (Fig. 4B), the expression of scoC-lacZ was reduced in the glnA mutants (Table 3, compare strains TSU2 and TSU31with TSU2G and TSU31G, respectively), whereas glnA deletion did not affect scoC-lacZ expression in strains TU32G, TU33G, and TU34G, carrying the 5′ end points of −144, −122, and −73, respectively, indicating that glnA deletion affects the DNA region between positions −167 and −144. A computer search revealed a sequence similar to the TnrA recognition sequence, TGTNANAWWWTMTNACA (44), located in this DNA region (CTGGAGAAAACCTTACA [underlined nucleotides match the requirement], see also Fig. 4B). To examine whether this sequence is involved in the regulation of scoC expression, we constructed strain TSU2M, in which the putative TnrA target sequence in strain TSU2 was changed to CTGGCGTCGCCTGTTAC (underlined nucleotides were changed) and compared the scoC-lacZ expression levels in strain TSU2M and its glnA mutant, TSU2MG. As shown in Table 3, the expression of scoC-lacZ in strain TSU2MG was not inhibited by the glnA mutation but was rather high compared to the level found in strain TSU2M. Disruption of tnrA did not affect the expression level (TSU2MT) but caused a decrease in the expression level in strain TSU2MG (glnA), suggesting participation of TnrA in the slight stimulation. Although the reason for this slight increase is not known at present, the results strongly suggest that the nucleotides that we changed are the constituents of the TnrA target involved in scoC regulation. It remains to be studied whether purified TnrA protein binds to this sequence.
TABLE 3.
Effect of deletion or sequence alteration of the scoC upstream region on scoC-lacZ expression
| Strain | 5′ End point | β-Galactosidase activity (Miller units)a |
|---|---|---|
| TSU2 | −422 | 8.7 |
| TSU2G (glnA) | −422 | 3.6 |
| TSU31 | −167 | 15 |
| TSU31G (glnA) | −167 | 5.8 |
| TSU32 | −144 | 41 |
| TSU32G (glnA) | −144 | 43 |
| TSU33 | −122 | 49 |
| TSU33G (glnA) | −122 | 53 |
| TSU34 | −73 | 41 |
| TSU34G (glnA) | −73 | 38 |
| TSU2 M | −422 | 26 |
| TSU2MG (glnA) | −422 | 39 |
| TSU2MT (tnrA) | −422 | 26 |
| TSU2MGT (glnA tnrA) | −422 | 27 |
| AY741L (no fusion) | 1.4 |
Cells were grown in SSMG as described in Materials and Methods. β- Galactosidase activities from T1 to T5 were determined, and the highest values attained at T3 (except for strain TSU2G) (see Fig. 3) are shown.
Inability of overexpressed degU to stimulate aprE expression in the glnA background.
We have previously shown that degU expression is stimulated by glnA deletion and that this is due to positive regulation of degU expression by TnrA (42). It is known that aprE expression is stimulated by the phosphorylated form of DegU (19). Thus, if the DegU protein produced in the glnA mutant is phosphorylated, aprE expression is expected to increase accordingly. To examine this possibility, we used scoC knockout mutants to eliminate the effect of glnA on scoC expression. The results in Table 4 show that under the conditions where glnA deletion caused a threefold increase in aprE expression in the scoC+ strains (compare strains OAM145 and AY145G), the level of aprE-lacZ expression in strain AY157G (scoC glnA) was much lower than that in strain OAM157 (scoC), indicating that the increased expression of degU in the glnA mutant does not contribute to positive regulation of aprE.
TABLE 4.
Effect of glnA, scoC, and degU mutations on aprE expression
| Strain | Relevant genotype | β-Galactosidase activity (Miller units)a |
|---|---|---|
| OAM145 | aprE-lacZ | 49 |
| AY145G | aprE-lacZ glnA | 156 |
| AY145U | aprE-lacZ degU::Nmr | 15 |
| OAM157 | aprE-lacZ scoC | 484 |
| AY157G | aprE-lacZ scoC glnA | 256 |
| AY157U | aprE-lacZ scoC degU | 40 |
| AY157GU | aprE-lacZ scoC degU glnA | 36 |
Cell growth and determination of β-galactosidase activities are described in the footnote a of Table 3. The highest values attained at either T3 or T4 are shown.
Since glnA disruption influences the expression of only the scoC and degU genes among the four DNA-binding factors that regulate aprE expression (see above), we expected that the effect of glnA deletion on aprE expression would not be seen in a scoC degU double mutant, and this was indeed the case (Table 4, compare strains AY157U and AY157GU).
These results suggested that although the expression level of degU is high in the glnA background, the level of functional DegU for aprE expression, i.e., the phosphorylated form of DegU, might be low. This notion prompted us to examine the factors that affect the expression of aprE through the DegS-DegU route.
Inhibition of degR expression by glnA deletion.
There are six such factors currently known (Fig. 1). Among these factors, degR and degQ exhibit larger effects than others in a single-copy state: disruption of degR and degQ resulted in 66 and 79% reduction in aprE-lacZ expression, respectively, in SSM, which we used in this study (25). To examine whether glnA deletion affects aprE expression through any of these genes, we determined the expression levels of their lacZ fusions in the glnA background. The results showed that there was no glnA effect on the expression of degQ, relA, tenA, and rapG, whereas less than 30% inhibition was observed for proB expression (data not shown). In contrast, the expression of degR-lacZ was greatly reduced in a glnA mutant, AY50G, compared to that in the wild-type strain ODM50 (Fig. 5). The expression level of degR-lacZ in the glnA background was restored to the wild-type level by an additional mutation in tnrA (AY50GT), indicating that the effect of glnA deletion is through TnrA. The tnrA mutation alone did not affect degR expression (AY50T).
FIG. 5.
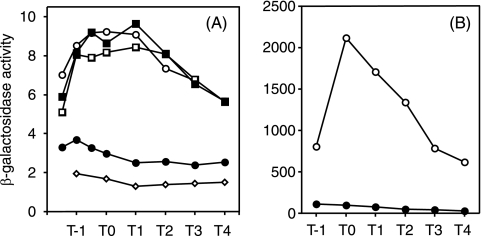
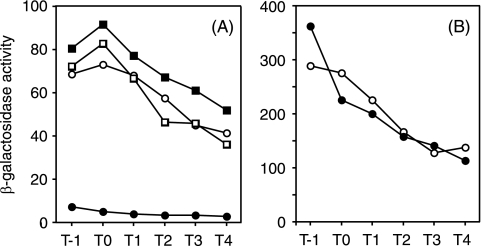
Effect of glnA disruption on degR-lacZ driven by σD (A)- and σA (B)-dependent RNA polymerase. (A) Open circles, ODM50 (wild type); solid circles, AY50G (glnA); open squares, AY50T (tnrA); solid squares, AY50GT (glnA tnrA). (B) Open circles, ODM612 (wild type); solid circles, AYM612 (glnA). β-Galactosidase activities were determined as described in Materials and Methods. The data set shown is from one of two experiments, and the variations of the enzyme levels between the experiments were within 15%.
The inhibitory effect of glnA deletion on degR expression was also observed when the upstream region of degR was deleted from position −422 (ODM50) to −52 (ODM20) (24) with respect to the transcriptional initiation site and also when extensive sequence alterations were introduced between positions −46 and −30, where there is a sequence showing a low similarity to the TnrA recognition sequence (data not shown). These results show that the sequence upstream of position −30 is not subject to regulation by GlnA and raised another possibility that transcription of degR driven by σD-RNA polymerase (24) might be affected. We have previously reported a strain, ODM 612, in which the recognition sequence of σD-RNA polymerase in the degR promoter is changed to that of σA-RNA polymerase and the sigD gene is deleted (24). When this strain was used to examine the effect of glnA deletion, no inhibition of degR-lacZ expression was observed (Fig. 5B), suggesting strongly that the glnA effect on degR expression is exerted via σD-dependent transcription.
Inhibition of sigD and hag expression by glnA deletion.
The above results prompted us to examine the effect of glnA deletion on the expression of the sigD gene, encoding the σD protein. As shown in Fig. 6A, sigD-lacZ expression was greatly reduced in the glnA mutant AY200G compared to the wild-type strain ODS200, and furthermore, the inhibition was lost by further addition of a tnrA deletion. It is apparent from these results that the inhibitory effect of glnA deletion on degR expression was caused by inhibition of sigD expression, which resulted in a reduction of the σD level.
It is known that the flagellin gene hag is transcribed by σD-RNA polymerase (3, 16). Therefore, if glnA deletion causes inhibition of sigD expression, it is expected that hag expression will also be inhibited by glnA deletion, and this was indeed the case, as shown in Fig. 6B. Furthermore, the inhibition of hag-lacZ expression was abolished by the introduction of the tnrA disruption mutation (data not shown), indicating that the glnA effect on hag expression is exerted through TnrA.
Regulation of aprE expression by glnA deletion through inhibition of scoC and degR expression.
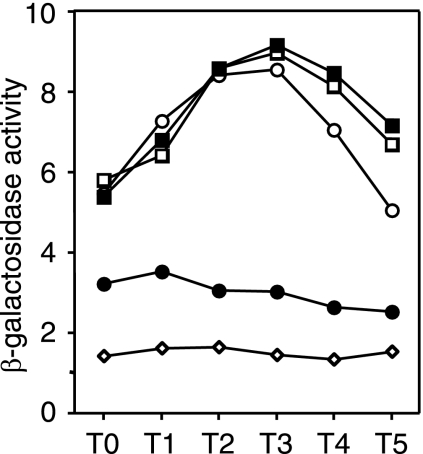
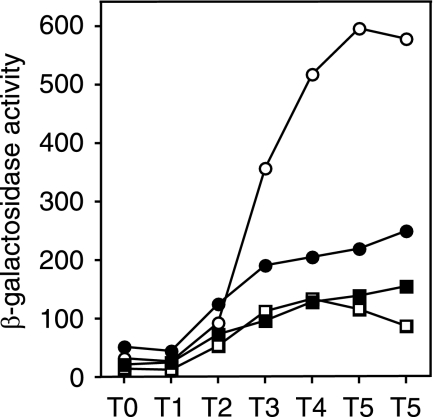
It was shown previously that enhanced expression of aprE by multicopy degR is caused by stabilization of phosphorylated DegU, possibly through inhibition of the dephosphorylation activity of DegS (20, 21). It thus appeared that deletion of glnA might reduce the functional activity of DegU through DegR, resulting in reduced aprE expression in the scoC background. As the inhibition of aprE expression by glnA deletion was not seen in the scoC degU background (Table 4), we presumed that the reduction of aprE expression in the scoC glnA strain compared the scoC mutant (Table 4) might be caused by a reduction in the level of phosphorylated DegU. To examine this possibility, we investigated the effect of deletion of degR on the expression of aprE in strains carrying scoC and scoC glnA mutations. If the negative glnA effect on aprE expression in the scoC background is exerted via degR, deletion of the degR gene would result in similar levels of aprE-lacZ expression in both the scoC and scoC glnA mutants. It was found that under the condition where glnA deletion caused 60% inhibition of aprE-lacZ expression in the scoC background (Fig. 7, compare OAM157 and AY157G), the inhibitory effect of glnA deletion was no longer seen in the scoC degR background (Fig. 7, compare AY157R and AY157GR). These results show that inhibition of degR by glnA deletion is responsible for the decrease in aprE expression in the scoC background.
FIG. 7.
Effect of glnA deletion on aprE-lacZ expression in the scoC and scoC degR background. Cells were grown under the same conditions as described in the legend to Fig. 2. Open circles, OAM157 (scoC); solid circles, AY157G (scoC glnA); open squares, AY157R (scoC degR); solid squares, AY157GR (scoC degR glnA). The data points are the means of values obtained from three determinations. The variations of the enzyme levels among the experiments were within 20%.
The above results show that the glnA effect on aprE expression was due to repression of scoC and degR expression and that the reduced expression of proB by glnA deletion (see above) was not involved. We have shown previously that a null mutation of proB resulted in 40% inhibition of aprE expression (25). Since this glnA effect on proB expression is small and, in addition, the effect of proB on aprE expression is indirect, i.e., through DegS-DegU (21), it is most likely that the inhibitory effect of proB on aprE expression in the glnA background was negligible, resulting in no difference in the aprE expression levels between strains AY157GR (scoC degR glnA) and AY157R (scoC degR) (Fig. 7).
DISCUSSION
It has been well documented that GlnA and TnrA are involved in nitrogen regulation and that the feedback-inhibited GlnA controls TnrA by complex formation, resulting in regulation of various cellular processes (6, 7, 39, 44). To examine whether the expression of the B. subtilis exocellular protease genes is under nitrogen regulation, we studied aprE expression in cells carrying a deletion in the glnA gene and indeed found that it is subject to nitrogen regulation, as demonstrated by the experiments in which glnA deletion caused an increase in aprE expression (Fig. 2A). It was also shown that the positive effect of glnA deletion was through inhibition of the expression of the negative regulator gene scoC (Fig. 3), and this was confirmed by primer extension analysis, deletion analysis of the upstream region of the scoC gene (Fig. 4), and sequence alteration of the putative TnrA binding site (Table 3).
It was shown previously that the expression of degU, encoding a positive regulator of aprE expression, was enhanced by glnA deletion (42), but this increase did not contribute to stimulation of aprE expression (Table 4). Among the regulators that affect aprE expression in a DegS-DegU-dependent manner, the expression of degR was severely inhibited by glnA deletion (Fig. 5). DegR stabilizes the phosphorylated form of DegU (20). Since degR expression was reduced in the glnA background, the inability of increased degU expression to enhance aprE expression was most likely due to the inactive (unphosphorylated) form of DegU. This notion was supported by the experiments in which there was no effect of glnA deletion on aprE expression in the scoC degR background (Fig. 7). These observations led us to conclude that aprE expression is under positive and negative nitrogen regulation by the GlnA-TnrA route and that the positive effect through scoC repression exceeds the negative effect through repression of degR expression.
In the cells carrying a deletion up to position −299 relative to the transcription start point of aprE, the expression of aprE was substantially reduced in the glnA background (AY147G) compared to its wild-type strain OAM147 (Fig. 2B), whereas there was no difference between strains AY241 and AY241G (glnA) carrying a deletion up to position −113 (Fig. 2C). These results can be explained on the basis of the presence or absence of the target sites of ScoC and DegU through which DegR exerts its effect. Previous deletion analyses have shown that the target sites of ScoC and DegU are located upstream of position −299 and between positions −164 and −113, respectively (11, 22), indicating that the DegU but not the ScoC target site is present in strains AY147 and AY147G. We conclude, therefore, that the decrease in aprE expression in AY147G is due to the negative effect of glnA deletion on degR expression that results in a decrease in the level of phosphorylated DegU.
We showed that the expression of degR and hag, both of which are transcribed by σD-RNA polymerase, was subject to regulation by the GlnA-TnrA route (Fig. 5A and 6B). In addition, the sigD gene, encoding the σD factor, was also found to be under the regulation of GlnA-TnrA (Fig. 6A). Since the DNA region upstream of position −30 relative to the transcription initiation site of degR is not involved in the GlnA-TnrA regulation (see above), it seems unlikely that the regulation is exerted by direct binding of TnrA to the regulatory region of degR. It seems more likely that the direct consequence of glnA deletion is repression of sigD expression, which then results in a decrease in the σD level, leading to inhibition of degR expression.
The sigD gene is transcribed by at least three promoters, PD-3, fla/che PA, and PsigD, and among them transcription only from the fla/che PA promoter supplies enough transcripts to support the expression of the hag gene (36). As glnA deletion causes a decrease in hag expression (Fig. 6B), it is possible that transcription from the fla/che PA promoter is the target of the GlnA-TnrA route. A future experiment will include study of the binding of the TnrA protein to this region as well as the putative target site upstream of scoC found in this study.
The two major global regulators TnrA and CodY play their roles according to the nitrogen status and the GTP level reflecting the energy in the cell, respectively (7, 31). Among the numerous genes regulated by these regulators, some exhibit regulation by both of them. These include ilv-leu (34) for the catabolic pathways of branched-chain amino acids, ureABC (37) for the degradation of urea, and gabP (5) for the transport of gamma-aminobutyrate. It was demonstrated previously that hag and the fla/che operon containing sigD are under the control of CodY (4, 17). We showed here that the expression of the hag and sigD genes is regulated by TnrA, indicating that these genes are new members of the group under the control of both the TnrA and Cod Y regulators.
The experimental condition that we used in this study, i.e., the absence of the glutamine synthetase gene glnA, may mimic the situation where the signal transduction through the GlnA-TnrA route is shut off. In other words, it may represent a condition in which TnrA is fully active due to the absence of feedback inhibition by GlnA (39). In this situation, it was found that aprE expression was stimulated by repression of the negative regulator gene, scoC, while the σD-dependent transcription of the hag and degR genes was repressed (Fig. 5 and 6B). Inhibition of hag expression by glnA deletion (Fig. 6B) will result in a reduction of the flagellin protein content in the cell, most likely leading to the generation of immobile cells. In an environment where cells secrete a large amount of proteases, it is conceivable that they stay there in order to take up the degradation products and that there is no need for them to elaborate flagella to swim away from the nutrients (18).
The above interpretation is contradictory, however, when the inhibitory effect of glnA deletion on degR expression is taken into consideration, since DegR is a positive regulator of aprE expression. The increased expression of degU and the decrease in degR expression may result in an increased level of unphosphorylated DegU. DegU is a molecular switch controlling the synthesis of degradative enzymes and competence development in its phosphorylated and unphosphorylated form, respectively (19). We speculate, therefore, that one consequence of glnA deletion might be stimulation of competence development, which may be useful for the cell to incorporate DNA for nutrients. A preliminary result has shown that glnA deletion affects comK expression (data not shown).
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science and Sports and Culture of Japan.
Footnotes
Published ahead of print on 27 February 2009.
REFERENCES
- 1.Amory, A., F. Kunst, E. Aubert, A. Klier, and G. Rapoport. 1987. Characterization of the sacQ genes from Bacillus licheniformis and Bacillus subtilis. J. Bacteriol. 169324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, U., I. Mandic-Mulec, and I. Smith. 1993. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 7139-148. [DOI] [PubMed] [Google Scholar]
- 3.Barilla, D., T. Caramori, and A. Galizzi. 1994. Coupling of flagellin gene transcription to flagellar assembly in Bacillus subtilis. J. Bacteriol. 1764558-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergara, F., C. Ibarra, J. Iwamasa, R. Aguilera, and L. M. Màrquez- Magaña. 2003. CodY is a nutritional repressor of flagellar gene expression in Bacillus subtilis. J. Bacteriol. 1853118-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferson, A. E., L. V. Wray, Jr., and S. H. Fisher. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22693-701. [DOI] [PubMed] [Google Scholar]
- 6.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence. Mol. Microbiol. 32223-232. [DOI] [PubMed] [Google Scholar]
- 7.Fisher, S. H., and M. Débarbouillé. 2002. Nitrogen source utilization and its regulation, p. 181-191. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 8.Gaur, N. K., J. Oppenheim, and I. Smith. 1991. The Bacillus subtilis sin gene, a regulator of alternate developmental processes, codes for a DNA-binding protein. J. Bacteriol. 173678-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata, M., M. Ogura, and T. Tanaka. 2001. Involvement of stringent factor RelA in expression of the alkaline protease gene aprE in Bacillus subtilis. J. Bacteriol. 1834648-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helman, J., and C. P. Moran. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 11.Henner, D. J., E. Ferrari, M. Perego, and J. A. Hoch. 1988. Location of the targets of the hpr-97, sacU32(Hy), and sacQ36(Hy) mutations in upstream regions of the subtilisin promoter. J. Bacteriol. 170296-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honjo, M., A. Nakayama, K. Furukawa, K. Kawamura, K. Ando, M. Hori, and Y. Furutani. 1990. A novel Bacillus subtilis gene involved in negative control of sporulation and degradative-enzyme production. J. Bacteriol. 1721783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallio, P. T., J. E. Fagelson, J. A. Hoch, and M. A. Strauch. 1991. The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein. J. Biol. Chem. 26613411-13417. [PubMed] [Google Scholar]
- 14.Kawachi, E., S. Abe, and T. Tanaka. 2005. Inhibition of Bacillus subtilis scoC expression by multicopy senS. J. Bacteriol. 1878526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi, K. 2007. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 66395-409. [DOI] [PubMed] [Google Scholar]
- 16.Mirel, D. B., and M. J. Chamberlin. 1994. The Bacillus subtilis flagellin gene (hag) is transcribed by the σ28 form of RN polymerase. J. Bacteriol. 1753095-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirel, D. B., W. F. Estacio, M. Mathieu, E. Olmsted, J. Ramirez, and L. M. Márquez-Magaña. 2000. Environmental regulation of Bacillus subtilis σD-dependent gene expression. J. Bacteriol. 1823055-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Msadek, T. 1999. When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol. 7201-207. [DOI] [PubMed] [Google Scholar]
- 19.Msadek, T., F. Kunst, and G. Rapoport. 1995. A signal transduction network in Bacillus subtilis includes DegS/DegU and ComP/ComA two-component systems, p. 447-471. In J. Hoch, and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, DC.
- 20.Mukai, K., M. Kawata-Mukai, and T. Tanaka. 1992. Stabilization of phosphorylated Bacillus subtilis DegU by DegR. J. Bacteriol. 1747954-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogura, M., M. Kawata-Mukai, M. Itaya, K. Takio, and T. Tanaka. 1994. Multiple copies of the proB gene enhance degS-dependent extracellular protease production in Bacillus subtilis. J. Bacteriol. 1765673-5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogura, M., A. Matsuzawa, H. Yoshikawa, and T. Tanaka. 2004. Bacillus subtilis SalA (YbaL) negatively regulates expression of scoC, which encodes the repressor for the alkaline exoprotease gene, aprE. J. Bacteriol. 1863056-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogura, M., K. Shimane, K. Asai, N. Ogasawara, and T. Tanaka. 2003. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol. Microbiol. 491685-1697. [DOI] [PubMed] [Google Scholar]
- 24.Ogura, M., and T. Tanaka. 1996. Transcription of Bacillus subtilis degR is σD dependent and suppressed by multicopy proB through σD. J. Bacteriol. 178216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogura, M., and T. Tanaka. 1997. Expression of alkaline protease gene in Bacillus subtilis mutants that lack positive regulatory genes degR, degQ, senS, tenA and proB. Biosci. Biotech. Biochem. 61372-374. [Google Scholar]
- 26.Pang, A. S., S. Nathoo, and S. L. Wong. 1991. Cloning and characterization of a pair of novel genes that regulate production of extracellular enzymes in Bacillus subtilis. J. Bacteriol. 17346-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priest, F. G. 1977. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol. Rev. 41711-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffer, P. J., J. Millet, and J. Aubert. 1965. Catabolite repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreier, H. J., S. W. Brown, K. D. Hirschi, J. F. Nomellini, and A. L. Sonenshein. 1989. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J. Mol. Biol. 21051-63. [DOI] [PubMed] [Google Scholar]
- 30.Shimane, K., and M. Ogura. 2004. Mutational analysis of the helix-turn-helix region of Bacillus subtilis response regulator DegU, and identification of cis-acting sequences for DegU in the aprE and comK promoters. J. Biochem. 136387-397. [DOI] [PubMed] [Google Scholar]
- 31.Sonenshein, A. L. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 5917-927. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 14279-83. [DOI] [PubMed] [Google Scholar]
- 33.Strauch, M. A., G. B. Spiegelman, M. Perego, W. C. Johnson, D. Burbulys, and J. A. Hoch. 1989. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J. 81615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tojo, S., T. Satomura, K. Morisaki, K.-I. Yoshida, K. Yoshida, K. Hirooka, and Y. Fujita. 2004. Negative regulation of the ilv-leu operon for biosynthesis of branched chain amino acids through the Bacillus subtilis global regulator TnrA. J. Bacteriol. 1867971-7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veening, J. W., O. A. Igoshin, R. T. Eijlander, R. Nijland, L. W. Hamoen, and O. P. Kuipers. 2008. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol. Syst. Biol. 41-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West, J. T., W. Estacio, and L. M. Márquez-Magaña. 2000. Relative roles of the fla/che PA, PD-3, and PsigD promoters in regulating motility and sigD expression in Bacillus subtilis. J. Bacteriol. 1824841-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray, L. V., Jr., A. E. Ferson, and S. H. Fisher. 1997. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J. Bacteriol. 1795494-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wray, L. V., Jr., A. E. Ferson, K. Rohrer, and S. H. Fisher. 1996. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1996. 938841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray, L. V., Jr., J. M. Zalieckas, and S. H. Fisher. 2001. Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA. Cell 107427-435. [DOI] [PubMed] [Google Scholar]
- 40.Yang, M., H. Shimotsu, E. Ferrari, and D. J. Henner. 1987. Characterization and mapping of the Bacillus subtilis prtR gene. J. Bacteriol. 169434-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-109. [DOI] [PubMed] [Google Scholar]
- 42.Yasumura, A., S. Abe, and T. Tanaka. 2008. Involvement of nitrogen regulation in Bacillus subtilis degU expression. J. Bacteriol. 1905162-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida, K.-I., K. Kobayashi, Y. Miwa, C.-M. Kang, M. Matsunaga, H. Yamaguchi, S. Tojo, M. Yamamoto, R. Nishi, N. Ogasawara, T. Nakayama, and Y. Fujita. 2001. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida, K.-I., H. Yamaguchi, M. Kinehara, Y. H. Ohki, Y. Nakaura, and Y. Fujita. 2003. Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol. Microbiol. 49157-165. [DOI] [PubMed] [Google Scholar]