Abstract
In the goldfish (Carassius auratus) the two endogenous forms of gonadotropin-releasing hormone (GnRH), namely chicken GnRH II ([His5,Trp7,Tyr8]GnRH) and salmon GnRH ([Trp7,Leu8]GnRH), stimulate the release of both gonadotropins and growth hormone from the pituitary. This control is thought to occur by means of the stimulation of distinct GnRH receptors. These receptors can be distinguished on the basis of differential gonadotropin and growth hormone releasing activities of naturally occurring GnRHs and GnRHs with variant amino acids in position 8. We have cloned the cDNAs of two GnRH receptors, GfA and GfB, from goldfish brain and pituitary. Although the receptors share 71% identity, there are marked differences in their ligand selectivity. Both receptors are expressed in the pituitary but are differentially expressed in the brain, ovary, and liver. Thus we have found and cloned two full-length cDNAs that appear to correspond to different forms of GnRH receptor, with distinct pharmacological characteristics and tissue distribution, in a single species.
It is well known that gonadotropin-releasing hormone (GnRH) is released from neurons in the hypothalamus to stimulate the release of gonadotropin(s) from the pituitary during the reproductive cycle. However, most vertebrates have two or more different GnRHs, which, in addition to regulating gonadotrope hormone secretion, regulate a number of extrapituitary functions (1). The presence of several forms of GnRH with effects in different tissues implies the existence of several cognate receptor types. To date, only a single GnRH receptor has been cloned and characterized in mammals (2) and in the catfish (3).
In goldfish (Carassius auratus), the brain and the pituitary contain two different forms of GnRH, [Trp7,Leu8]GnRH (salmon GnRH, sGnRH) and [His5,Trp7,Tyr8]GnRH (chicken GnRH II, cGnRH-II) (4, 5). These peptides stimulate growth hormone (GH) release from somatotrophs, in addition to stimulating release of the luteinizing hormone-like gonadotropin-II (GtH-II) release from gonadotrophs (6). It has been proposed that two different GnRH receptors are present in the goldfish pituitary, as there are functional differences in the stimulation of GtH-II release and GH release by different GnRH analogues (7). Furthermore, binding studies indicate the presence of two different types of GnRH receptor in goldfish brain and pituitary, as high-affinity–low-capacity and low-affinity–high-capacity GnRH binding sites in membrane preparations from these tissues (8, 9).
The description of two types of GnRH receptor in goldfish makes this species a useful model in which to investigate the molecular structure and function of GnRH receptor subtypes. In the present study we have cloned the cDNAs of two different full-length GnRH receptors, GfA and GfB, from goldfish pituitary and brain. They respectively share 71% and 82% identity with the catfish GnRH receptor, the only other nonmammalian GnRH receptor cloned to date. The goldfish GnRH receptors share lower identity with mammalian GnRH receptors. Pharmacological studies of these receptors transfected into COS cells revealed distinct differences in their ligand selectivity. In situ hybridization studies revealed that the receptors are both expressed in the goldfish pituitary, and that each has a unique pattern of expression in the goldfish brain, ovary, and liver.
MATERIALS AND METHODS
Peptides.
The following peptides were used in this study: The agonists GnRH, [His5,Trp7,Tyr8]GnRH (cGnRH-II), [Gln8]GnRH (chicken GnRH I, cGnRH-I); [dArg6,Trp7,Leu8]GnRH, [His5,dArg6,Leu8]GnRH, and [dArg6,Trp7,Tyr8]GnRH were prepared by solid-phase synthesis and purified by C18 reverse-phase chromatography. [Ser8]GnRH (sea bream GnRH), [Trp7,Leu8]GnRH (sGnRH), and [His8]GnRH were gifts from R. W. Roeske (Indiana University School of Medicine, Indianapolis). Thyrotropin-releasing hormone (TRH) and epinephrine were purchased from Sigma.
Cloning of Goldfish GnRH Receptors.
Degenerate PCR primers, JH5S and JH6A2, which straddle the transmembrane (TM) domains TM6 and TM7, respectively, were used to amplify the goldfish GnRH receptor from goldfish genomic DNA. The primers were as follows: JH5S, 5′-CTCGAATTCGGNATHTGGTAYTGGTT-3′; and JH6A2, 5′-ACACTCGAGCCRTADATNTRNGGRTC-3′ (where N = G, A, T, or C; R = G or A; D = A, G, or T; Y = T or C; and H = A, C, or T). The nucleotide sequences of two different GnRH receptors (GfA and GfB) were identified. This information was used to design gene-specific primers to amplify the remainder of these GnRH receptor sequences from total RNA extracted from goldfish pituitary and brain. This nucleotide sequence information was also used to design 35- and 36-mer oligonucleotide probes for in situ hybridization.
Adaptor primers were ligated onto cDNA constructed from goldfish pituitary and brain RNA by using the Marathon cDNA synthesis kit (CLONTECH). For the goldfish GfA receptor, gene-specific primers GFA 1AS (5′-AGCAGGTGTTCAGGTTCCCG-3′) and GFA 3AS (5′-CACGAAGAGCAGGTGATGGATGTACT-3′) were used in combination with the AP1 and AP2 primers (CLONTECH) to amplify the 5′ end of the GfA receptor from Marathon pituitary cDNA. Nucleotide sequence analysis of this PCR product showed that it contained the start codon for the GnRH receptor. The 3′ end of the GnRH receptor, extending from extracellular loop 3 to the C-terminal tail, was similarly cloned, by nested PCR amplification of goldfish pituitary cDNA with GFA 2S (5′-AGCCGGAGATGCTGAAGGTCA-3′) and GFA 4S (5′-ACATCCATCACCTGCTCTTCGTGTTC-3′), in combination with AP1 and AP2. One of these products contained the nucleotide sequence of the GfA receptor extending to a stop codon in the C-terminal tail. This nucleotide sequence information was used to design nested gene-specific primers to the noncoding 5′ and 3′ regions of the GfA receptor. Primers GFA 5′S (5′-CTCACTAGTGCGTTATAGAGGTGCAGA-3′) and GFA 5′0 (5′-ATCTGGATAATCAGGAATTTCTTG-3′) were used in combination with GFA 3′AS (5′-TAGTTCGAACCATCGCTTCATCGT-3′) and GFA 3′0 (5′-CTCAGAGTCTGGTTCATTATGGAG-3′) to amplify the full-length GfA receptor from goldfish pituitary cDNA. The nucleotide sequence of several clones, amplified by using both Klentaq (CLONTECH) and Pfu DNA polymerases was determined.
The 5′ end of the GfB receptor was cloned by PCR amplification on Marathon brain cDNA with primers GFB 1AS (5′AGCACGTGTTGAGATTGCCG-3′) and GFB 3AS (5′-GACGAAGAGAGCGTGATGGATGTATTC-3′) in combination with the AP1 and AP2 primers. Nucleotide sequence analysis showed that one of the PCR products contained the full-length 5′ coding region of the GfB receptor. This nucleotide sequence information was used to design two primers, GFB 5′1S (5′-CGAGTGGAGCATGTCTAGATGCTAATA-3′) and GFB 5′2S (5′-ACTGTCCGCATATTAGTGGAAATGAGG-3′), which were used in combination with the AP1 and AP2 primers to amplify the full-length GfB receptor from Marathon goldfish brain cDNA. The nucleotide sequence of four clones was determined.
Transient Transfection of COS-1 Cells.
The full-length GfA and GfB goldfish GnRH receptors were subcloned into pcDNA/Amp (Invitrogen), a mammalian expression vector. Twenty-four hours before transfection, COS-1 cells were plated at appropriate density on poly(dlysine)-coated dishes, in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum, penicillin (0.2 unit/ml), and streptomycin sulfate (100 μg/ml) (GIBCO/BRL). DNA was transfected in serum-free DMEM for 4 h by using an adapted DEAE-dextran method (10), with a 50-min chloroquine (200 μM) treatment and a 2-min 10% dimethyl sulfoxide shock (11). For inositol phosphate assays, 2 × 105 cells per well (12-well plates; Corning) were transfected with 2 μg of DNA. Cells were grown for 48 h after transfection, prior to each assay.
Inositol Phosphate Assay.
Transfected COS-1 cells were labeled overnight in 0.5 ml of medium 199 (GIBCO), containing 2% fetal calf serum and 2 μCi/ml myo-[2-3H]inositol (Amersham; 1 Ci = 37 GBq) as described by Millar et al. (12). Cells were washed twice for 5 min at 37°C in buffer I, followed by stimulation with GnRH, as required, in buffer I and 10 mM LiCl for 1 h at 37°C, with gentle agitation. Inositol phosphates were extracted with 10 mM formic acid for a minimum of 30 min at 4°C, as described by Berg et al. (13). Total inositol phosphates (monophosphate, bisphosphate, and trisphosphate) were separated on a Dowex ion-exchange column and eluted with 3 ml of 1 M ammonium formate/0.1 M formic acid, and radioactivity was determined. Data points were determined in duplicate, and data reduction was done by using graphpad prism. ED50 values are the mean of three separate experiments.
Probe Labeling, in Situ Hybridization, and Immunological Detection.
Five male and five female goldfish (30–40 g body weight) were used in the study. Goldfish brain, pituitary, and other tissues were fixed and processed for in situ hybridization as described previously (14). Nomenclature of forebrain nuclei was adopted from Peter and Gill (15).
Two antisense 35-mer and one sense 36-mer oligonucleotide primers were synthesized by GIBCO/BRL Canadian Life Technologies (Burlington, Ontario, Canada) for each receptor clone, as follows. Type A: antisense probe GfA 1a, 5′-ACTCGGGGGTGTCCTTCAGCATCTCCGGCTGG-3′; antisense probe GfA 2a, 5′-GCAGGTGTTCAGGTTCCCGAACACGAAGAGTAG-3′; and sense probe GfA 1s, 5′-AGCCGGAGATGCTGAAGGACACCCCCGAGTACA-3′. Type B: antisense probe GfB 1a, 5′-GTATTCTGGCATCACCTGCAGCATACGAGGCTGA-3′; antisense probe GfB 2a, 5′-GCAGCACGTGTTGAGATTGCCGAAGACGAAGAGAGC-3′; and sense probe GfB 1s, 5′-TCAGCCTCGTATGCTGCAGGTGATGCCAGAATACA-3′. Both antisense and sense probes were enzymatically labeled at their 3′ ends with terminal deoxynucleotidyltransferase (TdT) by incorporating a mixture of 2′-deoxyadenosine 5′-triphosphate (dATP) and digoxigenin-11–2′-deoxyuridine 5′-triphosphate (DIG-11-dUTP) according to specifications of the manufacturer and using the DIG Oligonucleotide Tailing Kit (Boehringer Mannheim). The procedure for in situ hybridization was adapted from Miller et al. (14). Hybridization signal detection was observed using the DIG Nucleic Acid Detection Kit (Boehringer Mannheim). Specificity of antisense probes was demonstrated by hybridizing adjacent tissue sections with sense probes. To immunocytochemically demonstrate which cells in the pituitary colocalize the antisense signals, adjacent tissue sections were exposed to rabbit anti-carp GtH-II and rabbit anti-carp GH, as previously described (16).
RESULTS
Primary Structures of Goldfish GnRH Receptors.
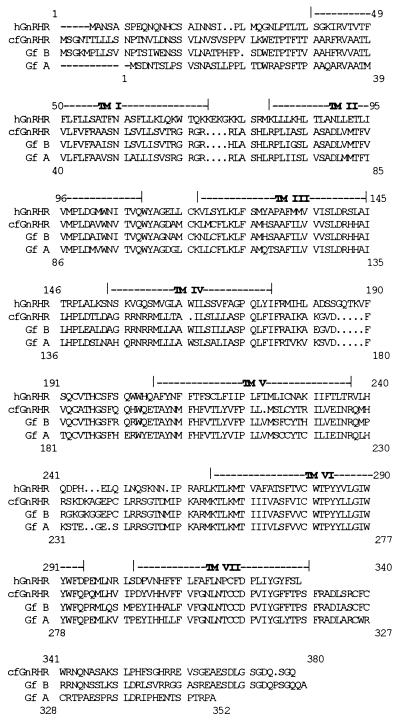
Two full-length putative goldfish GnRH receptor cDNAs, GfA and GfB, were identified in this study. Start codons were assigned on the basis of homology to the cloned GnRH receptors (2). Both cDNA clones encoded putative GnRH receptors with seven hydrophobic transmembrane domains and intracellular C-terminal tails, similar to the catfish GnRH receptor (3). Eight full-length clones of GfA were sequenced. Two differences in the amino acid sequence occurred when the consensus sequence of the eight clones was taken to be wild type. A C-to-T transition in transmembrane domain 3 (TM3) resulting in Ala125 being changed to a Val, and a C-to-T transition in extracellular loop II resulting in His195 being changed to a Tyr. All clones gave the same inositol phosphate response. Both Klentaq (CLONTECH) and Pfu (Stratagene) DNA polymerases were used to amplify these clones, and both polymerases have a high fidelity resulting from the 3′/5′ exonuclease activity (17). Four full-length clones of GfB were sequenced. The deduced coding regions of these revealed the presence of receptors with identical amino acid sequence, although differences in the nucleotide sequences did occur. GfA and GfB have a 71% amino acid identity, with highest identity in the transmembrane domains (Fig. 1). GfB has a higher identity to the catfish GnRH receptor (82%) than does the GfA receptor (71%). Both the GfA and GfB receptors share 43% identity with the human GnRH receptor.
Figure 1.
Sequence alignment of the goldfish GnRH receptor subtypes A and B to the human and catfish GnRH receptors. The putative transmembrane (TM) domains are indicated. cf, catfish; GfB, goldfish B; GfA, goldfish A; h, human. The two goldfish receptor subtypes A and B share 71% amino acid identity. The GfB GnRH receptor has an 82% amino acid identity to the catfish GnRH receptor, and the GfA subtype shows a lower identity of 71% to this receptor. All the fish receptors have amino acid identities of between 42% and 43% to the human GnRH receptor. Upper numbering refers to GfB, while lower numbering refers to GfA.
Pharmacological Characterization of Goldfish GnRH Receptors.
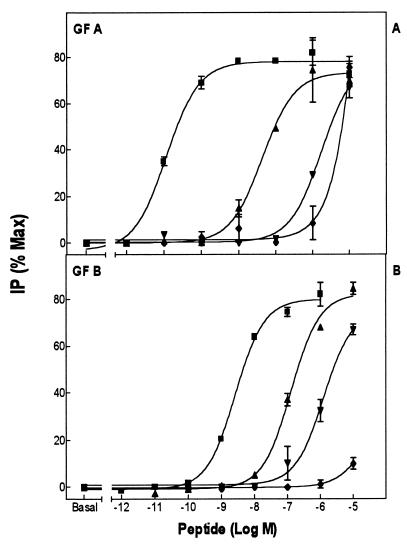
GnRH agonists stimulated inositol phosphate formation in COS-1 cells transfected with the GfA and GfB receptors, indicating that both receptors couple to Gq/G11 (Fig. 2 and Table 1). The ED50 values for GnRH analogues were generally higher for the GfB receptor compared with the GfA receptor. This could be due to a decreased receptor expression in the COS-1 cells or lower affinity. As we were unable to obtain satisfactory receptor binding to various iodinated peptides, including the most active peptides in Table 1, it was not possible to determine which factor is operative. Both GfA and GfB showed the same order of potencies for natural GnRHs, such that cGnRH-II > sGnRH > mammalian GnRH > sea bream GnRH. However, GfA showed a greater preference for cGnRH-II and a lesser preference for the other natural GnRHs than did GfB.
Figure 2.
Inositol phosphate production in COS-1 cells transiently transfected with the GfA (A) and GfB (B) GnRH receptor subtypes, after stimulation for 1 h with various GnRH agonists. ▾, mammalian GnRH; ▴, [Trp7,Leu8]GnRH (sGnRH); ■], [His5,Trp7,Tyr8]GnRH (cGnRH II); and ♦, [Ser8]GnRH (sea bream GnRH). For both receptor subtypes, cGnRH-II has the highest potency, followed by sGnRH, mammalian GnRH, and sea bream GnRH.
Table 1.
ED50 values of GnRH agonists for inositol phosphate production in COS-1 cells transiently transfected with the goldfish A and B GnRH receptor subtypes
| Peptide (common name) | GfA GnRH receptor
|
GfB GnRH receptor
|
||
|---|---|---|---|---|
| ED50, nM | Potency relative to sGnRH | ED50, nM | Potency relative to sGnRH | |
| [Trp7,Leu9]GnRH (sGnRH) | 4.6 ± 0.3 | 1 | 56 ± 20 | 1 |
| [His5,Trp7,Tyr8]GnRH (cGnRH-II) | 0.03 ± 0.01 | 153 | 3.4 ± 2 | 16.5 |
| Mammalian GnRH | 210 ± 69 | 0.02 | 2,850 ± 21 | 0.02 |
| [Ser8]GnRH (sea bream GnRH) | 684 ± 100 | 0.007 | >10,000 | >0.006 |
| [His8]GnRH | 11 ± 3 | 0.42 | >20,000 | >0.003 |
| [Tyr8]GnRH | 4.65 ± 1 | 1 | 640 ± 100 | 0.08 |
| [dArg6,Trp7,Tyr8]GnRH | 0.03 ± 0.01 | 153 | 0.8 ± 0.5 | 70 |
| [His5,dArg6,Leu8]GnRH | 3.3 ± 2 | 1.4 | 630 ± 100 | 0.08 |
| [dArg6,Trp7,Leu8]GnRH | 0.4 ± 0.2 | 11.5 | 21.7 ± 10 | 2.6 |
Data are calculated as the mean of three separate experiments.
There were differences between the two receptors in their relative potencies of GnRH agonists, with substitutions at position 8, relative to mammalian GnRH. While mammalian GnRH had a similar relative potency in both receptors, the GfB receptor did not recognize other GnRH agonists with variant amino acids in position 8, having a much lower potency for [His8]GnRH and [Tyr8]GnRH than the GfA receptor (Table 1). The ligand selectivity of the two receptors could be distinguished further in their response to superactive agonists. The GfB GnRH receptor had a much lower potency for [His5,dArg6,Leu8]GnRH compared with the GfA GnRH receptor, whereas they showed similar potencies for [dArg6,Trp7,Tyr8]GnRH and [dArg6,Trp7,Leu8]GnRH (Table 1).
The inositol phosphate response of the transfected COS-1 cells to treatment with different GnRH agonists was not a result of endogenous GnRH receptors. Inositol phosphate levels in untransfected COS-1 cells, stimulated with either cGnRH-II or sGnRH, did not rise above basal levels (data not shown). There was also no change above basal levels of inositol phosphate in COS-1 cells transfected with either the GfA or the GfB receptors, when stimulated with thyrotropin-releasing hormone or epinephrine.
In Situ Hybridization Results.
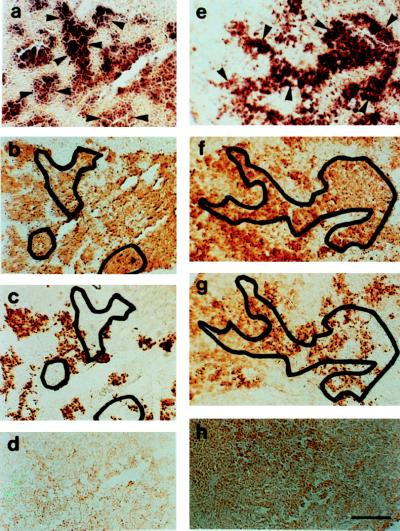
Antisense probes GfA 1a and GfB 1a demonstrated prominent in situ hybridization to pituitary cells in the proximal pars distalis of the goldfish pituitary (Fig. 3). The distribution of cells binding the probes overlapped with the distribution of gonadotrophs to a large extent. There was a minor overlap between the binding of both the probes with the distribution of somatotrophs.
Figure 3.
(a) In situ hybridization of antisense probe GfA 1a (dark purple staining) to the proximal pars distalis of the goldfish pituitary. To demonstrate the overlap of areas of hybridization with gonadotrophs and somatotrophs, selected areas (a) of hybridization (dark purple stain) indicated by arrowheads are projected onto adjacent sections immunocytochemically stained for GtH-II (brown stain; Fig. b) and growth hormone (brown stain; c). (d) Negative in situ hybridization reaction of sense probe to GfA to the goldfish pituitary. (e) In situ hybridization of antisense probe GfB1a (dark purple staining) to the goldfish pituitary. To demonstrate the overlap of areas of hybridization with gonadotrophs and somatotrophs, a selected area (e) of hybridization (dark purple stain) indicated by arrowheads is projected onto adjacent sections immunocytochemically stained for GtH-II (brown stain; f) and growth hormone (brown stain; g). (h) Negative in situ hybridization reaction of sense probe to GfB to the goldfish pituitary. (a–d) Female goldfish undergoing ovarian recrudescence, gonadosomatic index = 4.1%. (e–h) Mature male goldfish, gonadosomatic index = 2.3%. (Bar in h = 100 μm; all panels are the same magnification.)
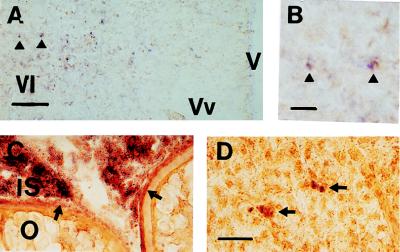
The GfA and GfB receptors were widely expressed in the brain (data to be presented elsewhere). A marked differential expression of the two receptors occurred in the area ventralis telencephali, where both GfA antisense probes (1a and 2a) hybridized to a small group of neuronal perikarya (Fig. 4 a and b). Antisense probes from the GfB receptor did not hybridize to this region (data not shown). The antisense probe GfA 1a demonstrated in situ hybridization to interstitial cells and the theca-granulosa cell layers surrounding oocytes of the goldfish ovary (Fig. 4c) and to isolated hepatocytes in the liver of female and male goldfish (Fig. 4d). The antisense probe GfB 1a did not hybridize with either ovarian or liver tissues (data not shown).
Figure 4.
(a) In situ hybridization of antisense probe GfA 2a (dark purple staining) to perikarya in the area ventralis telencephali of mature male goldfish, gonadosomatic index = 2.2%. Arrowheads indicate two perikarya shown at higher magnification in b. (Bar in a = 100 μm; bar in b = 20 μm.) (c) In situ hybridization of antisense probe GfA 1a (dark purple staining) to interstitial tissue (IS) of the goldfish ovary and the theca cell layer surrounding oocytes (arrowheads). (d) In situ hybridization of antisense probe GfA 1a (dark purple staining) to scattered hepatocytes (arrowheads) in the goldfish liver. (Bar in d = 20 μm; panels c–d are all the same magnification.) (c) Female goldfish, gonadosomatic index = 4.1%. (d) Male goldfish, gonadosomatic index = 2.3%. V, brain ventricle; Vl, area ventralis telencephali pars lateralis; Vv, area ventralis telencephali pars ventralis; IS, interstitial tissue; O, ovary.
DISCUSSION
We report here the full-length primary amino acid sequence of two GnRH receptor subtypes in a single species with distinct pharmacology and tissue distribution. Although the goldfish does have a tetraploid genome, it is evident that these two receptors arose from gene duplication that occurred before tetraploidization and that the receptors have different functions in teleosts. Three lines of evidence support this conclusion. The two subtypes, GfA and GfB, share only 71% amino acid identity. The mRNA transcripts for both the goldfish receptors show clear but different patterns of expression in the brain, ovary, and liver. Both of the goldfish GnRH receptors respond to natural forms of GnRH in COS-1 cells transfected with their cDNAs, but they show distinctive differences in stimulation of inositol phosphate production by GnRH agonists with substitutions at position 8. The relatively low amino acid homology and the different profiles of gene expression and receptor pharmacology indicate that the GnRH receptor has been duplicated in teleost fish evolution and has evolved into two functionally distinct receptors.
Our conclusion that the GnRH receptor has undergone a gene duplication early in teleost evolution is consistent with reports on other genes in goldfish. The cytochrome P450 aromotase gene (CYP19), which was duplicated early in teleost fish evolution, has given rise to two genes that are differentially expressed in brain and ovary of goldfish (18). The highly conserved synaptosome-associated protein, SNAP-25, was also duplicated in goldfish before tetraploidization of this species (19). The SnapA and SnapB loci share 91% amino acid identity. Subsequent tetraploidization in goldfish resulted in duplicate SnapB loci. The coding regions of the two SnapB loci differ by 23 nucleotides, which results in only a single amino acid substitution out of the 203 amino acids. Differences in the nucleotide sequence of the coding regions, between eight of the full-length GfA cDNAs and four of the GfB cDNAs clones, may be due to either gene polymorphism or the tetraploid condition of goldfish (20).
The two goldfish GnRH receptors share a number of features with the catfish GnRH receptor (3), as well as the chicken (Gallus domesticus) and frog (Xenopus laevis) GnRH receptors (B.E.T., T. R. Ott, J.P.H., R.P.M., and N.I., unpublished results; Y. Sun, N.I., J.P.H., P. Sharp, S. C. Sealfon, and R.P.M., unpublished results), which distinguishes them from the mammalian GnRH receptors. Mammalian GnRH receptors are characterized by the reciprocal exchange of Asn87 in TM2 and Asp318 in TM7, as compared with most G protein-coupled receptors (GPCRs) (21). In the two goldfish GnRH receptors and other nonmammalian GnRH receptors, the Asn87 has been replaced by Asp81 (GfA receptor) and Asp91 (GfB receptor). Since this arrangement leads to a loss of function in the mammalian GnRH receptor, there are presumably coordinated structural changes in the goldfish receptor that could shed light on the molecular mechanisms of receptor activation. The most striking structural difference of the goldfish and other nonmammalian GnRH receptors and their mammalian counterparts is the presence of a C-terminal tail that plays an important role in desensitization and internalization of GPCRs. Deletion of the C-terminal tail from the chicken GnRH receptor leads to a rapid decrease in the rate of internalization of the truncated receptor (22), whereas addition of the catfish GnRH receptor C-terminal tail to the rat GnRH receptor led to an increase in its rate of internalization, resulting in rapid desensitization of the chimeric receptor (23). Serines, which are potential phosphorylation sites [S(P)XXS*] are conserved in the C-terminal tails of the catfish and the two goldfish GnRH receptors (Ser336, Ser339 for GfA, Ser349, Ser352 for GfB GnRH receptors). A single cysteine in the C-terminal tail, Cys327 (GfA) or Cys340 (GfB) is also conserved in the catfish GnRH receptor, and may be a site of palmitoylation for anchoring the receptor to the cell membrane.
A number of key features are conserved between the mammalian and nonmammalian GnRH receptors. An Asn residue (Asn18) in the N-terminal domain of the human GnRH receptor, which has been shown to be glycosylated and important for receptor expression in mammalian GnRH receptors (24), is conserved in the GfA and GfB GnRH receptors (Asn12 and Asn23, respectively) and the catfish GnRH receptor (Asn23). Two additional glycosylation sites (Asn11 and Asn18) are conserved between the goldfish GfB receptor and the catfish GnRH receptors. The microdomain DRXXI/V on the cytoplasmic side of TM3, which is essential for efficient signal transduction and the conversion of the GnRH receptor from an inactive to active state (25), is also completely conserved.
Putative ligand contact sites, Asp98, Asn102, Lys121, and Glu301 (2) for the mouse GnRH receptor are conserved in the goldfish GfA and GfB receptors as Asp90/Asp100, Asn94/Asn104, Lys107/Lys117, and Glu290/Glu303, respectively. This conservation suggests the maintenance of the same ligand binding site in all vertebrates. However, the conservation of Glu290 and Glu303 is unexpected, as the mammalian Glu301 is responsible for selectivity for Arg in position 8 of GnRH, which is not discriminated in the goldfish GnRH receptors (7). The position of Pro in the motif SEP is switched to PEY for both the GfA and GfB GnRH receptors. The switch in the position of Pro may affect the orientation of the acidic side chain of Glu, such that contact with the Arg8 of GnRH is no longer possible.
Although both receptors showed the same order of potencies for natural forms of GnRH with cGnRH-II > sGnRH > cGnRH-I > sea bream GnRH, when transfected into COS-1 cells, there were significant differences in their ligand selectivity. The GfA GnRH receptor had greatest sensitivity to cGnRH-II (ED50 = 0.03 ± 0.01 nM), whereas sensitivity to sGnRH was about 100-fold less. Although the GfB receptor also had the greatest sensitivity to cGnRH-II (ED50 = 3.4 ± 2.0 nM), the sensitivity to sGnRH was only about 16-fold less. Furthermore, the two receptors also responded differentially to GnRH agonists with substitutions at position 8. The GfA receptor retained its affinity for agonists with position 8 substitutions, whereas the GfB receptor was unable to recognize GnRH agonists with His or Tyr substituted at position 8. The receptors also differed in their response to superactive agonists. Whereas both the GfA and GfB receptor retained high affinity for [dArg6,Trp7,Tyr8]GnRH and [dArg6,Trp7,Leu8]GnRH, only the GfB receptor had a low affinity for [His5,dArg6,Leu8]GnRH.
While there are marked differences in ligand selectivity between the GfA and GfB GnRH receptors, there is no clear correlation with the GtH- and GH-releasing activities of GnRH analogs shown in vivo. In a goldfish dispersed cell static culture system, cGnRH-II was more potent in releasing GtH-II than was sGnRH, but the two peptides were equipotent in releasing GH (26). On the basis of relative potencies for cGnRH-II and sGnRH, the GfA receptor is most similar to the GtH-II-releasing receptor. However, on the basis of affinities for ligands with substitutions at position 8, the GfA receptor is most similar to the GH-releasing receptor. Physiological studies on perfused goldfish pituitary fragments showed that His8, Leu8, and Tyr8 did not affect GH-releasing potency, but caused a significant decrease in GtH-releasing potency (7). The latter characteristic correlates well with the properties of the GfB GnRH receptor. This lack of clear correlation could be due to the different biological systems used to characterize these receptors. The physiological studies relied on the measurement of GtH and GH release in perifused goldfish pituitary fragments or dispersed cells, whereas these present studies relied on GfA and GfB receptors transiently transfected into transformed monkey kidney (COS-1) cells. It is possible that differences in coupling to second messenger systems in the transfected COS-1 cells compared with the native gonadotrophs and somatotrophs may account for the lack of clear correlation between these results.
There were also no major differences between the expression patterns of the GfA and GfB receptors in the pituitary, and the receptors did not show a significant differential expression relative to the distribution of gonadotrophs and somatotrophs. Probes GfA 1a and GfB 1a both demonstrated in situ hybridization with gonadotrophs and to a minor extent with somatotrophs in the goldfish pituitary.
Marchant et al. (6) demonstrated that GnRH peptides stimulate release of GH in the goldfish. Release of GH by GnRH has now been confirmed in common carp (27) and tilapia (28), but not in rainbow trout (29). Specific binding of gold-labeled sGnRH to goldfish somatotrophs has been demonstrated by electron microscopy (30), providing direct evidence of the presence of GnRH receptors. Antisense probes GfA 1a and GfB 1a both demonstrated in situ hybridization with a minor portion of somatotrophs. However, because the overlap is not complete with the somatotrophs, additional as-yet-unidentified GnRH receptor(s) may be expressed in this cell type, or the effects of GnRH on stimulating GH release from these cells are paracrine.
The GfA and GfB GnRH receptors were widely expressed in different regions in male and female goldfish brain (data presented elsewhere). An antisense probe to the GfA receptor demonstrated hybridization to the area ventralis telencephali, an area that is known to be involved in male spawning behavior (31). GnRH peptides have long been known to affect reproductive behavior in the mouse model (32). The septal region of mammals, which is homologous to the area ventralis telencephali, also demonstrates GnRH receptor binding (33); however, other areas of the telencephalon of mammals have higher levels of GnRH receptor binding and expression of GnRH receptor mRNA. This fact likely reflects the greater development of cortical function in mammals, with extensive involvement in reproductive behavior, as compared with the teleosts, in which the telencephalon is relatively undeveloped.
The GfA receptor was also expressed in the ovary and liver. In situ hybridization studies demonstrate expression of the GfA receptor in interstitial tissue of the ovary and the theca-granulosa cell layers surrounding oocytes. This finding confirms earlier observations of GnRH binding to goldfish ovary membrane preparations (8, 34) and the presence of GnRH peptides in the ovary of goldfish (35). The combined expression of GnRH peptides and GnRH receptors in the ovary provides evidence for an autocrine/paracrine function of GnRH peptides in the ovary. Expression of GnRH receptor mRNA has been demonstrated in granulosa-luteal cells in both humans and rats (36, 37).
The expression of the GfA receptor in the liver is consistent with the demonstration of GnRH binding on goldfish liver membrane preparations (8, 34). The present result demonstrating expression of the GfA receptor in isolated cells in the goldfish liver supports the idea that hepatocytes may be the origin of a GnRH-binding protein with a high specificity for native GnRH peptides that has been identified in goldfish serum (38).
Our demonstration of two distinct GnRH receptors in the goldfish pituitary, together with previous demonstrations of two ligands (4, 5), low- and high-affinity binding (7, 8), and differential release of GtH and GH (6) points to a complex interplay of ligands and receptors in the regulation of pituitary hormone secretion. While a counterpart appears not to exist in adult mammals, the stimulation of GH by GnRH in humans with different clinical disorders, including acromegaly, Klinefelter’s syndrome, schizophrenia, depression, and heroin addiction (6) and GnRH stimulation of GH in immature rats (39) suggests that the dual GnRH ligand and receptor arrangement in the goldfish may be ontogenetically operative in mammals. The marked differences in distribution of the two receptors in the ovary and brain of the goldfish is also a pointer to the possibility that the effects of GnRH on these tissues in mammals may be mediated by a receptor distinct from the single receptor described in the mammalian pituitary.
Acknowledgments
Faezah Davids and Mark Minshull assisted technically with the cloning of the GfA and GfB receptors. This work was supported by the South African Medical Research Council, the Foundation for Research and Development, the University of Cape Town, the Natural Sciences and Engineering Research Council of Canada, and the University of Alberta.
ABBREVIATIONS
- GnRH
gonadotropin-releasing hormone
- sGnRH
salmon GnRH
- cGnRH-II
chicken GnRH II
- GtH
gonadotropin
- GH
growth hormone, GfA, goldfish GnRH receptor subtype A
- GfB
goldfish GnRH receptor subtype B
- TM
transmembrane
Footnotes
References
- 1.King J A, Millar R P. In: GnRH Neurons: Gene to Behavior. Parhar I S, Sakuma Y, editors. Tokyo: Brain Shuppan; 1997. pp. 51–77. [Google Scholar]
- 2.Sealfon S C, Weinstein H, Millar R P. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- 3.Tensen C, Okuzawa K, Blomenrohr M, Rebers F, Leurrs R, Bogerd J, Schultz R, Goos H. Eur J Biochem. 1997;243:134–140. doi: 10.1111/j.1432-1033.1997.0134a.x. [DOI] [PubMed] [Google Scholar]
- 4.Yu K L, Sherwood N M, Peter R E. Peptides. 1988;9:625–630. doi: 10.1016/0196-9781(88)90174-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin X-W, Peter R E. Gen Comp Endocrinol. 1996;101:282–296. doi: 10.1006/gcen.1996.0031. [DOI] [PubMed] [Google Scholar]
- 6.Marchant T A, Chang J P, Nahorniak C S, Peter R E. Endocrinology. 1989;124:2509–2518. doi: 10.1210/endo-124-5-2509. [DOI] [PubMed] [Google Scholar]
- 7.Habibi H R, Peter R E, Nahorniak C S, Milton R C, Millar R P. Regul Pept. 1992;37:271–284. doi: 10.1016/0167-0115(92)90620-a. [DOI] [PubMed] [Google Scholar]
- 8.Habibi H R, Peter R E, Sokolowska M, Rivier J E, Vale W W. Biol Reprod. 1987;36:844–853. doi: 10.1095/biolreprod36.4.844. [DOI] [PubMed] [Google Scholar]
- 9.Habibi H R, Pati D. Fish Physiol Biochem. 1993;11:43–49. doi: 10.1007/BF00004549. [DOI] [PubMed] [Google Scholar]
- 10.Keown W A, Campbell C R, Kucherlapati R S. Methods Enzymol. 1990;185:527–537. doi: 10.1016/0076-6879(90)85043-n. [DOI] [PubMed] [Google Scholar]
- 11.Lopata M A, Cleveland D A, Sollner-Webb B. Nucleic Acids Res. 1984;12:5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar R P, Davidson J S, Flanagan C A, Wakefield I. In: Methods in Neurosciences: Receptor Molecular Biology. Sealfon S C, editor. San Diego: Academic; 1995. pp. 145–163. [Google Scholar]
- 13.Berg K A, Clarke W P, Chen Y, Ebersole B J, McKay R D, Maayani S. Mol Pharmacol. 1994;45:826–236. [PubMed] [Google Scholar]
- 14.Miller F D, Tetzlaff W, Bisby M A, Fawcett J W, Milner R. J Neurosci. 1989;9:1452–1463. doi: 10.1523/JNEUROSCI.09-04-01452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peter R E, Gill V E. J Comp Neurol. 1975;159:69–101. doi: 10.1002/cne.901590106. [DOI] [PubMed] [Google Scholar]
- 16.Himick B A, Golosinski A A, Jonsson A-C, Peter R E. Gen Comp Endocrinol. 1993;92:308–316. doi: 10.1006/gcen.1993.1146. [DOI] [PubMed] [Google Scholar]
- 17.Barnes W M. Proc Natl Acad Sci USA. 1994;91:2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callard G V, Tchoudakova A J. Steroid Biochem Mol Biol. 1997;61:387–392. doi: 10.1016/s0960-0760(97)80037-4. [DOI] [PubMed] [Google Scholar]
- 19.Risinger C, Larhammar D. Proc Natl Acad Sci USA. 1993;90:10598–10602. doi: 10.1073/pnas.90.22.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buth D G, Dowling T E, Gold T R. In: Cyprinid Fishes: Systematics, Biology and Exploitation. Winfield I J, Nelson J S, editors. London: Chapman & Hall; 1991. pp. 95–101. [Google Scholar]
- 21.Zhou W, Rodic V, Kitanovic S, Flanagan C A, Chi L, Weinstein H, Maayani S, Millar R P, Sealfon S C. J Biol Chem. 1995;270:18853–18857. doi: 10.1074/jbc.270.32.18853. [DOI] [PubMed] [Google Scholar]
- 22.Pawson A J, Katz A, Sun Y-M, Lopes J, Illing N, Millar R P, Davidson J S. J Endocrinol. 1998;156:R9–R12. doi: 10.1677/joe.0.156r009. [DOI] [PubMed] [Google Scholar]
- 23.Lin X, Janovick J A, Brothers S, Blomenrohr M, Bogerd J, Conn M P. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- 24.Davidson J S, Flanagan C A, Zhou W, Becker I I, Elario R, Emeran W, Sealfon S C, Millar R P. Mol Cell Endocrinol. 1995;107:241–245. doi: 10.1016/0303-7207(94)03449-4. [DOI] [PubMed] [Google Scholar]
- 25.Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme B J, Konvicka K, Chi L, Millar R P, Davidson J S, Weinstein H, Sealfon S C. J Biol Chem. 1998;273:10445–10453. doi: 10.1074/jbc.273.17.10445. [DOI] [PubMed] [Google Scholar]
- 26.Chang J P, Cook H, Freedman G L, Wiggs A J, Somoza G M, de Leew R, Peter R E. Gen Comp Endocrinol. 1990;77:256–273. doi: 10.1016/0016-6480(90)90310-i. [DOI] [PubMed] [Google Scholar]
- 27.Lin X-W, Lin H-R, Peter R E. Gen Comp Endocrinol. 1993;89:62–71. doi: 10.1006/gcen.1993.1009. [DOI] [PubMed] [Google Scholar]
- 28.Melamed P, Eliahu N, Levavi-Sivan B, Ofir M, Farchi-Pisanty O, Rentier-Delrue F, Smal J, Yaron Z, Naor Z. Gen Comp Endocrinol. 1995;97:13–30. doi: 10.1006/gcen.1995.1002. [DOI] [PubMed] [Google Scholar]
- 29.Blaise O, Le Bail P-Y, Weil C. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;110:133–141. [Google Scholar]
- 30.Cook H, Berkenbosch J W, Fernhout M J, Yu K-L, Peter R E, Chang J P, Rivier J E. Regul Pept. 1991;36:369–378. doi: 10.1016/0167-0115(91)90070-w. [DOI] [PubMed] [Google Scholar]
- 31.Kyle A L, Stacey N E, Peter R E. Behav Neural Biol. 1982;36:229–241. doi: 10.1016/s0163-1047(82)90855-x. [DOI] [PubMed] [Google Scholar]
- 32.Moss R L, McCann S M. Science. 1973;181:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- 33.Jennes L, Eyigor O, Janovick J A, Conn P M. Recent Prog Horm Res. 1997;52:475–491. [PubMed] [Google Scholar]
- 34.Pati D, Habibi H R. Am J Physiol. 1993;33:R227–R234. doi: 10.1152/ajpregu.1993.264.2.R227. [DOI] [PubMed] [Google Scholar]
- 35.Pati D, Habibi H R. Endocrinology. 1998;139:2015–2024. doi: 10.1210/endo.139.4.5877. [DOI] [PubMed] [Google Scholar]
- 36.Peng C, Fan N C, Ligier M, Väänänen J, Leung P C K. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- 37.Kogo H, Kudo A, Park M K, Mori T, Seiichiro K. J Exp Zool. 1995;272:62–68. doi: 10.1002/jez.1402720108. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y-P, Guo D C, Peter R E. Gen Comp Endocrinol. 1991;84:58–66. doi: 10.1016/0016-6480(91)90064-d. [DOI] [PubMed] [Google Scholar]
- 39.Andries M, Denef C. Peptides. 1995;16:527–532. doi: 10.1016/0196-9781(94)00217-t. [DOI] [PubMed] [Google Scholar]