Abstract
Pseudomonas syringae delivers virulence effector proteins into plant cells via an Hrp1 type III secretion system (T3SS). P. syringae pv. tomato DC3000 HrpP has a C-terminal, putative T3SS substrate specificity switch domain, like Yersinia YscP. A ΔhrpP DC3000 mutant could not cause disease in tomato or elicit a hypersensitive response (HR) in tobacco, but the HR could be restored by expression of HrpP in trans. Though HrpP is a relatively divergent protein in the T3SS of different P. syringae pathovars, hrpP from P. syringae pv. syringae 61 and P. syringae pv. phaseolicola 1448A restored HR elicitation and pathogenicity to DC3000 ΔhrpP. HrpP was translocated into Nicotiana benthamiana cells via the DC3000 T3SS when expressed from its native promoter, but it was not secreted in culture. N- and C-terminal truncations of HrpP were tested for their ability to be translocated and to restore HR elicitation activity to the ΔhrpP mutant. No N-terminal truncation completely abolished translocation, implying that HrpP has an atypical T3SS translocation signal. Deleting more than 20 amino acids from the C terminus abolished the ability to restore HR elicitation. HrpP fused to green fluorescent protein was no longer translocated but could restore HR elicitation activity to the ΔhrpP mutant, suggesting that translocation is not essential for the function of HrpP. No T3SS substrates were detectably secreted by DC3000 ΔhrpP except the pilin subunit HrpA, which unexpectedly was secreted poorly. HrpP may function somewhat differently than YscP because the P. syringae T3SS pilus likely varies in length due to differing plant cell walls.
Many proteobacterial pathogens use a type III secretion system (T3SS) as their primary mechanism to overcome and infect eukaryotic hosts. T3SSs are complex macromolecular machines that span both the bacterial cell envelope and host cell barriers to deliver proteins, commonly termed effectors, from the bacterial cytoplasm into the host cytoplasm (13, 19). After delivery into the host, effector proteins manipulate host cell function and suppress host defenses, allowing bacterial proliferation and disease development (6, 20). Bacteria that rely on T3SS to cause disease include plant pathogens such as Pseudomonas syringae, Ralstonia solanacearum, Erwinia and Xanthomonas species and animal pathogens in the genera Yersinia, Salmonella, Shigella, Escherichia, and Pseudomonas. While the repertoire of effectors delivered by a given T3SS is unique, the T3SS machinery is more universal (13). T3SS includes a core set of eight conserved proteins. These proteins, which are also conserved in bacterial flagellar biogenesis machines, make up the multiringed base structure, or basal body, that spans the bacterial membranes and cell wall. T3SS machines are also comprised of less-conserved and unique proteins that vary between systems. These include regulatory proteins that orchestrate construction of the machine and the extracellular components that function to translocate effectors across host barriers.
The extracellular portion of the T3SS is comprised of the pilus or needle appendage (in plant or animal pathogens, respectively), which acts as a conduit for effector delivery, and the translocon complex, which creates the pore in the host cell membrane. These substructures vary between different T3SSs; presumably these external structures have adapted to allow different bacteria to infect different types of host cells. For Yersinia enterocolitica to infect macrophage cells, the T3SS needle must be a particular length (∼58 nm) to bridge the lipopolysaccharides extending from the bacterial outer membrane and reach the host cell membrane (35). Several other animal pathogens have T3SS needles of a defined length (48). Enteropathogenic Escherichia coli also has an additional extension beyond the needle called the EspA filament that functions to span the mucous layer found outside enterocyte cells (13). In plant pathogens, however, the extracellular gap between a bacterium and a plant cell includes a thick plant cell wall that is variable in width between plant species. Consequently, plant pathogenic Pseudomonas syringae has a pilus that can measure over 1 μm in vitro (25).
Another major difference between the T3SS machineries of animal and plant pathogens is their translocon complexes. In animal pathogens, these are typically comprised of three essential proteins, but there is growing evidence that plant pathogen translocons employ diverse, functionally redundant components (28). There is growing interest in understanding the regulatory players that orchestrate the construction of diverse machinery. It is hypothesized that the assembly of the T3SS must involve several tightly regulated steps that allow secretion of the required components, followed by that of effectors upon completion. Of particular interest here is the control of pilus/needle subunit secretion, which is necessary when the pilus/needle is being constructed but would presumably compete with translocon and effector secretion after the T3SS is complete.
We study the model plant pathogen P. syringae pv. tomato (Pto) DC3000, the causal agent of bacterial speck of tomato and Arabidopsis thaliana (8). DC3000 has a T3SS that delivers ca. 28 effectors and is essential for pathogenesis (11, 12, 30, 43). The P. syringae T3SS is encoded by hrp and hrc genes (hypersensitive response and pathogenicity/conserved), which are located in a pathogenicity island on the chromosome (4). hrc genes encode the conserved core components present in every T3SS. hrp genes encode T3SS components that are divergent or unique to P. syringae and enterobacterial plant pathogens, which also possess Hrp1 class T3SS (13). In contrast, plant pathogenic Ralstonia and Xanthomonas spp. have Hrp2 class T3SS, as indicated by several different Hrp proteins and distinct regulatory systems.
To better understand the T3SS machinery, we previously conducted a survey of the hrp genes of P. syringae pv. syringae (Psy) 61 to complete the inventory of all those encoding proteins capable of traveling the T3SS into plant cells when expressed from a constitutive promoter (39). We hypothesized that these proteins might aid in pilus or translocon construction or regulate the construction process. HrpP was one protein found to be a T3SS substrate and important for secretion and translocation of the model effector AvrPto. Importantly, HrpP is related to a well-studied protein from Yersinia enterocolitica, YscP, which is a T3SS-secreted protein and a regulator responsible for switching the T3SS from secreting needle subunits to secreting effector proteins (15, 38, 47). It has also been shown that secretion of YscP into the culture medium is not essential for the switch function and that there may be two type III secretion signals embedded in YscP (2).
The phenotype of a yscP mutant is unregulated secretion of the needle subunit, no secretion of effectors, and production of needles of indeterminate length. The switching phenotype requires a domain at the C terminus of YscP called the type III secretion substrate specificity switch (T3S4) domain, which is a conserved feature unifying its homologs (1). YscP has been proposed to act as a molecular ruler because the length of the YscP protein is directly correlated with the length of the Ysc needle (26). According to this model, when the needle has reached its proper length, YscP signals to the T3SS machinery to stop secreting needle subunits and begin secreting effector proteins. However, other functional models have been hypothesized for homologs of YscP. A recent study of the Salmonella enterica serovar Typhimurium YscP homolog InvJ showed that an invJ mutant lacked an inner rod. When the inner rod protein PrgJ was overexpressed, the length of the needle decreased relative to that of the wild type, leading the researchers to conclude that InvJ controls the inner rod, which in turn controls needle length (33). Recent evidence in Yersinia has lent more support to this model. YscP was found to negatively control secretion of YscI, the inner rod protein (51). Also, certain YscI mutations affected needle assembly but not effector secretion, implying that YscI may be a key player in substrate switching. Little is known about HrpB, the inner rod homolog in P. syringae (22), other than that the protein can be translocated into plant cells and is essential for T3SS function (39).
Other models for length control/substrate switching have been proposed, such as the “C-ring cup model” in flagella, which was based on the observation that certain mutations in proteins that make up the inner membrane C ring of the basal body lead to shorter hooks (the flagellar equivalent of the needle), thus suggesting that C-ring capacity controls hook length (32). A more recent, flagellar “molecular-clock” model suggests that because overexpression of hook subunits leads to longer hooks and hook polymerization-defective mutants make shorter hooks, hook polymerization initiates a countdown, and the timing, in cooperation with the YscP homolog FliK, determines final hook length (34).
HrpP is considered a member of the YscP/FliK family due mostly to the presence of a T3S4 domain at its C terminus. HrpP is also proline rich (10.6%), which is considered a characteristic of the family. The most striking feature of HrpP is its small size; the protein is 189 amino acids, compared with YscP from Y. enterocolitica, which is 453 amino acids and 8.4% proline. We were intrigued by how HrpP functions in P. syringae to regulate a pilus that can measure several hundred nanometers in length. Also, unlike animal pathogen needles and flagellar hooks, the pilus of P. syringae is predicted to be indeterminate in length, based on the fact that plant cell walls vary in width between species (40).
We hypothesized that HrpP would be a main player in regulating pilus construction in P. syringae by allowing the system to make the transition between secretion of pilus subunits and secretion of translocon or effector proteins, though perhaps by a novel mechanism. In this study, we more precisely define the role of HrpP in the P. syringae T3SS. We show that HrpP is a T3SS substrate in DC3000, is translocated into plant cells at levels equivalent to those of effectors, and is essential for the function of the T3SS. Though it is highly translocated and variable, we found that HrpP from different P. syringae pathovars could complement the DC3000 hrpP mutant. Analysis of truncations of HrpP and an impassible HrpP-green fluorescent protein (GFP) fusion suggests that it has structural similarities to YscP, but surprisingly, HrpP was found to be required for full secretion of the pilus subunit HrpA as well as for translocation of HrpB.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Pto strains were grown at 28°C in King's B (KB) medium or Hrp-inducing minimal medium (HMM) (24, 27), and Escherichia coli strains were grown at 37°C in Luria-Bertani medium or Terrific broth for plasmid isolation. Antibiotics were used at the following concentrations when required: kanamycin, 50 μg/ml; rifampin, 10 μg/ml; tetracycline, 20 μg/ml; and gentamicin (Gm), 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source |
|---|---|---|
| E. coli strains | ||
| DH5α | F−supE44 Δ(lacZYA-argF)U169 (φ80dlacZΔM15) hsdR17(rK− mK+) recA1 endA1 ΔgyrA96 thi-1 relA1 deoR; Nalr | Life Technologies |
| HB101 | F−thi-1 hsdS20(rB− mB−) supE44 recA13 ara-14 leuB6 proA2 lacY1 galK2 rpsL20 (Smr) xyl-5 mtl-1 | Promega |
| TOP10 | F−mcrA (mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Smr) endA1 nupG | Invitrogen |
| P. syringae pv. tomato strains | ||
| DC3000 | Wild type; Rifr | 14 |
| CUCPB5453 | DC3000 ΔhrpP | This study |
| CUCPB5112 | DC3000 ΔhrcC::Kmr | 50 |
| CUCPB5113 | DC3000 ΔhrcQBRSTU::ΩSpr/Smr | 5 |
| Plasmids | ||
| pRK2013 | Conjugation helper plasmid; Kmr | 17 |
| pCPP5301 | pCPP5215 lacZ; Tcr | 28 |
| pENTR/SD/D-TOPO | Gateway entry vector; Shine-Dalgarno sequence; Kmr | Invitrogen |
| pENTR/D/D-TOPO | Gateway entry vector; Kmr | Invitrogen |
| pCPP5602 | pENTR/D/D-TOPO derivative carrying 1-kb flanks of hrpP gene from DC3000 | This study |
| pCPP5603 | pENTR/D/D-TOPO derivative carrying 1-kb flanks of hrpP gene from DC3000; FRT-Gmr | This study |
| pCPP5209 | FRT-Gmrgus cassette plasmid; Apr | 28 |
| pCPP5604 | pCPP5301 derivative with hrpP deletion construct; FRT-Cmr | This study |
| pCPP5264 | pCPP5215 inducibly expressing Flp recombinase; Tcr | 28 |
| pCPP5451 | pENTR/SD/D-TOPO derivative carrying hrpP from Pto DC3000 | This study |
| pCPP5699 | pENTR/SD/D-TOPO derivative carrying hrpP from Pph 1448A | This study |
| pCPP5123 | pENTR/SD/D-TOPO derivative carrying hrpP from Psy 61 | 39 |
| pCPP5695 | pENTR/D/D-TOPO derivative carrying hrpP and hrp box promoter from Pto DC3000 | This study |
| pCPP5774 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 2 to 20 from Pto DC3000 | This study |
| pCPP5776 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 2 to 40 from Pto DC3000 | This study |
| pCPP5894 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 2 to 67 from Pto DC3000 | This study |
| pCPP5895 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 2 to 92 from Pto DC3000 | This study |
| pCPP5896 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 120 to 192 from Pto DC3000 | This study |
| pCPP5775 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 150 to 192 from Pto DC3000 | This study |
| pCPP5777 | pENTR/SD/D-TOPO derivative carrying hrpP deletion codons 170 to 192 from Pto DC3000 | This study |
| pCPP5897 | pENTR/SD/D-TOPO derivative carrying hrpP-gfp | This study |
| pCPP5686 | pENTR/SD/D-TOPO derivative carrying hrpB from Pto DC3000 | This study |
| pBS46 | Mobilizable, pBBR PnptII expression Gateway destination vector, makes C-terminal HA fusions; Gmr Cmr | Bryan Swingle |
| pBS60 | pBS46 derivative lacking Gateway cassette | Bryan Swingle |
| pCPP5295 | pBBR Gateway RfB CT-cya; Gmr Cmr | 28 |
| pCPP5296 | pBBR Gateway RfB CT-HA; Gmr Cmr | This study |
| pCPP5371 | pBBR Gateway PavrPto RfB CT-cya; Gmr Cmr | 37 |
| pCPP5372 | pBBR Gateway PavrPto RfB CT-HA; Gmr Cmr | 37 |
| pCPP5697 | pCPP5295 derivative containing hrpP and hrp box promoter from Pto DC3000 | This study |
| pCPP5653 | pCPP5296 derivative containing hrpP and hrp box promoter from Pto DC3000 | This study |
| pCPP5312 | pCPP5295 derivative containing avrPto and hrp box promoter from Pto DC3000 | 28 |
| pCPP5716 | pCPP5371 derivative containing hopA1 from Pto DC3000 | 37 |
| pCPP5898 | pCPP5295 derivative containing hrpW1 and hrp box promoter from Pto DC3000 | This study |
| pCPP5614 | pBS46 derivative carrying hrpP from Pto DC3000 | This study |
| pCPP5701 | pBS46 derivative carrying hrpP from Pph 1448A | This study |
| pCPP5700 | pBS46 derivative carrying hrpP from Psy 61 | This study |
| pCPP5780 | pBS46 derivative carrying hrpP deletion codons 2 to 20 from Pto DC3000 | This study |
| pCPP5899 | pBS46 derivative carrying hrpP deletion codons 2 to 40 from Pto DC3000 | This study |
| pCPP5900 | pBS46 derivative carrying hrpP deletion codons 2 to 67 from Pto DC3000 | This study |
| pCPP5901 | pBS46 derivative carrying hrpP deletion codons 2 to 92 from Pto DC3000 | This study |
| pCPP5902 | pBS46 derivative carrying hrpP deletion codons 120 to 192 from Pto DC3000 | This study |
| pCPP5903 | pBS46 derivative carrying hrpP deletion codons 150 to 192 from Pto DC3000 | This study |
| pCPP5781 | pBS46 derivative carrying hrpP deletion codons 170 to 192 from Pto DC3000 | This study |
| pCPP5778 | pCPP5371 derivative carrying hrpP deletion codons 2 to 20 from Pto DC3000 | This study |
| pCPP5904 | pCPP5371 derivative carrying hrpP deletion codons 2 to 40 from Pto DC3000 | This study |
| pCPP5905 | pCPP5371 derivative carrying hrpP deletion codons 2 to 67 from Pto DC3000 | This study |
| pCPP5906 | pCPP5371 derivative carrying hrpP deletion codons 2 to 92 from Pto DC3000 | This study |
| pCPP5907 | pCPP5371 derivative carrying hrpP deletion codons 120 to 192 from Pto DC3000 | This study |
| pCPP5908 | pCPP5371 derivative carrying hrpP deletion codons 150 to 192 from Pto DC3000 | This study |
| pCPP5779 | pCPP5371 derivative carrying hrpP deletion codons 170 to 192 from Pto DC3000 | This study |
| pCPP5909 | pCPP5371 derivative carrying hrpP-gfp | This study |
| pCPP5911 | pCPP5372 derivative carrying hrpP-gfp | This study |
| pCPP5688 | pCPP5371 derivative carrying hrpB from Pto DC3000 | This study |
DNA and protein manipulation techniques.
Plasmids were isolated from E. coli using the Qiagen spin miniprep kit (Qiagen). Genomic DNA was isolated using the Wizard genomic DNA purification kit (Promega). DNA enzyme reaction cleanups were conducted using the Clean-up & Concentrator kit (Zymo Research). hrpP genes, truncated hrpP genes, and hrpB from Pto DC3000, Psy 61, and P. syringae pv. phaseolicola (Pph) 1448A were cloned using PCR primers that allow directional TOPO cloning into the pENTR/SD/D-TOPO or pENTR/D/D-TOPO vectors (Invitrogen) (Table 2). The gfp gene was fused to hrpP by SOEing PCR as previously described (21). All pENTR clones were sequence confirmed. Through an LR recombination reaction using the Gateway system (Invitrogen), hrpP, hrpB, and hrpP derivatives were cloned into pBS46, pCPP5295, or pCPP5371. Each recombination reaction mixture was transformed into chemically competent E. coli TOP10. The resulting clones were conjugated into P. syringae with the aid of E. coli HB101 harboring the helper plasmid pRK2013. DNA sequencing was conducted at the Cornell University Biotechnology Resource Center using an Applied BioSystems 3730×l DNA analyzer. Sequences were analyzed using the Vector NTI software package (Invitrogen). To confirm production of cloned HrpP and its derivatives, Pseudomonas strains carrying expression plasmids were grown overnight in 5 ml KB broth or HMM, centrifuged, and resuspended in 500 μl water. Cells were boiled, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblot analysis as previously described (49) using antihemagglutinin (anti-HA) (Roche), anti-Cya (Santa Cruz Biotechnology), or anti-GFP (BD Biosciences) antibodies, followed by secondary antibody conjugated to alkaline phosphatase.
TABLE 2.
DNA oliogonucleotides used in this study
| Designation | Primer name | Sequence |
|---|---|---|
| P2218 | DCKpn1hrpO for | TAATGGTACCCGGTGCGGTCATCGTCAC |
| P2219 | DCTOPOhrpO rev | CACCCGATCACCGCGCTCTACA |
| P2220 | DchrcQa for | GGCGAGCGTCAAGCGCAATT |
| P2221 | DCKpn1hrcQ-a rev | TAATGGTACCATCATGCATGAAGACCTT |
| P2203 | DCTOPOhrpP for | CACCATGACCGCACCGATCAA |
| P2204 | DChrpP rev | TGCTGAAAGGTCTTCATG |
| P2365 | DChrpPhrpbox for | CACCATCGCCAGGCGCAGGTGGAA |
| P2380 | 1448ATOPOhrpP for | CACCATGACCGCACCGATCAAA |
| P2381 | 1448AhrpP rev | TGCTGCAAGATCTTCATGCAGG |
| P2417 | DCTOPOdel2-20 for | CACCATGGTCGAGCGGCCAGCCACA |
| P2419 | DChrpPdel170-189 rev | GTCGCGGCATGCGCCCGT |
| P2420 | DChrpPdel2-40 for | CACCATGGGTGGCCTGGGCCGTGGC |
| P2421 | DChrpPdel448-end rev | CAACTGCACGCTCCAGCGGTT |
| P2427 | DChrpPdelta120-end rev | GTCAGGCTGATCCGGCAGG |
| P2425 | DChrpPdel2-67 for | CACCATGAGTGCCGACGGCATGTTCTTTT |
| P2426 | DChrpPdel2-92 for | CACCATGATAGCCTTTCCGGTA |
| P2448 | GfphrpP for | GCATGAAGACCTTTCAGCAGTGAGCAAGGGCGAGGAG |
| P2449 | Gfp rev | CTTGTACAGCTCGTCCATGCCGA |
| P2446 | HrpPgfp rev | GCTCCTCGCCCTTGCTCACTGCTGAAAGGTCTTCATG |
| P2239 | TOPODChrpBfor | CACCGTGACCATTTCCCAACTC |
| P2363 | DChrpBrev | CTGCAGGTTGGTCAACTTGT |
Construction of hrpP mutant in DC3000.
A nonpolar, unmarked deletion in the hrpP gene was constructed using methods described previously (28). One kilobase of either flank of the hrpP gene from DC3000 was amplified using primers P2218/2219 and P2220/2221 (Table 2), ligated together through an introduced KpnI site and cloned into pENTR/D/D-TOPO to create pCPP5602. pCPP5602 was digested with KpnI, and a Gmr-FLP recombination target (FRT) cassette (amplified from pCPP5209) was introduced at this site, creating pCPP5603. pCPP5603 was LR recombined with pCPP5301, creating pCPP5604. pCPP5604 was conjugated into DC3000, and bacteria were subcultured in KB broth to allow for chromosomal-marker exchange. After recombination into the chromosome, the Gmr-FRT cassette was evicted by introduction of pCPP5264. The hrpP deletion mutation was confirmed by PCR and sequencing.
Cya reporter assay for translocation.
Cells from 2-day-old KB plates of P. syringae carrying HrpP-Cya or HrpB-Cya hybrids were resuspended in 5 mM morpholinoethanesulfonic acid (pH 5.5) to an optical density at 600 nm (OD600) of 0.3 and infiltrated with a blunt syringe into the leaves of 3-week-old Nicotiana benthamiana plants. Plants were kept at 24°C. Seven hours postinoculation, leaf discs (0.32 cm2) were collected, pulverized in liquid nitrogen, and resuspended in 300 μl of 0.1 M HCl. The samples were frozen at −20°C until assayed. Samples were assayed for cyclic AMP (cAMP) with the direct cAMP enzyme immunoassay kit according to the manufacturer's instructions (Assay Designs). Protein levels in each sample were determined through the Bradford method (7), and cAMP levels were reported as picomoles of cAMP per microgram of total protein.
Plant assays.
For hypersensitive-response (HR) assays, Pseudomonas strains from 2-day-old KB plates were resuspended in 10 mM MgCl2 to an OD600 of 0.5 and infiltrated into Nicotiana tabacum cv. Xanthi, using a blunt syringe. Plants were maintained at 24°C in the laboratory with supplemental lighting, and scored for visual collapse after 48 h. For tomato virulence assays, strains from 2-day-old KB plates were resuspended in 10 mM MgCl2. Solanum lycopersicum cv. Moneymaker plants were vacuum infiltrated with a suspension of 5 × 104 CFU/ml, 10 mM MgCl2, and 0.02% (vol/vol) Silwet. Bacterial concentrations of the inoculum were verified by plate count. Plants were maintained at 21°C, 60% humidity, and 16-h days. At 3, 4, and 6 days postinfiltration, symptoms were visually assessed, and leaf discs were taken from three representative leaves per plant and shaken in 10 mM MgCl2 and 100 mM sucrose for 3 h at 12°C. Bacterial suspensions were plated onto selective media, and colonies were counted to assess bacterial growth.
In vitro secretion assays.
Pseudomonas strains were grown overnight in 8 ml KB broth at 30°C, pelleted by centrifugation and resuspended in 1 ml HMM supplemented with 0.2% fructose. Bacteria were directly added to 100 ml HMM to an OD600 of 0.15 and grown with shaking at 22°C to an OD600 of 0.3. Cultures were centrifuged at 5,200 × g for 15 min, and the bacterial pellet was collected and resuspended in protein sample buffer for the “cell pellet” fraction. The top 40 ml of supernatant was spun at 20,800 × g for 40 min. The top 25 ml of supernatant was then added to 5 ml trichloroacetic acid, shaken, and incubated at 4°C overnight to precipitate protein. Protein was sedimented by centrifugation at 20,800 × g for 40 min, and the pellet was resuspended in protein sample buffer for the “supernatant” fraction. Cell pellet and supernatant fractions were separated by SDS-PAGE and subjected to immunoblot analysis as previously described (49) using anti-AvrPto, anti-HrpW1, anti-HrpA, anti-HrpZ1, anti-HopA1, anti-HA (Roche), or anti-Cya (Santa Cruz) antibodies, followed by secondary antibody conjugated to alkaline phosphatase. For two-dimensional electrophoresis (2DE), lawns of DC3000 and mutant derivatives were grown overnight at 30°C on KB plates with the appropriate antibiotics. Bacteria were harvested from the plates by resuspension in 5 ml HMM followed by brief vortexing. Bacteria were directly added to 100 ml HMM (supplemented with 0.2% fructose and 0.2% mannitol) to an OD600 of 0.3, and grown with shaking at 22°C to an OD600 of 0.5. Supernatant proteins from 500 ml HMM cultures were collected as described above but were resuspended in 7 M urea, 2 M thiourea, and 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} buffer. Protein was mixed with 1% ampholyte (Bio-Rad) and 50 mM dithiothreitol and allowed to passively rehydrate 17-cm, pH 3 to 10, nonlinear IPG strips (Bio-Rad). The strips were subjected to isoelectric focusing, followed by second-dimension separation of proteins by SDS-PAGE (12% acrylamide). Acrylamide gels were stained with Sypro ruby protein stain (Bio-Rad) and visualized using UV light. Gels were visually compared to Pto DC3000 2DE gel maps found at http://www.leelab.org/resources/syringae/index.html. 2DE gel maps were prepared from proteins that were collected from supernatants of HMM cultures in a manner similar to that described above.
RESULTS
HrpP is essential for DC3000 to cause disease in tomato and elicit an HR in tobacco.
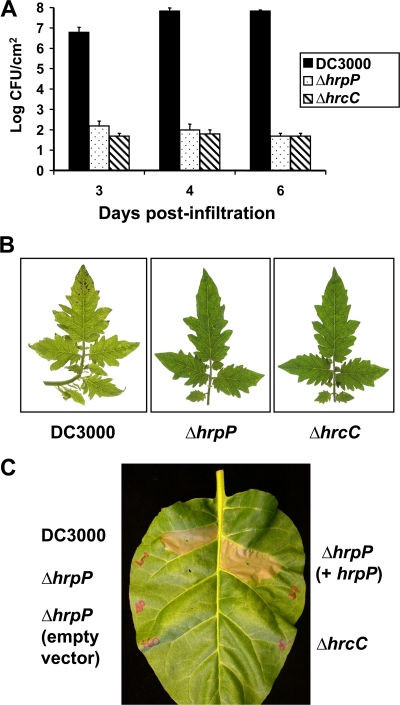
To assess the function of HrpP in Pto DC3000, we generated the mutant CUCPB5453 (DC3000 ΔhrpP), a DC3000 derivative with an unmarked, nonpolar deletion of the entire hrpP open reading frame. The genomic region was sequenced to verify its integrity, as hrpP is the first gene in an essential T3SS operon. To first determine if hrpP is required for virulence, DC3000 ΔhrpP was vacuum infiltrated into Solanum lycopersicum (tomato) cv. Moneymaker, a host for DC3000. DC3000 ΔhrpP grew equally as poorly as the type III mutant DC3000 ΔhrcC (CUCPB5112) (Fig. 1A) and produced no visible symptoms (Fig. 1B).
FIG. 1.
A DC3000 ΔhrpP mutant is avirulent in tomato and fails to elicit an HR in N. tabacum. (A) Wild-type DC3000, DC3000 ΔhrpP (CUCPB5453), and DC3000 ΔhrcC (CUCPB5112 T3SS−) were vacuum infiltrated into Solanum lycopersicum cv. Moneymaker plants at 5 × 104 CFU/ml. Levels of inoculum were confirmed by dilution plate counts. At 3, 4, and 6 days postinfiltration (dpi), bacteria were recovered from leaf discs by shaking into 10 mM MgCl2 and 100 mM sucrose, and cell numbers were assessed by plate counts and calculated as CFU/cm2. (B) One representative leaf from tomato plants infected with each strain at 6 dpi was photographed. (C) DC3000 ΔhrpP (CUCPB5453) was infiltrated into N. tabacum at 5 × 108 CFU/ml, along with wild-type DC3000, DC3000 ΔhrcC (CUCPB5112 T3SS−), and DC3000 ΔhrpP carrying either an empty vector (pBS60) or a vector expressing HrpP-HA driven by the nptII promoter (pCPP5614). Leaves were scored for visual collapse at 48 h postinfection. One representative leaf is shown.
To test if this mutant could be complemented by reintroduction of the hrpP gene, pCPP5614 (expressing HrpP-HA) was introduced into DC3000 ΔhrpP. The strains were then tested for their ability to elicit the HR in tobacco, which is dependent on effector translocation. DC3000 ΔhrpP(pCPP5614) was infiltrated into N. tabacum cv. Xanthi along with DC3000, DC3000 ΔhrcC, and DC3000 ΔhrpP. DC3000 ΔhrpP was unable to elicit an HR in tobacco (Fig. 1C), which is in agreement with previous findings in Psy 61 (39). HR was restored by introduction of pCPP5614 expressing HrpP-HA, indicating the mutant is defective only in hrpP. In both compatible and incompatible interactions with plants, DC3000 ΔhrpP performs like a T3SS-negative strain, indicating that hrpP is an essential component of the P. syringae T3SS.
HrpP from multiple P. syringae pathovars can complement DC3000 ΔhrpP for HR and virulence.
HrpP is the fifth most variable protein encoded by the hrp/hrc cluster when comparing the three representative P. syringae pathovars, Pto DC3000, Psy B728a, and Pph 1448A. The more variable components are the pilus subunit HrpA, the harpin/translocator HrpZ1, HrpF, and HrcQB (18, 28, 39). These proteins have all been shown to be extracellular, except HrcQB, which has a hypervariable N terminus and is related to SpaO, a type III secreted protein of Salmonella enterica (16, 29). The amino acid identity between the HrpP proteins of DC3000 and B728a is 68.4%, that between the HrpP proteins of DC3000 and 1448A is 65.8%, and that between the HrpP proteins of B728a and 1448A is 72.1%. The finding that HrpP is relatively variable prompted us to explore whether HrpP has evolved in certain pathovars for specific host plants or whether it interacts with another more variable part of the type III machinery. It was previously found that the hrp/hrc cluster from Psy 61 can complement a DC3000 hrp/hrc cluster mutant for tomato virulence, but not Arabidopsis virulence (18), indicating that some T3SS machinery components may be better adapted for different host species.
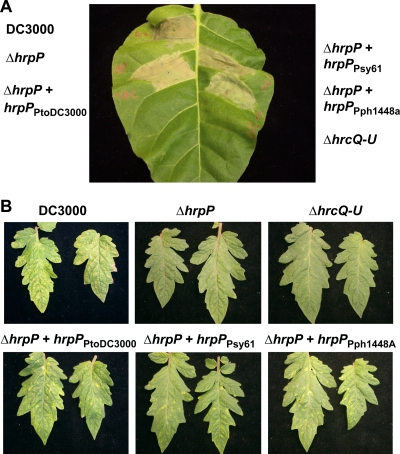
hrpP was cloned from Pph 1448A and Psy 61, which we have used in past studies, and is 94.2% identical to hrpP from Psy B728a. Plasmids expressing either HrpPPsy61 (pCPP5700) or HrpPPph1448A (pCPP5701) from an nptII promoter were introduced into DC3000 ΔhrpP and tested to see if they could complement the mutant as well as hrpP from DC3000(pCPP5614). Strains were infiltrated into N. tabacum as described above and scored for HR at 48 h. DC3000 ΔhrpP expressing HrpPPsy61 and HrpPPph1448A consistently elicited as strong an HR as did the mutant expressing HrpPPtoDC3000 (Fig. 2A). To test how these strains performed in a compatible interaction, Moneymaker tomato plants were vacuum infiltrated as described above. Both hrpP alleles complemented as well as hrpPPtoDC3000 for disease symptoms (Fig. 2B). These results indicate that HrpP from different P. syringae pathovars can function in a DC3000 background, and therefore can properly interact with the DC3000 T3SS and any potential host factors.
FIG. 2.
HrpP from different P. syringae pathovars can complement DC3000 ΔhrpP for HR in tobacco and disease in tomato. (A) DC3000 ΔhrpP (CUCPB5453) carrying a plasmid expressing from the nptII promoter HrpP-HA from either pathovar tomato (PtoDC3000), pathovar syringae (Psy61), or pathovar phaseolicola (Pph1448A) (pCPP5614, pCPP5700, and pCPP5701, respectively) was infiltrated into N. tabacum at 5 × 108 CFU/ml along with wild-type DC3000, DC3000 ΔhrpP, and DC3000 ΔhrcQRSTU (CUCPB5113). Leaves were scored for visual collapse at 48 h postinfiltration (hpi). One representative leaf is shown. (B) The same strains were vacuum infiltrated into Solanum lycopersicum cv. Moneymaker plants at 5 × 104 CFU/ml. Plants were visually scored for bacterial speck disease at 6 dpi. Two representative leaves for each strain are shown.
HrpP is translocated into plant cells at levels comparable to those of type III effectors when expressed from its hrp promoter but is not secreted in culture.
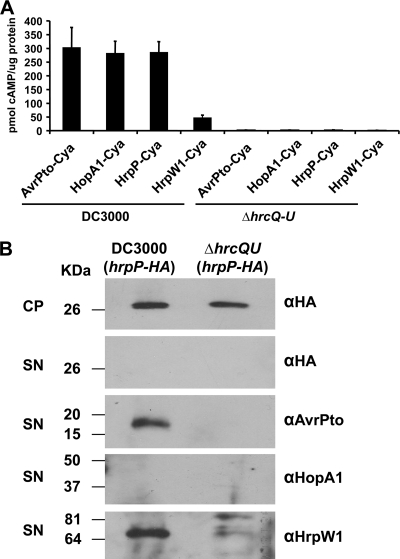
Previously, we found that HrpP from P. syringae pv. syringae 61 was translocated into N. benthamiana cells in a type III-dependent manner when expressed from a vector tac promoter (39). We wanted to verify that HrpP from Pto DC3000 is also a type III substrate, but more importantly, that it is translocated into plant cells under more natural levels of production. In past studies, we have successfully used the Cya reporter system to ascertain if a protein is a type III substrate or effector by fusing the protein of interest to Cya, a protein from Bordetella pertussis that converts ATP to cAMP in the presence of calmodulin, which is present only in eukaryotic cells (42, 46). hrpP from DC3000 was cloned, with the genomic region immediately upstream containing its hrp box promoter, in plasmid pCPP5697. pCPP5697 was transformed into both wild-type DC3000 and the DC3000 ΔhrcC mutant CUCPB5112, used as a T3SS-negative control. Production of HrpP-Cya in HMM was verified by Western blotting (data not shown). To compare HrpP to other known type III substrates, DC3000 expressing the harpin/translocator HrpW1-Cya and the effectors AvrPto-Cya and HopA1-Cya was included in the experiment. HrpW1 is hypothesized to be a component of the T3SS translocon complex and has low levels of translocation relative to those of true effectors such as AvrPto and HopA1 (11, 28). All proteins were expressed from their native hrp promoters. Strains were infiltrated into N. benthamiana leaves, sampled at 7 h postinfiltration, and assayed for cAMP. The high cAMP levels caused by DC3000(pCPP5697) indicate that HrpP-Cya is translocated into plant cells at levels similar to those of the true effectors AvrPto and HopA1 when expressed from its hrp promoter (Fig. 3A), indicating that HrpP may enter the plant cytoplasm during infection.
FIG. 3.
HrpP-Cya is translocated into N. benthamiana cells by DC3000 at levels comparable to those of type III effectors when expressed from its native hrp promoter, but it is not secreted in culture. (A) pCPP5297 (HrpP-Cya), pCPP5716 (HopA1-Cya), pCPP5312 (AvrPto-Cya), and pCPP5898 (HrpW1-Cya) were introduced into wild-type DC3000 or the T3SS− derivative DC3000 ΔhrcQRSTU (CUCPB5113). Strains were infiltrated into N. benthamiana at 3 × 108 CFU/ml. Leaf discs were excised at 7 h postinfiltration and assayed for cAMP (using the Assay Designs direct cyclic AMP kit). Total protein was measured by the Bradford method. Values are the means and standard deviation from triplicate samples. The experiment was repeated three times with similar results. (B) DC3000 and DC3000 ΔhrcQRSTU (CUCPB5113) carrying pCPP5614 (expressing HrpP-HA from the nptII promoter) were grown in HMM from an OD600 of 0.15 to 0.3. Cell pellet and supernatant fractions were separated by centrifugation. Proteins were separated by SDS-PAGE, transferred to the polyvinylidene difluoride (PVDF) membrane, and subjected to immunoblot analysis using the indicated antibodies (indicated by α) and secondary antibodies conjugated to alkaline phosphatase. CP, cell pellet; SN, supernatant.
Due to its high level of translocation, we wanted to assess the level of HrpP secretion in culture. HrpP C-terminally tagged with HA peptide was expressed from pCPP5614 from a constitutive nptII promoter in DC3000 and DC3000 ΔhrcQRSTU (CUCPB5113), a T3SS-negative strain. The nptII promoter was used to express HrpP in this assay to maximize the chance of detecting in vitro secretion. Strains were grown in HMM to induce type III secretion and were separated into cell pellet and supernatant fractions. Proteins were separated by SDS-PAGE and subjected to Western analysis. Regardless of the high levels of HrpP-HA accumulation in the cell pellet, no HrpP-HA was ever detected in supernatant fractions (Fig. 3B). HopA1 was also not detected in these same supernatant fractions. With a few exceptions, including AvrPto, most P. syringae type III effectors are not detectably secreted into HMM. On the other hand, HrpW1 was highly secreted into HMM though it is poorly translocated into plant cells, which is a noted characteristic of translocators in P. syringae (Fig. 3A and B) (28). The lack of HrpP secretion in culture but high translocation in planta, similar to a true effector, may indicate a biological role for HrpP entering the plant cell during infection.
HrpP has an atypical T3SS translocation signal and requires the putative T3S4 domain for function.
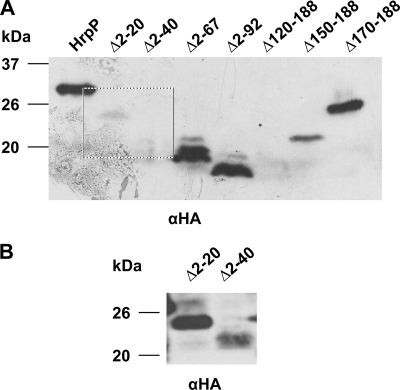
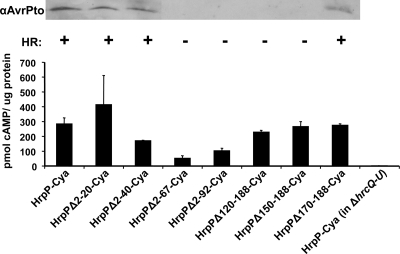
To further investigate the function of HrpP translocation, N-terminally truncated versions of HrpP were constructed and tested for their ability to be translocated as well as to complement DC3000 ΔhrpP for HR elicitation. The N terminus of T3SS substrates typically contains the signal necessary for secretion through the pathway, and N-terminal truncations eliminate type III secretability (36, 45). Amino acids 2 to 20, 2 to 40, 2 to 67, and 2 to 92 were deleted from HrpP, and the derivatives (HrpPΔ2-20, HrpPΔ2-40, HrpPΔ2-67, and HrpPΔ2-92, respectively) were tested for their ability to be translocated when fused to Cya and expressed from an hrp promoter. All truncated genes produced proteins of the proper size (Fig. 4A and B). In all cases, there was significant cAMP accumulation, indicating some translocation of the truncated protein (Fig. 5). Even when devoid of its N terminus, HrpP retains the ability to travel the T3SS pathway, indicating that it has an atypical translocation signal and possibly a unique way of interacting with the T3SS machinery.
FIG. 4.
Production of truncated versions of HrpP in DC3000 ΔhrpP. (A) DC3000 ΔhrpP carrying plasmids expressing (from the nptII promoter) full-length and truncated versions of HrpP-HA was grown overnight in KB broth to an OD600 of 1.0. Cells were collected by centrifugation and lysed, and cellular proteins were separated by SDS-PAGE, transferred to the PVDF membrane, and subjected to immunoblot analysis using an anti-HA antibody (αHA) and secondary antibody conjugated to alkaline phosphatase. (B) Boxed portion of the Western blot from panel A (lanes 2 and 3) overexposed to show the production of HrpPΔ2-20-HA and HrpPΔ2-40-HA.
FIG. 5.
N- and C-terminal truncations of HrpP reveal an atypical T3SS translocation signal and a C terminus that is important for function in promoting DC3000 secretion of AvrPto-Cya and elicitation of the HR in tobacco leaves. For the immunoblot data at the top of the figure, DC3000 ΔhrpP (CUCPB5453) carrying plasmids expressing full-length and truncated versions of HrpP (as described in the legend to Fig. 4) was grown in HMM from an OD600 of 0.3 to 0.5. Cell pellets and supernatant fractions were separated by centrifugation. Supernatant proteins were separated by SDS-PAGE, transferred to the PVDF membrane, and subjected to immunoblot analysis using an anti-AvrPto antibody and secondary antibody conjugated to alkaline phosphatase. For the HR tests in the middle of the figure, the same DC3000 ΔhrpP derivatives were infiltrated into N. tabacum at 5 × 108 CFU/ml. Leaves were scored for visual collapse at 48 hpi. +, HR; −, no HR. For the Hrp-Cya translocation tests at the bottom of the figure, full-length and truncated versions of hrpP were fused to Cya, in pCPP5371, which provides expression from the AvrPto promoter, and introduced into DC3000 or DC3000 ΔhrcQRSTU as indicated. Strains were infiltrated into N. benthamiana at 3 × 108 CFU/ml. Leaf discs were taken at 7 hpi and assayed for cAMP (Assay Designs direct cyclic AMP kit). Total protein was measured by the Bradford method. Values are the means and standard deviation from triplicate samples. The experiment was repeated three times with similar results.
To assess the functionality of these proteins, HrpP derivatives were expressed from an nptII promoter and tested for their ability to restore to the DC3000 ΔhrpP mutant HR elicitation in tobacco leaves and AvrPto secretion in culture. We chose these assays because HR elicitation is a general test for effector translocation and AvrPto secretion is a specific test for pathway switching to a representative, native effector. HrpPΔ2-20-HA and HrpPΔ2-40-HA can complement DC3000 ΔhrpP for HR elicitation and AvrPto secretion, indicating that the very N terminus of HrpP may not be essential for function (Fig. 5).
To assess the importance of the C terminus of HrpP, C-terminal truncations were also constructed and tested for complementation of DC3000 ΔhrpP and their own translocation. The C terminus of HrpP is the location of the T3S4 domain, a signature of the YscP/FliK family of proteins to which HrpP belongs (1). This large region is believed to be the primary functional domain in these proteins and, in HrpP, is predicted to span amino acids 101 to 184 (1). We deleted amino acids 170 to 188, 150 to 188, and 120 to 188 from HrpP. All truncated genes produced proteins of the proper size with the exception of HrpPΔ120-188-HA, which was not detectable (Fig. 4A). When fused to Cya, all C-terminal truncations were translocated at levels equivalent to those of HrpP-Cya, but only HrpPΔ170-188-HA could complement DC3000 ΔhrpP for HR elicitation and AvrPto secretion (Fig. 5). This indicates that the deletion of only 20 to 40 amino acids from the C terminus hinders the function of HrpP in HR elicitation and potentially disrupts substrate switching and the T3S4 domain. As expected, the C terminus has no role in the translocation of HrpP.
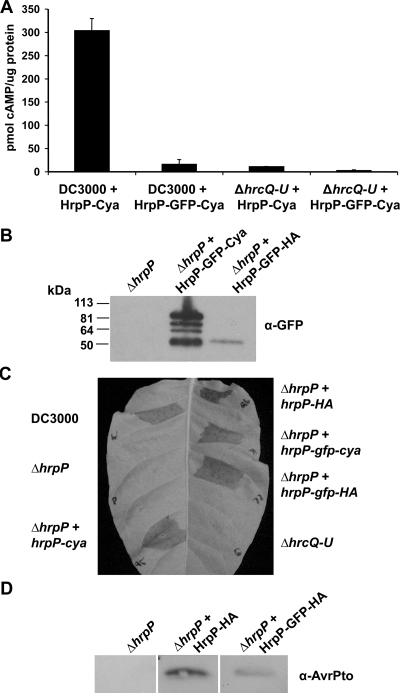
An HrpP-GFP hybrid protein cannot be translocated but can still restore HR elicitation and AvrPto secretion to the DC3000 hrpP mutant.
Because no N-terminal truncation of HrpP eliminated its translocation into plants, we were not able to assess the importance of HrpP translocation for its function. We therefore took the approach of fusing GFP, a protein that folds rapidly in the cytoplasm and cannot travel the T3SS (3), to the C terminus of HrpP. We tested the translocation of this hybrid protein following the construction of an HrpP-GFP-Cya translational fusion in pCPP5909. We found that DC3000 expressing HrpP-GFP-Cya gave very little cAMP signal compared to HrpP-Cya, indicating that this protein was blocked from translocation (Fig. 6A). Production of this protein was confirmed by immunoblotting (Fig. 6B). To test its functionality, we introduced HrpP-GFP-Cya or HrpP-GFP-HA (pCPP5911) into DC3000 ΔhrpP and assayed for HR elicitation in comparison to that of HrpP-Cya or HrpP-HA (pCPP5653), all expressed from hrp promoters. All HrpP derivatives complemented the hrpP mutant for HR elicitation equally (Fig. 6C). They also complemented equally well for speck disease in tomato (data not shown). Furthermore, HrpP-HA and HrpP-GFP-HA restored to the DC3000 ΔhrpP mutant the ability to secrete AvrPto in culture (Fig. 6D), and repeated experiments revealed no consistent difference in their ability to restore secretion. Thus, although HrpP-GFP is not translocated, it still retains enough functionality to allow P. syringae to elicit an HR and secrete AvrPto. We cannot however rule out the possibility, though it is not evident by immunoblot analysis (Fig. 6B), that GFP could be cleaved off in the bacterial cytoplasm, thus liberating HrpP to perform its function and be delivered into the plant cytoplasm.
FIG. 6.
An HrpP-GFP fusion cannot be translocated but can still restore to the DC3000 ΔhrpP mutant the ability to elicit HR in tobacco and to secrete AvrPto in culture. (A) DC3000 and DC3000 ΔhrcQRSTU (CUCPB5113) expressing HrpP-GFP-Cya (pCPP5909) or HrpP-Cya (pCPP5697) from the vector-provided hrpP promoter were infiltrated into N. benthamiana at 3 × 108 CFU/ml. Leaf discs were excised at 7 hpi and assayed for cAMP (Assay Designs direct cyclic AMP kit). Total protein was measured by the Bradford method. Values are the means and standard deviation from triplicate samples. The experiment was repeated three times with similar results. (B) DC3000 ΔhrpP expressing HrpP-GFP-Cya or HrpP-GFP-HA (pCPP5911) were grown overnight in HMM to an OD600 of 1.0. Cells were collected by centrifugation and lysed, and cellular proteins were separated by SDS-PAGE, transferred to the PVDF membrane, and subjected to immunoblot analysis using an anti-GFP antibody and secondary antibodies conjugated to alkaline phosphatase. (C) DC3000, DC3000 ΔhrcQRSTU (CUCPB5113), and DC3000 ΔhrpP (CUCPB5453) and DC3000 ΔhrpP expressing HrpP-GFP-Cya (pCPP5909), HrpP-Cya (pCPP5697), HrpP-GFP-HA (pCPP5911), or HrpP-HA (pCPP5653) were infiltrated into N. tabacum at 5 × 108 CFU/ml. Leaves were scored for visual collapse at 48 hpi. One representative leaf is shown. (D) Immunoblot analysis, performed as described in the legend for Fig. 5, indicates that AvrPto is secreted in culture by DC3000 ΔhrpP expressing HrpP-HA and HrpP-GFP-HA.
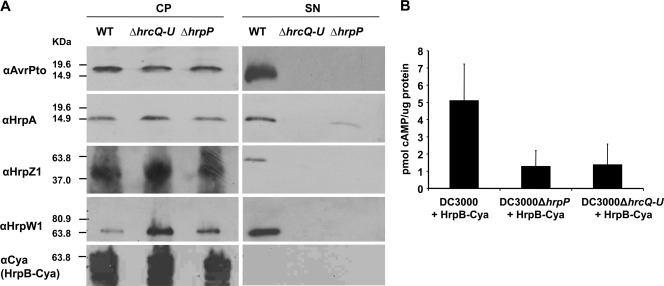
The hrpP mutant secretes only small amounts of the pilus subunit HrpA.
Though we found hrpP to be required for HR and disease in planta, and for AvrPto secretion in culture, it was important to ask if the DC3000 hrpP mutant could secrete any T3SS substrates. Due to HrpP's similarities to YscP from Yersinia, which is required for secretion of effectors but not the needle subunit YscF or the inner rod YscI, we hypothesized that DC3000 ΔhrpP would robustly or excessively secrete the pilus subunit HrpA and/or the inner rod protein HrpB. DC3000, DC3000 ΔhrpP, and DC3000 ΔhrcQRSTU (T3SS−) were cultured in HMM to induce type III secretion. Cell pellet and supernatant fractions were then collected as described above and separated by SDS-PAGE, and subjected to Western analysis using antibodies to HrpA, the harpin/translocators HrpW1 and HrpZ1, and the effector AvrPto. To assess HrpB secretion, an HrpB-Cya fusion was expressed from pCPPP5688, and cell fractions were probed with an anti-Cya antibody. All proteins were secreted by DC3000 in a type III-dependent manner but not by DC3000 ΔhrpP (Fig. 7A), with the exception of HrpB-Cya, which was not secreted by either strain. HrpA, however, was detectable at low levels in the supernatant of DC3000 ΔhrpP, indicating that hrpP may not be completely essential for the secretion of HrpA. Contrary to what was expected though, levels of HrpA secretion in DC3000 ΔhrpP were never equal to or greater than those in wild-type DC3000.
FIG. 7.
The hrpP mutant secretes small amounts of the pilus subunit HrpA and fails to secrete AvrPto, HrpZ1, HrpW1, or HrpB-Cya and fails to translocate HrpB-Cya. (A) Wild-type DC3000, DC3000 ΔhrcQRSTU (CUCPB5113), and DC3000 ΔhrpP (CUCPB5453) were grown in HMM from an OD600 of 0.15 to 0.3. Cell pellet and supernatant fractions were separated by centrifugation. Proteins were separated by SDS-PAGE, transferred to the PVDF membrane, and subjected to immunoblot analysis using the indicated antibodies and secondary antibodies conjugated to alkaline phosphatase. CP, cell pellet; SN, supernatant. Bacteria in the bottom-most panel contained pCPP5688, which expresses HrpB-Cya from a hrp promoter. All other panels involved proteins expressed from native genes in the DC3000 genome. (B) Cya reporter translocation assay, performed as described in the legend for Fig. 3A, shows that HrpB-Cya translocation into N. benthamiana cells is impaired in DC3000 ΔhrpP. Values are the means and standard deviations from triplicate samples. The experiment was repeated three times with similar results.
Though HrpB-Cya was not secreted by wild-type DC3000, it was found in a previous study to be weakly translocated into plant cells (39). It was necessary to ask, then, if HrpB-Cya could be translocated by DC3000 ΔhrpP. In support of previous findings, expression of HrpB-Cya from pCPP5688 led to type III-dependent cAMP accumulation with DC3000 infection, but not with DC3000 ΔhrpP, indicating that HrpB is also not translocated by the hrpP mutant (Fig. 7B). Considering DC3000 ΔhrpP poorly secretes the pilus subunit and fails to secrete translocator proteins, it is likely that the translocation process in planta is not efficient in this mutant.
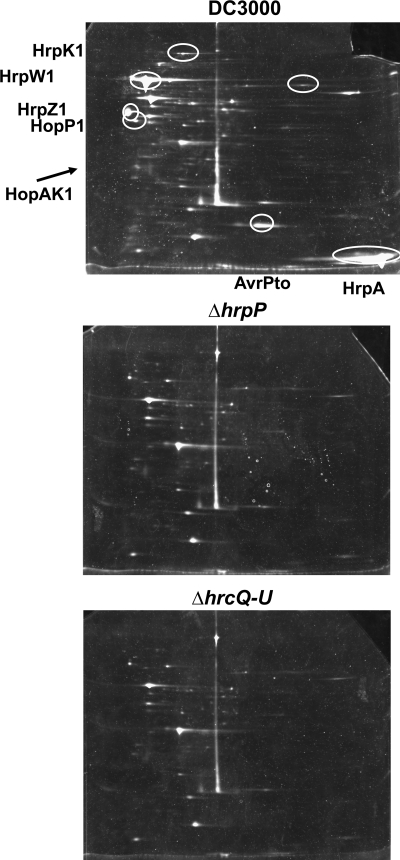
We next asked whether DC3000 ΔhrpP could secrete any other type III substrates. Using 2DE to separate all secreted proteins in this strain, we were able to ask without bias whether the hrpP mutant was oversecreting or unaffected in secretion of any substrate. DC3000, DC3000 ΔhrpP, and DC3000 ΔhrcQRSTU (T3SS−) were grown in HMM, and the secreted proteins were collected from culture supernatants as described above. Secreted proteins were separated first by isoelectric focusing, followed by SDS-PAGE, and then stained with the Sypro ruby protein stain (Bio-Rad). Though most effector proteins (with the exception of AvrPto) are poorly secreted in HMM and cannot be visualized this way, most hypothesized “early” substrates (pilin and translocators) are secreted in abundance in HMM (28). Visual inspection comparing the profile of proteins secreted by wild-type DC3000 to the profile of those secreted by DC3000 ΔhrcQRSTU revealed the presence of several T3SS-dependent substrates (Fig. 8). Most importantly, the spot pattern of DC3000 ΔhrpP was identical to that of DC3000 ΔhrcQRSTU. DC3000 ΔhrpP shared no T3SS-dependent secreted proteins with wild-type DC3000, and no proteins were present in the supernatant of DC3000 ΔhrpP only. Also, our 2DE gels appear to be in agreement with the Pto DC3000 HMM secretome determined and released by the Lee research group (http://www.leelab.org/resources/syringae/index.html). All T3SS-secreted proteins found in their work were apparent in our analysis by comparing molecular weights, isoelectric points, and relative locations. This approach confirms that hrpP is important for secretion of all type III secreted proteins that can be visualized in vitro.
FIG. 8.
The DC3000 ΔhrpP mutant secretes no other T3SS substrate. The wild-type DC3000, DC3000 ΔhrpP (CUCPB5453), and DC3000 ΔhrcQRSTU (CUCPB5113) strains were grown in HMM as described for Fig. 7. Supernatant proteins were separated first by isoelectric focusing on a pH-3 to -10 nonlinear gradient, followed by SDS-PAGE. Gels were stained with Sypro ruby protein stain (Bio-Rad) and visualized with UV light. One representative gel for each strain is shown. T3SS substrates secreted by DC3000 are indicated by the white circles.
DISCUSSION
Though we found that HrpP has many similarities to YscP, such as a predicted and important T3S4 domain, high-proline content, ability to travel the T3SS, and an atypical T3SS translocation signal, and that its secretion through the pathway is not entirely essential for its function, there is a clear difference in its role in P. syringae. The finding that an hrpP mutant secretes only small amounts of HrpA, the T3SS pilus subunit, is surprising. A yscP mutant constitutively secretes needle subunits, and therefore, it is believed that YscP functions as a switch between secretion of needle subunits and secretion of effectors. If the pilus in P. syringae and other plant pathogens has the same functional structure as the needle in animal pathogens, we would expect the hrpP mutant to secrete HrpA in an unregulated manner. The finding that the secretion of HrpA is somewhat dependent on HrpP is therefore puzzling but seems to suggest that HrpP does not regulate the pilus secretion step during T3SS construction. Additionally, our failure to find another type III substrate that is secreted by the hrpP mutant, assayed by both Western and more-comprehensive 2D analysis, makes it difficult to speculate about the function of this protein. If it does function as a switch, but in a different step of the construction process, we would have expected to have found a substrate whose secretion did not require HrpP. HrpB, the inner rod protein, whose homolog YscI is secreted by the yscP mutant and has recently been implicated in the switching process, was not secreted by the hrpP mutant, though it was also not secreted by wild-type DC3000. This observation also suggests a fundamental difference between P. syringae and previously studied systems.
It is possible that HrpP, regardless of its similarities to YscP, does not function as a switch at all and has evolved a different but obviously necessary function in P. syringae. Further work will be needed to generate functional hypotheses for HrpP. However, there remains the fact that HrpP has intriguing similarities to other T3S4-containing family members. Besides the presence of the T3S4 domain, which truncation mutants revealed was almost entirely necessary for function, HrpP, like YscP, may have multiple secretion signals and enter the T3SS pathway in a nonconventional way. Also, YscP is known to interact with the inner membrane component YscU, which contains a conserved autocleavage motif important for substrate switching (15, 44). The YscU homolog in P. syringae, HrcU, also has this conserved motif (data not shown).
Based on the Cya reporter assay and expression from its hrp promoter, HrpP is translocated into N. benthamiana at very high levels. HrpP is also not secreted in culture, like most effector proteins, and therefore might be classified as a “late” substrate, supporting the idea that there could be biological relevance to its translocation into plants. However it is paradoxical that HrpP could be translocated into plant cells at the same time as effectors and yet be essential for an early stage of T3SS machinery construction. We were able to introduce HrpP from different pathovars into DC3000 ΔhrpP and show that they can function in HR elicitation and disease development. This suggests that HrpP does not have a specific function in a given host species.
We also created a translocation-deficient version of HrpP by fusing it to GFP, and showed this can still function in HR elicitation. This indicates that translocation of HrpP may not be essential for complete function. Because elicitation of the HR is a simple way to determine whether type III translocation is occurring and is a very sensitive assay, it is possible that HrpP-GFP was not fully functional and that a subtle defect in type III secretion did exist that we did not detect. Though we cannot eliminate this possibility, we did observe that HrpP-GFP-HA was able to restore AvrPto secretion to the DC3000 ΔhrpP mutant. In this regard, it is noteworthy that a secretion-deficient mutant of YscP was still able to secrete effectors, though it was not able to control needle length, and therefore, was still a partially functioning protein (1). Also, given that past work predicted two secretion signals in YscP, the reason for and the importance of secretion of this protein are still enigmatic. The high level of HrpP translocation could also be an artifact of plasmid-borne expression, though this does not occur in our system with translocator proteins, which are believed to function at the plant plasma membrane and are translocated at only low levels when expressed from hrp promoters (28).
Recently, in the plant pathogen Xanthomonas campestris pv. vesicatoria, the HrpP homolog HpaC was found to be important for the secretion of effector and translocon proteins but not that of the pilus subunit HrpE or the protein HrpB2, which was in fact oversecreted by an hpaC mutant (31). Although HrpB2 is not in the Pfam family that contains YscI of Yersinia spp. and HrpB of P. syringae and other plant pathogens with an Hrp1 T3SS, for several reasons, HrpB2 is a good candidate for the inner rod of Xanthomonas and other plant pathogens with an Hrp2 T3SS: (i) HrpB2 is a secreted protein (41); (ii) HrpB2 is essential for T3SS function (41); (iii) the size of HrpB2 (13.6 kDa) is comparable to that of YscI (12.6 kDa) and HrpB (13.0 kDa); (iv) the location of hrpB2 appears syntenic with that of yscI in Yersinia (yscI, yscJ, yscK, yscL). That is, the P. syringae homologs of these ysc genes occur in the same arrangement (hrpB, hrcJ, hrpD, hrpE), and in the corresponding region in X. campestris pv. vesicatoria (hrpB2, hrcJ, hrpB4, hrpB5), hrcJ and hrpB5 are homologs of yscJ and yscL, respectively (9, 23).
Given these observations, apparent differences in the function of HrpP and HpaC are striking. In contrast to HrpP, HpaC is not secreted (10). Whereas an hpaC mutant strongly secretes HrpB2 (31), an hrpP mutant neither secretes nor translocates HrpB. Also, an hrpP mutant is strongly reduced in secretion of the pilus protein (31), whereas an hpaC mutant is unaffected. On the other hand, HrpP and HpaC share the characteristic of being much smaller than YscP, InvJ, FliK, and other T3S4 domain proteins associated with the production of fixed-length T3SS/flagellar appendages. The smaller size of the HrpP and HpaC proteins, contrasted with their association with having appendages of potentially much longer and indeterminate lengths, argues against these proteins functioning as molecular rulers. It remains unknown how pilus length and substrate switching from pilin to effector are controlled in plant pathogens, but obviously, HrpP functions differently from its animal pathogen homologs and differently from Xanthomonas HpaC. The Hrp1 T3SS and Hrp2 T3SS may have independently evolved the capacity to penetrate plant cell walls, and the surprising differences between the phenotypes of hrpP and hpaC mutants may reflect independent adaptations of the respective T3S4 domain proteins to the problem of regulating T3SS traffic when pathway construction is contingent upon variable host barriers.
Acknowledgments
We thank Bryan Swingle for the construction of pBS60 and pBS46 and Brian Kvitko for the construction of pCPP5898 and pCPP5296. We also thank Cynthia Damasceno and Jocelyn Rose for technical help with 2DE and David J. Schneider for helpful discussions regarding inner rod proteins.
This work was supported by NSF grant MCB-0544066.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Agrain, C., I. Callebaut, L. Journet, I. Sorg, C. Paroz, L. J. Mota, and G. R. Cornelis. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 5654-67. [DOI] [PubMed] [Google Scholar]
- 2.Agrain, C., I. Sorg, C. Paroz, and G. R. Cornelis. 2005. Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol. Microbiol. 571415-1427. [DOI] [PubMed] [Google Scholar]
- 3.Akeda, Y., and J. E. Galan. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437911-915. [DOI] [PubMed] [Google Scholar]
- 4.Alfano, J. R., A. O. Charkowski, W.-L. Deng, J. L. Badel, T. Petnicki-Ocwieja, K. van Dijk, and A. Collmer. 2000. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. Proc. Natl. Acad. Sci. USA 974856-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badel, J. L., R. Shimizu, H.-S. Oh, and A. Collmer. 2006. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant-Microbe Interact. 1999-111. [DOI] [PubMed] [Google Scholar]
- 6.Block, A., G. Li, Z. Q. Fu, and J. R. Alfano. 2008. Phytopathogen type III effector weaponry and their plant targets. Curr. Opin. Plant Biol. 11396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 8.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Vanaken, T. V. Feldblyum, M. D'Ascenzo, W.-L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 10010181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Büttner, D., and U. Bonas. 2002. Getting across-bacterial type III effector proteins on their way to the plant cell. EMBO J. 215313-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büttner, D., C. Lorenz, E. Weber, and U. Bonas. 2006. Targeting of two effector protein classes to the type III secretion system by a HpaC- and HpaB-dependent protein complex from Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 59513-527. [DOI] [PubMed] [Google Scholar]
- 11.Chang, J. H., J. M. Urbach, T. F. Law, L. W. Arnold, A. Hu, S. Gombar, S. R. Grant, F. M. Ausubel, and J. L. Dangl. 2005. A high-throughput, near-saturating screen for type III effector genes from Pseudomonas syringae. Proc. Natl. Acad. Sci. USA 1022549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collmer, A., J. L. Badel, A. O. Charkowski, W.-L. Deng, D. E. Fouts, A. R. Ramos, A. H. Rehm, D. M. Anderson, O. Schneewind, K. van Dijk, and J. R. Alfano. 2000. Pseudomonas syringae Hrp type III secretion system and effector proteins. Proc. Natl. Acad. Sci. USA 978770-8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4811-825. [DOI] [PubMed] [Google Scholar]
- 14.Cuppels, D. A. 1986. Generation and characterization of Tn5 insertion mutations in Pseudomonas syringae pv. tomato. Appl. Environ. Microbiol. 51323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edqvist, P. J., J. Olsson, M. Lavander, L. Sundberg, A. Forsberg, H. Wolf-Watz, and S. A. Lloyd. 2003. YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J. Bacteriol. 1852259-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadouloglou, V. E., A. P. Tampakaki, N. M. Glykos, M. N. Bastaki, J. M. Hadden, S. E. Phillips, N. J. Panopoulos, and M. Kokkinidis. 2004. Structure of HrcQB-C, a conserved component of the bacterial type III secretion systems. Proc. Natl. Acad. Sci. USA 10170-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figurski, D., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 761648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouts, D. E., J. L. Badel, A. R. Ramos, R. A. Rapp, and A. Collmer. 2003. A Pseudomonas syringae pv. tomato DC3000 Hrp (type III secretion) deletion mutant expressing the Hrp system of bean pathogen P. syringae pv. syringae 61 retains normal host specificity for tomato. Mol. Plant-Microbe Interact. 1643-52. [DOI] [PubMed] [Google Scholar]
- 19.Galán, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 20.Göhre, V., and S. Robatzek. 2008. Breaking the barriers: microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 46189-215. [DOI] [PubMed] [Google Scholar]
- 21.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 7761-68. [DOI] [PubMed] [Google Scholar]
- 22.Huang, H.-C., R.-W. Lin, C.-J. Chang, A. Collmer, and W.-L. Deng. 1995. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged colinearly with Yersinia ysc homologs. Mol. Plant-Microbe Interact. 8733-746. [DOI] [PubMed] [Google Scholar]
- 23.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huynh, T. V., D. Dahlbeck, and B. J. Staskawicz. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 2451374-1377. [DOI] [PubMed] [Google Scholar]
- 25.Jin, Q., and S. Y. He. 2001. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science 2942556-2558. [DOI] [PubMed] [Google Scholar]
- 26.Journet, L., C. Agrain, P. Broz, and G. R. Cornelis. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 3021757-1760. [DOI] [PubMed] [Google Scholar]
- 27.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44301-307. [PubMed] [Google Scholar]
- 28.Kvitko, B. H., A. R. Ramos, J. E. Morello, H.-S. Oh, and A. Collmer. 2007. Identification of harpins in Pseudomonas syringae pv. tomato DC3000, which are functionally similar to HrpK1 in promoting translocation of type III secretion system effectors. J. Bacteriol. 1898059-8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J., H. Ochman, E. A. Groisman, E. F. Boyd, F. Solomon, K. Nelson, and R. K. Selander. 1995. Relationship between evolutionary rate and cellular location among the Inv/Spa invasion proteins of Salmonella enterica. Proc. Natl. Acad. Sci. USA 927252-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindeberg, M., S. Cartinhour, C. R. Myers, L. M. Schechter, D. J. Schneider, and A. Collmer. 2006. Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains. Mol. Plant-Microbe Interact. 191151-1158. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz, C., S. Schulz, T. Wolsch, O. Rossier, U. Bonas, and D. Buttner. 2008. HpaC controls substrate specificity of the Xanthomonas type III secretion system. PLoS Pathog. 4e1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makishima, S., K. Komoriya, S. Yamaguchi, and S. I. Aizawa. 2001. Length of the flagellar hook and the capacity of the type III export apparatus. Science 2912411-2413. [DOI] [PubMed] [Google Scholar]
- 33.Marlovits, T. C., T. Kubori, M. Lara-Tejero, D. Thomas, V. M. Unger, and J. E. Galan. 2006. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441637-640. [DOI] [PubMed] [Google Scholar]
- 34.Moriya, N., T. Minamino, K. T. Hughes, R. M. Macnab, and K. Namba. 2006. The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J. Mol. Biol. 359466-477. [DOI] [PubMed] [Google Scholar]
- 35.Mota, L. J., L. Journet, I. Sorg, C. Agrain, and G. R. Cornelis. 2005. Bacterial injectisomes: needle length does matter. Science 3071278. [DOI] [PubMed] [Google Scholar]
- 36.Mudgett, M. B., O. Chesnokova, D. Dahlbeck, E. T. Clark, O. Rossier, U. Bonas, and B. J. Staskawicz. 2000. Molecular signals required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants. Proc. Natl. Acad. Sci. USA 9713324-13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh, H.-S., B. H. Kvitko, J. E. Morello, and A. Collmer. 2007. Pseudomonas syringae lytic transglycosylases coregulated with the type III secretion system contribute to the translocation of effector proteins into plant cells. J. Bacteriol. 1898277-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne, P. L., and S. C. Straley. 1999. YscP of Yersinia pestis is a secreted component of the Yop secretion system. J. Bacteriol. 1812852-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos, A. R., J. E. Morello, S. Ravindran, W.-L. Deng, H.-C. Huang, and A. Collmer. 2007. Identification of Pseudomonas syringae pv. syringae 61 type III secretion system Hrp proteins that can travel the type III pathway and contribute to the translocation of effector proteins into plant cells. J. Bacteriol. 1895773-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose, J. 2003. The plant cell wall. Blackwell Press, Ltd., Oxford, United Kingdom.
- 41.Rossier, O., G. Van den Ackerveken, and U. Bonas. 2000. HrpB2 and HrpF from Xanthomonas are type III-secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol. 38828-838. [DOI] [PubMed] [Google Scholar]
- 42.Schechter, L. M., K. A. Roberts, Y. Jamir, J. R. Alfano, and A. Collmer. 2004. Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schechter, L. M., M. Vencato, K. L. Jordan, S. E. Schneider, D. J. Schneider, and A. Collmer. 2006. Multiple approaches to a complete inventory of Pseudomonas syringae pv. tomato DC3000 type III secretion system effector proteins. Mol. Plant-Microbe Interact. 191180-1192. [DOI] [PubMed] [Google Scholar]
- 44.Sorg, I., S. Wagner, M. Amstutz, S. A. Muller, P. Broz, Y. Lussi, A. Engel, and G. R. Cornelis. 2007. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 263015-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sory, M.-P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 9211998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sory, M.-P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14583-594. [DOI] [PubMed] [Google Scholar]
- 47.Stainier, I., S. Bleves, C. Josenhans, L. Karmani, C. Kerbourch, I. Lambermont, S. Totemeyer, A. Boyd, and G. R. Cornelis. 2000. YscP, a Yersinia protein required for Yop secretion that is surface exposed, and released in low Ca2+. Mol. Microbiol. 371005-1018. [DOI] [PubMed] [Google Scholar]
- 48.Tampakaki, A. P., V. E. Fadouloglou, A. D. Gazi, N. J. Panopoulos, and M. Kokkinidis. 2004. Conserved features of type III secretion. Cell. Microbiol. 6805-816. [DOI] [PubMed] [Google Scholar]
- 49.van Dijk, K., D. E. Fouts, A. H. Rehm, A. R. Hill, A. Collmer, and J. R. Alfano. 1999. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J. Bacteriol. 1814790-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei, W., A. Plovanich-Jones, W.-L. Deng, A. Collmer, H.-C. Huang, and S. Y. He. 2000. The gene coding for the Hrp pilus structural protein is required for type III secretion of Hrp and Avr proteins in Pseudomonas syringae pv. tomato. Proc. Natl. Acad. Sci. USA 972247-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood, S. E., J. Jin, and S. A. Lloyd. 2008. YscP and YscU switch the substrate specificity of the Yersinia type III secretion system by regulating export of the inner rod protein YscI. J. Bacteriol. 1904252-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]