Abstract
The Enterococcus faecalis class IIa bacteriocin MC4-1 encoded by the sex pheromone-responding, multiple-antibiotic resistance plasmid pAMS1 exhibits “siblicidal” (sibling-killing) activity under certain conditions. Stabs of plasmid-containing cells on solid medium containing lawns of bacteria of the same (plasmid-containing) strain give rise to zones of inhibition. If the plasmid-containing host also produces gelatinase, bacteriocin cannot be detected.
Class IIa bacteriocins are relatively small, heat-stable proteins able to lethally permeabilize target membranes (6). We recently reported that Enterococcus faecalis MC4 encodes a class IIa bacteriocin, MC4-1, on a 130-kb conjugative, pheromone (cCF10)-responding plasmid, pAMS1, that also confers resistance to chloramphenicol, streptomycin, and tetracycline (11). Interestingly, the expression of MC4-1 bacteriocin and immunity to the bacteriocin are not detectable in the MC4 host but can be detected after the transfer of pAMS1 into E. faecalis JH2-2. Indeed, the original MC4 host exhibits sensitivity to bacteriocin from JH2-2/pAMS1 strains, a phenomenon which suggested the term “retrocidal.” We also found that when the plasmid is transferred into the nonisogenic strain OG1SS or its isogenic relative OG1RF, bacteriocin expression and immunity are not detected (11). The bacteriocin and immunity genes (designated bacA and bacB, respectively; GenBank accession no. EU047916) were cloned and sequenced and found to represent an operon associated with a sigma 70-like promoter. It was concluded that the differences observed among the plasmid hosts involve a factor present in some hosts but not others.
In the present study, we examine further the nature of the differences between pAMS1-containing strains. Because the two types of plasmid hosts (MC4 and the OG1 derivatives) in which we were not able to detect bacteriocin expression and immunity are gelatinase producers (JH2-2 does not produce gelatinase), we now examine bacteriocin expression in a number of additional hosts that either produce or do not produce gelatinase. Gelatinase production in E. faecalis has been reported previously to be regulated by an autoinducer (quorum-sensing agent) and is upregulated and secreted at high levels as cells approach stationary growth phase (19, 22).
One of the strains we examined is a well-defined, gelatinase-negative derivative of OG1RF, strain TX5128, which contains an insertion within the gelatinase determinant (26). The other hosts were a collection of clinical E. faecalis isolates (23, 25). The results of plate assays (11) indicate that MC4-1 bacteriocin activity is detectable in gelatinase-negative strains but not gelatinase-producing strains. Interestingly, in the course of these studies, we also observed that pAMS1-containing cells, when used as indicators in lawns, do not express immunity to bacteriocin produced from colonies or stabs of the same strain. These and related transcription data are presented below.
The bacterial strains and plasmids used are listed in Table 1. Unless otherwise mentioned, culture conditions and methods were as described previously (11). The conjugative plasmid pAMS1 encoding bacteriocin MC4-1 was introduced into clinical strains by filter mating with donor SFJ1 (11). Prior to filter mating, those strains without a selectable antibiotic resistance marker (the clinical isolates) were transformed with pVA749 to be erythromycin resistant as described previously (10). RNA isolation and reverse transcriptase quantitative PCR (RT-QPCR) procedures were essentially as described previously (1) but modified by using TaqMan probes (obtained from Applied Biosystems through the Assays-by-Design service) instead of SYBR green.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description and/or relevant characteristic(s)a | Expressionb of:
|
Reference or sourcec | |
|---|---|---|---|---|
| Gelatinase | Bacteriocin MC4-1 | |||
| E. faecalis strains | ||||
| MC4 | Root canal isolate; contains pAMS1 | + | − | 9 |
| FA2-2 | Rfr Far | − | NA | 4 |
| SFF2 | FA2-2/pAMS1 | − | + | 11 |
| JH2-2 | Rfr Far | − | NA | 15 |
| SFJ1 | JH2-2/pAMS1 | − | + | 11 |
| SF411 | JH2-2/pAMS411 | − | + | 11 |
| SF412 | JH2-2/pAMS412 | − | + | 11 |
| JH2SS | Smr Spr | − | NA | 27 |
| SFJS1 | JH2SS/pAMS1 | − | + | 11 |
| OG1RF | Rfr Far | + | NA | 7 |
| SFOR2 | OG1RF/pAMS1 | + | − | 11 |
| OG1SS | Smr Spr | + | NA | 12 |
| SFOS2 | OG1SS/pAMS1 | + | − | 11 |
| TX5128 | Gel− derivative of OG1RF; Rfr Far | − | NA | 26 |
| SFTX2 | TX5128/pAMS1 from SFJS1 × TX5128 filter mating | − | + | This study |
| SFTX3 | TX5128/pAMS1 from SFOS2 × TX5128 filter mating | − | + | This study |
| GS18 | Root canal isolate | − | NA | 25 |
| GS18e | Emr | − | NA | This study |
| GS18e/pAMS1 | − | + | This study | |
| GS25 | Root canal isolate | − | NA | 25 |
| GS25e | Emr | − | NA | This study |
| GS25e/pAMS1 | − | + | This study | |
| E5 | Oral isolate | − | NA | 24 |
| E5e | Emr | − | NA | This study |
| E5e/pAMS1 | − | + | This study | |
| AA-OR4 | Oral isolate | − | NA | 23 |
| AA-OR4e | Emr | − | NA | This study |
| AA-OR4e/pAMS1 | − | + | This study | |
| GS3 | Root canal isolate | + | NA | 25 |
| GS3e | Emr | + | NA | This study |
| GS3e/pAMS1 | + | − | This study | |
| GS6 | Root canal isolate | + | NA | 25 |
| GS6e | Emr | + | NA | This study |
| GS6e/pAMS1 | + | − | This study | |
| GS16 | Root canal isolate | + | NA | 25 |
| GS16e | Emr | + | NA | This study |
| GS16e/pAMS1 | + | − | This study | |
| E6 | Oral isolate | + | NA | 24 |
| E6e | Emr | + | NA | This study |
| E6e/pAMS1 | + | − | This study | |
| ER5/1 | Root canal isolate | + | NA | 16 |
| ER5/1e | Emr | + | NA | This study |
| ER5/1e/pAMS1 | + | − | This study | |
| Enterococcus faecium 409 | NA | NA | UMH | |
| Listeria monocytogenes ATCC 19115 | NA | NA | ATCC | |
| Plasmids | ||||
| pAMS1 | Cmr Smr Tcr; encodes bacteriocin MC4-1 and response to pheromone cCF10 | 11 | ||
| pAMS411 | Emr; PCR-generated bacAB clone; orientation in vector pAMS749-12 opposite to pAMS412 | 11 | ||
| pAMS412 | Emr; PCR-generated bacAB clone; orientation in vector pAMS749-12 opposite to pAMS411 | 11 | ||
| pVA749 | Emr | 17 | ||
Gel, gelatinase; Cm, chloramphenicol; Em, erythromycin; Fa, fusidic acid; Rf, rifampin; Sm, streptomycin; Sp, spectinomycin; Tc, tetracycline.
When used as a producer with indicators E. faecium 409 and L. monocytogenes ATCC 19115, as described previously (11). +, positive for expression; −, negative for expression; NA, not applicable.
ATCC, American Type Culture Collection; UMH, University of Michigan Hospital.
Bacteriocin MC4-1 activity in E. faecalis strains harboring pAMS1 was observed for gelatinase-negative hosts but not for gelatinase-positive hosts.
The transfer of pAMS1 to the gelatinase-negative E. faecalis strain TX5128, but not to the isogenic gelatinase-positive strain OG1RF, generated the ability to express bacteriocin activity against the original host MC4, as well as against a number of other strains containing the plasmid (Table 2). It was similarly observed that the transfer of pAMS1 to additional gelatinase-negative strains, but not to gelatinase-positive strains, resulted in bacteriocin activity (Tables 1 and 2). A reasonable explanation for the absence of bacteriocin activity in gelatinase-positive strains is that gelatinase, a secreted metalloendopeptidase, degrades the secreted bacteriocin MC4-1. Indeed, the capacity of gelatinase to inhibit bacteriocin MC4-1 activity extracellularly could be demonstrated in an agar diffusion assay (Fig. 1A).
TABLE 2.
Spectrum of activity of MC4-1a bacteriocin from gelatinase-negative and gelatinase-positive strains
| Indicator strain | Bacteriocin activity from gelatinase-negative strain:
|
Bacteriocin activity from gelatinase-positive strain:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SFJ1 | SFJS1 | SFF2 | SFTX3b | E5e/pAMS1 | AA-OR4e/pAMS1 | GS18e/pAMS1 | GS25e/pAMS1 | MC4c | SFOR2b | SFOS2b | |
| Gelatinase-negative strains | |||||||||||
| SFJ1 | − | − | − | (+) | − | − | − | − | (+) | (+) | (+) |
| SFJS1 | + | − | (+) | (+) | − | − | − | − | (+) | (+) | (+) |
| SFF2 | − | − | − | − | − | − | − | − | (+) | (+) | (+) |
| SFTX3 | + | + | + | + | + | + | + | + | (+) | − | − |
| E5e/pAMS1 | + | + | + | + | + | + | + | + | (+) | − | − |
| AA-OR4e/pAMS1 | + | + | + | + | + | + | + | + | (+) | − | − |
| GS18e/pAMS1 | + | + | + | + | + | + | + | + | (+) | (+) | (+) |
| GS25e/pAMS1 | + | + | + | + | + | + | + | + | (+) | − | − |
| Gelatinase-positive strains | |||||||||||
| MC4 | + | + | + | + | + | + | + | + | − | − | (+) |
| SFOR2 | + | + | + | + | + | + | + | + | (+) | − | − |
| SFOS2 | + | + | + | + | + | + | + | + | (+) | − | − |
Activity was evaluated by agar diffusion assays as described previously (11). −, no zone of inhibition (lawn grows up to, and under, producer); +, zone of inhibition; (+), very small zone of inhibition.
Bacteriocin MC4-1 from SFOR2 and SFOS2 is not detected (and immunity to MC4-1 activity is not shown). However, an unrelated activity that is characteristic of the OG1 family is expressed by SFTX3, SFOR2, and SFOS2 (11).
Bacteriocin MC4-1 from pAMS1 in MC4 is not detected (and immunity to MC4-1 activity is not shown); however, at least one additional bacteriocin activity is expressed (11).
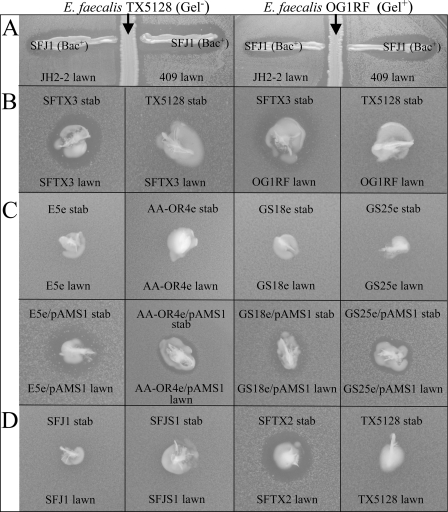
FIG. 1.
Expression of bacteriocin MC4-1 and sibling killing. (A) Inhibition of bacteriocin MC4-1 activity by gelatinase. Central inocula of isogenic strains TX5128 (gelatinase negative [Gel−]) (left) and OG1RF (gelatinase positive [Gel+]) (right) on Todd-Hewitt broth (Becton, Dickinson and Co., Sparks, MD) agar were incubated for 24 h before the inoculation of the agar with susceptible indicator lawns (dilute broth cultures swabbed onto the agar surface) and the bacteriocin producer E. faecalis SFJ1. Indicator lawns are E. faecalis JH2-2 and E. faecium 409. Clear strips alongside TX5128 and OG1RF are areas not inoculated. Zones of inhibition created by SFJ1 extend up to TX5128 growth. However, zones of inhibition produced by SFJ1 were absent within the approximate area of gelatinase diffusion extending from OG1RF (as measured under similar conditions on gelatin-containing agar) (data not shown). (B) Gelatinase-negative SFTX3 (TX5128/pAMS1) as a producer kills siblings used as indicators on solid medium. SFTX3 is not killed by plasmid-free TX5128. SFTX3, but not the plasmid-free TX5128, exhibits bacteriocin activity against the gelatinase-positive OG1RF. (C) Nonisogenic clinical isolates without and with pAMS1 (upper and lower rows, respectively). Only when carrying pAMS1 do producers kill siblings used as indicators on solid medium. (D) Hosts from the JH2 family (SFJ1 [JH2-2/pAMS1] and SFJS1 [JH2SS/pAMS1]) exhibit no sibling killing. However, following the transfer of the plasmid from SFJS1 to the plasmid-free TX5128, sibling killing by the transconjugant SFTX2 occurs. Sibling killing by TX5128 before the acquisition of the plasmid is not observed.
Sibling killing of pAMS1-containing strains.
Gelatinase-negative strains carrying the plasmid (Table 1) exhibited unexpected sibling killing, or “siblicide” (Fig. 1B and C; Table 2). Strains carrying pAMS1, when sparsely inoculated as indicator lawns, did not express immunity to bacteriocin from a stab (densely inoculated) culture of the same strain (i.e., a zone of inhibition with characteristic resistant microcolonies [11] was observed). An interpretation of this phenomenon is that there is a difference in expression depending on the conditions of growth. During the early growth of the lawn, cells multiply relatively freely, approximating exponential growth. On the other hand, bacteriocin producers tested as a dense inoculum (stab) on plates rapidly experience limiting growth conditions, well before indicator lawns reach an equivalent state. Sibling killing may therefore be ascribed to the absence of immunity during the growth of the lawns, a view supported by data presented below. The notion that the early development of lawns approximates exponential growth is also consistent with the view that gelatinase is not expressed during this time and is thus not interfering with sensitivity to bacteriocin-producing stabs (Fig. 1B).
Transcripts of bacAB appear at significantly lower levels in cells in early exponential growth than in cells in late exponential growth, regardless of the gelatinase phenotype.
We hypothesized that the sibling-killing phenomenon reflects variation in the expression of bacAB dependent on the growth phase, with producer cultures (stabs) on agar representing cells under limiting growth conditions and indicators grown as lawns experiencing exponential growth. Although growth on solid surfaces clearly involves a number of different parameters compared to growth in broth, transcriptional analyses are more easily conducted under the latter condition. Thus, we compared bacAB transcript levels from early and late exponential states as well as stationary states of cells representing both gelatinase-negative (SFJ1 and SFTX3) and gelatinase-positive (MC4, SFOS2, and SFOR2) strains by RT-QPCR. Transcript levels in both gelatinase-negative and gelatinase-positive strains were significantly increased (range of increase, 4.6-fold [for SFTX3] to 7.5-fold [for SFJ1]) at late exponential stage compared to those at early exponential stage (Fig. 2A). When culture supernatant from cells (SFJ1) in the late exponential state was added to cells growing in early exponential phase, analyses did not reveal a significant increase in bacAB transcripts after 10 and 30 min (Fig. 2B). This finding is consistent with the absence of involvement of an autoinducer (quorum-sensing) agent in the increased transcription of bacAB during late exponential growth.
FIG. 2.
RT-QPCR analyses. TaqMan primer sequences were designed to target the middle third of the bacB open reading frame, the second (downstream) gene of the bacAB operon (BACB-F, GCAAGTCTATAAAAAAATAGATAGCGAAAAATATCCA, and BACB-R, CTCCCAATTCAATTAATAACTTTTCATCATTTCCT), and the 16S rRNA gene (EF16S-F, GAAACAGGTGCTAATACCGCATAAC, and EF16S-R, CAGCGACACCCGAAAGC). Probe sequences were as follows: BACB FAM, CAATGTAGATATAGTTCACCAATTTA, and EF16S FAM, CATGCGGCATAAACT. Forty-five cycles were performed using an ABI Prism 7500 sequence detection system, bacAB expression was normalized to 16S rRNA gene expression, and changes (n-fold) in transcript levels relative to those at early exponential phase (A) and baseline (B) were determined using the threshold cycle (ΔΔCT) method with software from Applied Biosystems. Experiments were performed in quadruplicate on two separate occasions. (A) Comparisons of different stages of growth. Results from experiments with hosts that are gelatinase negative (SFJ1 and SFTX3) and gelatinase positive (MC4, SFOS2, and SFOR2) are shown. The data are expressed as changes (n-fold; means ± standard errors of the means) in bacAB transcript levels at late exponential and stationary stages of growth relative to those at early exponential stage (set at 1). Strains were grown in 50 ml of Todd-Hewitt broth with gentle shaking, and aliquots were obtained at early exponential phase (optical density at 650 nm, 0.1), late exponential phase (optical density at 650 nm, 0.8), and 2 h into stationary phase and at 24 h. Transcript levels at late exponential phase were significantly higher than those at other stages in SFJ1, SFTX3, SFOS2, and SFOR2 (P < 0.001) and MC4 (P < 0.01) (values from repeated measures were subjected to two-way analyses of variance with Bonferroni posttests). (B) Results of a test for autoinduction. Changes (n-fold) in bacAB transcript levels following 10- or 30-min exposure of early-exponential-phase SFJ1 cultures to 20% (vol/vol) filter-sterilized culture supernatant (CS) from late-exponential-phase cultures of SFJ1 (JH2-2/pAMS1) or JH2-2 relative to the baseline level (set at 1).
Siblicide among JH2 family hosts is undetected.
Our initial report (11) on pAMS1 showed that when the plasmid is present in certain E. faecalis strains (e.g., MC4 and OG1RF), not only is bacteriocin MC4-1 production undetectable, but these strains also appear to be sensitive to bacteriocin produced by pAMS1-containing derivatives of JH2-2. The present data explain the basis of these observations and show a consistent correlation between the expression of gelatinase by the host and the inability to detect bacteriocin activity. The presence of gelatinase was found to prevent bacteriocin activity (Fig. 1A), an observation consistent with the fact that gelatinase is known to hydrolyze a variety of peptide bonds (18). (Note that gelatinase-positive E. faecalis hosts do not block the detection of bacteriocins in general, as many are readily observed to be bacteriocinogenic [see, e.g., reference 25].) This finding raised the question of why the original gelatinase-positive strains exhibited sensitivity to bacteriocin producers, since generally the presence of the plasmid would be expected to confer immunity. We then found that plasmid-containing gelatinase-negative strains (e.g., SFTX3 [TX5128/pAMS1] and four nonisogenic clinical isolates carrying pAMS1) exhibited the ability to kill siblings used as indicator lawns on solid medium, a phenomenon we call siblicide (Fig. 1B and C). This result was surprising, since our earlier study showed that the gelatinase-negative bacteriocin producer SFJ1 (JH2-2/pAMS1) does not produce zones of inhibition when the same strain is used as an indicator. For reasons that are not yet clear, the host-plasmid combination with at least some members of the JH2 family (JH2-2, JH2SS, and FA2-2) results in apparent resistance to bacteriocin (Fig. 1D) (although certain members of the JH2 family exhibit sensitivity to other members, e.g., in the case of SFJ1 versus SFJS1 [Table 2]). Consistent with this view is the observation that when the plasmid is transferred from SFJ1 (JH2-2/pAMS1) into gelatinase-negative hosts (Fig. 1B and C), transconjugant strains exhibit sibling killing. Additionally, we have observed that the previously described bacAB clones SF411 (JH2-2/pAMS411) and SF412 (JH2-2/pAMS412) (11) can indeed exhibit sibling killing (data not shown), suggesting that a pAMS1 determinant not present in the clone or a gene dosage effect may be involved in conferring resistance to siblicide in the JH2-related background. An alternative explanation for the difference seen in JH2 family hosts is that the growth of the cells as lawns results in earlier upregulation of bacteriocin and immunity, creating insensitivity to bacteriocin.
Concluding remarks.
We have shown here that pAMS1-containing, gelatinase-negative strains, other than those involving the JH2 family, exhibit bacteriocin sensitivity or sibling killing. The explanation would appear to relate to a difference in the expression of the bacteriocin-immunity operon, dependent on growth conditions. Our data are consistent with a regulatory effect at the level of transcription, as the number of transcripts detected in cells during early exponential growth in broth was significantly lower than that in cells approaching a stationary state. We believe that this finding relates to the view that in the early stages of growth as a lawn on solid medium, the cells more closely approximate a steady state (i.e., exponential growth) than the stabs producing bacteriocin. Under such conditions, bacteriocin, as well as immunity, is not expressed or is greatly reduced. Of course, there are likely to be numerous factors operating that differentially affect growth in or on liquid or solid medium, as well as differences between early growth as a lawn and that as a stab or colony. While transcriptional differences (between early and late exponential phases) in broth ranged from 4.6- to 7.5-fold (Fig. 2A), it is conceivable that additional regulatory events occurring at a posttranscriptional level may significantly magnify such differences with respect to the ultimate amounts of bacteriocin and immunity proteins produced. We note that the system is not currently sensitive enough to directly measure bacteriocin activity against E. faecalis in culture supernatants during various stages of growth.
A number of bacteriocin systems have been reported to exhibit regulation, with different levels of expression occurring under different growth conditions (e.g., different media, temperatures, and redox potentials) and/or in different growth phases (20, 21). Some class IIa systems (e.g., in certain strains of Lactobacillus plantarum, Lactobacillus sakei, and Enterococcus faecium) involve quorum-sensing mechanisms with peptide autoinducers being encoded close to, or as part of, an operon with the bacteriocin-immunity determinants and/or related export and sensing genes (5, 8, 21). The precursors of the autoinducers have been reported to have leader sequences with a double-glycine processing signal that resembles that of leaders of their cognate bacteriocins and that relates to a dedicated processing-export system. The corresponding regulated operons usually contain direct repeats located within the −40 to −80 regions of their promoters, a trait frequently associated with regulated genes (21). In the case of the bacteriocin MC4-1 system, the inspection of approximately 1.1 kb upstream and 2.5 kb downstream of the bacAB operon revealed no evidence of linked determinants that encode an export system, a sensing system, or an autoinducer peptide, although direct repeats are present within the −40 to −80 region of the operon (11). It is also noteworthy that the MC4-1 precursor does not have a double-glycine signal at the processing site and appears to make use of a general secretion system of the host (11). These points suggest that a specific autoinduction (quorum-sensing) mechanism is not involved in the regulation of MC4-1 expression and that the regulation observed is reflective of other activities that are sensitive to growth conditions or environmental factors. In fact, we are not aware of any reports thus far on specific quorum-sensing systems in E. faecalis involving class IIa bacteriocins, and we were unable to detect such a phenomenon related to MC4-1 (Fig. 2B).
To our knowledge, the observation of bacteriocin-related sibling killing on solid medium has not been reported previously. In the case of certain bacteriocin producers (e.g., L. plantarum C11 [5]), there are reports that the Bac+ phenotype can be lost completely upon extensive dilution during growth in certain media. The production of bacteriocin is not regained, even under stationary conditions, unless the cells are exposed during exponential growth to small amounts of spent culture medium (i.e., containing autoinducer) from Bac+ cultures (5). While exhibiting the Bac− phenotype, the cells also lack immunity, as observed when they are exposed to purified bacteriocin in liquid suspensions. This observation is not comparable to one of stabs or colonies on solid medium giving rise to a zone of killing on lawns of siblings. It does seem surprising, however, that siblicide of the nature we present here has not been reported previously, insofar as those bacteriocin systems known to be regulated by autoinduction (quorum sensing) might have been anticipated to exhibit such a phenomenon. Perhaps there are additional factors that prevent such behavior in those cases, or the particular systems may simply not have been tested in this manner.
Finally, in light of the present results, the definition of the term retrocidal plasmid can be broadened from our earlier definition, in which host-specific factors in the plasmid-bearing cell are involved in the expression of bacteriocin/immunity, to one in which the host per se is not necessarily related. Thus, any plasmid able to give rise to sibling killing would be a retrocidal plasmid. Of significant interest is the apparent relationship of this phenomenon to aspects of bacterial development in natural environments where cells may be growing on surfaces. One can envision situations in which certain cells, after attaching to a surface and giving rise to a colony, turn on or upregulate the production of bacteriocin activity able to kill siblings that have not yet been as successful in establishing firm occupancy, thereby resulting in less competition at subsequent stages of development. Conceivably, analogous behavior could be involved in other aspects of differentiation, such as those that occur during biofilm formation. Recent analyses of Streptococcus pneumoniae have identified “fratricidal” behavior, whereby the development of competence in certain members of a bacterial population is associated with a subpopulation of cells that undergo a lysis step involved in releasing DNA (available for transformation), as well as certain potential virulence proteins unable to be otherwise secreted (3, 14). Whereas the involvement of bacteriocin activity has been inferred, the system appears to be significantly different from that described in the present study. However, these observations, as well as those involved in a “cannibalization” phenomenon associated with sporulation in Bacillus subtilis (2, 13), illustrate that sibling killing, or bacterial fratricide, may represent behaviors commonly associated with development in the bacterial world.
Acknowledgments
This work was supported by a grant from the University of Michigan Office of the Vice President for Research.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Appelbe, O. K., and C. M. Sedgley. 2007. Effects of prolonged exposure to alkaline pH on Enterococcus faecalis survival and specific gene transcripts. Oral Microbiol. Immunol. 22169-174. [DOI] [PubMed] [Google Scholar]
- 2.Claverys, J. P., and L. S. Havarstein. 2007. Cannibalism and fratricide: mechanisms and raisons d'etre. Nat. Rev. Microbiol. 5219-229. [DOI] [PubMed] [Google Scholar]
- 3.Claverys, J. P., B. Martin, and L. S. Havarstein. 2007. Competence-induced fratricide in streptococci. Mol. Microbiol. 641423-1433. [DOI] [PubMed] [Google Scholar]
- 4.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 1521220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diep, D. B., L. S. Havarstein, and I. F. Nes. 1995. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol. Microbiol. 18631-639. [DOI] [PubMed] [Google Scholar]
- 6.Drider, D., G. Fimland, Y. Hechard, L. M. McMullen, and H. Prevost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 753479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eijsink, V. G., M. B. Brurberg, P. H. Middelhoven, and I. F. Nes. 1996. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J. Bacteriol. 1782232-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabricius, L., G. Dahlen, S. E. Holm, and A. J. Moller. 1982. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand. J. Dent. Res. 90200-206. [DOI] [PubMed] [Google Scholar]
- 10.Flannagan, S. E., and D. B. Clewell. 1991. Conjugative transfer of Tn916 in Enterococcus faecalis: trans activation of homologous transposons. J. Bacteriol. 1737136-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flannagan, S. E., D. B. Clewell, and C. M. Sedgley. 2008. A “retrocidal” plasmid in Enterococcus faecalis: passage and protection. Plasmid 59217-230. [DOI] [PubMed] [Google Scholar]
- 12.Franke, A. E., and D. B. Clewell. 1981. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J. Bacteriol. 145494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Pastor, J. E., E. C. Hobbs, and R. Losick. 2003. Cannibalism by sporulating bacteria. Science 301510-513. [DOI] [PubMed] [Google Scholar]
- 14.Guiral, S., T. J. Mitchell, B. Martin, and J. P. Claverys. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. USA 1028710-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E. M., S. E. Flannagan, and C. M. Sedgley. 2006. Coaggregation interactions between oral and endodontic Enterococcus faecalis and bacterial species isolated from persistent apical periodontitis. J. Endod. 32946-950. [DOI] [PubMed] [Google Scholar]
- 17.Macrina, F. L., J. A. Tobian, K. R. Jones, R. P. Evans, and D. B. Clewell. 1982. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene 19345-353. [DOI] [PubMed] [Google Scholar]
- 18.Makinen, P. L., D. B. Clewell, F. An, and K. K. Makinen. 1989. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain OG1-10). J. Biol. Chem. 2643325-3334. [PubMed] [Google Scholar]
- 19.Nakayama, J., Y. Cao, T. Horii, S. Sakuda, A. D. Akkermans, W. M. de Vos, and H. Nagasawa. 2001. Gelatinase biosynthesis-activating pheromone: a peptide lactone that mediates a quorum sensing in Enterococcus faecalis. Mol. Microbiol. 41145-154. [DOI] [PubMed] [Google Scholar]
- 20.Nes, I. F., D. B. Diep, and H. Holo. 2007. Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 1891189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nes, I. F., and V. G. H. Eijsink. 1999. Regulation of group II peptide bacteriocin synthesis by quorum-sensing mechanisms, p. 175-192. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, DC.
- 22.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J. Bacteriol. 1833372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedgley, C., G. Buck, and O. Appelbe. 2006. Prevalence of Enterococcus faecalis at multiple oral sites in endodontic patients using culture and PCR. J. Endod. 32104-109. [DOI] [PubMed] [Google Scholar]
- 24.Sedgley, C. M., S. L. Lennan, and D. B. Clewell. 2004. Prevalence, phenotype and genotype of oral enterococci. Oral Microbiol. Immunol. 1995-101. [DOI] [PubMed] [Google Scholar]
- 25.Sedgley, C. M., A. Molander, S. E. Flannagan, A. C. Nagel, O. K. Appelbe, D. B. Clewell, and G. Dahlen. 2005. Virulence, phenotype and genotype characteristics of endodontic Enterococcus spp. Oral Microbiol. Immunol. 2010-19. [DOI] [PubMed] [Google Scholar]
- 26.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 1781416-1420. [DOI] [PubMed] [Google Scholar]
- 27.Tomich, P. K., F. Y. An, and D. B. Clewell. 1980. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J. Bacteriol. 1411366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]