Abstract
Type I fimbriae in Salmonella enterica serovar Typhimurium are surface appendages that facilitate binding to eukaryotic cells. Expression of the fim gene cluster is known to be regulated by three proteins—FimW, FimY, and FimZ—and a tRNA encoded by fimU. In this work, we investigated how these proteins and tRNA coordinately regulate fim gene expression. Our results indicate that FimY and FimZ independently activate the PfimA promoter which controls the expression of the fim structural genes. FimY and FimZ were also found to strongly activate each other's expression and weakly activate their own expression. FimW was found to negatively regulate fim gene expression by repressing transcription from the PfimY promoter, independent of FimY or FimZ. Moreover, FimW and FimY interact within a negative feedback loop, as FimY was found to activate the PfimW promoter. In the case of fimU, the expression of this gene was not found to be regulated by FimW, FimY, or FimZ. We also explored the effect of fim gene expression on Salmonella pathogenicity island 1 (SPI1). Our results indicate that FimZ alone is able to enhance the expression of hilE, a known repressor of SPI1 gene expression. Based on our results, we were able to propose an integrated model for the fim gene circuit. As this model involves a combination of positive and negative feedback, we hypothesized that the response of this circuit may be bistable and thus a possible mechanism for phase variation. However, we found that the response was continuous and not bistable.
Type I fimbriae in Salmonella enterica serovar Typhimurium are proteinaceous surface appendages that carry adhesions specific for mannosylated glycoproteins (9). Type I fimbriae are involved in serovar Typhimurium pathogenicity by facilitating the binding to and invasion of intestinal epithelial cells (43). In orally inoculated mice, a wild-type strain has been shown to cause more infections and deaths than a fim mutant strain (18). A fim mutant has also been shown to exhibit severalfold weaker binding to HEp-2 and HeLa cells, and the defect in binding could be restored by complementing the fim system on a plasmid (4). Apart from type I fimbriae, mutations in different Salmonella fimbrial systems—lpf, pef, and agf—have all also been shown to greatly reduce virulence in mice (47). These systems appear to work synergistically in order to facilitate colonization of the ileum (5). In serovar Typhimurium, the fim gene cluster possesses all of the genes necessary for type I fimbrial production. This gene cluster is composed of six structural genes, three regulators, and a tRNA specific for rare arginine codons (AGA and AGG). The structural genes fimA, fimI, fimC, fimD, fimH, and fimF are all expressed in one transcript from the PfimA promoter (26, 36-38). The regulators fimZ, fimY, and fimW are all expressed from independent promoters (44, 46, 48). The tRNA encoded by fimU is located at one end of the cluster and is required for the effective translation of the regulatory genes that all carry rare arginine codons (42).
Type I fimbriation is environmentally regulated with fim gene expression favored in static liquid medium, whereas growth on solid medium inhibits expression (17). Moreover, serovar Typhimurium cultures in fimbriae-inducing conditions contain cells in both fimbriated and nonfimbriated states (35). While the regulation of fim gene expression has been studied extensively in Escherichia coli, far less is known about the regulation in serovar Typhimurium (1, 27). In particular, despite homology between the structural genes for type I fimbriae in E. coli and serovar Typhimurium, their expression is regulated in completely different manners. No homologs of E. coli regulators, FimB and FimE, are present in serovar Typhimurium (24, 28). Also, the serovar Typhimurium PfimA promoter is inactive in E. coli, indicating that the PfimA promoter is regulated by different factors in these two organisms (48). In serovar Typhimurium, the expression of the structural genes is regulated by three transcription factors, FimY, FimZ, and FimW (44, 46, 48). Both FimZ and FimY are essential for the expression of the structural genes from the PfimA promoter (48). In particular, the deletion of either the fimY or fimZ gene reduces expression from the PfimA promoter and prevents serovar Typhimurium from making type I fimbriae. FimZ has been shown to bind the PfimA promoter and promote transcription (13, 48). FimY, on the other hand, is thought to facilitate the activation of the PfimA promoter, as direct binding has not been observed (44). FimW is a negative regulator of fim gene expression (45). FimW has also been suggested to autoregulate its expression, as enhanced PfimW activity has been observed in the ΔfimW mutant. In DNA-binding assays, FimW was not observed to bind any of the fim promoters. However, FimW was found to interact with FimZ in a LexA-based two-hybrid system in E. coli (45). Thus, a possible mechanism for FimW-mediated repression may be that it binds FimZ and prevents it from activating transcription. However, an analysis of the FimW amino acid sequence predicts that it has a DNA-binding domain. Moreover, it is related to a broad range of prokaryotic transcription factors, with its closest relatives being BpdT from Rhodococcus spp. and an uncharacterized response regulator, TodD, from Pseudomonas putida (29, 30). Thus, FimW may also act by an alternate mechanism involving DNA binding.
In addition to these transcription factors, the fimU tRNA also plays a role in fim gene expression (42). All three regulators—FimZ, FimY, and FimW—contain a number of the rare arginine codons, AGA and AGG, recognized by the fimU tRNA. In the case of FimY, ΔfimU mutants have been shown to be nonfimbriated due to the inefficient translation of fimY mRNA. This translational regulation results from FimY having three rare arginine codons within its first 14 amino acids. The phenotypic effect of the ΔfimU mutation could, however, be overcome by expressing fimU from a plasmid or by changing these three rare arginine codons in fimY to ones more efficiently translated.
As a pathogen, serovar Typhimurium invades host cells by a process in which effector proteins are injected into the target cells with the help of the Salmonella pathogenicity island 1 (SPI1) type III secretion system (12, 14). SPI1 gene expression is regulated by a number of proteins, with the critical activator being HilA (2). The expression of hilA, in turn, is regulated by three AraC-like transcriptional activators, hilC, hilD, and rtsA (19, 21, 22, 32, 40, 41). HilD activity is controlled by HilE; this protein binds HilD and is thought to prevent it from activating the PhilA promoter (6, 8). FimY and FimZ have been previously shown to regulate SPI1 gene expression by repressing hilA expression through their activation of the PhilE promoter (7).
In this work, we investigated the gene circuit regulating fim expression. Using genetic approaches, we found that FimZ and FimY activate each other's expression and that each protein can independently activate the PfimA promoter. Moreover, FimZ and FimY were found to be weak autoactivators. Our data also suggest that FimW-mediated repression occurs at the level of fimY transcription. With regard to fimU, we found that none of the fim regulatory genes had any effect on its transcription. As the fim gene circuit involves a combination of positive and negative feedback, we tested whether induction was bistable. However, we found the cell population responded homogeneously when induced. Finally, we looked at the link between the fim and SPI1 gene circuits and found that the PhilE promoter is activated solely by FimZ. Collectively, these results allow us to propose an integrated model for the regulation of the fim gene circuit in serovar Typhimurium.
MATERIALS AND METHODS
General techniques and growth conditions.
All culture experiments were performed in Luria-Bertani (LB) broth (10 g/liter tryptone, 5 g/liter yeast extract, and 10 g/liter NaCl) at 37°C unless otherwise noted. Antibiotics were used at the following concentrations: ampicillin at 100 μg/ml, chloramphenicol at 20 μg/ml, and kanamycin at 40 μg/ml. All experiments involving the growth of cells carrying pKD46 were performed at 30°C as previously described (15). Loss of the helper plasmid pKD46 was achieved by growth in nonselective conditions on LB agar at 42°C. The removal of the antibiotic cassette from the FLP recombinant target (FRT)-chloramphenicol/kanamycin-FRT insert was obtained by the transformation of pCP20 into the respective strain and selection on ampicillin at 30°C. The loss of the helper plasmid pCP20 was obtained by growth at 42°C under nonselective conditions on LB agar (10). Integrations into the λattB sites of the serovar Typhimurium and E. coli genomes were done using the helper plasmid pInt-ts as described previously (25). The loss of the helper plasmid pInt-ts was obtained by growth at 42°C under nonselective conditions. Primers were purchased from IDT, Inc. Enzymes were purchased from New England Biolabs and Fermentas and used according to the manufacturer's recommendations.
Strain and plasmid construction.
All bacterial strains and plasmids used in this study are described in Tables 1 and 2, respectively. All serovar Typhimurium strains are isogenic derivatives of strain 14028 (American Type Culture Collection [ATCC]). The generalized transducing phage of serovar Typhimurium P22 HT105/1int-201 was used in all transductional crosses (16).
TABLE 1.
Strains used during this study
| Strain | Genotype or characteristica |
|---|---|
| 14028 | Wild-type serovar Typhimurium |
| CR311 | ΔfimY::FRT cm FRT |
| CR312 | ΔfimZ::FRT cm FRT |
| CR313 | ΔfimYZ::FRT cm FRT |
| CR314 | ΔfimW::FRT cm FRT |
| CR315 | ΔfimU::FRT cm FRT |
| CR316 | ΔfimU::FRT |
| CR317 | 14028 attλ::pVenus::PfimA venus |
| CR318 | 14028 attλ::pVenus::PfimY venus |
| CR319 | 14028 attλ::pVenus::PfimZ venus |
| CR320 | 14028 attλ::pVenus::Pfimw venus |
| CR321 | 14028 attλ::pVenus::PfimU venus |
| CR322 | ΔfimY::FRT |
| CR323 | ΔfimY::FRT attλ::pVenus::PfimA venus |
| CR324 | ΔfimY::FRT attλ::pVenus::PfimY venus |
| CR325 | ΔfimY::FRT attλ::pVenus::PfimZ venus |
| CR326 | ΔfimY::FRT attλ::pVenus::Pfimw venus |
| CR327 | ΔfimY::FRT attλ::pVenus::PfimU venus |
| CR328 | ΔfimZ::FRT |
| CR329 | ΔfimZ::FRT attλ::pVenus::PfimA venus |
| CR330 | ΔfimZ::FRT attλ::pVenus::PfimY venus |
| CR331 | ΔfimZ::FRT attλ::pVenus::PfimZ venus |
| CR332 | ΔfimZ::FRT attλ::pVenus::Pfimw venus |
| CR333 | ΔfimZ::FRT attλ::pVenus::PfimU venus |
| CR334 | ΔfimYZ::FRT |
| CR335 | ΔfimYZ::FRT attλ::pVenus::PfimA venus |
| CR336 | ΔfimYZ::FRT attλ::pVenus::PfimY venus |
| CR337 | ΔfimYZ::FRT attλ::pVenus::PfimZ venus |
| CR338 | ΔfimYZ::FRT attλ::pVenus::Pfimw venus |
| CR339 | ΔfimYZ::FRT attλ::pVenus::PfimU venus |
| CR340 | ΔfimW::FRT |
| CR341 | ΔfimW::FRT attλ::pVenus::PfimA venus |
| CR342 | ΔfimW::FRT attλ::pVenus::PfimY venus |
| CR343 | ΔfimW::FRT attλ::pVenus::PfimZ venus |
| CR344 | ΔfimW::FRT attλ::pVenus::Pfimw venus |
| CR345 | ΔfimW::FRT attλ::pVenus::PfimU venus |
| CR346 | 14028 attλ::pVenus::PhilE venus |
| CR347 | ΔfimYZ::FRT attλ::pVenus::PhilE venus |
| CR348 | ΔfimYZ::FRT ΔfimW::FRT attλ::pVenus::PfimY venus |
All Salmonella strains are isogenic derivatives of the serovar Typhimurium strain 14028. Strains are from this study except for the wild-type 14028, which is from the American Type Culture Collection.
TABLE 2.
Plasmids used during this study
| Plasmid | Relevant characteristics | Source or referencea |
|---|---|---|
| pKD46 | bla PBAD gam beto exo pSC101 oriTS | 15 |
| pCP20 | bla cat λcI857 λPRflp pSC101 oriTS | 10 |
| pKD3 | bla FRT cm FRT oriR6K | 15 |
| pKD4 | bla FRT kan FRT oriR6K | 15 |
| plnt-ts | bla lnt oriR6K | 29 |
| pQE80L | bla lacIq ColE1 | Qiagen |
| pAH125 | kan lacZ attλ oriR6K | 29 |
| pVenus | kan venus attλ oriR6K | |
| PfimA-Venus | kan PfimA venus attλ oriR6K | |
| PfimY-Venus | kan PfimY venus attλ oriR6K | |
| PfimZ-Venus | kan PfimZ venus attλ oriR6K | |
| Pfimw-Venus | kan Pfimw venus attλ oriR6K | |
| PfimU-Venus | kan PfimU venus attλ oriR6K | |
| PhilE-Venus | kan PhilE venus attλ oriR6K | |
| pPROTet.E | cm PLtetO-1ori ColE1 | Stratagene |
| pSS012 (pPROTet.E tetR) | cm PLtetO-1tetR ori ColE1 | 45 |
| pSS039 (pFimY) | cm PLtetO-1fimY tetR ori ColE1 | |
| pSS040 (pFimZ) | cm PLtetO-1fimZ tetR ori ColE1 | |
| pSS041 (pFimW) | cm PLtetO-1fimW tetR ori ColE1 | |
| pSS042 (pFimY*) | cm PLtetO-1fimY* tetR ori ColE1 |
Plasmids are from this study unless specified otherwise.
The plasmids pKD3 and pKD4 were used as templates to generate scarred FRT mutants as described previously (14). The ΔfimYZ mutant was made using primers SS105F and SS105R. The ΔfimZ mutant was made using primers SS105FII and SS105F. The ΔfimY mutant was made using the primers SS105RII and SS105R. The ΔfimW mutant was made using primers SS152F and SS152R. The ΔfimU mutant was made using the primers SS165F and SS165R. All mutations were checked by PCR using primers that bound outside the deleted region. Prior to the removal of the antibiotic resistance marker, the constructs resulting from this procedure were moved into a clean wild-type background (14028) by P22 transduction.
In order to construct the fluorescent Venus reporter plasmid (34), PCR was used to amplify Venus from pBS7 using primers LC294F and LC296R. The resulting PCR product was used as a template with primers LC295F and LC296R to add three out-of-frame stop codons and a synthetic Shine-Dalgarno sequence before the Venus start codon. The resultant PCR product was then digested with EcoRI and HindIII and subcloned into the EcoRI and HindIII cut sites of pQE80L (Qiagen), yielding pQE80L-Venus. The plasmid pQE80L was digested with EcoRI and NheI, and the fragment was cloned into the conditional-replication, integration, and modular (CRIM) plasmid pAH125 digested with EcoRI and NheI (25). The resulting CRIM plasmid was called pVenus. Venus transcriptional fusions were made by amplifying the promoter of interest and then cloning these PCR fragments into the multiple cloning site of pVenus. The fimA transcriptional fusion was made using primers SS104F and SS104R. The fimY transcriptional fusion was made using primers SS037F and SS037R. The fimZ transcriptional fusion was made using primers SS103F and SS103R. The fimW transcriptional fusion was made using primers SS154F and SS154R. The fimU transcriptional fusion was made using primers SS162F and SS162R. The hilE transcriptional fusion was made using primers SS024F and SS024R. The PCR fragments were then digested with KpnI and EcoRI (sequences underlined) and cloned into the multiple cloning site of the pVenus vector. The resulting transcriptional fusions were integrated into the serovar Typhimurium and E. coli chromosomes at the λattB site using λInt produced from the CRIM helper plasmid pInt-ts, thus creating single-copy transcriptional fusions. In the case of serovar Typhimurium, the integrated plasmid was moved into different mutant strains by P22 transduction.
Expression plasmids for fimY, fimZ, and fimW were made by cloning the respective gene into the multiple cloning site of pPROTet.E (Clontech) under the control of a strong promoter, PLTetO-1, resulting in plasmids pFimY, pFimZ, and pFimW (33). The plasmid pFimZ was made first by amplifying the fimZ gene using the primers SS106F and SS106R. The PCR product was then digested with EcoRI and KpnI and cloned into pPROTet.E. The plasmid pFimY was made by amplifying the fimY gene using the primers SS107F and SS107R. The PCR product was then digested with SalI and BamHI and cloned into pPROTet.E. The plasmid pFimW was made by amplifying the fimW gene using the primers SS160F and SS160R. The PCR product was then digested with EcoRI and HindIII and cloned into pPROTet.E. In order to mutate the first three arginine rare codons at positions 7, 9, and 14 in fimY, primers SS162F and SS107R were used to amplify fimY with the rare arginine codons mutated to consensus arginine codons. The resulting PCR product was used as a template with primers SS167F and SS107R. The amplified product was digested with EcoRI and BamHI and cloned into the multiple cloning site of pPROTet.E. The plasmid is called pFimY*.
In our expression plasmids, in the absence of TetR, the PLTetO-1 promoter is constitutively active. To regulate the expression levels from the PLTetO-1 promoter, the tetR gene was also cloned downstream of the gene target into the plasmids as previously described (39). In this arrangement, in the absence of the inducer anhydrotetracycline (aTc), expression from the promoter is inhibited due to TetR. The inhibition, however, is relieved upon the addition of 100 ng/ml of aTc, and expression from the PLTetO-1 promoter then takes place. All constructs were sequenced prior to transforming into the wild-type and mutant strains. The sequences for all the primers used in this study are given in Table 3.
TABLE 3.
List of primers used in the study
| Primer | Sequence | Characteristic |
|---|---|---|
| SS105F | TGT CCG TTA TTG TGG CTC CCG AAC GAT AAT TCG CCG GGA GGA TGG TAG TGT GGG GTC TCC | fimYZ knockout primer |
| SS105R | ATC AAT CAG TTT CTT TAA TAT TTC ACC ATG ATT CAC CTG CCA TGG GAA TTA GCC ATG GTC C | fimYZ knockout primer |
| SS105FII | TTA TAA AAC GAA GGA CGC ATA ACA GTC TGA GGC ATA CAA ATG GGA ATT AGC CAT GGT CC | fimZ knockout primer |
| SS105RII | GTA ATT TCT TAA AAA ATC TTA TTC ACC AAA ACG TTA CTT CGA TGG TAG TGT GGG GTC TCC | fimY knockout primer |
| SS152F | TAT TTC ACC ATG ATT CAC CTG CCG TGT AGG ATA TTT TTT TGT GTA GGC TGG AGC TGC TTC | fimW knockout primer |
| SS152R | GGT GAG ATA TTT CGT AAG CCT TGT AAA AAG TTA AGT GAG TCA TAT GAA TAT CCT CCT TAG | fimW knockout primer |
| SS165F | CTC GCG TTT CGT CTA CAC GAA GTC TTC ACT TCA CAA GGC GGT GTA GGC TGG AGC TGC TTC | fimU knockout primer |
| SS165R | GGA AAA TAA GGA GGA AAT AAA GAA GCG TAA CAC GTT GAT TCA TAT GAA TAT CCT CCT TAG | fimU knockout primer |
| LC294F | GAT TAA CTT TAT AAG GAG GAA AAA CAT ATG AGT AAA GGA GAA GAA CTT TTC | venus gene |
| LC296R | ATA AAG CTT TTA TTT GTA TAG TTC ATC CAT GCC ATG | venus gene |
| LC295F | CTC GAA TTC CCT AAC TAA CTA AAG ATT AAC TTT ATA AGG AGG A | venus gene |
| SS104F | TTT GGT ACC AAA TCT GTG AGG CCG GAT TG | fimA promoter |
| SS104R | GGG GAA TTC GTA GAG GTC ATT AAT TTA TG | fimA promoter |
| SS037F | TCT GGT ACC AAA ATA TAT TAG AGT TAA CC | fimY promoter |
| SS037R | AAA GAA TTC CCC TGC GTG GTA CGC TGC GC | fimY promoter |
| SS103F | TTT GGT ACC ATA AAA CCT CCG CTA TAA CA | fimZ promoter |
| SS103R | GGG GAA TTC CCA TAA TGA TAA CAG ATG CA | fimZ promoter |
| SS154F | GGG GGT ACC GGA TTC GAA CCT GCG ACC CA | fimW promoter |
| SS154R | GGG GAA TTC TTT TCC GGG TAA TTT CTT CA | fimW promoter |
| SS162F | GGG GGT ACC CGT TTC GCT TAA ATG ATA AC | fimU promoter |
| SS162R | GGG GAA TTC CTA TCC AAC TGA GCT AAG GG | fimU promoter |
| SS024F | AAG GGT ACC ATG ACG TTG CGT AGC GTT GG | hilE promoter |
| SS024R | GGT GAA TTC GAA AGA ACG TTC CAT TTT CC | hilE promoter |
| SS106F | GGG GAA TTC TAA CAG TCT GAG GCA TAC AA | fimZ gene |
| SS106R | TTT GGT ACC TTA CAA TAA TTC GTG TGA TT | fimZ gene |
| SS107F | CCT GTC GAC ATA TTA GAG CAA TGG AAA A | fimY gene |
| SS107R | CCC GGA TCC TTA AAA AAT GTC GTG GAA AG | fimY gene |
| SS160F | ATA GAA TTC GCC GTG TAG GAT ATT TTT TT | fimW gene |
| SS160R | ATA AAG CTT TTA TTA CTT ACT GAG TAA GAA ATG AAG G | fimW gene |
| SS162F | GGG GGT ACC ATG CGC AGC GTA CCA CGC CGG GAA CGA CAC CGC CGT TTA CGA AAT GCT AA | fimY* gene |
| SS167F | GGG GAA TTC TTT ATA AGG AGG AAA AAC ATA TGC GCA GCG TAC CAC GCC GG | fimY* gene |
Fluorescence assays.
As an indirect measure of gene expression, end-point and dynamic measurements of the fluorescent reporter system were made using a Tecan Safire2 microplate reader. For fluorescence end-point measurements, 1 ml culture was grown at 37°C overnight and then subcultured 1:1,000 in fresh medium and grown in static conditions for 24 h at 37°C. A total of 100 μl of the culture was then transferred to a 96-well microplate, and the relative fluorescence and optical density at 600 nm (OD600) measured. The fluorescence readings were normalized with the OD600 to account for cell density. For time course measurements, overnight cultures at 37°C were subcultured to an OD of 0.05 in fresh medium and allowed to grow to an OD of 0.15. A total of 100 μl of the culture was then transferred to a 96-well microplate and overlaid with 25 μl of oil to prevent evaporation. The temperature was maintained at 37°C, and fluorescence and OD readings were taken every 5 min. All experiments were done in triplicate and average values with the standard deviations reported.
Single-cell measurements were done similarly by growing the cells in noninducing conditions with vigorous shaking at 37°C. Overnight cultures were subcultured to an OD of 0.05 in fresh medium (LB) and grown in inducing conditions of high oxygen and no shaking at 37°C. Samples were collected at different time points by spinning the cells down, resuspending them in phosphate-buffered saline supplemented with chloramphenicol (34 μg/ml) to stop all translation and arrest the cells in their respective state, and finally storing on ice. All flow cytometry experiments were performed on a BD LRS II system (BD Biosciences). The data extraction and analysis for the flow cytometry experiments were done using FCS Express version 3 (De Novo Software).
RESULTS
FimZ and FimY are activators and FimW is a repressor of fim gene expression.
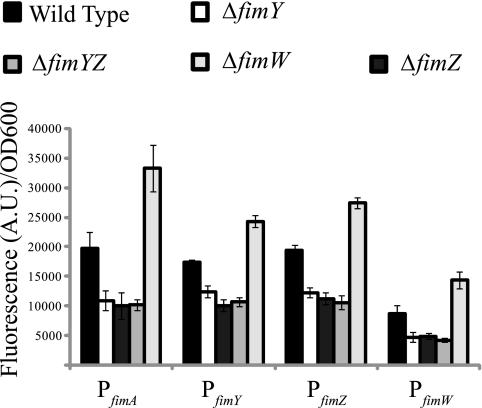
FimZ and FimY have previously been reported as activators of fim gene expression in serovar Typhimurium (44, 48). Both have also been reported as essential for fimbriation, as the deletion of either one results in the loss of expression from the PfimA promoter (49). To understand the roles of FimZ and FimY in the fim gene circuit, we measured expression from the PfimA, PfimZ, PfimY, and PfimW promoters in the wild type and the ΔfimZ, ΔfimY, ΔfimYZ, and ΔfimW mutants (Fig. 1). Chromosomally integrated Venus transcriptional reporters were employed as indirect measures of promoter activities (34). In the cases of all four promoters, activity levels were found to be about two times less active in the ΔfimZ, ΔfimY, and ΔfimYZ mutants than in the wild type. For all four promoters, note that no further reduction in promoter activity was observed in the double mutant. In a ΔfimW mutant, the activities of all four promoters were approximately two times higher than the wild-type levels. While these results agree with previously published data regarding the fim system in serovar Typhimurium, they still do not tell us how FimW, FimY, and FimZ individually contribute to PfimA activation.
FIG. 1.
FimY and FimZ are activators and FimW is a repressor of fim gene expression. Shown is a comparison of the PfimA, PfimY, PfimZ, and PfimW promoter activities in the wild type and the ΔfimY, ΔfimZ, ΔfimYZ, and ΔfimW mutants. Data are averages of the results from three experiments. Each experiment was done in triplicate.
FimY and FimZ are strong activators of each other's expression and weak activators of their own expression.
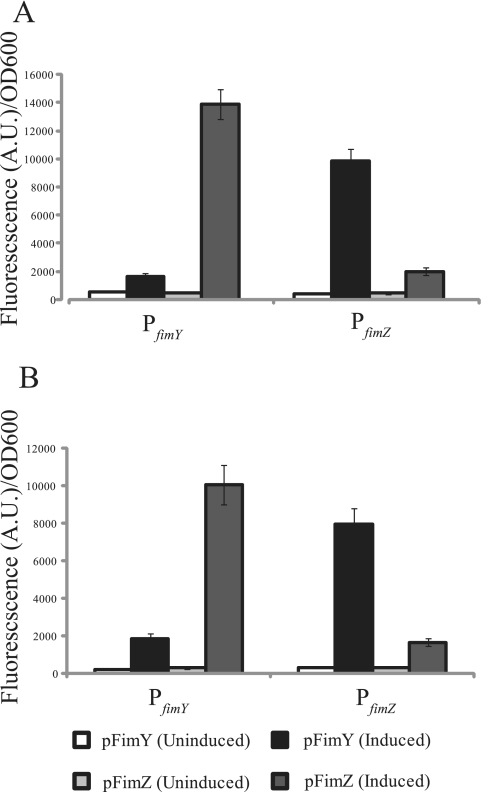
To determine the relative effect of FimY and FimZ on fim gene expression, the PfimY and PfimZ promoter activities were measured in a ΔfimYZ mutant in which either FimZ or FimY was expressed from a strong, aTc-inducible promoter on a plasmid (see Materials and Methods). Using this system, we found that expressing FimZ in the ΔfimYZ mutant led to a more than 10-fold increase in PfimY activity (Fig. 2A). Likewise, expressing FimY in the ΔfimYZ mutant led to about a 10-fold increase in PfimZ levels. In addition to their ability to activate each other's promoters, FimY and FimZ were found to increase expression from their own promoters roughly threefold.
FIG. 2.
FimY and FimZ are strong activators of each other's expression and also weak autoactivators. Shown is a comparison of the PfimY and PfimZ promoter activities in a serovar Typhimurium ΔfimYZ mutant (A) and E. coli (B) in which FimY and FimZ are independently expressed from an aTc-inducible promoter on a plasmid. Note that tetR is also expressed from this plasmid in order to achieve aTc-inducible expression. Data are averages of the results from three experiments. Each experiment was done in triplicate.
Even though E. coli makes type I fimbriae, the serovar Typhimurium fim promoters by themselves are inactive in this organism. Therefore, we performed an identical set of experiments with E. coli using the serovar Typhimurium proteins and promoters. Overall, the results were identical to those for serovar Typhimurium (Fig. 2B). In particular, FimZ expression led to a more than 10-fold increase in PfimY promoter activity, and FimY expression led to a 10-fold increase in PfimZ activity. Both FimZ and FimY were again found to weakly activate expression from their own promoters. The goal of these experiments was to remove the effect of any serovar Typhimurium-specific regulatory mechanisms, thus allowing us to more confidently conclude that the observed results are due to direct interactions. Collectively, these results show that FimY and FimZ strongly activate each other's expression and weakly activate their own expression. This cross-regulation also explains why both FimY and FimZ are required for strong PfimA promoter activity, as the expression of each is dependent on the other.
FimZ and FimY can independently activate expression from the PfimA promoter.
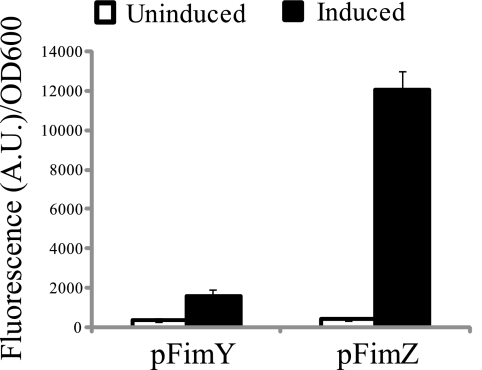
Next, we looked at how FimZ and FimY independently affected PfimA expression. To investigate this problem, we measured PfimA promoter activity in a ΔfimYZ mutant in which either FimY or FimZ was expressed using the aTc-inducible system. FimZ expression was found to strongly (>15-fold) activate the PfimA promoter, whereas FimY could only weakly (more than twofold) activate it (Fig. 3). We also performed these experiments with E. coli with similar results (data not shown). Based on these results, we conclude that FimZ and FimY can both independently activate the PfimA promoter. In the case of FimY, the weak activation of the PfimA promoter is likely due to its strong dependence on fimU tRNA (see below) (42).
FIG. 3.
FimY and FimZ can independently activate expression from the PfimA promoter. Shown is a comparison of PfimA promoter activities in a ΔfimYZ mutant in which FimY and FimZ are independently expressed from an aTc-inducible promoter on a plasmid. Data are averages of the results from three experiments. Each experiment was done in triplicate.
FimY activates the PfimW promoter, and FimW represses the PfimY promoter.
FimW has previously been observed to repress fim gene expression (45). Consistent with these results, we observed that PfimA, PfimW, PfimY, and PfimZ promoter activities were all elevated in a ΔfimW mutant (Fig. 1). To understand the mechanism of FimW-mediated repression, we first sought to identify the proteins that regulate expression from the PfimW promoter. To answer this question, we measured the level of expression from the PfimW promoter in a ΔfimYZ mutant in which FimW, FimY, and FimZ were independently expressed using the aTc-inducible system. In the case of FimW and FimZ, expression had no effect on PfimW promoter activity (data not shown). However, in the case of FimY, we observed a significant increase in PfimW promoter activity (1,052 ± 381 relative fluorescence units [RFU]/OD [uninduced] versus 14,718 ± 1,032 RFU/OD [induced]). Similar results were also obtained when these experiments were performed with E. coli (data not shown). To identify the regulatory targets of FimW, we measured the expression of the PfimA, PfimZ, and PfimY promoters in a ΔfimW ΔfimYZ mutant in which FimW was expressed using the aTc-inducible system. In the cases of the PfimA and PfimZ promoters, we found that FimW expression had no effect. However, in the case of the PfimY promoter, FimW expression led to about a threefold decrease in PfimY activity (7,462 ± 319 RFU/OD [uninduced] versus 2,781 ± 188 RFU/OD [induced]). Based on these results, we conclude that FimY activates expression from the PfimW promoter and that FimW represses expression from the PfimY promoter.
The PfimU promoter is not regulated by FimW, FimY, or FimZ.
Both fimY and fimZ contain rare arginine codons (AGA and AGG) and need fimU, a tRNA specific for rare arginine codons, for effective translation. In a ΔfimU mutant, PfimA activity was less than 10-fold compared to the wild-type levels (wild type, 16,723 ± 1,173 RFU/OD; the ΔfimU mutant, 1,389 ± 261 RFU/OD). The expression of FimY in the ΔfimU mutant using the aTc-inducible system, however, did not increase PfimA activity (988 ± 319 [uninduced] versus 1,343 ± 166 [induced]). Replacing the rare arginine codons in the fimY gene with consensus ones did restore PfimA activity to the wild-type levels (817 ± 73 RFU/OD [uninduced] versus 11,294 ± 462 RFU/OD [induced]). These experiments are consistent with previously published results (45) and indicate that fimU is essential for effective fimY translation.
As fimU has a strong effect on PfimA promoter activity, we hypothesized that it may be subject to regulation by the other proteins within the circuit. To test this hypothesis, we measured PfimU promoter activity in different regulatory mutants. Contrary to our hypothesis, we did not observe any change in PfimU promoter activity in any mutant (wild type, 26,717 ± 1,381 RFU/OD; the ΔfimZ mutant, 28,991 ± 2,164 RFU/OD; the ΔfimY mutant, 25,884 ± 1,983 RFU/OD; the ΔfimYZ mutant, 26,516 ± 1,772 RFU/OD; and the ΔfimW mutant, 24,829 ± 2,073 RFU/OD). Likewise, we did not observe any change in PfimU promoter activity when FimW, FimY, and FimZ were expressed using the aTc-inducible system in wild-type serovar Typhimurium or E. coli (data not shown). Based on these results, we conclude that the PfimU promoter is not regulated by any fim protein.
FimZ alone is able to regulate SPI1 gene expression.
Previous studies have shown that both FimY and FimZ regulate SPI1 expression through their activation of the PhilE promoter (7). HilE, in turn, is known to bind HilD and repress the HilD-mediated activation of the PhilA, PhilC, PrtsA, and PhilD promoters (6, 20). To test which protein activates the PhilE promoter, we independently expressed FimY and FimZ in a ΔfimYZ mutant using the aTc-inducible system and then measured the level of expression from the PhilE promoter. Of the two, only FimZ was found to affect PhilE expression (1,089 ± 421 RFU/OD [uninduced] versus 17,654 ± 2,234 RFU/OD [induced]). Similar results were also observed for E. coli (data not shown).
We note that these results are contrary to those previously reported, for which it was shown that both FimY and FimZ were necessary for activation of the PhilE promoter (7). One possible explanation for the discrepancy involves how the two gene products were selectively expressed. In the original study, a DNA fragment containing the fimYZ gene cluster was cloned onto a plasmid and expressed using the tetracycline promoter. To study their relative effects, each gene was selectively inactivated using a universal translational terminator. As part of the PfimY promoter and the whole PfimZ promoter were left intact in their construct, transcriptional inference may have occurred between the various promoters. In our design, we selectively cloned each gene and then expressed it from an inducible promoter, eliminating any potential interfering effects from having the native promoters still present.
Dynamics of fim gene expression.
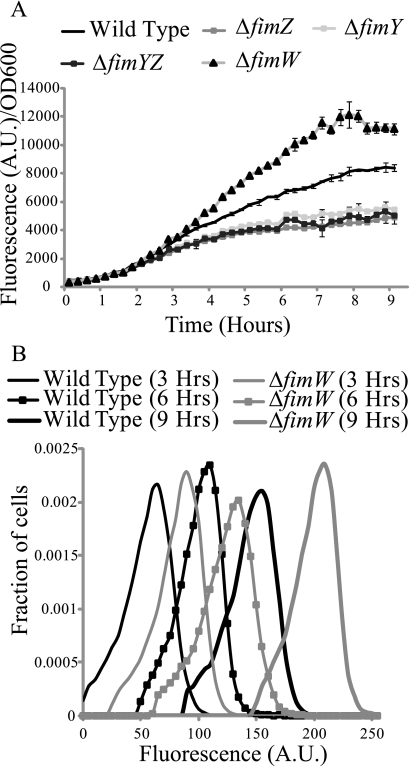
Finally, we wished to investigate the dynamics of fim gene expression. We first measured PfimA promoter activity in the wild type and the ΔfimY, ΔfimZ, ΔfimYZ, and ΔfimW mutants using a microplate reader (Fig. 4A). Consistent with our end-point measurements, we found that the PfimA promoter was weakly expressed in the ΔfimY, ΔfimZ, and ΔfimYZ mutants. Likewise, expression was enhanced in a ΔfimW mutant. Note that the microplate experiments tell us only about the average response of the population and nothing about how individual cells are behaving. To test whether the cells were responding homogeneously, we also performed single-cell measurements of PfimA promoter activity at selected times in the wild type and a ΔfimW mutant using flow cytometry (Fig. 4B). Our results indicate that individual wild-type and ΔfimW mutant cells are responding homogeneously with respect to PfimA promoter activity at all times tested. In other words, we did not observe any phase variation or heterogeneity with regard to PfimA promoter activity in our kinetic experiments.
FIG. 4.
Dynamics of PfimA promoter activity. (A) Population average PfimA activity as a function of time in the wild type and the ΔfimY, ΔfimZ, ΔfimYZ, and ΔfimW mutants. Data are averages of the results from a single experiment with an average of six independent cultures. The experiment was repeated thrice, and identical results were observed. (B) Histogram of single-cell PfimA promoter activity at selected times in the wild type and a ΔfimW mutant. Single-cell measurements of promoter activity were obtained using flow cytometry. Population distribution data are from a single experiment. The experiment was repeated thrice, and identical results were observed (data not shown).
DISCUSSION
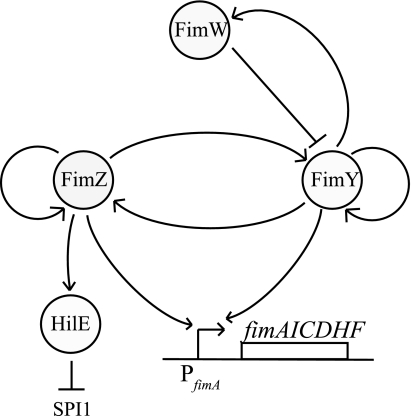
In this work, we investigated the regulatory gene circuit controlling the expression of type I fimbriae in serovar Typhimurium. Using genetic approaches, we demonstrated that FimY and FimZ independently activate the PfimA promoter. Of the two, FimZ was found to be the dominant activator. We also found that FimY and FimZ strongly activate each other's expression and weakly activate their own expression. In addition to these two positive regulators, a third regulator, FimW, is known to repress fim gene expression. We found that FimW negatively regulates fim gene expression by repressing expression from the PfimY promoter. Furthermore, FimW participates in a negative feedback loop as FimY was found to enhance PfimW expression. Interestingly, these results suggest that FimY is both an activator and a repressor of fim gene expression, as it can directly activate the PfimZ, PfimY, and PfimA promoters and indirectly repress them by enhancing FimW expression. In addition to these regulators, type I fimbriation is also dependent on the expression of a rare arginine codon tRNA, fimU. However, our results showed that the PfimU promoter is not regulated by FimY, FimZ, or FimW. The results suggest that fimU does not play a role in the internal regulation of the circuit. Finally, we demonstrated that the previously observed coordinate regulation of SPI1 gene expression by the fim gene circuit (7) occurs through the activation of hilE expression by FimZ. Based on these results, we are able to propose the following model for the fim gene circuit in serovar Typhimurium (Fig. 5).
FIG. 5.
Model for the type I fimbria gene circuit in serovar Typhimurium.
According to our model, the induction of the fim circuit begins with the activation of the PfimY and PfimZ promoters, resulting in small amounts of fimY and fimZ being expressed. FimY and FimZ then rapidly accumulate in the cell due to the positive feedback loop formed by the cross-activation of the PfimY and PfimZ promoters by these two proteins. The expression of the type I fimbrial structural genes from the PfimA promoter commences when the concentration of FimY and FimZ within the cell rises beyond a critical level. These two regulators can independently activate the PfimA promoter; however, their expression is correlated, as each activates the other's expression. Moreover, FimY and FimZ protein expression levels are controlled by a negative feedback loop involving FimW. In this loop, FimY activates the expression of the PfimW promoter, and FimW represses the expression of the PfimY promoter. We hypothesize that this negative feedback loop involving FimW prevents the runaway expression of FimY and FimZ arising from their participation in interacting positive feedback. Specifically, we hypothesize that when FimY and FimZ reach their optimum expression levels, the FimW negative feedback loop is activated and halts expression from the PfimY and PfimZ promoters.
While our model for the fim circuit explains internal regulation, it still does not explain how the circuit is activated. In particular, we do not know which factors induce the PfimY and PfimZ promoters. We suspect that these factors activate both promoters, as each alone exhibits some activity in a ΔfimYZ mutant (Fig. 1). In addition to these factors, another open question concerns whether fimU plays a role in regulating circuit dynamics. While it is tempting to speculate that fimU expression is tuned in response to environmental signals and thus affects circuit dynamics, more likely this gene is constitutively expressed like other tRNAs.
Our results also indicate that the FimW-mediated inhibition of fim gene expression is through repression of the PfimY promoter. Earlier reports suggested that FimW binds FimZ and somehow inhibits the FimZ-dependent activation of fim promoters (45). Moreover, FimW was not found to bind to the PfimW promoter. Based on these results, FimW would appear to repress the PfimY promoter by preventing FimZ from activating it. However, we found that FimW is able to repress the PfimY promoter in the absence of FimZ. Our results would suggest that FimW directly binds the PfimY promoter and represses transcription independently of FimZ. Consistent with our model, FimW has a C-terminal LuxR-type helix-turn-helix DNA domain (SM00421) (31). However, at this time we have no direct experimental support for such a mechanism. Moreover, an equally likely hypothesis is that repression by FimW is indirect. Further experiments are clearly required to determine the mechanism of FimW-mediated repression and distinguish between these different putative models.
A final unanswered question concerns the role of the positive and negative feedback loops in the fim gene circuit. Our initial hypothesis was that these feedback loops would result in bistability. In particular, interacting positive and negative feedback loops are known to be sufficient ingredients for bistability (23). This bistability could potentially explain the phase variation observed in type I fimbriation during growth in inducing conditions (35). To test whether the fim circuit exhibited bistability, we measured PfimA activity at single-cell resolution as a function of time. Contrary to our initial hypothesis, we did not observe a heterogeneous or switch-like response in induction, the telltale indicator of bistability. Rather, we observed a continuous or rheostat-like response in both the wild type and a ΔfimW mutant (3). One possibility is that there is a lack of correlation between fim gene expression and the production of type I fimbria in serovar Typhimurium (11). Another is that the environment may dictate the response characteristics. For example, under conditions different from those used in our study, Duguid and coworkers observed subpopulations of cells expressing type I fimbriae, indicative of phase variation (17). With these in mind, we hypothesize that the bacteria exhibit type I fimbria phase variation under specific environmental conditions and that the regulation of this process involves posttranscriptional mechanisms as well.
Acknowledgments
We thank James Slauch and Philip Aldridge for strains and technical advice.
This work was partially supported by grants from the National Science Foundation and the National Institutes of Health.
Footnotes
Published ahead of print on 13 February 2009.
REFERENCES
- 1.Abraham, J. M., C. S. Freitag, J. R. Clements, and B. I. Eisenstein. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 825724-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18715-727. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor, E., T. J. Silhavy, and M. Goulian. 2004. Continuous control in bacterial regulatory circuits. J. Bacteriol. 1867618-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler, A. J., R. M. Tsolis, and F. Heffron. 1996. Contribution of fimbrial operons to attachment to and invasion of epithelial cell lines by Salmonella typhimurium. Infect. Immun. 641862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumler, A. J., R. M. Tsolis, and F. Heffron. 1997. Fimbrial adhesins of Salmonella typhimurium. Role in bacterial interactions with epithelial cells. Adv. Exp. Med. Biol. 412149-158. [PubMed] [Google Scholar]
- 6.Baxter, M. A., T. F. Fahlen, R. L. Wilson, and B. D. Jones. 2003. HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect. Immun. 711295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter, M. A., and B. D. Jones. 2005. The fimYZ genes regulate Salmonella enterica serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect. Immun. 731377-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddicker, J. D., B. M. Knosp, and B. D. Jones. 2003. Transcription of the Salmonella invasion gene activator, hilA, requires HilD activation in the absence of negative regulators. J. Bacteriol. 185525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan, K., S. Falkow, R. A. Hull, and S. I. Hull. 1985. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J. Bacteriol. 162799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1589-14. [DOI] [PubMed] [Google Scholar]
- 11.Clegg, S., L. S. Hancox, and K.-S. Yeh. 1996. Salmonella typhimurium fimbrial phase variation and FimA expression. J. Bacteriol. 178542-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collazo, C. M., and J. E. Galan. 1997. The invasion-associated type-III protein secretion system in Salmonella—a review. Gene 19251-59. [DOI] [PubMed] [Google Scholar]
- 13.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 623381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darwin, K. H., and V. L. Miller. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, R. W., D. Botsein, and J. R. Roth. 1980. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Duguid, J. P., E. S. Anderson, and I. Campbell. 1966. Fimbriae and adhesive properties in Salmonellae. J. Pathol Bacteriol. 92107-138. [DOI] [PubMed] [Google Scholar]
- 18.Duguid, J. P., M. R. Darekar, and D. W. Wheater. 1976. Fimbriae and infectivity in Salmonella typhimurium. J. Med. Microbiol. 9459-473. [DOI] [PubMed] [Google Scholar]
- 19.Eichelberg, K., W. D. Hardt, and J. E. Galan. 1999. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol. Microbiol. 33139-152. [DOI] [PubMed] [Google Scholar]
- 20.Ellermeier, C. D., J. R. Ellermeier, and J. M. Slauch. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 57691-705. [DOI] [PubMed] [Google Scholar]
- 21.Ellermeier, C. D., and J. M. Slauch. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1855096-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahlen, T. F., N. Mathur, and B. D. Jones. 2000. Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 2825-35. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell, J. E. 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14140-148. [DOI] [PubMed] [Google Scholar]
- 24.Gally, D. L., J. Leathart, and I. C. Blomfield. 1996. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 21725-738. [DOI] [PubMed] [Google Scholar]
- 25.Haldimann, A., and B. L. Wanner. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 1836384-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancox, L. S., K. S. Yeh, and S. Clegg. 1997. Construction and characterization of type 1 non-fimbriate and non-adhesive mutants of Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 19289-296. [DOI] [PubMed] [Google Scholar]
- 27.Klemm, P. 1984. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur. J. Biochem. 143395-399. [DOI] [PubMed] [Google Scholar]
- 28.Klemm, P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 51389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labbe, D., J. Garnon, and P. C. Lau. 1997. Characterization of the genes encoding a receptor-like histidine kinase and a cognate response regulator from a biphenyl/polychlorobiphenyl-degrading bacterium, Rhodococcus sp. strain M5. J. Bacteriol. 1792772-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau, P. C., Y. Wang, A. Patel, D. Labbe, H. Bergeron, R. Brousseau, Y. Konishi, and M. Rawlings. 1997. A bacterial basic region leucine zipper histidine kinase regulating toluene degradation. Proc. Natl. Acad. Sci. USA 941453-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letunic, I., R. R. Copley, B. Pils, S. Pinkert, J. Schultz, and P. Bork. 2006. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 34257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas, R. L., and C. A. Lee. 2001. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1832733-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz, R., and H. Bujard. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 251203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai, T., K. Ibata, E. S. Park, M. Kubota, K. Mikoshiba, and A. Miyawaki. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2087-90. [DOI] [PubMed] [Google Scholar]
- 35.Old, D. C., and J. P. Duguid. 1970. Selective outgrowth of fimbriate bacteria in static liquid medium. J. Bacteriol. 103447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piknova, L., E. Kaclikova, D. Pangallo, B. Polek, and T. Kuchta. 2005. Quantification of Salmonella by 5′-nuclease real-time polymerase chain reaction targeted to fimC gene. Curr. Microbiol. 5038-42. [DOI] [PubMed] [Google Scholar]
- 37.Purcell, B. K., J. Pruckler, and S. Clegg. 1987. Nucleotide sequences of the genes encoding type 1 fimbrial subunits of Klebsiella pneumoniae and Salmonella typhimurium. J. Bacteriol. 1695831-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossolini, G. M., P. Muscas, A. Chiesurin, and G. Satta. 1993. Analysis of the Salmonella fim gene cluster: identification of a new gene (fimI) encoding a fimbrin-like protein and located downstream from the fimA gene. FEMS Microbiol. Lett. 114259-265. [DOI] [PubMed] [Google Scholar]
- 39.Saini, S., J. D. Brown, P. D. Aldridge, and C. V. Rao. 2008. FliZ is a posttranslational activator of FlhD4C2-dependent flagellar gene expression. J. Bacteriol. 1904979-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schechter, L. M., S. M. Damrauer, and C. A. Lee. 1999. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol. 32629-642. [DOI] [PubMed] [Google Scholar]
- 41.Schechter, L. M., and C. A. Lee. 2001. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol. Microbiol. 401289-1299. [DOI] [PubMed] [Google Scholar]
- 42.Swenson, D. L., K. J. Kim, E. W. Six, and S. Clegg. 1994. The gene fimU affects expression of Salmonella typhimurium type 1 fimbriae and is related to the Escherichia coli tRNA gene argU. Mol. Gen. Genet. 244216-218. [DOI] [PubMed] [Google Scholar]
- 43.Tavendale, A., C. K. Jardine, D. C. Old, and J. P. Duguid. 1983. Haemagglutinins and adhesion of Salmonella typhimurium to HEp2 and HeLa cells. J. Med. Microbiol. 16371-380. [DOI] [PubMed] [Google Scholar]
- 44.Tinker, J. K., and S. Clegg. 2000. Characterization of FimY as a coactivator of type 1 fimbrial expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 683305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tinker, J. K., and S. Clegg. 2001. Control of FimY translation and type 1 fimbrial production by the arginine tRNA encoded by fimU in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 40757-768. [DOI] [PubMed] [Google Scholar]
- 46.Tinker, J. K., L. S. Hancox, and S. Clegg. 2001. FimW is a negative regulator affecting type 1 fimbrial expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 183435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Velden, A. W., A. J. Baumler, R. M. Tsolis, and F. Heffron. 1998. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Infect. Immun. 662803-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh, K. S., L. S. Hancox, and S. Clegg. 1995. Construction and characterization of a fimZ mutant of Salmonella typhimurium. J. Bacteriol. 1776861-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh, K. S., J. K. Tinker, and S. Clegg. 2002. FimZ binds the Salmonella typhimurium fimA promoter region and may regulate its own expression with FimY. Microbiol. Immunol. 461-10. [DOI] [PubMed] [Google Scholar]