Abstract
For the ornithine fermentation pathway, described more than 70 years ago, genetic and biochemical information are still incomplete. We present here the experimental identification of the last four missing genes of this metabolic pathway. They encode l-ornithine racemase, (2R,4S)-2,4-diaminopentanoate dehydrogenase, and the two subunits of 2-amino-4-ketopentanoate thiolase. While described only for the Clostridiaceae to date, this pathway is shown to be more widespread.
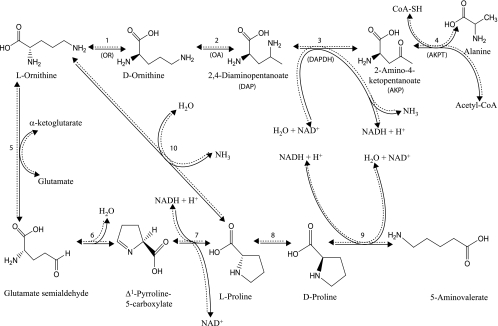
The catabolism of ornithine by anaerobic bacteria can be accomplished through the Stickland reaction, the main chemical reaction by which Clostridium sporogenes obtains its energy (16, 17). The Stickland reaction usually involves one amino acid which acts as an electron donor while another acts as an electron acceptor, as described for Clostridium sporogenes (16, 20), Clostridium botulinum (4, 5), and Clostridium sticklandii (6, 7). However, l-ornithine, as a single substrate, is converted into both an electron donor and acceptor and metabolized in a way similar to the Stickland reaction: it is oxidized to acetate, alanine, and ammonia (oxidative pathway) and reduced to 5-aminovalerate through the formation of proline (reductive pathway) (Fig. 1). This study focuses on the oxidative degradation pathway, starting with the conversion of l-ornithine to the d isomer by ornithine racemase (OR) (EC 5.1.1.12) (Fig. 1, step 1) (2). d-Ornithine is next converted to (2R,4S)-2,4-diaminopentanoate (DAP) through the action of d-ornithine aminomutase (OA) (EC 5.4.3.5) (Fig. 1, step 2), an adenosylcobalamine and pyridoxal phosphate (PLP)-dependent enzyme (3, 14). DAP then undergoes a NAD+- or NADP+-dependent oxidative deamination by DAP dehydrogenase (DAPDH) (EC 1.4.1.12) (Fig. 1, step 3), leading to 2-amino-4-ketopentanoate (AKP) (13, 18). This compound is metabolized by AKP thiolase (AKPT), a PLP-dependent enzyme, through a thiolytic cleavage with coenzyme A (CoA) to form acetyl-CoA and alanine (Fig. 1, step 4) (9).
FIG. 1.
The ornithine fermentation pathway. Enzymes involved are OR (encoded by or-5) (EC 5.1.1.12) in step 1, OA (encoded by oraS and oraE) (EC 5.4.3.5) in step 2, DAPDH (encoded by or-1) (EC 1.4.1.12) in step 3, AKPT (encoded by or-2 and or-3) in step 4, ornithine transaminase (EC 2.6.1.13) in step 5, spontaneous in step 6, pyrroline-5-carboxylate reductase (EC 1.5.1.2) in step 7, proline racemase (EC 5.1.1.4) in step 8, d-proline reductase (EC 1.21.4.1) in step 9, and ornithine cyclodeaminase (EC 4.3.1.12) in step 10.
Although the proteins of this oxidative pathway were characterized biochemically 30 years ago for C. sticklandii, only the genes corresponding to the two subunits of OA (oraS and oraE) have been identified to date (3). In this article, we present the analysis of genes which are colocalized with oraS and oraE and which are hypothesized to be involved in the conversion of l-ornithine to d-ornithine, the oxidative deamination of DAP, and the thiolytic cleavage of AKP. The proteins encoded by these genes were purified and their enzymatic activity characterized, which made it possible to reconstitute the whole oxidative branch of the l-ornithine fermentation pathway in vitro. The occurrence of this oxidative metabolic pathway in bacterial genomes which have been sequenced to date is discussed.
Searching for candidate genes.
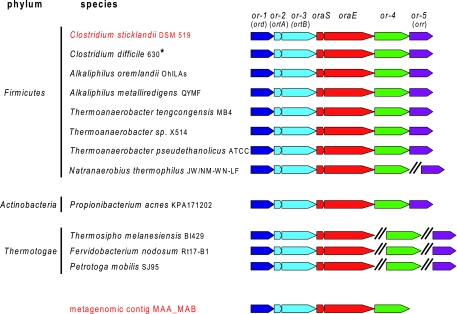
Since genes coding for enzymes involved in the same metabolic pathway are often colocalized on the bacterial chromosome, the genomic context of oraS and oraE (the only known genes of the pathway) was explored in the following: (i) the ∼1,100 sequenced bacterial genomes, using the Integrated Microbial Genomes interface (http://img.jgi.doe.gov); (ii) the assembled contigs from a metagenomics resource from the anaerobic digester of a wastewater treatment plant (12); and (iii) the genome of C. sticklandii, which was recently sequenced in our laboratory. Analyses of the latter two resources were performed using the microbial genome annotation system MaGe (19). The genomic contexts of bacterial genomes and a metagenomic sequence possessing the oraS and oraE genes are shown in Fig. 2. At least five genes, including oraS and oraE, are found in synteny (from or-1 to oraE). Moreover, the synteny conservation is extended to two additional genes (or-4 and or-5) in eight of the genomes. This conserved genomic neighborhood suggests that the genes flanking oraS and oraE could be involved in the oxidative degradation of l-ornithine. DAPDH (Fig. 1, step 3) from C. sticklandii has been described as a homodimer with a subunit molecular mass of about 36 kDa (13). Interestingly, one of the genes of the cluster, or-1 (Fig. 2), encodes a protein with a computed molecular mass of 38 kDa. This protein contains a predicted N-terminal NAD(P)+-binding Rossmann fold, consistent with dehydrogenase activity. These data therefore support or-1 as a promising candidate. Concerning AKPT (Fig. 1, step 4), the only available biochemical data concern its stimulation by PLP (9). The protein encoded by the gene or-3 (Fig. 2) presents (weak) similarities with PLP-dependent enzymes, such as threonine, tryptophan, and cysteine synthases. Moreover, the InterProScan software program classifies this protein as a “tryptophan synthase β-subunit-like PLP-dependent enzyme.” Therefore, the or-3 gene seemed to be an interesting candidate for encoding the AKPT activity. Nevertheless, the conserved presence of a small gene (or-2) just upstream of the AKPT gene candidate suggests by analogy with tryptophan synthase that AKPT may be composed of two different subunits encoded by genes or-2 and or-3. Finally, OR (Fig. 1, step 1) has been described by Chen et al. as a PLP-dependent enzyme with a subunit molecular mass of 46.8 kDa (2). The or-5 gene is present in the conserved gene cluster in eight organisms, including C. sticklandii (Fig. 2), and is predicted to encode a putative amino acid/alanine racemase. Its homology with known alanine racemases is weak but reaches 26% in the N-terminal region, which includes the PLP fixation site. However, the alanine racemase consensus PLP attachment site (prosite) is not present, and the C-terminal part of the or-5 protein shares no similarity with known racemases. This suggests that or-5 may encode a new type of racemase. In spite of the slight size difference between the protein encoded by or-5 and the one characterized by Chen et al. (39.4 versus 46.8 kDa), it was nevertheless considered the most promising candidate for OR activity.
FIG. 2.
Gene organization around oraS and oraE in complete sequenced genomes of 11 putative ornithine-fermenting bacteria (black) and in the sequence resources where the candidate genes have been cloned: the metagenomic contig MAA_MAB from an anaerobic digester of a wastewater treatment plant and the genome of ornithine-fermenting C. sticklandii (red). The symbol “//” indicates that the or-4 and or-5 genes are located elsewhere in the genome in these organisms. *, the draft genomes of eight additional C. difficile strains exhibit ornithine clusters which are identical to that in the completed 630 strain.
Cloning and purification of candidate proteins.
The coding sequences of OA (Fig. 1, step 2), DAPDH (Fig. 1, step 3), and AKPT (Fig. 1, step 4) were amplified by PCR on the EJ0ADIGA411YI15 fosmid DNA from our metagenomic collection of sequences, using the following primers (Sigma Genosys): α-OA-fwd, 5′-CAAAGGCAAAGTGATTTTGATAAG-3′; β-OA-rev, 5′-TCAATTTTCATTTGAGGTGGCC-3′; DAPDH-fwd, 5′-GGTAAAAACGTAAAGA-3′; DAPDH-rev, 5′-TTAAAAACCATTTGAGATAGATC-3′; β-AKPT-fwd, 5′-GCAAAAGACACATCCTACGAC-3′; β-AKPT-rev, 5′-TCACAATCTCACTCCCAAGTC- 3′; and α-AKPT-fwd, 5′-ATGAGTGAGAAATGCAAAAAAGATG-3′.
The coding sequence of OR (Fig. 1, step 1) was amplified by PCR on C. sticklandii DNA with the following primers: OR-fwd, 5′-TATCCTAAAATTACAATAGATATT-3′; OR-rev, 5′-TTAAAGAAGTTCTTTTTCAATATAT-3′.
The amplified sequences were inserted into the Invitrogen pEXP5-NT/TOPO vector according to the manufacturer's protocol, and the sequences of the resulting plasmids were verified. Cell cultures, cell extracts, and nickel affinity purifications were conducted as previously reported (10). OA and DAPDH were further purified by ion exchange using a MonoQ 5/50 GL column (Amersham Biosciences). OR, DAPDH, and AKPT were submitted to gel filtration on a Superdex 200 10/300 GL column (Amersham Biosciences). Chromatographic conditions have been described previously (10). The purified proteins were stored at −80°C. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the Invitrogen NuPAGE system.
Characterization of DAPDH.
DAPDH catalyzes the oxidative deamination of DAP, leading to the formation of AKP (Fig. 1, step 3). The apparent molecular mass of the product of the gene or-1, 38 kDa (not shown), is consistent with its calculated value: 38,926 Da. According to gel filtration experiments (not shown) and earlier studies carried out by Somack and Costilow (13), the putative DAPDH is a homodimer. The natural substrate of DAPDH, (2R,4S)-DAP, was obtained by enzymatic synthesis from d-ornithine. The reaction was carried out in 20 ml Tris-HCl (50 mM) (pH 9.0)-10 mM dithiothreitol in the presence of 3 μM adenosylcobalamine, 40 μM PLP, and 90 μg of purified OA. The reaction mixture was stirred at room temperature for 42 h in an N2 atmosphere, and the reaction was terminated by the addition of 200 μl of 13 M trifluoroacetic acid. After centrifugation and filtration, the solution was lyophilized and purified by high-performance liquid chromatography. After several lyophilizations and filtrations on Sephadex LH20, purified (2R,4S)-DAP was obtained and analyzed by nuclear magnetic resonance (D2O, 500 MHz) and mass spectrometry. The putative DAPDH was first assayed to estimate the stoichiometry of the products formed by its enzymatic reaction. NADH was monitored spectrophotometrically ( ɛ340 = 6,300 M−1·cm−1) (21). The enzyme was incubated in the presence of 800 μM (2R,4S)-DAP and 2 mM NAD+ in 50 mM Tris-HCl (pH 9.0)-0.2 M NaCl for 10 min. The A340 was recorded, the enzymatic reaction stopped with 1% (vol/vol) trifluoroacetic acid and neutralized with 5 M K2CO3, and NH3 assayed according to the method of Bailey et al. (1). Results show that for 91 ± 1 μM NADH formed, 88 ± 1 μM of NH3 is released (average of two replicates), proving that DAPDH catalyzes the oxidative deamination of DAP. In a second set of experiments, kinetic constants of DAPDH for NAD+ and NADP+ were evaluated, since the enzyme can function with both nucleotides as hydrogen acceptors, as described for C. sticklandii (13, 18). The assays were performed by varying the NAD+ or NADP+ concentration at a saturating concentration of (2R,4S)-DAP. The kinetic parameters were obtained from duplicate experiments by nonlinear analysis of initial rates using the software program SigmaPlot 9.0 (Systat Software, Inc.). Results show that the enzyme studied here is a NAD+-preferring enzyme, since it is about 1,120 times more efficient (as indicated by the ratios between kcat/Km NAD+ and kcat/Km NADP+) with NAD+ (Table 1).
TABLE 1.
Kinetic parameters of purified enzymes involved in anaerobic oxidative degradation of l-ornithine
| Enzyme | Substrate | Michaelian parameter
|
Non-Michaelian parametere
|
||||
|---|---|---|---|---|---|---|---|
| Km (μM) | kcat (s−1) | kcat/Km (s−1·M−1) | S50 (μM) | Vmax (s−1) | n | ||
| DAPDH | NAD+a | 16 ± 1 | 34 ± 0.7 | 2.1 106 | |||
| NADP+a | 2,560 ± 280 | 5 ± 0.5 | 2.0 103 | ||||
| (2R,4S)-DAPb | 109 ± 4 | 51.6 ± 0.6 | 1.24 ± 0.04 | ||||
| (2R,4R)-DAPb | 1,800 ± 200 | 2.8 ± 0.1 | 1.77 ± 0.25 | ||||
| OR | l-Ornithine | 520 ± 20 | 1,660 ± 30 | 3.2 106 | |||
| AKPT | CoA-SHc | 18 ± 3 | 3.5 ± 0.2 | 1.9 105 | |||
| AKPd | 35 ± 6 | 3.4 ± 0.2 | 9.7 104 | ||||
The (2R,4S)-DAP concentration was 800 μM.
The NAD+ concentration was 2 mM.
The AKP concentration was 350 μM.
The CoA-SH concentration was 80 μM.
S50 is the substrate concentration showing half-maximal velocity, n is the Hill coefficient, and Vmax is the maximal velocity.
Although it was previously established that the product of the ornithine mutase reaction is (2R,4S)-DAP (14), it was nevertheless decided to assess the ability of DAPDH to metabolize (2R,4R)-DAP. (2R,4R)-DAP was synthesized from the synthetic intermediate tert-butyl (3R)-5-methyl-2-oxopyrrolidin-3-ylcarbamate produced from d-serine by Alfa Chimica. This intermediate was purified by high-performance liquid chromatography to furnish the two separated diastereoisomers tert-butyl (3R,5R)-5-methyl-2-oxopyrrolidin-3-ylcarbamate and tert-butyl (3R,5S)-5-methyl-2-oxopyrrolidin-3-ylcarbamate. After acidic treatment (HCl [6 M, 4 h, 80°C]), the two desired enantiomers (2R,4S)-DAP and (2R,4R)-DAP were obtained as bishydrochloride salts and assigned by nuclear magnetic resonance comparison with the (2R,4S)-DAP obtained enzymatically. These experiments revealed that although the enzyme is more efficient with (2R,4S)-DAP, both diastereoisomers can be metabolized (Table 1). The higher catalytic activity observed in the presence of (2R,4S)-DAP may be due to a lower activation energy needed to yield its transition state compared to that for (2R,4R)-DAP. Although kinetics of DAPDH with a fixed concentration of (2R,4S)-DAP and increasing NAD+ and NADP+ concentrations were hyperbolic, kinetics with a fixed NAD+ concentration and various concentrations of (2R,4S)-DAP and (2R,4R)-DAP were sigmoidal. Values of v (initial rate of reaction) at various substrate concentrations were fit using nonlinear regression according to the general Hill equation, v = (Vmax Sn)/(S50n + Sn), where S50 is the substrate concentration showing half-maximal velocity, n is the Hill coefficient, and Vmax is the maximal velocity. The resulting kinetic parameters are presented in Table 1. Binding of a molecule of ligand to one subunit of the α2 dimer may cause a conformational change that increases the binding affinity of the ligand for the second subunit, leading to positive homotropic cooperativity. DAPDH undergoes allosteric activation by its own substrate, but it is negatively regulated by its product, AKP, and by both the end products of the oxidative metabolic pathway, acetyl-CoA and d-alanine. The initial rate of the reaction conducted with 2 mM NAD+ and 100 μM (2R,4S)-DAP was 70%, 30%, or 45% inhibited in the presence of 1 mM AKP, acetyl-CoA, or d-alanine, respectively.
Characterization of AKPT.
The first attempts to characterize AKPT activity were conducted with the product of or-3. Since no enzymatic activity could be detected, it was considered that AKPT might be composed of subunits encoded by distinct genes and acting as a complex. Because or-2 is always found next to the AKPT candidate gene (or-3), it was expected that these two genes could encode the α and β subunits of AKPT, respectively. The two genes were then cloned as a polycistronic sequence in pEXP5-NT/TOPO for recombinant production. Under these conditions, the hexahistidine tag was inserted only into the putative α subunit of the complex. SDS-PAGE analysis after immobilized metal affinity chromatography revealed the presence of two purified polypeptide chains with apparent molecular masses around 14 and 50 kDa (not shown), consistent with the calculated masses of 13,930 and 51,450 Da for the putative α and β subunits, respectively. The copurification of the polypeptide chain deprived of the hexahistidine sequence using an Ni2+ affinity column strengthens the hypothesis that AKPT is composed of distinct subunits. But according to SDS-PAGE analysis, the absence of a His-tagged sequence on the putative β subunit led to its substoichiometric proportion. To purify the α and β subunits in equimolar proportions, gel filtration experiments were therefore conducted on the mixture containing the two purified proteins. A total of 300 μg of putative AKPT in 50 mM Tris-HCl (pH 8.0)-150 mM NaCl was loaded onto the column. A peak with an apparent mass of 136 kDa, consistent with a functional heterotetrameric α2β2 structure, was isolated. SDS-PAGE analysis revealed that the protein fractions recovered contained both α and β subunits in apparent stoichiometric proportions and purified to apparent homogeneity (not shown). AKPT activity was thus assayed in the forward direction, following the production of acetyl-CoA (Fig. 1, step 4). The formation of the thioester bond of acetyl-CoA could be continuously monitored (ɛ232 = 4,500 M−1·cm−1) (15). The reaction was carried out in 50 mM Tris-HCl (pH 8.0) in the presence of 40 μM PLP, 80 μM CoA-SH, and 1 mM AKP, confirming the functionality of the purified thiolase. Experiments indicated that, in agreement with the data of Jeng et al. (9), AKPT activity is strictly PLP dependent (not shown). The kinetic constants of AKPT for AKP and CoA-SH were evaluated by varying the concentration of one substrate while keeping the other substrate at the saturating concentration. The kinetic parameters were obtained from duplicate experiments by nonlinear analysis of initial rates. Results are presented in Table 1. The identity of the compound detected at 232 nm was further analyzed using citrate synthase. Briefly, AKP was allowed to react with CoA-SH in the presence of AKPT to accumulate acetyl-CoA. Twenty-five micrograms of AKPT was incubated in 100 μl 50 mM Tris-HCl (pH 8.0) in the presence of 40 μM PLP, 80 μM CoA-SH, and 1 mM AKP for 1 h. The reaction was stopped by the addition of 1% (vol/vol) trifluoroacetic acid and neutralized with 5 M K2CO3, and the mixture was further incubated with 450 μM dithionitrobenzoate to deplete free CoA-SH. The depletion of the free CoA-SH pool was continuously monitored at 412 nm through the formation of the thionitrobenzoate ion until a plateau was reached. The addition of 0.4 U of citrate synthase and 1 mM oxaloacetate led to a new strong increase in absorption, confirming the presence of acetyl-CoA synthesized by AKPT. Alanine has been described to be the other reaction product, but its conformation has never been established, while it is expected to be the d isomer (9). Indeed, d-ornithine is the substrate for ornithine mutase (14), and there is no evidence for further steric rearrangements of the α-amino group during dehydrogenase and thiolase reactions. The identification of the isomer conformation was experimentally established, taking advantage of the reversibility of the AKPT reaction (Fig. 1, step 4). A 2.5-μg amount of AKPT was incubated in 100 μl 50 mM Tris-HCl (pH 8.1), containing 40 μM PLP and 450 μM dithionitrobenzoate, in the presence of 2 mM acetyl-CoA and 2 mM d-alanine. The reaction was initiated by the addition of d-alanine and monitored continuously at 412 nm. The formation of CoA-SH was observed only in the presence of the d isomer as the substrate and not in that of the l isomer (not shown). The substrate specificity of the enzyme was investigated in a similar way. Several amino acids and esters of CoA were tested for thiolase activity. The enzyme was incubated as follows: (i) in the presence of acetyl-CoA and of each proteogenic amino acid (with the exception of cysteine) in its d conformation; (ii) in the presence of d-alanine and of a CoA ester (propionyl-CoA, crotonyl-CoA, succinyl-CoA, butyryl-CoA, or malonyl-CoA). Under these conditions, the formation of CoA-SH was never observed, suggesting a strict substrate specificity. AKPT has no significant homology with any known enzyme family. Homologs of AKPT (>57% identity over the whole length of the protein) were found only in genomes containing all the genes of the pathway, which are expected to ferment this amino acid. In the other organisms, the maximum percentage identity falls to ∼26%, with other PLP-dependent enzymes, such as tryptophan synthase.
Characterization of OR.
OR catalyzes the conversion of l- to d-ornithine (Fig. 1, step 1). The His-tagged recombinant putative OR has a calculated molecular mass consistent with its apparent mass in SDS-PAGE (41,763.9 Da and ∼43 kDa, respectively). The protein was purified as a homodimer by gel filtration (not shown). The UV/visible spectrum of the purified protein revealed an absorption maximum at 420 nm, suggesting the presence of tightly bound PLP (not shown). These data can be linked to the work of Chen et al., who described the purification of dimeric and PLP-dependent OR with an apparent mass of 46.8 kDa on an SDS-PAGE gel for C. sticklandii (2). Here, the OR activity was first demonstrated by NADH formation in a coupled enzymatic assay with excess OA and DAPDH. The pyridine nucleotide reduction was triggered by the addition of l-ornithine, proving the functionality of the enzyme. Preliminary experiments with saturating concentrations of l-ornithine were conducted to set up an assay with adequate amounts of enzymes. Particular care was given to the handling of the racemase due to its instability upon dilution (∼40% activity lost within 2 h). Under these conditions, the rate of formation of NADH was strictly proportional to the amount of OR (with amounts of enzyme ranging from 0.24 to 1.43 ng). The coupled assay was conducted using 0.24 ng OR, 20 μg OA, 5.5 μg DAPDH, 2 mM NAD+, 3 μM adenosylcobalamine, and 40 μM PLP in 100 μl 50 mM Tris-HCl, pH 8.5. The kinetic parameters of OR were determined from triplicate experiments by nonlinear analysis of initial rates and are shown in Table 1. Data are close to those previously reported (2): Km = 770 μM; kcat = 980 s−1. To further characterize the enzyme, its substrate specificity was assessed. Five mM of l and d enantiomers of all proteogenic amino acids plus ornithine were incubated in 100 μl 10 mM Tris-HCl (pH 8.5) in the presence of 10 μM PLP and 8 μg OR for 3 h. l and d enantiomers of amino acids were separated on a Sumichiral OA-6100 column (150 by 4.6 mm, 5 μm; Sumika) using an isocratic gradient (1 mM CuSO4 plus 2% acetonitrile) with a flow rate of 1 ml/min. Compounds were detected by a photodiode array (Thermo-Fisher) between 200 and 600 nm. Results indicated that OR racemizes preferentially basic amino acids; for lysine, arginine, and ornithine, the reaction reached equilibrium with around 50% conversion for each enantiomer. Three other amino acids, serine, asparagine, and alanine, were also converted, but at a lower rate, with a maximum of only 10% serine conversion within the time course of the experiment (data not shown). The conversion of d- to l-alanine was further confirmed by a spectrophotometric assay coupling the formation of l-alanine to NAD+ reduction in the presence of l-alanine dehydrogenase. These results are thus in disagreement with the work of Chen et al. (2), where a strict specificity for ornithine was reported. Finally, these discrepancies (the slight difference in molecular mass but especially the marked difference in substrate specificity) suggest that the racemase presented here could be different from the one previously reported.
In vitro reconstitution of the l-ornithine degradation pathway.
A coupled enzymatic assay, combining purified OR, OA, DAPDH, and AKPT, was set up to generate acetyl-CoA from l-ornithine. The overall reaction was first monitored through the reduction kinetics of NAD+ and stopped with 1% (vol/vol) trifluoroacetic acid at equilibrium. The end products of the oxidative degradation pathway, acetyl-CoA and alanine, along with the intermediates DAP and AKP, were then characterized by liquid chromatograph (LC)-mass spectrometry analysis. Experiments were carried out using an LTQ/Orbitrap coupled to an Accela LC system (Thermo-Fisher). Intermediates of the oxidative ornithine degradation pathway were detected in the positive mode by full-scan mass analysis from m/z 50 to 1,000 at a resolving power of 15,000 at m/z = 400. Compounds were detected using a heated electrospray ionization source (150°C) at 4 kV with sheath, auxiliary, and sweep gases set at 40, 35, and 5 arbitrary units, respectively. The chromatographic columns were thermostated at 35°C. Ornithine, DAP, and AKP were separated with a Hypercarb column (150 by 2.1 mm, 5 μm; Thermo-Fisher) using an isocratic gradient (95% water containing 0.1% formic acid plus 5% 2-propanol) with a flow rate of 0.2 ml/min. Acetyl-CoA was detected with a Hypersil Gold C18 column (100 by 2.1 mm, 1.9 μm; Thermo-Fisher). A mobile-phase gradient was used with a flow rate of 0.4 ml/min in which mobile phase A consisted of 50 mM ammonium acetate adjusted to pH 9.0 and mobile phase B consisted of acetonitrile. The gradient started at 100% A for 5 min, followed by a linear gradient at 100% B for 5 min and finally 5 min at 100% B. Alanine was separated with a Luna NH2 column (100 by 2.1 mm, 3 μm; Phenomenex). A mobile-phase gradient was used with a flow rate of 0.2 ml/min in which the mobile phases were the same as those used for the separation of acetyl-CoA. The gradient started at 85% B for 2 min, followed by a linear decreasing gradient from 85 to 5% B in 10 min and finally 10 min at 5% B. Ornithine was detected at m/z 133.0970 (accuracy, 1.0 ppm) at a retention time of 1.92 min and could be separated from DAP, detected at m/z 133.0969 (accuracy, 1.7 ppm) at a retention time of 2.37 min. AKP was detected at m/z 132.0654 (accuracy, 0.7 ppm). Acetyl-CoA was detected at m/z 810.1316 (accuracy, 1.8 ppm) and alanine at m/z 90.0544 (accuracy, 5.5 ppm). In conclusion, LC-mass spectrometry analyses confirmed the functionality of such a reconstituted metabolic pathway.
In this study, we have identified the four last missing genes implicated in the anaerobic oxidative degradation of l-ornithine. We demonstrated that these genes, named orr, ord, ortA, and ortB (Fig. 2), code for OR, DAPDH, and AKPT (α and β subunits) activities, respectively. Of the seven genes colocalized in most of the putative ornithine-fermenting bacteria, six (ord, ortA, ortB, oraS, oraE, and orr) (Fig. 2) have been experimentally associated with a function. Concerning the remaining gene, or-4 (between oraE and orr), its product shares 37% amino acid identity with the product of mutL. The mutL gene is generally located between the mutS and mutE genes, which encode the two subunits of the adenosylcobalamine-dependent glutamate mutase. While the function of MutL is not known, it has been suggested that it might be a reactivating factor for glutamate mutase (11). In this case, or-4 may have a similar role for the adenosylcobalamine-dependent OA. As expected, the genes of the oxidative ornithine fermentation pathway, experimentally found in C. sticklandii, are also found in other members of the Clostridiaceae, including the nosocomial pathogen Clostridium difficile, in which Stickland reactions and amino acid metabolism are critical for growth and toxin production (8). Interestingly, we have found that two additional phylogenetically distant phyla, the Actinobacteria and the Thermotogae, contain these genes. These data indicate that these organisms might ferment ornithine and thus that this pathway is certainly not restricted to the Firmicutes.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this article have been submitted to the GenBank/EBI data bank with the accession numbers CU695246, CU695247, CU695248, and CU695250.
Acknowledgments
This work was supported by the Commissariat à l'Energie Atomique (CEA) and the Consortium National de Recherche en Génomique (CNRG).
We are grateful to Alexandra Calteau, Annett Kreimeyer, and Georges Cohen for their helpful discussions. We thank Eric Pelletier for bioinformatic support concerning the metagenomics resource and Susan Cure for reading the manuscript.
Footnotes
Published ahead of print on 27 February 2009.
REFERENCES
- 1.Bailey, J., E. T. Bell, and J. E. Bell. 1982. Regulation of bovine glutamate dehydrogenase. The effects of pH and ADP. J. Biol. Chem. 2575579-5583. [PubMed] [Google Scholar]
- 2.Chen, H. P., C. F. Lin, Y. J. Lee, S. S. Tsay, and S. H. Wu. 2000. Purification and properties of ornithine racemase from Clostridium sticklandii. J. Bacteriol. 1822052-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, H. P., S. H. Wu, Y. L. Lin, C. M. Chen, and S. S. Tsay. 2001. Cloning, sequencing, heterologous expression, purification, and characterization of adenosylcobalamin-dependent D-ornithine aminomutase from Clostridium sticklandii. J. Biol. Chem. 27644744-44750. [DOI] [PubMed] [Google Scholar]
- 4.Costilow, R. N. 1962. Fermentative activities of control and radiation-“killed” spores of Clostridium botulinum. J. Bacteriol. 841268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day, L. E., and R. N. Costilow. 1964. Physiology of the sporulation process in Clostridium botulinum. II. Maturation of forespores. J. Bacteriol. 88695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer, J. K., and R. N. Costilow. 1970. 2,4-Diaminovaleric acid: an intermediate in the anaerobic oxidation of ornithine by Clostridium sticklandii. J. Bacteriol. 10177-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyer, J. K., and R. N. Costilow. 1968. Fermentation of ornithine by Clostridium sticklandii. J. Bacteriol. 961617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson, S., M. Calos, A. Myers, and W. T. Self. 2006. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. J. Bacteriol. 1888487-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeng, I. M., R. Somack, and H. A. Barker. 1974. Ornithine degradation in Clostridium sticklandii; pyridoxal phosphate and coenzyme A dependent thiolytic cleavage of 2-amino-4-ketopentanoate to alanine and acetyl coenzyme A. Biochemistry 132898-2903. [DOI] [PubMed] [Google Scholar]
- 10.Kreimeyer, A., A. Perret, C. Lechaplais, D. Vallenet, C. Medigue, M. Salanoubat, and J. Weissenbach. 2007. Identification of the last unknown genes in the fermentation pathway of lysine. J. Biol. Chem. 2827191-7197. [DOI] [PubMed] [Google Scholar]
- 11.Mori, K., R. Bando, N. Hieda, and T. Toraya. 2004. Identification of a reactivating factor for adenosylcobalamin-dependent ethanolamine ammonia lyase. J. Bacteriol. 1866845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelletier, E., A. Kreimeyer, S. Bocs, Z. Rouy, G. Gyapay, R. Chouari, D. Riviere, A. Ganesan, P. Daegelen, A. Sghir, G. N. Cohen, C. Medigue, J. Weissenbach, and D. Le Paslier. 2008. “Candidatus Cloacamonas acidaminovorans”: genome sequence reconstruction provides a first glimpse of a new bacterial division. J. Bacteriol. 1902572-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somack, R., and R. N. Costilow. 1973. 2,4-Diaminopentanoic acid C 4 dehydrogenase. Purification and properties of the protein. J. Biol. Chem. 248385-388. [PubMed] [Google Scholar]
- 14.Somack, R., and R. N. Costilow. 1973. Purification and properties of a pyridoxal phosphate and coenzyme B 12 dependent D-ornithine 5,4-aminomutase. Biochemistry 122597-2604. [DOI] [PubMed] [Google Scholar]
- 15.Stadtman, E. R. 1957. Preparation and assay of acyl coenzyme A and other thiol esters; use of hydroxylamine. Methods Enzymol. 3931-941. [Google Scholar]
- 16.Stickland, L. H. 1934. Studies in the metabolism of the strict anaerobes (genus Clostridium): the chemical reactions by which Cl. sporogenes obtains its energy. Biochem. J. 281746-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stickland, L. H. 1935. Studies in the metabolism of the strict anaerobes (genus Clostridium): The oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. Biochem. J. 29889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda, Y., and H. C. Friedmann. 1970. Ornithine metabolism by Clostridium sticklandii. Oxidation of ornithine to 2-amino-4-ketopentanoic acid via 2,4-diaminopentanoic acid; participation of B12 coenzyme, pyridoxal phosphate, and pyridine nucleotide. J. Biol. Chem. 2455914-5926. [PubMed] [Google Scholar]
- 19.Vallenet, D., L. Labarre, Z. Rouy, V. Barbe, S. Bocs, S. Cruveiller, A. Lajus, G. Pascal, C. Scarpelli, and C. Medigue. 2006. MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res. 3453-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods, D. D. 1936. Studies in the metabolism of the strict anaerobes (genus Clostridium): further experiments on the coupled reactions between pairs of amino-acids induced by Cl. sporogenes. Biochem. J. 301934-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegenhorn, J., M. Senn, and T. Bucher. 1976. Molar absorptivities of beta-NADH and beta-NADPH. Clin. Chem. 22151-160. [PubMed] [Google Scholar]