Abstract
Pseudomonas aeruginosa is an important cause of pulmonary infection in cystic fibrosis (CF). Its correct identification ensures effective patient management and infection control strategies. However, little is known about how often CF sputum isolates are falsely identified as P. aeruginosa. We used P. aeruginosa-specific duplex real-time PCR assays to determine if 2,267 P. aeruginosa sputum isolates from 561 CF patients were correctly identified by 17 Australian clinical microbiology laboratories. Misidentified isolates underwent further phenotypic tests, amplified rRNA gene restriction analysis, and partial 16S rRNA gene sequence analysis. Participating laboratories were surveyed on how they identified P. aeruginosa from CF sputum. Overall, 2,214 (97.7%) isolates from 531 (94.7%) CF patients were correctly identified as P. aeruginosa. Further testing with the API 20NE kit correctly identified only 34 (59%) of the misidentified isolates. Twelve (40%) patients had previously grown the misidentified species in their sputum. Achromobacter xylosoxidans (n = 21), Stenotrophomonas maltophilia (n = 15), and Inquilinus limosus (n = 4) were the species most commonly misidentified as P. aeruginosa. Overall, there were very low rates of P. aeruginosa misidentification among isolates from a broad cross section of Australian CF patients. Additional improvements are possible by undertaking a culture history review, noting colonial morphology, and performing stringent oxidase, DNase, and colistin susceptibility testing for all presumptive P. aeruginosa isolates. Isolates exhibiting atypical phenotypic features should be evaluated further by additional phenotypic or genotypic identification techniques.
The accurate identification of Pseudomonas aeruginosa is a critical component of cystic fibrosis (CF) patient management. Once established within CF lungs, P. aeruginosa is rarely eradicated, leading to increased treatment requirements and an accelerated decline in pulmonary function, quality of life, and life expectancy (10, 13, 27). Emerging evidence indicates that aggressive antipseudomonal therapy at the time of initial acquisition may eliminate P. aeruginosa, preventing the development of chronic infection for months or even years (37). Similarly, separating patients with P. aeruginosa from other CF patients may reduce the spread of multiple-antibiotic-resistant strains capable of person-to-person transmission (16). Such strategies are contingent upon the early and correct identification of these organisms (30).
While there is much emphasis on misidentifying P. aeruginosa as another species (39), less attention is paid to falsely identifying other species as P. aeruginosa. Nevertheless, accurate identification of P. aeruginosa is important, as this may avoid prolonged and sometimes unnecessary antibiotic treatments, which could select for other antibiotic-resistant pathogens (6). Similarly, in CF clinics where cohort isolation is practiced as an infection control measure, false identification could mean exposure of the CF patient to potentially transmissible bacteria (2, 17, 28, 33).
While most clinical strains of P. aeruginosa are easily identified, respiratory isolates from patients with CF can present a taxonomic challenge (15, 24). Phenotypic identification of P. aeruginosa from patients with CF is often complicated by slow growth, auxotrophic metabolic activity, loss of pigment production, multiple antibiotic resistance, atypical colonial morphology, and development of mucoid exopolysaccharide (14, 25). Commercial identification platforms are also considered unreliable (18, 21, 39). Moreover, CF respiratory secretions may contain other nonfermenting gram-negative bacilli, such as Achromobacter, Stenotrophomonas, and Burkholderia species, which can further impede the identification of P. aeruginosa (29, 32, 35, 39).
Although several molecular strategies have been developed recently (1, 35, 39), most clinical microbiology laboratories still identify P. aeruginosa by traditional phenotypic techniques. However, there are few published data describing the frequency at which bacterial species in CF sputum are falsely identified as P. aeruginosa by phenotypic methods. In this study, we used P. aeruginosa-specific duplex real-time (PAduplex) PCR assays, phenotypic analysis, amplified rRNA gene restriction analysis (ARDRA), and partial 16S rRNA gene sequence analysis to assess the rate and extent of misidentification of P. aeruginosa isolates in CF sputum by Australian clinical microbiology laboratories.
MATERIALS AND METHODS
Study group and initial routine identification.
This study was part of an ongoing national multicenter prevalence study of P. aeruginosa clonal complexes in Australian CF clinics and was approved by human research ethics committees at each of the participating centers. Following informed consent, sputum samples were collected from patients attending routine outpatient appointments and during hospital admissions between September 2007 and September 2008.
Seventeen clinical microbiology laboratories servicing 10 large CF centers participated in the study. Nine were located in the capital cities of five Australian states, and another eight were in regional areas of one state (Table 1). The laboratories were surveyed and asked to describe the phenotypic, and any genotypic, methods they used to identify P. aeruginosa in CF sputum specimens.
TABLE 1.
Submitting laboratory, number of isolates submitted, and rates of misidentification for each participating laboratory and CF center
| Submitting laboratorya | Location of laboratoryb | No. of isolates submitted | No. of misidentifications | % Misidentification | CF centerc |
|---|---|---|---|---|---|
| A1 | R | 16 | 0 | 0.0 | A |
| A2 | R | 9 | 0 | 0.0 | A |
| A3 | R | 3 | 0 | 0.0 | A |
| A4 | R | 3 | 0 | 0.0 | A |
| A5 | R | 3 | 0 | 0.0 | A |
| A6 | R | 3 | 0 | 0.0 | A |
| A7 | C | 246 | 18 | 7.3d | A |
| A8 | R | 3 | 0 | 0.0 | A |
| A9 | C | 9 | 0 | 0.0 | B |
| A10 | C | 864 | 15 | 1.7 | C |
| A11 | C | 228 | 2 | 0.9 | D/Ee |
| A12 | R | 63 | 0 | 0.0 | B |
| B1 | C | 258 | 10 | 3.9 | F |
| C1 | C | 99 | 6 | 6.1 | G |
| D1 | C | 174 | 1 | 0.6 | H |
| D2 | C | 72 | 0 | 0.0 | I |
| E1 | C | 214 | 1 | 0.5 | J |
| Total | 2,267 | 53 | 2.3 | 10 |
Letters denote different Australian states, and numbers denote different clinical microbiology laboratories.
R, laboratory located in a regional area of Australia; C, laboratory located in a capital city of Australia.
Letters denote different CF centers.
API 20NE analysis of all potential P. aeruginosa isolates was initiated during the study. The misidentification rate prior to regular API analysis (n = 105 isolates, with eight negative results) was 7.6%; the misidentification rate after introduction of regular API analysis (n = 141, with 10 negative results) was 7.1% (χ2 = 0.25; P = 0.88).
Represents two CF centers submitting isolates to a single microbiology laboratory.
For the purposes of this study, laboratory staff selected three P. aeruginosa isolates cultured from each sputum specimen. Selection was based upon colony morphology (nonmucoid versus mucoid). Isolates were identified in accordance with local CF-related P. aeruginosa testing procedures. Overall, 561 CF patients (416 adults aged ≥18 years of age) provided 757 sputum specimens (188 patients gave two specimens 6 months apart, and 8 patients provided a second specimen after transfer to another center), from which 2,267 P. aeruginosa isolates were selected. Until transportation to a research laboratory, the isolates were stored at either −80°C in Protect vials (Oxoid Australia Pty. Ltd., Adelaide, Australia) or room temperature on nutrient agar slopes.
Specimen processing.
P. aeruginosa isolates were sent to one of two CF microbiology research laboratories, where they were processed by one of three scientists (T.K., K.R., and H.H.), using standardized techniques. Isolates were subcultured onto nutrient agar (Oxoid Australia Pty. Ltd.), checked for purity, and stored in 15% glycerol (Sigma-Aldrich Pty. Ltd., Castle Hill, Australia) at −80°C until being tested further.
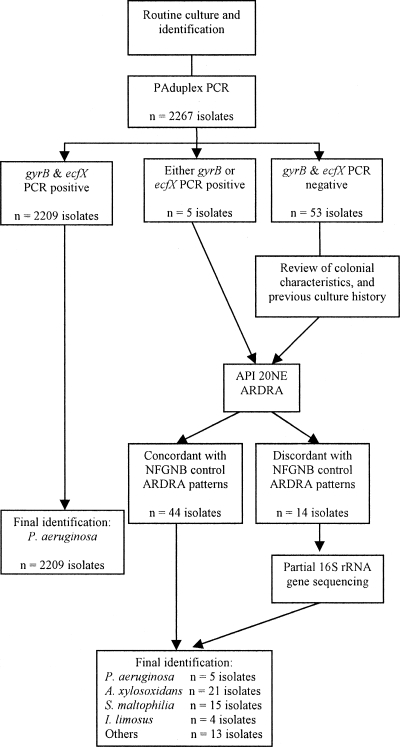
As outlined in Fig. 1, following DNA extraction, each isolate underwent PAduplex PCR (1). Isolates that produced amplicons for both PAduplex PCR targets were categorized as P. aeruginosa. Those showing negative or discordant PAduplex PCR results for the ecfX and gyrB targets had further testing by phenotypic methods and ARDRA. Finally, isolates with discordant PAduplex PCR results or those not reliably identified by ARDRA underwent partial 16S rRNA gene sequence analysis.
FIG. 1.
Algorithm for confirming Pseudomonas aeruginosa isolates. API, analytical profile index; ARDRA, amplified rRNA gene restriction analysis; NFGNB, nonfermenting gram-negative bacilli; PAduplex PCR, Pseudomonas aeruginosa-specific duplex PCR.
DNA extraction.
Bacterial isolates were suspended in 1 ml of 0.9% NaCl (Baxter Healthcare, NSW, Australia) and centrifuged at 10,970 × g for 3 min. The supernatant was thereafter removed, and the pellet was resuspended in 200 μl of 0.1 M Tris buffer (Sigma-Aldrich) and 40 μl of 10 mg/ml lysozyme (Sigma). Following incubation at 37°C for 1 hour, DNA from each lysate was extracted using the DX Universal Liquid Sample CorProtocol on a CAS-1820 X-Tractor gene instrument (Corbett Life Sciences, Sydney, Australia) as per the manufacturer's instructions.
PAduplex PCR analysis.
PAduplex PCR targeting the ecfX and gyrB genes was performed on each isolate as described previously (1). Each 25-μl reaction mix comprised 12.5 μl of Qiagen Quantitect probe master mix (Qiagen Pty. Ltd., Doncaster, Australia), 10 pmol of each primer (GeneWorks Pty. Ltd., SA, Australia), 4 pmol of each TaqMan probe (GeneWorks Pty. Ltd., Hindmarsh, Australia), and 2 μl of DNA template. Thermal cycling and amplicon detection was performed using an ABI Prism 7500 detection system (Applied Biosystems, Scoresby, Australia).
ARDRA.
ARDRA was performed as described previously (20, 34). Briefly, each 25-μl PCR mix contained 5 μl of template DNA, 2.5 μl of 10× Qiagen PCR buffer (Qiagen Pty. Ltd.), 1.5 mM MgCl2, a 20 mM concentration of each deoxynucleoside triphosphate (GE Healthcare, Australia), 1 U of Taq DNA polymerase (Qiagen Pty. Ltd.), 11.8 μl of H2O, and 20 pmol (each) of the oligonucleotide primers fD1 and rD1 (GeneWorks Pty. Ltd.). Thermal cycling was performed in a Bio-Rad Mycycler personal thermal cycler (Bio-Rad Laboratories Pty. Ltd., Gladesville, Australia). Restriction enzyme digestion (37°C for 1 hour) was performed using the enzymes DdeI and MspI (Roche Diagnostics Australia Pty. Ltd., Castle Hill, Australia). Following agarose gel electrophoresis (3% MS agarose [Roche Diagnostics Australia Pty. Ltd.] at 100 V for 60 min), each isolate's ARDRA profile was compared to those produced by a collection of commonly encountered CF and related nonfermenting gram-negative control strains (7, 20, 34). The control strain panel consisted of P. aeruginosa LMG 6395, Stenotrophomonas maltophilia LMG 957, Achromobacter xylosoxidans LMG 1863, Burkholderia cepacia LMG 1222, Burkholderia multivorans LMG 18822, Burkholderia stabilis LMG 14294, Burkholderia vietnamiensis LMG 18836, Burkholderia ambifaria LMG 19467, Burkholderia gladioli LMG 11626, B. gladioli LMG 2121, Ralstonia pickettii LMG 5942, Ralstonia solanacearum LMG 2299, Cupriavidus necator LMG 1199, Pandoraea norimbergensis LMG 18379, and Inquilinus limosus wild type. Isolates that exhibited ARDRA profiles identical to those of the control strains were identified as those particular species. Isolates that exhibited an atypical ARDRA profile compared to the control strains were analyzed by partial 16S rRNA gene sequencing.
Partial 16S rRNA gene sequencing.
Initial 16S rRNA gene PCR primers, reagents, and conditions were identical to those described for the ARDRA assay. Sequencing PCR (i.e., forward and reverse) of the undigested amplicons was performed using the oligonucleotide primers fD1 and rD1 (34) and the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions. Postreaction cleanup was undertaken using the Agencourt CleanSEQ sequencing purification system (Beckman Coulter Australia Pty. Ltd., Gladesville, Australia) according to the manufacturer's instructions. Capillary separation was achieved using an AB3730xl genetic analyzer (Applied Biosystems).
16S rRNA gene sequences were compared with those available in the GenBank, EMBL, and DDBJ databases. The overall quality of each sequence was verified by Sequence Scanner v1.0 (Applied Biosystems) software, and sequence editing was performed using BioEdit Sequence Alignment Editor, version 7.0.5.3. The identity of each edited sequence was analyzed with BLASTN. Isolates were allocated to a genus and/or species if the sequence yielded a similarity score of ≥98%.
Phenotypic identification.
Bacterial isolates were tested with an API 20NE V7.0 kit (bioMérieux Australia Pty. Ltd., Baulkam Hills, Australia) according to the manufacturer's instructions. Oxidase activity was checked with 1% tetramethyl p-phenylenediamine (Oxoid Australia Pty. Ltd., Osborne Park, Australia). Isolates that produced a violet to purple pigment within 10 to 15 s were recorded as oxidase positive. False-positive reactions for oxidase-negative isolates were also observed and recorded at 30 s. Interpretation of the oxidase and API results was performed after 48 h of incubation, using APILAB PLUS software (version 3.3.3). Pigmentation, colony characteristics (mucoid or nonmucoid), and colistin susceptibility were also recorded for each isolate analyzed by the API 20NE system. Susceptibility to colistin sulfate (Oxoid Australia Pty. Ltd.) was determined following Clinical Laboratory Standards Institute disk diffusion guidelines and interpretative breakpoints (8).
Retrospective analysis of patients with misidentified isolates.
A retrospective review of the respiratory culture history for each patient with an isolate misidentified as P. aeruginosa was also performed. Patients were recorded as “previously infected with a misidentified isolate” if the misidentified species confirmed in this study had been found in their sputum within two previous years of the study's sample collection.
Statistical analysis.
All patient-specific data were stored in a customized database (Filemaker Pro Advanced 9.0v3; Claris Corporation, Santa Clara, CA), and analysis was performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL). Comparison between categorical variables used χ2 and Spearman correlation. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Study group and initial routine identification.
Each participating laboratory provided details of how they identified P. aeruginosa isolated from CF sputum (Table 2). Eleven (65%) laboratories used a combination of basic phenotypic traits, five employed Vitek (1 or 2), and another introduced API 20NE testing during the study. Thirteen laboratories reported using Vitek (1 or 2) or API 20NE testing when atypical isolates were encountered (isolates lacking phenotypic characteristics typical of P. aeruginosa, such as colony morphology and growth at 42°C). Molecular identification of P. aeruginosa was not regularly performed by any laboratory, although five reported its application for atypical isolates.
TABLE 2.
Frequency and nature of methods used to identify Pseudomonas aeruginosa by the 17 participating clinical microbiology laboratories
| Identification system | No. of laboratories
|
|
|---|---|---|
| Routine usea | Occasional useb | |
| Oxidase activity | 17 | |
| Colony morphology | 17 | |
| Pigmentation | 7 | |
| Growth at 42°C | 11 | |
| Colistin susceptibility | 4c | |
| Previous culture history | 2 | |
| Vitek 1 | 2 | 1 |
| Vitek 2 | 3 | 3 |
| API 20NE | 1d | 9e |
| Fluorescence on centrimide agar | 1 | |
| C390 susceptibility | 1 | 1 |
| Chromogenic agar | 2 | |
| Arginine hydrolysis | 1 | |
| Fatty acid analysis | 1 | |
| Molecular identification | 5 | |
Routinely used to identify all potential P. aeruginosa isolates.
Occasionally used when isolates lacked typical phenotypic features of P. aeruginosa.
CLSI disk diffusion testing was performed in three laboratories, and CLSI agar dilution testing was used by one laboratory.
Routine API 20NE use was initiated during the course of the study.
Includes one laboratory which routinely used Vitek 2 for all potential isolates and API 20NE testing when atypical isolates arose.
PAduplex analysis.
Of the 2,267 isolates, 2,209 (97.4%) were confirmed as P. aeruginosa by PCR (both gyrB and ecfX targets were present), leaving 5 (0.2%) with discordant results (only one of the PCR targets detected) and 53 (2.3%) with negative results (neither target identified) by the PAduplex PCR assay (Table 3). Of the isolates that were negative for one or both of the PAduplex PCR targets, no amplification product was detected.
TABLE 3.
PAduplex PCR, API 20NE, ARDRA, and partial 16S rRNA gene sequencing results for the 58 isolates that were discordant or negative by the PAduplex PCR assay
| Group | No. of isolates | No. of patients | Result
|
Final identification | |||||
|---|---|---|---|---|---|---|---|---|---|
| PAduplex PCR
|
Oxidasea | API 20NE | ARDRA | Partial 16S rRNA gene sequencing | |||||
| gyrB | ecfX | ||||||||
| 1 | 3 | 1 | − | − | + | Alcaligenes faecalis (59.3%); low discrimination level | A. xylosoxidans | Not performed | Achromobacter xylosoxidans |
| 13 | 8 | − | − | + | Achromobacter xylosoxidans (94.5%); excellent or good identification | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| 1 | 1 | − | − | + | Burkholderia pseudomallei (81.7%); very good identification to the genus level | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| 1 | 1 | − | − | + | Mannheimia hemolytica/Pastuerella trehalosi (92.9%); good identification | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| 1 | 1 | − | − | + | Pseudomonas stutzeri (76.0%); doubtful profile | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| 1 | 1 | − | − | + | Unacceptable profile | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| 1 | 1 | − | − | + | Comamonas testosteroni/Pseudomonas alcaligenes (57.4%); low discrimination level | A. xylosoxidans | Not performed | Achromobacter xylosoxidans | |
| Total | 21 | 14 | |||||||
| 2 | 5 | 3 | − | − | − | Stenotrophomonas maltophilia (99.9%); excellent or very good identification | S. maltophilia | Not performed | Stenotrophomonas maltophilia |
| 6 | 2 | − | − | − | Stenotrophomonas maltophilia (99.9%); doubtful profile | S. maltophilia | Not performed | Stenotrophomonas maltophilia | |
| 1 | 1 | − | − | − | Unacceptable profile | S. maltophilia | Not performed | Stenotrophomonas maltophilia | |
| 1 | 1 | − | − | − | Weeksella virosa/Empedobacter brevis (64.0%); low discrimination level | S. maltophilia | Not performed | Stenotrophomonas maltophilia | |
| 2 | 1 | − | − | − | Stenotrophomonas maltophilia (99.9%); excellent identification | Indeterminate | Stenotrophomonas maltophilia (100%); 815 bp | Stenotrophomonas maltophilia | |
| Total | 15 | 8 | |||||||
| 3 | 1 | 1 | + | − | + | Burkholderia cepacia (99.9%); good identification, possibility of B. gladioli | P. aeruginosa | Pseudomonas aeruginosa (100%); 723 bp | Pseudomonas aeruginosa |
| 2 | 1 | + | − | + | Pseudomonas aeruginosa (97.9%); acceptable identification | P. aeruginosa | Pseudomonas aeruginosa (100%); 787 bp | Pseudomonas aeruginosa | |
| 1 | 1 | + | − | + | Unacceptable profile | P. aeruginosa | Pseudomonas aeruginosa (100%); 782 bp | Pseudomonas aeruginosa | |
| 1 | 1 | − | + | + | Pseudomonas aeruginosa (99.9%); very good identification | P. aeruginosa | Pseudomonas aeruginosa (100%); 870 bp | Pseudomonas aeruginosa | |
| 2 | 1 | − | − | + | Pseudomonas fluorescens (99.9%); very good identification | Indeterminate | Pseudomonas fluorescens/synxantha/reactans (100%); 827bp | Pseudomonas spp. | |
| 1 | 1 | − | − | + | Pseudomonas stutzeri (99.2%); very good identification | Indeterminate | Pseudomonas mendocina (100%); 793 bp | Pseudomonas mendocina | |
| Total | 8 | 6 | |||||||
| 4 | 1 | 1 | − | − | − | Pseudomonas luteola (92.0%); acceptable identification | Indeterminate | Pantoea agglomerans (100%); 867 bp | Pantoea agglomerans |
| 4 | 2 | − | − | + | Sphingomonas paucimobilis (96.8%); good identification | I. limosus | Not performed | Inquilinus limosus | |
| 3 | 1 | − | − | + | Rhizobium radiobacter (99.9%); very good identification | Indeterminate | Rhizobium radiobacter (100%); 864 bp | Rhizobium radiobacter | |
| 2 | 1 | − | − | + | Watersia paucula (47.6%); low discrimination level | Indeterminate | Cupriavidus respiraculi (100%); 824 bp | Cupriavidus respiraculi | |
| 2 | 2 | − | − | − | Unacceptable profile | Indeterminate | Serrata marcescens (100%); 751 bp | Serrata marcescens | |
| 1 | 1 | − | − | − | Burkholderia cepacia (99.9%); excellent identification, possibility of B. gladioli | B. gladioli | Not performed | Burkholderia gladioli | |
| 1 | 1 | − | − | + | Methylobacterium mesophilicum (84.5%); acceptable identification, possibility of Ochrobactrum anthropi | Indeterminate | Ralstonia mannitolilytica (100%); 803 bp | Ralstonia mannitolilytica | |
| Total | 14 | 9 | |||||||
A positive oxidase test was recorded only if an isolate produced a violet to purple pigment within 10 to 15 s.
Phenotypic analysis.
Of the 58 isolates with discordant or negative PAduplex PCR results, 39 (67%) had a positive oxidase reaction within the prescribed 15 s (Table 3). False-positive reactions were seen for 8 (42%) of the 19 oxidase-negative isolates. API results successfully identified (excellent to acceptable profiles) the following species in 38 (66%) isolates: A. xylosoxidans, Mannheimia haemolytica/Pasteurella trehalosi, S. maltophilia, B. cepacia, P. aeruginosa, Pseudomonas fluorescens, Pseudomonas stutzeri, Pseudomonas luteola, Sphingomonas paucimobilis, and Methylobacterium mesophilicum (Table 3). Of the remaining 20 isolates, 1 had a very good identification to the genus level and 19 had API results with low discrimination or doubtful or unacceptable profiles (Table 3).
Among the 58 isolates, 48 (74%) had nonmucoid colonies and 10 had mucoid colonies. Thirty (52%) isolates had nonmucoid and nonpigmented colony morphotypes. Thirteen isolates exhibited yellow- or pale brown-pigmented nonmucoid colonies; one mucoid and five nonmucoid colonies had green pigmentation, while nine were nonpigmented mucoid colonies. Ten (17%) isolates were resistant to colistin.
ARDRA.
Of the 58 isolates analyzed by ARDRA, 44 (76%) showed DdeI and MspI restriction patterns identical to those of one of the control strains (Table 3). A. xylosoxidans (21 isolates), S. maltophilia (13 isolates), I. limosus (4 isolates), and B. gladioli (1 isolate) were confirmed by ARDRA. All five isolates with discordant PAduplex PCR results were identified as P. aeruginosa. Overall, ARDRA failed to reliably identify 14 (24%) isolates.
16S rRNA gene sequencing.
Partial 16S rRNA gene sequencing was performed on the 14 isolates with indeterminate ARDRA profiles and the 5 discordant isolates by PAduplex PCR assay (Table 3). The mean sequence length after manual editing was 809 nucleotides (range, 723 to 870 nucleotides).
Seventeen of 19 (89%) isolates were identified to an individual genus and species. Forward and reverse sequences obtained from each of these isolates exhibited 100% homology with those available in the GenBank, EMBL, or DDBJ database, including sequences for the following species: S. maltophilia, Cupriavidus respiraculi, Serratia marcescens, Ralstonia mannitolilytica, Pseudomonas mendocina, Pantoea agglomerans, and P. aeruginosa (Table 3). Two isolates with 100% homology with sequences for P. fluorescens, Pseudomonas synxantha, and Pseudomonas reactans were identified as Pseudomonas spp. (Table 3).
Comparison of molecular and API 20NE analysis.
API 20NE results for 34 (59%) of the 58 isolates were consistent with ARDRA and/or partial 16S rRNA gene sequence analysis (Table 3). Of the 38 isolates adequately identified by API testing (excellent to acceptable profiles), only 29 (76%) were confirmed by molecular testing. Altogether, just 13 (62%) A. xylosoxidans and 7 (47%) S. maltophilia isolates were successfully identified by API testing. All four I. limosus isolates had mucoid colonies and were misidentified by API testing as S. paucimobilis (≥96.8%; good identification).
Final identification and evaluation of the misidentified isolates.
Virtually all (2,214/2,267 isolates [97.7%]) bacterial isolates submitted by clinical microbiology laboratories were correctly identified as P. aeruginosa. Misidentification rates ranged from 0.0% to 7.3% (Table 1) and were higher for laboratories processing larger numbers of isolates for the study (r = 0.88; P < 0.0001). Overall, 30 (19 adults) of the 561 (5.3%) CF patients had isolates misclassified as P. aeruginosa. While all adults were known to be chronically infected with P. aeruginosa, for five children the culture results were falsely diagnosed as a new P. aeruginosa infection. Of the 30 patients, 12 provided specimens on two separate occasions, although only 1 of the 12 (8.3%) had isolates misidentified in both samples. Nevertheless, when prior microbiology data for the 30 patients were reviewed from the preceding 2 years, 12 (40%) had the misidentified species cultured on at least one other occasion.
Table 3 shows that A. xylosoxidans (40%), S. maltophilia (28%), and I. limosus (7.5%) were the most commonly misidentified species. The other misidentified isolates were three Rhizobium radiobacter isolates, two each of Pseudomonas spp., C. respiraculi, and S. marcescens, and one isolate each of B. gladioli, P. mendocina, and P. agglomerans.
All A. xylosoxidans isolates had nonpigmented, nonmucoid colony morphology and were oxidase positive and susceptible to colistin. Each S. maltophilia isolate was oxidase negative within the prescribed 10 to 15 s, although 53% had a false-positive reaction at 30 s. Their colony morphology was nonmucoid, with either yellow (n = 9), pale brown (n = 3), or no (n = 3) pigmentation. Thirteen (87%) S. maltophilia isolates were susceptible to colistin. In contrast, all I. limosus isolates produced highly mucoid, nonpigmented colonies, and along with B. gladioli, R. mannitolilytica, and S. marcescens isolates, they demonstrated resistance to colistin.
Of the misidentified isolates, 66% originated from laboratories relying predominantly upon basic phenotypic tests to identify P. aeruginosa. These isolates included A. xylosoxidans (n = 15), S. maltophilia (n = 11), R. radiobacter (n = 3), Pseudomonas spp. (n = 2), and one each of B. gladioli, I. limosus, P. mendocina, and P. agglomerans.
DISCUSSION
Identifying P. aeruginosa clinical isolates is usually straightforward and easily accomplished by simple phenotypic tests. However, accurate identification of P. aeruginosa strains from CF sputum is made difficult when chronic infection becomes established, as strains undergo a series of phenotypic changes during adaptation to the CF lung (14, 26). Furthermore, patients may harbor several other phenotypically similar species, including A. xylosoxidans, S. maltophilia, Burkholderia spp., Ralstonia spp., Pandoraea spp., and I. limosus (14). Accurate differentiation of these organisms can critically influence patient management (11, 35, 39). Several recent studies have assessed the accuracy of identification practices for CF-related nonfermenting gram-negative bacilli. However, most have focused upon either commercial and molecular assays or pathogens other than P. aeruginosa (4, 23, 32, 39).
The present study showed that when Australian clinical microbiology laboratories identified bacterial isolates from CF sputum as P. aeruginosa, they were correct on almost 98% of occasions. When the laboratories were wrong, the three most common species misidentified as P. aeruginosa were A. xylosoxidans, S. maltophilia, and I. limosus. These three species are regarded as emerging pathogens, although their pathogenic role in CF lung disease is unclear and subject to controversy, especially for patients already chronically infected with P. aeruginosa (3, 9, 19, 31, 36, 38). Fortunately, none of the misidentified bacteria included recognized gram-negative bacillary CF pathogens from the B. cepacia complex (12). Nevertheless, five children received arguably unnecessarily prolonged and aggressive antimicrobial therapy and may have accidentally been exposed to patients infected with dominant P. aeruginosa clonal complexes (2, 17, 28, 33, 37).
Recent reports highlight difficulties with using standard phenotypic identification techniques to differentiate P. aeruginosa from A. xylosoxidans (9, 32). In the present study, misidentified A. xylosoxidans isolates had a nonmucoid and nonpigmented colony appearance and were susceptible to colistin. Provided there was adherence to a stringent time interval, all S. maltophilia isolates detected in this study were oxidase negative. False-positive oxidase reactivity among S. maltophilia isolates has previously been implicated as contributing to misidentification of B. cepacia complex isolates (5). Extracellular DNase activity is another important phenotypic feature which can help to differentiate this species from other gram-negative bacteria (14). Therefore, stringent oxidase testing and testing for DNase may reduce misidentification of S. maltophilia.
I. limosus has only recently been reported for a small number of patients with CF (3, 7, 38). Its phenotypic characteristics include failure to grow on MacConkey agar, colistin resistance, a predominantly mucoid colony appearance, growth on blood agar at 42°C, and classification as either S. paucimobilis or Agrobacterium radiobacter by API 20NE testing (7). The four I. limosus isolates reported here had these features and exhibited previously reported ARDRA profiles (7). These isolates were collected from two female patients (aged 20 and 36 years) whose forced expiratory volume in 1 s percent predicted (FEV1%) values were 78.4 and 91.9%, respectively, in two different centers. One patient was coinfected with P. aeruginosa, Staphylococcus aureus, and Aspergillus fumigatus, and the other was coinfected with P. aeruginosa. To our knowledge, this is the first published account of this species in Australian CF patients. Of interest, three of these isolates collected from one patient were submitted by a laboratory which did not employ routine colistin susceptibility testing. The introduction of routine colistin susceptibility testing and follow-up molecular testing for resistant isolates may reduce misidentification of I. limosus.
Molecular identification strategies for identifying CF-related bacterial isolates have been reported previously (11, 29, 34, 39). In this study, PAduplex PCR provided definitive results (concordant positive or negative results for both the ecfX and gyrB targets) for 2,262 (99.8%) isolates. Of those with negative results, all were subsequently confirmed by ARDRA and/or sequencing to represent a genus and species other than P. aeruginosa. The five isolates with discordant PAduplex PCR results were all identified as P. aeruginosa. Reasons for the incomplete reactivity of these isolates are unexplained, but we intend to perform sequence analysis, as variation within the ecfX and gyrB sequences may be responsible, highlighting the importance of performing PCR assays targeted at multiple genomic sites, thereby reducing the potential for false-negative results (40).
API 20NE results for just over half of the misidentified isolates were consistent with molecular testing results, confirming the inadequacies of commercial identification systems for nonfermenting gram-negative bacilli (18, 21, 39). One laboratory that relied on oxidase testing, colony morphology, and growth at 42°C introduced routine API 20NE analysis during the study period, but this did not improve misidentification rates.
A study strength was its prospective and multicenter design. There were significant differences in misidentification rates between large laboratories in capital cities and smaller regional laboratories from a single state. Some of the differences are likely to be related to differences in the numbers of samples submitted for testing. The results, however, represent the range of diagnostic accuracies in identifying P. aeruginosa isolates from the sputa of CF patients in Australian clinical microbiology laboratories and are likely to be representative of practices in this region. The study also has several limitations. The primary focus was to investigate isolates identified by clinical microbiology laboratories as P. aeruginosa. Isolates reported as species other than P. aeruginosa were not submitted and were outside the scope of the study. It is likely that some P. aeruginosa isolates were misidentified as other species, but this has been reviewed elsewhere (23, 32, 39). Second, selection of colonies for analysis by morphological criteria may have introduced a sample bias, although this is likely to have been minimized by the sample size. Third, we reviewed identification procedures used by each laboratory, but this analysis did not account for other factors which may impact misidentification rates (e.g., time and resource constraints, local laboratory staff experience, and numbers of staff handling the samples in the clinical laboratories). Finally, all isolates that exhibited positive PAduplex PCR results were identified as P. aeruginosa. This study has not assessed the specificity of PAduplex PCR, but in earlier work no cross-reactivity using the ecfX and gyrB targets was noted (1, 22, 29).
To our knowledge, this study represents the most extensive analysis of misidentification rates for P. aeruginosa isolates collected from patients with CF. It demonstrated very low rates of misidentification among P. aeruginosa isolates collected from a broad cross section of CF patients, suggesting that most isolates are accurately identified in the clinical laboratory. Furthermore, within individual patients, microbiology laboratories will often have previously identified the organism misdiagnosed as P. aeruginosa. In this study, A. xylosoxidans, S. maltophilia, and I. limosus were the most commonly misidentified species. Performance of culture history review, attention to colony morphology, and undertaking stringent oxidase testing and DNase and colistin susceptibility analysis should be considered for all presumptive P. aeruginosa isolates. Those isolates exhibiting atypical phenotypic characteristics should be evaluated further by additional phenotypic or genotypic identification techniques.
Acknowledgments
We are grateful to all of the CF center directors, CF research coordinators, and clinical microbiology laboratories at each of the study sites. Participating laboratories included Pathology Queensland (PQ)—Central; PQ—The Prince Charles Hospital; PQ—Townsville Hospital; PQ—Mackay Hospital; PQ—Cairns Hospital; PQ—Toowoomba Hospital; PQ—Bundaberg Hospital; PQ—Nambour Hospital; PQ—Maryborough Hospital; PQ—Gold Coast Hospital; Microbiology Department, Mater Health Services Pathology; Microbiology Department, Sullivan Nicolaides Pathology; Microbiology Department, PathWest Laboratory Medicine, QEII Medical Centre; Infectious Diseases Laboratory, Institute of Medical and Veterinary Science; Department of Microbiology and Infectious Diseases, Women's and Children's Hospital; Microbiology & Infectious Diseases, Royal Children's Hospital Melbourne; and Department of Microbiology, Central Sydney Laboratory Service.
Additional ACPinCF Investigators included Adam Jaffe, Sydney Children's Hospital; Tonia Douglas, Princess Margaret Hospital for Children; Peter Cooper, The Children's Hospital at Westmead; Gerard Ryan, Sir Charles Gairdner Hospital; David Reid, Royal Hobart Hospital; Peter Wark, John Hunter Adult Hospital; Bruce Whitehead, John Hunter Children's Hospital; Hugh Greville, Royal Adelaide Hospital; David Serisier, Mater Adult Hospital; Carolyn Dakin, Mater Children's Hospital; Iain Feather, Gold Coast Hospital; Darrell Price, Gold Coast Hospital; James Martin, Women's and Children's Hospital; John Wilson, The Alfred Hospital; and David Armstrong, Monash Medical Centre.
This work was supported by National Health and Medical Research Council project grant 455919, the Australian Cystic Fibrosis Research Trust, The Prince Charles Hospital Foundation, and Rotary Australia.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Anuj, S. N., D. M. Whiley, T. J. Kidd, S. C. Bell, C. E. Wainwright, M. D. Nissen, and T. P. Sloots. 2009. Identification of Pseudomonas aeruginosa by a duplex real-time polymerase chain reaction assay targeting the ecfX and the gyrB genes. Diagn. Microbiol. Infect. Dis. 63127-131. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. S., G. M. Nixon, R. Carzino, A. Bigham, J. B. Carlin, R. M. Robins-Browne, and K. Grimwood. 2002. Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am. J. Respir. Crit. Care Med. 166983-987. [DOI] [PubMed] [Google Scholar]
- 3.Bittar, F., A. Leydier, E. Bosdure, A. Toro, M. Reynaud-Gaubert, S. Boniface, N. Stremler, J. C. Dubus, J. Sarles, D. Raoult, and J. M. Rolain. 2008. Inquilinus limosus and cystic fibrosis. Emerg. Infect. Dis. 14993-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosshard, P. P., R. Zbinden, S. Abels, B. Boddinghaus, M. Altwegg, and E. C. Bottger. 2006. 16S rRNA gene sequencing versus the API 20 NE system and the VITEK 2 ID-GNB card for identification of nonfermenting gram-negative bacteria in the clinical laboratory. J. Clin. Microbiol. 441359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdge, D. R., M. A. Noble, M. E. Campbell, V. L. Krell, and D. P. Speert. 1995. Xanthomonas maltophilia misidentified as Pseudomonas cepacia in cultures of sputum from patients with cystic fibrosis: a diagnostic pitfall with major clinical implications. Clin. Infect. Dis. 20445-448. [DOI] [PubMed] [Google Scholar]
- 6.Burns, J. L. 2002. Emergence of new pathogens in CF: the devil we know or the devil we don't know? J. Pediatr. 140283-284. [DOI] [PubMed] [Google Scholar]
- 7.Chiron, R., H. Marchandin, F. Counil, E. Jumas-Bilak, A. M. Freydiere, G. Bellon, M. O. Husson, D. Turck, F. Bremont, G. Chabanon, and C. Segonds. 2005. Clinical and microbiological features of Inquilinus sp. isolates from five patients with cystic fibrosis. J. Clin. Microbiol. 433938-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing. Seventeenth informational supplement. Document M100-S17. CLSI, Wayne, PA.
- 9.De Baets, F., P. Schelstraete, S. Van Daele, F. Haerynck, and M. Vaneechoutte. 2007. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J. Cyst. Fibros. 675-78. [DOI] [PubMed] [Google Scholar]
- 10.Emerson, J., M. Rosenfeld, S. McNamara, B. Ramsey, and R. L. Gibson. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 3491-100. [DOI] [PubMed] [Google Scholar]
- 11.Ferroni, A., I. Sermet-Gaudelus, E. Abachin, G. Quesne, G. Lenoir, P. Berche, and J. L. Gaillard. 2002. Use of 16S rRNA gene sequencing for identification of nonfermenting gram-negative bacilli recovered from patients attending a single cystic fibrosis center. J. Clin. Microbiol. 403793-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangolias, D. D., E. Mahenthiralingam, S. Rae, J. M. Raboud, A. G. Davidson, R. Wittmann, and P. G. Wilcox. 1999. Burkholderia cepacia in cystic fibrosis. Variable disease course. Am. J. Respir. Crit. Care Med. 1601572-1577. [DOI] [PubMed] [Google Scholar]
- 13.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168918-951. [DOI] [PubMed] [Google Scholar]
- 14.Gilligan, P. H., D. L. Kiska, and M. D. Appleman. 2006. Cumitech 43, Cystic fibrosis microbiology, vol. 43. ASM Press, Washington, DC.
- 15.Govan, J. R., A. R. Brown, and A. M. Jones. 2007. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2153-164. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths, A. L., K. Jamsen, J. B. Carlin, K. Grimwood, R. Carzino, P. J. Robinson, J. Massie, and D. S. Armstrong. 2005. Effects of segregation on an epidemic Pseudomonas aeruginosa strain in a cystic fibrosis clinic. Am. J. Respir. Crit. Care Med. 1711020-1025. [DOI] [PubMed] [Google Scholar]
- 17.Johansen, H. K., S. M. Moskowitz, O. Ciofu, T. Pressler, and N. Hoiby. 2008. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. J. Cyst. Fibros. 7391-397. [DOI] [PubMed] [Google Scholar]
- 18.Joyanes, P., M. del Carmen Conejo, L. Martinez-Martinez, and E. J. Perea. 2001. Evaluation of the VITEK 2 system for the identification and susceptibility testing of three species of nonfermenting gram-negative rods frequently isolated from clinical samples. J. Clin. Microbiol. 393247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanellopoulou, M., S. Pournaras, H. Iglezos, N. Skarmoutsou, E. Papafrangas, and A. N. Maniatis. 2004. Persistent colonization of nine cystic fibrosis patients with an Achromobacter (Alcaligenes) xylosoxidans clone. Eur. J. Clin. Microbiol. Infect. Dis. 23336-339. [DOI] [PubMed] [Google Scholar]
- 20.Kidd, T. J., S. C. Bell, and C. Coulter. 2003. Genomovar diversity amongst Burkholderia cepacia complex isolates from an Australian adult cystic fibrosis unit. Eur. J. Clin. Microbiol. Infect. Dis. 22434-437. [DOI] [PubMed] [Google Scholar]
- 21.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavenir, R., D. Jocktane, F. Laurent, S. Nazaret, and B. Cournoyer. 2007. Improved reliability of Pseudomonas aeruginosa PCR detection by the use of the species-specific ecfX gene target. J. Microbiol. Methods 7020-29. [DOI] [PubMed] [Google Scholar]
- 23.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 1171661-1665. [DOI] [PubMed] [Google Scholar]
- 24.Miller, M. B., and P. H. Gilligan. 2003. Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J. Clin. Microbiol. 414009-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller. 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 26.Murray, T. S., M. Egan, and B. I. Kazmierczak. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 1983-88. [DOI] [PubMed] [Google Scholar]
- 27.Nixon, G. M., D. S. Armstrong, R. Carzino, J. B. Carlin, A. Olinsky, C. F. Robertson, and K. Grimwood. 2001. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 138699-704. [DOI] [PubMed] [Google Scholar]
- 28.O'Carroll, M. R., M. W. Syrmis, C. E. Wainwright, R. M. Greer, P. Mitchell, C. Coulter, T. P. Sloots, M. D. Nissen, and S. C. Bell. 2004. Clonal strains of Pseudomonas aeruginosa in paediatric and adult cystic fibrosis units. Eur. Respir. J. 24101-106. [DOI] [PubMed] [Google Scholar]
- 29.Qin, X., J. Emerson, J. Stapp, L. Stapp, P. Abe, and J. L. Burns. 2003. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. J. Clin. Microbiol. 414312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratjen, F. 2006. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 12428-432. [DOI] [PubMed] [Google Scholar]
- 31.Ronne Hansen, C., T. Pressler, N. Hoiby, and M. Gormsen. 2006. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J. Cyst. Fibros. 5245-251. [DOI] [PubMed] [Google Scholar]
- 32.Saiman, L., Y. Chen, S. Tabibi, P. San Gabriel, J. Zhou, Z. Liu, L. Lai, and S. Whittier. 2001. Identification and antimicrobial susceptibility of Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. J. Clin. Microbiol. 393942-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott, F. W., and T. L. Pitt. 2004. Identification and characterization of transmissible Pseudomonas aeruginosa strains in cystic fibrosis patients in England and Wales. J. Med. Microbiol. 53609-615. [DOI] [PubMed] [Google Scholar]
- 34.Segonds, C., T. Heulin, N. Marty, and G. Chabanon. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 372201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spilker, T., T. Coenye, P. Vandamme, and J. J. LiPuma. 2004. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 422074-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talmaciu, I., L. Varlotta, J. Mortensen, and D. V. Schidlow. 2000. Risk factors for emergence of Stenotrophomonas maltophilia in cystic fibrosis. Pediatr. Pulmonol. 3010-15. [DOI] [PubMed] [Google Scholar]
- 37.Treggiari, M. M., M. Rosenfeld, G. Retsch-Bogart, R. Gibson, and B. Ramsey. 2007. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr. Pulmonol. 42751-756. [DOI] [PubMed] [Google Scholar]
- 38.Wellinghausen, N., A. Essig, and O. Sommerburg. 2005. Inquilinus limosus in patients with cystic fibrosis, Germany. Emerg. Infect. Dis. 11457-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wellinghausen, N., J. Kothe, B. Wirths, A. Sigge, and S. Poppert. 2005. Superiority of molecular techniques for identification of gram-negative, oxidase-positive rods, including morphologically nontypical Pseudomonas aeruginosa, from patients with cystic fibrosis. J. Clin. Microbiol. 434070-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiley, D. M., S. B. Lambert, S. Bialasiewicz, N. Goire, M. D. Nissen, and T. P. Sloots. 2008. False-negative results in nucleic acid amplification tests—do we need to routinely use two genetic targets in all assays to overcome problems caused by sequence variation? Crit. Rev. Microbiol. 3471-76. [DOI] [PubMed] [Google Scholar]