Abstract
During encystment of Azotobacter vinelandii, a family of alkylresorcinols (ARs) and alkylpyrones (APs) are synthesized. In the mature cyst, these lipids replace the membrane phospholipids and are also components of the layers covering the cyst. In this study, A. vinelandii strains unable to synthesize ARs were isolated after mini-Tn5 mutagenesis. Cloning and nucleotide sequencing of the affected loci revealed the presence of the transposons within the arsA gene of the previously reported arsABCD gene cluster, which encodes a type I fatty acid synthase. A mutant strain (SW-A) carrying an arsA mutation allowing transcription of arsBCD was constructed and shown to be unable to produce ARs, indicating that the ArsA protein is essential for the synthesis of these phenolic lipids. Transcription of arsA was induced 200-fold in cells undergoing encystment, but only 14-fold in aged cultures of A. vinelandii, in accordance with AR synthesis and cyst formation percentages under the two conditions. Although it was previously reported that the inactivation of arsB abolishes AR synthesis and results in a failure in encystment, the arsA mutants were able to form cysts resistant to desiccation. These data indicate that ARs play a structural role in the exine layer of the cysts, but they are not essential for either cyst formation or for desiccation resistance.
Azotobacter vinelandii is a nitrogen-fixing soil bacterium that undergoes differentiation to form cysts resistant to desiccation. A mature cyst consists of a contracted cell known as the central body that is surrounded by a capsule made up of a thin laminated outer layer, called the exine, and a thicker inner layer, the intine (23).
The polysaccharide alginate is also a major component of the cyst capsule and is essential for the differentiation process, since mutations in alginate biosynthetic genes abrogate the formation of cysts resistant to desiccation (5, 16). In A. vinelandii ATCC 9046 inactivation of algU, the gene coding for the sigma factor AlgU (σE), impairs alginate synthesis and cyst formation (18), as this sigma factor is required for full expression of the alginate biosynthetic genes algD and algC (8, 15, 18). Besides its role in the expression of alg genes, AlgU has been suggested to have an additional role in encystment (18). A. vinelandii UW136 (3), a derivative of the nonmucoid strain OP, also unable to produce alginate and to form cysts resistant to desiccation, was found to have a natural insertion within algU (15). Complementation of this strain with a wild-type algU gene restored alginate biosynthesis and the ability to produce mature cysts (18).
Other components of the cyst capsule are the lipids alkylresorcinols (ARs) and alkylpyrones (APs). ARs are phenolic lipids common to plants but rare in bacteria. Induction of encystment results in the synthesis of ARs and APs that replace the membrane phospholipids and are components of the exine. 5-n-Heneicosylresorcinol and 5-n-tricosylresorcinol (known as AR1) and their galactoside derivatives (known as AR2), are the main alkylresorcinols synthesized (20). Recently, the gene cluster arsABCD, involved in the synthesis of these compounds, was identified (7). ArsA and ArsD constitute a fatty acid synthase responsible for the synthesis and direct transfer of the C22 to C26 fatty acids that serve as substrates for ArsB and ArsC (17). ArsB and ArsC are type III polyketide synthases which synthesize alkylresorcinols and alkylpyrones, respectively, by two or three extensions of the C22 to C26 fatty acids with malonyl coenzyme A (7). To investigate the role of ARs in encystment, an arsB mutant derived from strain OP (4) was constructed and shown to impair synthesis of alkylresorcinols (7). Electron microscopy of the asrB mutant induced for encystment showed that it was unable to produce cysts. Thus, Funa et al. (7) concluded that phenolic lipids are essential for the formation of mature cysts. However, the OP strain used by these authors is impaired in alginate biosynthesis due to an algU::IS mutation (15) (accession numbers AAF18261 ZP_00415083 and ZP_00415083); therefore, it is expected to be impaired in the formation of mature cysts. In addition, desiccation resistance was not determined for the OP and its arsB mutant derivative, and ARs are presumed to contribute to the desiccation resistance of cysts (22). The aim of this work was to determine the role of these phenolic lipids in the formation and resistance of the cysts.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. A. vinelandii cells were grown on Burk's medium (11) supplemented with 2% sucrose (BS) or 0.2% n-butanol (BBOH) as carbon sources. Liquid cultures were carried out in 125-ml flasks containing 25 ml of medium in a rotatory shaker at 250 rpm and 30°C. Inocula for all experiments were grown on BS, washed twice with Burk's medium without carbon source (Burk's buffer), and transferred to the indicated medium. Escherichia coli strains were grown at 37°C on Luria-Bertani medium. Antibiotic concentrations routinely used were as follows: nalidixic acid, 20 μg/ml; rifampin, 10 μg/ml; tetracycline, 10 μg/ml; spectinomycin, 25 μg/ml; gentamicin, 0.25 μg/ml.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference(s) |
|---|---|---|
| A. vinelandii strains | ||
| OP | Natural algU mutant of strain O, nonmucoid | 4, 15 |
| UW136 | Natural rifampin-resistant mutant of OP, nonmucoid | 3 |
| SW136 | algU+ derivative of UW136, mucoid | This work |
| OV8 | SW136 with an arsA::Tn5SSgusA40 insertion, mucoid | This work |
| OV11 | SW136 with an arsA::Tn5SSgusA40 insertion, mucoid | This work |
| SW-A | SW136 with an arsA::Gmr insertion, mucoid | This work |
| SW-AP | SW136 with an arsA::Gmr insertion opposite to arsA, mucoid | This work |
| SW-B | SW136 with an arsB::Gmr insertion, mucoid | This work |
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 9 |
| S17-1 λpir | thi pro hsdR hsdM+recA RP4 2-Tc::Mu-Km::Tn7(Tpr/Smr) λ-pir | 27 |
| Plasmids | ||
| pCAM140 | Vector containing the mTn5SSgusA40 for mutagenesis, (promoterless gusA for transcriptional fusions) | 27 |
| pBluescript KS(+) | Plasmid for subcloning DNA | Stratagene |
| pMOSBlue | Plasmid for cloning PCR products | Amersham |
| pBSL141 | Source of gentamicin resistance cassette | 1, 6 |
| pSMU85 | pBluescript KS(+) carrying algU from A. vinelandii ATCC 9046 | 18 |
| pOV8 | pBluescript KS(+) carrying 7-kb PstI fragment containing Tn5SSgusA40 insertion of OV8 | This work |
| pOV11 | pBluescript KS(+) carrying 7-kb PstI fragment containing Tn5gusA40 insertion of OV11 | This work |
| pSMarsA-Gm | pMOSBlue derivative carrying an arsA::Gmr insertion | This work |
| pSMarsA-GmP | pMOSBlue derivative carrying an arsA::Gmr insertion opposite to arsA | This work |
| pYRC1n | pMOSBlue derivative carrying an arsB::Gmr insertion | This work |
DNA manipulations.
Standard procedures for isolation of total genomic DNA, restriction endonuclease digestion, agarose gel electrophoresis, purification of DNA from agarose, DNA ligations, and transformation of E. coli were carried out as described by Sambrook et al. (24). DNA sequences were determined by the dideoxy chain termination method (25). For Southern blot analysis, DNA samples were digested with the indicated restriction endonucleases and DNA fragments were separated in a 1% agarose gel and blotted as described by Sambrook et al. (24). The radioactive probes were prepared by random priming using the Rediprime DNA labeling system (GE Healthcare).
Construction of A. vinelandii strain SW136.
Strain UW136 (3) is a rifampin-resistant derivative of A. vinelandii OP (4). Both strains are nonmucoid due to the presence of an insertion sequence (IS) within algU (15). To be able to study the encystment phenotype of mutants affected in alkylresorcinol synthesis, we constructed strain SW136, a UW136 derivative carrying a wild-type algU gene, as follows. Plasmid pSMU85 (18), carrying a wild-type algU gene from the mucoid A. vinelandii strain ATCC 9046 (accession number AAF18265), was transformed into strain UW136. A mucoid derivative (strain SW136) generated by a double recombination event was isolated and confirmed by PCR analysis to carry the wild-type algU copy by using the primers 5′-GGACATCATGCTGAAAGTG-3′ and 5′-CATGCTCCTCCTCAGCG-3′.
Transposon mutagenesis and identification of mutants affected in AR synthesis.
Mutagenesis of A. vinelandii SW136 was carried out using E. coli S17-1 λ-pir containing the promoter-probe minitransposon mTn5SSgusA40, as described previously (27). The mini-Tn5 mutant library obtained was stained for alkylresorcinol visualization as follows. A. vinelandii mutants were grown for 5 days on Burk's medium containing 0.2% n-butanol. The petri dishes were then sprayed with a solution of 0.5% Fast Blue B in 5% acetic acid. AR-producing colonies turned dark red after a few minutes of reaction with the staining solution.
Determination of alkylresorcinols.
Phenolic lipids were extracted with acetone for 20 min at room temperature in closed tubes. After centrifugation, the acetone extract was removed and a second extraction was done with acetone for 12 h at room temperature. The resulting extracts were mixed and used for the spectrophotometric determination of alkylresorcinols, with the use of Fast Blue B as previously described (26). Orcinol was used as a standard. The protein content of the cells used for AR determinations was quantified by the method of Lowry et al. (14).
Encystment and resistance to desiccation.
Cyst formation was induced by transferring washed vegetative cells grown on BS for 24 h to plates with BBOH medium (encystment induction medium) (23). After 5 days of incubation at 30°C, the cells were suspended in Burk's medium without carbon source (Burk's buffer). To disaggregate the cysts, the cell suspensions were dispersed with a sonicator (Virsonic 60) at 4 W (power output). Six pulses (4 s on, 30 s off) were applied. The tubes were kept in ice throughout the treatments. To determine the effect of sonication on cyst viability, treated and untreated controls of the parental strain (SW136) were included. Desiccation resistance assays were carried out as described previously (5). Approximately 106 CFU of each strain were applied to Millipore 0.2-mm-pore-size membranes and placed in sterile tubes. The cells on the filters were desiccated at 30°C for the indicated times. Surviving cells, quantified by viable count, were considered mature cysts.
Light and electron microscopy.
An optical microscope (Olympus EX41) was used to observe vegetative cells and cysts of A. vinelandii. To differentiate the cysts, Fast Blue B staining was used to color the alkylresorcinol lipids in the layers of cysts. For this staining the cells were grown for 5 days in the appropriate medium, and samples of the culture were placed on a microscope slide and stained with a solution of 0.5% Fast Blue B in 5% acetic acid for 10 min. Electron microscopy was carried out using cells grown on BBOH for 5 days, as previously reported (16).
Insertional inactivation of the ars genes.
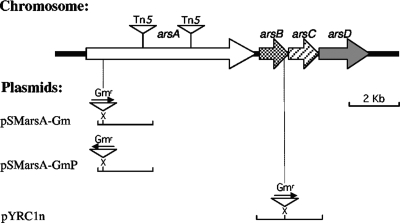
arsA and arsB mutants were obtained by gene disruption with antibiotic resistance cassettes. DNA fragments containing the target genes were obtained by PCR, using DNA from A. vinelandii UW136. Primers arsA6 (5′-GCCAAAGCAAATCTAAAGG-3′) and arsA5 (5′-CAATCGCAATCCTGGAGTC-3′) were used for the amplification of a fragment of arsA, and primers arBC1 (5′-CACGGTTGAGGTTTTTCC-3′) and arBC2 (5′-GGACTCCACCTCGTAGAC-3′) were used for the amplification of arsB. The resulting fragments were cloned in the pMOSBlue vector. The arsA and arsB genes were disrupted by insertion of a gentamicin cassette (Gmr) from plasmid pBSL141 (1, 6). Restriction analysis was used to determine the orientation of the Gmr resistance cassette. The cassette inserted in the same orientation as the inactivated gene (insertion allowing transcription of downstream genes) was selected for arsB::Gmr. For the arsA::Gmr insertion, both orientations were used. The physical map depicting the plasmids with the corresponding DNA fragments contained and the location of the restriction sites used for the insertion of the antibiotic cassette is shown in Fig. 1. The corresponding arsA and arsB mutants of A. vinelandii (Table 1) were obtained by gene replacement, transforming strain SW136 with plasmids pSMarsA-Gm, pSMarsA-GmP, and pYRC1n (Fig. 1) and selecting for transformants resistant to 0.5 μg/ml gentamicin. The double-crossover events were confirmed by PCR analysis.
FIG. 1.
Physical map of the A. vinelandii ars chromosomal region. The fragments contained in the corresponding plasmids are illustrated. The arrows represent genes. Restriction sites relevant for gene disruption are shown. Triangles represent insertions of either antibiotic resistance cassettes or the mTn5SSgusA40 transposon.
Real-time PCR.
Total RNA extraction was performed as reported by Barry et al. (2). To eliminate genomic DNA, RNA was treated with DNase (DNA-free; Ambion) and its concentration measured by the 260 nm/280 nm absorbance ratio. cDNA was synthesized using 200 ng of total RNA, the Revert Aid H first strand cDNA synthesis kit (Fermentas Inc.), and a mixture of the specific DNA reverse primers ribL9-1Ds (5′-CGGTGATGGTGATTTCCAGT-3′), gyrA2Ds (5′-TCCTCGTCGTCGAATAGCTC-3′), and arsBlw (5′-AAGGCATAGGCGGACAGC-3′). The cDNA obtained was used as template for real-time PCRs. Real-time PCR was performed with the ABI Prism 7000 sequence detection system (Perkin-Elmer/Applied Biosystems) using SYBR green PCR master mix (Perkin-Elmer/Applied Biosystems). Amplification conditions were 10 min at 95°C and a two-step cycle of 95°C for 15 s and 60°C for 60 s for a total of 40 cycles. The sizes of all amplimers were 100 to 101 bp. Primers ribL9-1US (5′-AGCCCGTCGTGTCGAACT-3′) and ribL9-1DS were used for the amplification of rplI; gyrA2US (5′-CGTGATGCTGATCAAGTTGG-3′) and gyrA2DS were used for gyrA; and arsBup (5′-ATGAGCAGTCCCCACAACG-3′) and arsBlw were used for arsB. The final primer concentration was 250 nM. All real-time PCRs were performed in triplicate for each gene of each strain, and very similar values were obtained (differences of <0.3 standard deviations). After amplification, melting curve analysis was performed. The levels of the gyrA and rplI mRNAs (coding for the A subunit of the DNA gyrase and the 50S ribosomal protein L9, respectively) were used as internal controls in the same samples to normalize the results obtained for arsB mRNA among the tested strains. A nontemplate control of each reaction was included for each gene. The quantification technique used to analyze data was the 2−Δ,ΔCT method reported by Livak and Shmittgen (13). Reproducibility of the whole procedure was determined by performing cDNA synthesis and real-time PCR experiments from two separate RNAs extracted for each strain. Similar results were obtained for the transcription of all measured genes in the repetitions and with the two different internal controls (gyrA and rplI) used for the normalization.
RESULTS
Construction of a mucoid strain of A. vinelandii and visualization of AR production on plates.
To be able to study the encystment phenotype of mutants affected in alkylresorcinol synthesis, we constructed, as described in Materials and Methods, strain SW136, a UW136 derivative carrying a wild-type algU gene from strain ATCC 9046 in its chromosome. As expected, A. vinelandii SW136 produced alginate (data not shown) and therefore was able to form mature cysts, whereas the parental strain UW136 was unable to encyst (Table 2).
TABLE 2.
Characteristics and desiccation resistance of cysts of different A. vinelandii strains
| Strain | Genotype | AR production | Mucoidy | Resistance to desiccation (%) at:
|
|
|---|---|---|---|---|---|
| 5 days | 45 days | ||||
| SW136 | algU+ | + | + | 5.0 ± 1.1 | 0.3 ± 0.1 |
| OV11 | algU+arsA::Tn5 | − | + | 3.9 ± 0.4 | 0.4 ± 0.1 |
| SW-B | algU+arsB::Gmr | − | + | 5.7 ± 1.0 | 0.3 ± 0.1 |
| UW136 | algU mutant, Rifr | + | − | <0.01 | ND |
| OP | algU mutant | + | − | <0.01 | ND |
Resistance to desiccation for 5 or 45 days of cysts induced on Burk's medium with n-butanol as the sole carbon source. Values are the means of three determinations ± standard deviations. ND, not determined.
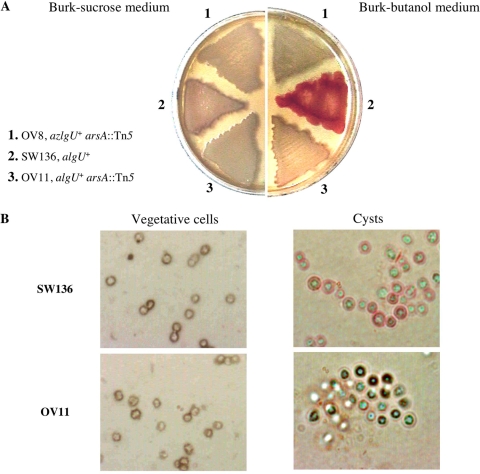
A. vinelandii synchronously encysts and produces alkylresorcinols when vegetative cells of this bacterium are transferred to plates of Burk's minimal medium supplemented with 0.2% n-butanol as the sole carbon source (encystment induction medium [12]). For visualization of cells producing ARs, vegetative cells of SW136 were transferred to BBOH medium. Plates containing BS medium (vegetative growth medium) were also inoculated. After 5 days of incubation, the plates were sprayed with a solution containing 0.5% Fast Blue in 5% acetic acid. Colonies grown in BBOH (cysts) developed a red color, whereas colonies grown on BS remained white (Fig. 2A, sector 2). ARs were quantified from SW136 cells isolated from these plates. Less than 0.1 μg of ARs per mg of protein was detected from SW136 grown on BS plates, whereas 4.6 μg of ARs per mg of protein was present in SW136 cells grown in BBOH. When observed under the light microscope (Fig. 2B), the red color was mostly distributed in the most external part of the cells induced to encyst, in agreement with the localization of these lipids in the exine layer and the cell membrane (21). No staining was observed in vegetative cells.
FIG. 2.
Staining of alkylresorcinols produced by A. vinelandii. (A) Staining of A. vinelandii mutant OV8 (1), SW136 (2), and mutant OV11 (3), grown on petri dishes containing Burk-sucrose medium (vegetative growth) or Burk-butanol medium (encystment induction). (B) Light microscopy (bright field) of Fast Blue B-stained vegetative cells and cysts of A. vinelandii SW136 and OV11. In all cases the cells were grown for 5 days.
Isolation of mutants impaired in ARs production.
Random mini-Tn5 mutagenesis of strain SW136 was carried out as described in Materials and Methods. A total of 1,000 strains resistant to spectinomycin were isolated and screened for the production of ARs. Two mutants, OV8 and OV11, unable to develop the red color characteristic of the wild-type strain SW136 when induced for encystment were identified (Fig. 2A, sectors 1 and 3). When observed under the microscope, the OV11 cells that were induced to encyst and treated with the Fast Blue B reagent showed the same morphology as the SW136 cysts. However, no staining of the cyst capsule was obtained (Fig. 2B). When quantified, no AR synthesis was detected in OV8 and OV11, confirming the inability of these strains to produce these phenolic lipids.
PstI DNA fragments containing the mini-Tn5 mutations from strains OV8 and OV11 were cloned into plasmid pBluescript KS (Stratagene). The resultant plasmids, pOV8 and pOV11, were used to determine the location of the mini-Tn5 by sequencing across the transposon insertion junction. In strains OV8 and OV11, the transposon was found to lie within the arsA gene (Fig. 1), which codes for a type I fatty acid synthase and heads the arsABCD putative operon identified by Funa et al. (7). Analysis of the GC content of the arsA sequence and its upstream sequence using FramePlot (10) suggested the start codon is probably 246 bp upstream from the one considered in the annotation (accession number ZP_00418324; http://www.jgi.doe.gov). In the OV8 strain the mini-Tn5 is inserted 3,017 nucleotides downstream of the putative start codon, whereas in strain OV11 the mini-Tn5 was inserted after nucleotide 4906.
Polarity of the asrA::Tn5 mutation.
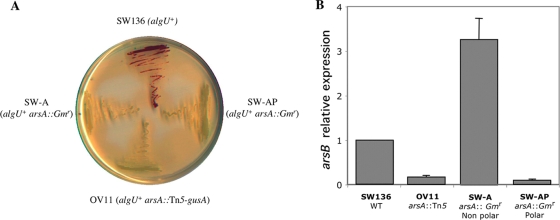
The arsABCD genes are likely to constitute an operon, since the pair arsB and asrC and the pair arsC and arsD overlap by 4 nucleotides in each case. In addition, no promoter consensus sequences were identified in the 83-nucleotide intergenic arsA-arsB sequence (SoftBerry BPROM program; http://linux1.softberry.com/berry.phtml). Because a mutation in arsB was shown to impair ARs synthesis (7), the question of whether the inability of the OV11 and OV8 mutants to produce ARs was due to polarity of the arsA::Tn5 mutations on arsB was raised. In A. vinelandii, the insertion of the Ω-Km cassette from plasmid pHP45Ω-Km (6) into a gene in the same direction of its transcription produces mutations allowing transcription of the downstream genes in the same operon (16). Strains SW-A and SW-AP, which contain an asrA::Ω-Gm insertion with the cassette oriented in the same or in the opposite direction of arsA, respectively, were constructed as described in Materials and Methods. Both mutants were impaired in ARs synthesis (Fig. 3A). The effects of these insertions, and of the mini-Tn5 insertion of OV11, on the expression of the genes downstream were confirmed by determining the relative content of arsB mRNA with respect to the parental strain SW136 by real-time reverse transcription-PCR. As shown in Fig. 3B, the insertions in SW-AP and OV8 negatively affected arsB expression. However, the arsB mRNA level in strain SW-A was even higher than that of the wild-type strain. These data confirmed that at least arsAB constitutes an operon and that the protein encoded by arsA is essential for the synthesis of alkylresorcinols.
FIG. 3.
Effects of different arsA gene insertions on alkylresocinol synthesis and on the expression of arsB. (A) Alkylresorcinol staining of SW136 and different asrA mutants. The cells were induced to encyst on Burk-butanol medium for 5 days. (B) Effects of different arsA insertions on the expression of arsB, measured by real-time reverse transcription-PCR. The levels of the arsB transcripts were measured under encystment-inducing conditions and were normalized according to the level of the gyrA mRNA. The data are presented as fold changes of mRNA levels of OV11, SW-A, and SW-AP mutant strains relative to those of the parental strain (SW136). These data represent the means of triplicates, and the error bars represent the standard deviations.
Analysis of arsA transcription.
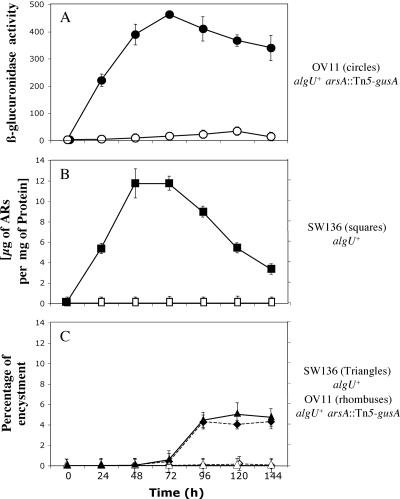
A. vinelandii synthesizes alkylresorcinols under encysting conditions but not in vegetative cells (21). In the OV8 and OV11 mutant strains, the mini-Tn5 promoter-probe transposon is inserted in the direction of arsA transcription, thus allowing transcription of the gusA reporter gene from the promoter transcribing arsA. We examined transcription of arsA by measuring β-glucuronidase activity in strain OV11 grown in BS (vegetative cells) and under encystment induction conditions in BBOH (Fig. 4A). In a parallel culture we also determined the corresponding AR production of strain SW136 (Fig. 4B). The corresponding encystment percentages of SW136 and OV11 were also determined and are shown in Fig. 4C. A small increase in the activity of the reporter gene product (14-fold) was observed in BS medium, when the culture aged and a small percentage of cysts formed (less than 0.001%), whereas a 200-fold induction was observed in BBOH, where 4 to 5% of the cells formed mature cysts. The induction of arsA expression correlated with AR accumulation up to a maximum content of 11.7 μg per mg of protein.
FIG. 4.
Expression of arsA, AR synthesis, and encystment in two different media, Burk-sucrose medium (vegetative growth; open symbols) or Burk-butanol (encystment induction medium; closed symbols). (A) β-Glucoronidase activity of strain OV11 containing an arsA::Tn5-gusA reporter fusion. (B) Accumulation of alkylresorcinols over time in A. vinelandii SW136. (C) Percentages of encystment of strains SW136 and OV11, measured as desiccation resistance for 5 days. The inocula were incubated for 24 h on liquid Burk-sucrose, washed with Burk's medium with no carbon source, and transferred to plates with the corresponding medium (at time zero). These data are the means of triplicates, and the error bars represent the standard deviations. One unit of β-glucoronidase activity corresponds to 1 nmol of substrate 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid hydrolyzed per min per mg of protein.
Encystment phenotype of the arsA mutant.
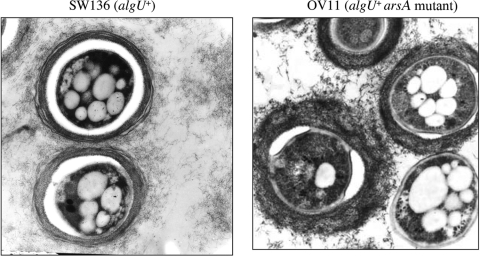
To study the role of ARs in encystment we induced the isogenic strain SW136 and mutant derivatives OV8, OV11, and strain OP to encyst. Cell aggregation was observed with the cysts obtained from OV8 and OV11 strains. The cyst aggregates formed made it difficult to accurately determine the number of cells remaining viable after desiccation. Thus, the cyst suspension was sonicated to break the aggregates (see Materials and Methods). Although no aggregation was obtained with SW136, OP, or UW136 strains, they were also sonicated as controls to show that the sonication treatment used did not break the cysts. The OV8 and OV11 mutant cysts were able to survive desiccation for 5 days in a percentage similar to that observed for the SW136 cysts (Table 2). Strains OP and UW136 were unable to produce cysts resistant to desiccation. The results suggest that the lack of ARs did not abolish cyst formation or the capacity of the cysts to survive desiccation for 5 days. To test the role of ARs in the survival with a lack of water for longer periods of time, we left dried cysts of SW136 and OV11 strains for 45 days at 30°C. The percentages of survival of these strains were similar (Table 2). Electron microscopy of cysts of the arsA mutant OV11 (whose mini-Tn5 insertion is polar on arsB) revealed capsulated cysts, although their exine layer showed a disorganization of its dark laminar structures compared with the cysts developed by the wild-type strain (Fig. 5). Since this phenotype is different from that reported by Funa et al. (7) for an arsB mutant, we constructed strain SW-B, an SW136 derivative carrying an arsB mutation. Similar to the arsA mutants, when induced for encystment the SW-B strain formed cell aggregates and produced cysts resistant to desiccation (Table 2).
FIG. 5.
Electron micrographs of A. vinelandii cysts of strains SW136 and OV11 (algU+ arsA mutant) 5 days after induction on Burk-butanol medium.
DISCUSSION
We developed a simple method to stain cells that produce ARs. This method can be used in other organisms to detect the presence of phenolic lipids. It allowed us to distinguish the cysts under the microscope and to screen for mutants unable to produce ARs. Two of these mutants were shown to carry mutations in arsA, the gene encoding a type I fatty acid synthase (7). Miyanaga et al. (17) showed, in vitro and in a reconstituted system in E. coli, that ArsA, together with ArsD, is a fatty acid synthase responsible for the synthesis and transfer of the acyl substrates to the active sites of ArsB and ArsC for the synthesis of alkylresorcinols and alkylpyrones, respectively (17). This study confirms the essentiality of ArsA activity for alkylresorcinol synthesis in A. vinelandii, as an arsA mutation allowing transcription of downstream genes completely impaired AR synthesis.
The expression of the arsA gene is cyst specific, in accordance with the synthesis of ARs. The amount of arsA mRNA is low in BS cultures, where most cells are vegetative, and it is slightly increased in aging cultures, where a low percentage of cysts are formed (0.001%). In BBOH medium, a condition promoting a higher encystment percentage (5%), its transcription was induced 200-fold. Thus, AR synthesis is controlled at the transcriptional level.
Electron microscopy of the arsB mutant induced to encyst, reported by Funa et al. (7), showed that the lack of ARs severely impaired exine formation. Thus, those authors concluded that the phenolic lipid synthesis is essential for cyst formation in A. vinelandii. However, inactivation of arsB was carried out in the nonmucoid strain OP, also named UW (4), which is unable to produce alginate due to an insertion element present in the algU gene (15) (accession numbers AAF18261 ZP_00415083 and ZP_00415083). Electron microscopy of the arsA mutants induced to differentiate revealed that they formed cysts with a disorganized exine, in agreement with previous reports showing ARs are components of the exine layer (21). This altered morphology of the capsule shows that the ARs play a structural role in the cysts. The agglutination phenotype observed in the arsA mutants could be related to their altered exine structure, suggesting that the presence of ARs in the exine contributes to the segregation of the cysts formed during the differentiation process. The replacement of phospholipids by alkylresorcinols in the membrane of the cysts has been considered to contribute to the desiccation resistance of these cells (7, 22). However, our results show that, under the conditions tested, ARs are not essential for the cysts to resist desiccation, since the cysts of the arsA mutants were able to survive desiccation similar to the SW136 strain (Table 2). Thus, the results show that although ARs play a structural role in the capsule of the mature cyst, they are not essential for cyst formation or for desiccation resistance. Differences between the arsB mutant phenotype reported by Funa et al. (7) and the ars mutants constructed in this study are probably due to the inability of strain OP, the one considered by those authors as wild type, to produce alginate as a consequence of the insertion within its algU gene. Here we have shown that strain OP is unable to form genuine mature cysts resistant to desiccation, in accordance with its lack of the AlgU sigma factor. The severe effect on survival to dryness observed for this strain or the nonmucoid strain UW136 shows that alginate is much more important than ARs for the cysts to withstand desiccation.
Formation of fragile cyst-like structures in the nonmucoid OP strain have been reported (19). However, these structures were reported to have a distinct exine layer composed of membrane-like plates which were probably composed of ARs and APs. The impairment in AR synthesis, together with their lack of alginate, could explain the severely impaired exine observed by Funa et al. (7) in the cyst-like cells of the arsB mutant.
In summary, we have demonstrated here that AR lipids are not essential for either cyst formation or desiccation resistance.
Acknowledgments
This work was supported by grant U47781-Q from CONACyT.
We thank Josefina Guzmán for technical support.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Alexeyev, M. F., I. N. Shokolenko, and T. P. Croughan. 1995. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 16063-67. [DOI] [PubMed] [Google Scholar]
- 2.Barry, T., S. Geary, S. Hannify, C. MacGearailt, M. Shalloo, D. Heery, F. Gannon, and R. Powell. 1992. Rapid mini-preparations of total RNA from bacteria. Nucleic Acids Res. 204940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, P. E., and W. J. Brill. 1977. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J. Bacteriol. 130954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush, J. A., and P. W. Wilson. 1959. A Non-gummy chromogenic strain of Azotobacter vinelandii. Nature 184381.13815135 [Google Scholar]
- 5.Campos, M., J. M. Martínez-Salazar, L. Lloret, S. Moreno, C. Núñez, G. Espín, and G. Soberón-Chávez. 1996. Characterization of the gene coding for GDP-mannose dehydrogenase (algD) from Azotobacter vinelandii. J. Bacteriol. 1781793-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52147-154. [DOI] [PubMed] [Google Scholar]
- 7.Funa, N., H. Ozawa, A. Hirata, and S. Horinouchi. 2006. Phenolic lipid synthesis by type III polyketide synthases is essential for cyst formation in Azotobacter vinelandii. Proc. Natl. Acad. Sci. USA 1036356-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaona, G., C. Núñez, J. B. Goldberg, A. S. Linford, R. Nájera, M. Castañeda, J. Guzmán, G. Espín, and G. Soberón-Chávez. 2004. Characterization of the Azotobacter vinelandii algC gene involved in alginate and lipopolysaccharide production. FEMS Microbiol. Lett. 238199-206. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa, J., and K. Hotta. 1999. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 174251-253. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy, C., R. Gamal, R. Humphrey, J. Ramos, K. Brigle, and D. Dean. 1986. The nifH, nifM and nifN genes of Azotobacter vinelandii: characterization by Tn5 mutagenesis and isolation from pLARF1 gene banks. Mol. Gen. Genet. 205318-325. [Google Scholar]
- 12.Lin, L. P., and H. L. Sadoff. 1968. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J. Bacteriol. 952336-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 14.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 15.Martínez-Salazar, J. M., S. Moreno, R. Nájera, J. C. Boucher, G. Espín, G. Soberón-Chávez, and V. Deretic. 1996. Characterization of the genes coding for the putative sigma factor AlgU and its regulators MucA, MucB, MucC, and MucD in Azotobacter vinelandii and evaluation of their roles in alginate biosynthesis. J. Bacteriol. 1781800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mejia-Ruiz, H., S. Moreno, J. Guzman, R. Najera, R. Leon, G. Soberon-Chavez, and G. Espin. 1997. Isolation and characterization of an Azotobacter vinelandii algK mutant. FEMS Microbiol. Lett. 156101-106. [DOI] [PubMed] [Google Scholar]
- 17.Miyanaga, A., N. Funa, T. Awakawa, and S. Horinouchi. 2008. Direct transfer of starter substrates from type I fatty acid synthase to type III polyketide synthases in phenolic lipid synthesis. Proc. Natl. Acad. Sci. USA 105871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno, S., R. Najera, J. Guzman, G. Soberon-Chavez, and G. Espin. 1998. Role of alternative sigma factor algU in encystment of Azotobacter vinelandii. J. Bacteriol. 1802766-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page, W. J. 1983. Formation of cyst-like structures by iron-limited Azotobacter vinelandii strain UW during prolonged storage. Can. J. Microbiol. 291110-1118. [Google Scholar]
- 20.Reusch, R. N., and H. L. Sadoff. 1979. 5-n-Alkylresorcinols from encysting Azotobacter vinelandii: isolation and characterization. J. Bacteriol. 139448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reusch, R. N., and H. L. Sadoff. 1981. Lipid metabolism during encystment of Azotobacter vinelandii. J. Bacteriol. 145889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reusch, R. N., and H. L. Sadoff. 1983. Novel lipid components of the Azotobacter vinelandii cyst membrane. Nature 302268-270. [DOI] [PubMed] [Google Scholar]
- 23.Sadoff, H. L. 1975. Encystment and germination in Azotobacter vinelandii. Bacteriol. Rev. 39516-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 25.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tluscik, F., A. Kozubek, and W. Mejbaum-Katzenellenbogen. 1981. Alkylresorcinols in rye (Secale cereale L.) grains. VI. Colorimetric micromethod for the determination of alkylresorcinols with the use of diazonium salt, Fast Blue B. Acta Soc. Bot. Pol. 50645-651. [Google Scholar]
- 27.Wilson, K. J., A. Sessitsch, J. C. Corbo, K. E. Giller, A. D. Akkermans, and R. A. Jefferson. 1995. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology 1411691-1705. [DOI] [PubMed] [Google Scholar]