Abstract
The rapid identification of mycobacteria from culture is of primary importance for the administration of empirical antibiotic therapy and for the implementation of public health measures, yet there are few commercially available assays that can easily and accurately identify the mycobacteria in culture in a timely manner. Here we report on the development of a multiplex, real-time PCR assay that can identify 93% of the pathogenic mycobacteria in our laboratory in two parallel reactions. The mycobacteria identified by this assay include the Mycobacterium tuberculosis complex (MTC), the M. avium complex (MAC), the M. chelonae-M. abscessus group (MCAG), the M. fortuitum group (MFG), and M. mucogenicum. The primer targets included the 16S rRNA gene and the internal transcribed spacer. The assay was initially validated with a repository of reference strains and was subsequently tested with 314 clinical cultures identified by the AccuProbe assay or high-performance liquid chromatography. Of the 314 cultures tested, multiplex, real-time PCR produced congruent results for 99.8% of the 1,559 targets evaluated. The sensitivity and the specificity were each 99% or greater for MTC (n = 96), MAC (n = 97), MCAG (n = 68), and M. mucogenicum (n = 9) and 95% and 100%, respectively, for MFG (n = 19). We conclude that this multiplex, real-time PCR assay is a useful diagnostic tool for the rapid and accurate identification of MTC and clinically relevant nontuberculous mycobacteria.
Mycobacterium tuberculosis, the causative agent of tuberculosis, is among the leading infectious causes of death in developing nations (23). In resource-rich countries, where tuberculosis is not endemic, nontuberculous mycobacteria (NTM) are responsible for the majority of mycobacterial infections in both immunocompromised and immunocompetent individuals (14). The clinical gravity of mycobacterial infections necessitates rapid isolation by culture and timely identification by the appropriate diagnostic assays. Rapid identification not only serves to focus empirical antibiotic therapy and thus avoid unnecessary drug exposure but also may aid with the appropriate respiratory isolation and prevention of secondary cases (15).
Few molecular methods commercially available in the United States easily and rapidly identify mycobacteria in culture. Although the AccuProbe assay by Gen-Probe (San Diego, CA) is sufficient for the identification of isolates of the Mycobacterium tuberculosis complex (MTC) and the M. avium complex (MAC) (37), it lacks probe sets specific for rapidly growing mycobacteria, such as the M. chelonae-M. abscessus group (MCAG) and the M. fortuitum group (MFG), which make up approximately 30% of the pathogenic mycobacteria in the Clinical Microbiology Laboratory of Stanford Hospital (Stanford, CA). MCAG consists of M. chelonae, M. abscessus, and M. immunogenum; MFG consists of M. fortuitum, M. senegalense, M. farcinogenes, M. porcinum, M. septicum, M. peregrinum, and M. alvei (11). For these isolates, other methods, such as biochemical reactions (6, 33), high-performance liquid chromatography (HPLC) (13), and DNA sequencing (17), are required for identification. To address the need for a simple and rapid molecular assay with a broader identification scope, we developed a multiplex, real-time PCR assay that can identify 93% of the pathogenic mycobacterial isolates (both slowly growing and rapidly growing) recovered in the Clinical Microbiology Laboratory of Stanford Hospital. This assay simplifies the identification of MTC and MAC and also accommodates the identification of rapidly growing mycobacteria. Although conventional PCR-based and singleplex, real-time PCR assays for the identification of MTC and NTM have previously been reported (3, 7, 18, 19, 25, 29, 30, 32, 35), multiplex, real-time PCR assays with simple interpretative criteria have not been developed. A multiplex, real-time PCR assay for the identification of 18 mycobacterial species, including MTC and rapidly growing mycobacteria, was recently described (21); however, the complex nature of this assay may preclude its use in the routine laboratory. The only simple application of PCR for the identification of rapidly growing mycobacteria consists of PCR-restriction analysis (8, 36). Multiplex, real-time PCR has several crucial advantages over conventional PCR. Besides the ease of use and rapid availability of results, it also eliminates the need for postamplification handling and thus the potential contamination of the laboratory.
Here we describe the development and validation of a multiplex, real-time PCR assay for the simple identification of MTC and clinically relevant NTM.
MATERIALS AND METHODS
Mycobacterial strains.
The reference strains used in this study are shown in Table 1. A total of 314 mycobacterial cultures recovered from 255 distinct clinical specimen types from 233 patients at the Stanford Hospital Clinical Microbiology Laboratory between 2005 and 2007 were included in the validation study. Except for isolates of the MTC, these isolates were from consecutive cultures. For MTC, additional isolates were included to ensure the presence of adequate numbers. A total of 254 of these were subcultured on Middlebrook 7H11/Middlebrook 7H11 selective biplates (Hardy Diagnostics, Santa Maria, CA). The remaining 60 represented primary mycobacterium growth indicator tube (MGIT) 960 cultures (Becton Dickinson, Franklin Lakes, NJ). In addition, 32 M. kansasii isolates from 26 patients (6 from Stanford Hospital and 20 from the California Department of Health Services, Richmond, CA) were included. All isolates had been identified to the species or complex level by the AccuProbe assay (Gen-Probe) or HPLC at the Focus Diagnostics reference laboratory (Cypress, CA) or the California Department of Health Services.
TABLE 1.
Reference strains used in this study
| Organism | ATCC strain no. | Genus Tm (°C)a |
|---|---|---|
| M. abscessus | 19977b | 80.6 |
| M. africanum | 25420b | 81.9 |
| M. avium subsp. avium | 25291b | 81.2 |
| M. avium subsp. paratuberculosis | BAA-968 | 81.9 |
| M. bovis Ravenel | 35720 | 81.6 |
| M. bovis BCG Pasteur | 35734 | 82.0 |
| M. chelonae | 35752b | 80.6 |
| M. fortuitum subsp. fortuitum | 6841b | 81.4 |
| M. gordonae | —c | 82.3 |
| M. intracellulare | 13950b | 81.4 |
| M. kansasii | 12478b | 81.7 |
| M. marinum | — | 81.7 |
| M. mucogenicum | 49650 | 82.3 |
| M. parafortuitum | 19686b | 82.5 |
| M. phlei | 10142 | 81.8 |
| M. scrofulaceum | 19981b | 81.7 |
| M. simiae | — | 82.5 |
| M. terrae | — | 81.5 |
| M. tuberculosis H37Rv | 27294b | 81.8 |
| M. xenopi | 19250b | 82.0 |
Tm for mycobacterial genus amplicon.
Type strain organism.
—, from the Stanford Hospital collection.
DNA extraction.
Bacteria growing on Middlebrook 7H11 agar were harvested with a 1-μl loop and resuspended in 0.5 ml of molecular-grade water (Sigma-Aldrich, St. Louis, MO). MGIT cultures were tested for contamination and purity by subculturing them to blood agar and Middlebrook 7H11 agar, respectively. A 0.5-ml volume of contaminant-free MGIT culture was sedimented at 12,600 × g for 2 min and resuspended in 0.5 ml of molecular-grade water. Both sample types were heated in a 95°C water bath for 10 min and centrifuged for 2 min before an aliquot of the supernatant was removed for the real-time PCR assay and sequencing.
Primers and primer design.
Primers targeting the following were designed by importing and aligning the target sequences by using the Clone Manager Professional Suite (Science and Education Central, Cary, NC): the internal transcribed spacers (ITSs) of MTC and MCAG; the 16S rRNA genes of MAC, MFG, and M. mucogenicum; the region of the 16S rRNA gene common to all members of the genus Mycobacterium; the insertion sequence IS1311 of M. avium (9); and the DT1 sequence of M. intracellulare (31). Primer selection was facilitated by determination of the regions of difference that would selectively and differentially amplify targets of interest. The list of real-time PCR primers for ribosomal targets and their corresponding amplicon lengths and melting temperature (Tm) values are provided in Table 2. The forward and reverse primers for IS1311 were 5′-CGATTTGGAGTTGCGGATT-3′ and 5′-GGAACACATACGGGAAGTGC-3′, respectively, and those for DT1 were 5′-CGCGAACCTTCCACAATG-3′ and 5′-GTGGTGCCTCAGGCTAGTTG-3′, respectively. The reverse primer specific for the genus Mycobacterium, also known as primer 264, was adopted from a previous study (17).
TABLE 2.
Primers used in this study
| Primer set | Primer name | Target gene | Primer sequence | Amplicon length (bp) | Tm (°C)a |
|---|---|---|---|---|---|
| Reaction 1 | M. avium FWD-01 | 16S | CCTCAAGACGCATGTCTTC | 299 | 86.2 ± 0.04 |
| M. intracellulare FWD-02 | 16S | GACCTTTAGRCGCATGTCTTT | 301 | ||
| MAC REV-02 | 16S | ACCTACCGTCAATCCGAGAA | |||
| MTC FWD-01 | ITS | GCGAGAGCCGGGTGCATG | 47 | 77.0 ± 0.04 | |
| MTC REV-01 | ITS | AACAGTGTGTTGGTGGCCAA | |||
| AFB genus FWD-06 | 16S | CCGCAAGRCTAAAACTCAAA | 149 | 80.2 to 82.9c | |
| AFB genus REV-01b | 16S | TGCACACAGGCCACAAGGGA | |||
| Reaction 2 | MCAG FWD-01 | ITS | TAAGGAGCACCATTTCCCAG | 128 | 77.4 ± 0.1 |
| MCAG REV-01 | ITS | CGACGTTTTGCCGACTACC | |||
| MFG FWD-01 | 16S | CCACGCGCTTCATGGTGT | 286 | 85.8 ± 0.1 | |
| MFG FWD-02 | 16S | CCGCGCTCTTCATGGGGT | 286 | ||
| MFG FWD-03 | 16S | ACCACGCATTTCATGGTGT | 287 | ||
| MFG REV-01 | 16S | ACTTGCGCTTCGTCCCTAT | |||
| AFB genus FWD-06 | 16S | CCGCAAGRCTAAAACTCAAA | 149 | 80.2 to 82.9c | |
| AFB genus REV-01b | 16S | TGCACACAGGCCACAAGGGA |
The product Tm values represent means ± 95% confidence intervals.
Also known as primer 264; adopted from a previous study (17).
MFG, 81.5 ± 0.1; M. mucogenicum, 82.4 ± 0.2.
Real-time PCR.
Each 25-μl PCR mixture contained 1.25 μl of 10 μM multiplexed primer mix (Table 2), 3.75 μl molecular-grade water, 12.5 μl 2× FastStart Sybr green master mixture (Roche Diagnostics, Indianapolis, IN), and 7.5 μl extracted mycobacterial DNA. The reactions were performed on a SmartCycler II real-time PCR instrument (Cepheid, Sunnyvale, CA), and amplification was monitored by measurement of the Sybr green fluorescence. The PCR program included a 95°C activation step for 5 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s and measurement of the fluorescence. Following the last cycle, the instrument generated a melting curve by ramping from 60°C to 95°C at 0.2°C/s and continuously measuring the fluorescence. The melting curves displayed the negative first derivative of the DNA melting profile.
Analysis.
Reactions with amplification cycle threshold values of between 20 and 35 were considered successful and were analyzed with DNA melting curves. Amplified products in the melting curve plot were analyzed for their intensity and Tm. Peaks with intensities of less than or equal to 20 units were considered negative, those with intensities of greater than or equal to 40 units were considered positive, and those with intensities of between 20 and 40 units were considered indeterminate. In reaction 1, peaks with Tm values of 77°C, 80.2 to 82.9°C, and 86.2°C represented MTC, the genus Mycobacterium, and MAC, respectively. In reaction 2, peaks with Tm values of 77.4°C, 80.2 to 82.9°C, and 85.8°C represented MCAG, the genus Mycobacterium, and MFG or M. mucogenicum, respectively. The MFG-specific primers in reaction 2 amplified both MFG and M. mucogenicum; thus, the latter organism was distinguished from MFG on the basis of the higher Tm of its genus Mycobacterium amplicon (82.4°C and 81.5°C, respectively). Because of the higher efficiencies of the primers specific for a complex or group compared with efficiencies of the primers specific for the Mycobacterium genus, the genus peak can be minor or absent in the presence of a complex- or group-specific target.
16S rRNA gene sequencing.
Three microliters of genomic DNA was amplified in a 50-μl reaction mixture consisting of 0.5 μM of universal primers TGGAGAGTTTGATCCTGGCTCAG and AAGGAGGTGATCCATCCGCA and 1× HotStarTaq Plus Master Mix (Qiagen Inc., Valencia, CA). The PCR was performed on a PE GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA). The PCR conditions included a 95°C activation step for 5 min, followed by 35 cycles of 95°C for 40 s, 60°C for 30 s, and 72°C for 2 min and a final 72°C elongation step for 10 min. Eight microliters of the PCR mixture was visualized on a 1% agarose gel for the presence of a 1.5-kb amplicon. Cycle sequencing was performed in separate reaction volumes consisting of 2 pM of primers GTTTGATYMTGGCTCAG, TGCCAGCAGCCGCGGTAA, GGACTACCAGGGTATCTAAT, and TACCGCGGCTGCTGGCAC plus 2 μl of BigDye Terminator mixture, 3 μl of 5× BigDye Terminator buffer (Perkin-Elmer Applied Biosystems, Foster City, CA), and 10 μl of the diluted (1:6 in water) PCR mixture. The cycling conditions included 25 cycles at 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. The sequencing products were purified with a BigDye Xterminator purification kit and were separated on an ABI 3730 genetic analyzer (Applied Biosystems). The DNA sequences were assembled with Lasergene software (DNAStar, Madison, WI) and were compared to those in the LeBIBI database (http://umr5558-sud-str1.univ-lyon1.fr/lebibi/lebibi.cgi). A distance score of 0% to less than 1% was used as the criterion for species identification.
Statistical analysis.
The sensitivity, specificity, and accuracy of the multiplex, real-time assay were calculated. Confidence intervals were calculated by using the Student's t test.
RESULTS
We developed a multiplex, real-time PCR assay that can identify 93% of the pathogenic mycobacteria recovered in the Clinical Microbiology Laboratory of Stanford Hospital. The assay was designed to detect the two most common slowly growing mycobacteria, MTC and MAC, in one tube, and the three most common rapidly growing mycobacteria, MCAG, MFG, and M. mucogenicum, in a second tube. In addition, to a control for the DNA extraction and reaction setup, each primer mixture also contained a pair of primers specific for the mycobacterial genus. The primer targets in the assay included the ITSs for MTC and MCAG and the 16S rRNA genes for MAC, MFG, M. mucogenicum, and the genus Mycobacterium. The amplicons were detected with the reporter dye Sybr green, and their specificities were determined by performing a DNA melting curve analysis.
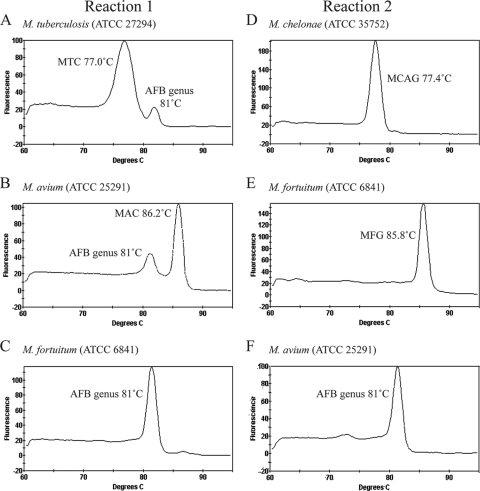
Initial assessment of the PCR primers was done in singleplex, real-time PCR tubes with DNA templates from a collection of mycobacterial reference strains (Table 1). The sensitivities and the specificities of the individual primer sets were confirmed (data not shown). Subsequent evaluation of the primers in multiplex reactions (Table 2) showed that only the expected PCR products were amplified from each reference strain (Fig. 1 and data not shown). For example, in reaction 1, a DNA template from M. tuberculosis produced a major peak with a Tm of 77°C, which corresponded to MTC, and a smaller peak with a Tm of 81°C, which corresponded to the genus Mycobacterium (Fig. 1A). Similarly, DNA from M. avium resulted in a major peak with a Tm of 86.5°C and a smaller peak with a Tm of 81°C, which corresponded to MAC and the genus Mycobacterium, respectively (Fig. 1B). Evaluations with all other NTM resulted in a single peak for the genus Mycobacterium with Tm values ranging from 80.2 to 82.9°C (Fig. 1C and Table 1). In reaction 2, DNA from M. fortuitum and M. chelonae produced major peaks with Tm values of 86°C and 77.5°C, which corresponded to MFG and MCAG, respectively (Fig. 1D and E). Except for M. mucogenicum, which is distinguished from MFG on the basis of its Mycobacterium genus Tm, all other mycobacterial species produced only a single peak for the genus Mycobacterium in reaction 2 (Fig. 1F and data not shown). The higher efficiencies of complex- or group-specific primers compared with those of the Mycobacterium genus-specific primers were demonstrated in the melting curve plots for M. tuberculosis, M. avium, M. fortuitum, and M. chelonae (Fig. 1). The occasional absence of the peak for the Mycobacterium genus (Fig. 1D and E) did not affect the interpretation of the results; if a PCR test reports the presence of a specific target, the target for the genus Mycobacterium must inherently be present.
FIG. 1.
Melting curve analysis of amplicons obtained by multiplex, real-time PCR allows the identification of mycobacteria. Representative reaction 1 melting curves for M. tuberculosis (A), M. avium (B), and M. fortuitum (C) and representative reaction 2 melting curves for M. chelonae (D), M. fortuitum (E), and M. avium (F) are shown. Peaks corresponding to specific products are labeled.
To evaluate the performance of our multiplex, real-time assay with clinical isolates, we blindly tested 314 isolates recovered from 255 distinct clinical specimens from 233 patients. The isolates consisted of 254 cultures grown on Middlebrook 7H11 agar and 60 primary MGIT cultures. For comparison, the organisms in the cultures were identified by the AccuProbe assay or HPLC at a reference laboratory and consisted of 96 MTC isolates, 98 MAC isolates, 68 MCAG isolates, 19 MFG isolates, 10 M. mucogenicum isolates, and 23 other mycobacteria, including 3 M. kansasii isolates (Table 3). Of the 1,570 potential PCR targets for MTC, MAC, MFG or M. mucogenicum, MCAG, and the genus Mycobacterium evaluated by the multiplex, real-time PCR, the results for 11 targets, including 1 target for MAC, 1 target for M. mucogenicum, and 9 targets for the genus Mycobacterium, were excluded due to indeterminate results by PCR. However, the exclusion of only three of these results precluded isolate identification because the results for the rest of the targets included indeterminate genus targets which did not interfere with isolate identification in the second reaction. Of the remaining 1,559 targets evaluated, multiplex, real-time PCR gave congruent results for 99.8% of the targets. Multiplex, real-time PCR had sensitivities of 100% for MTC, MCAG, and M. mucogenicum (after the exclusion of one indeterminate result); 99% for MAC; and 95% for MFG (Table 3). The specificities were 100% for MTC, MAC, MCAG, and MFG and 99.7% for M. mucogenicum (Table 3). The sensitivities for the MGIT cultures were 100% for MTC (n = 12), MAC (n = 30), MCAG (n = 5), MFG (n = 2), and M. mucogenicum (n = 1). The specificity for the MGIT cultures was 100% for all organisms. The organisms in the two cultures misidentified by multiplex, real-time PCR were further investigated by 16S rRNA sequencing. The MFG isolate misidentified as M. mucogenicum was identified as M. alvei by 16S rRNA sequencing. M. alvei is a member of the MFG (1) and therefore was truly misidentified by PCR. However, the MAC isolate identified as another acid-fast bacillus (AFB) (Table 3) by PCR was shown to be M. paraffinicum by sequencing. This mycobacterium, which shares 99% sequence homology with M. scrofulaceum (20, 34), was misidentified as MAC by the AccuProbe assay.
TABLE 3.
Comparison of multiplex, real-time PCR results with AccuProbe or HPLC results for identification of 314 clinical isolates of mycobacteria
| PCR result | No. of isolates with the following result:
|
|||||
|---|---|---|---|---|---|---|
| MTC by probe | MAC by probe | MCAG by HPLC | MFG by HPLC | M. mucogenicum by HPLC | Other AFB by HPLC | |
| MTC | 96 | 0 | 0 | 0 | 0 | 0 |
| MAC | 0 | 96 | 0 | 0 | 0 | 0 |
| MCAG | 0 | 0 | 68 | 0 | 0 | 0 |
| MFG | 0 | 0 | 0 | 18 | 0 | 0 |
| M. mucogenicum | 0 | 0 | 0 | 1a | 9 | 0 |
| Other AFBb | 0 | 1c | 0 | 0 | 0 | 22 |
| Indeterminated | 0 | 1 | 0 | 0 | 1 | 1 |
Identified as M. alvei by 16S rRNA sequencing.
AFB for which PCR amplified only the mycobacterial genus target.
Identified as M. paraffinicum by 16S rRNA sequencing.
The eight genus targets that did not interfere with isolate identification are not shown.
In addition to evaluating ribosomal targets, we also tested multiplexed primers targeting IS1311 and DT1 for the identification of M. avium and M. intracellulare, respectively (9, 31). The IS1311- and DT1-specific primers were 76% sensitive for the identification of the MAC isolates. The DT1-specific primers failed to identify 13 isolates shown to be M. intracellulare by use of the 16S rRNA-specific primers.
Additionally, since M. kansasii isolates make up a significant fraction of the NTM in certain geographical locations, such as the southern region of the United States (4, 38), we tested the specificity of our multiplex, real-time PCR assay with an additional 32 M. kansasii clinical isolates. In both reactions 1 and 2, only the Mycobacterium genus peak with Tm values ranging from 81.3 to 82.5°C was detected. None of these isolates was falsely identified as one of our target organisms (MTC, MAC, MCAG, MFG, or M. mucogenicum).
DISCUSSION
The rapid identification of mycobacteria in culture serves an important role in clinical decision making and in public health algorithms. For example, at our institution, an immunocompromised patient with risk factors for tuberculosis and a culture positive for mycobacteria would be treated with antibiotics active against M. tuberculosis and NTM and placed in respiratory isolation until the organism was identified. Thus, rapid identification of the organism not only would shorten unnecessary antibiotic exposure but also could prevent the financially and emotionally costly placement of an individual in respiratory isolation (16). Similarly, the rapid identification of the organism in cultures of lymph node tissue specimens from children would determine whether they would be treated medically for M. tuberculosis lymphadenitis (2) or whether they would undergo surgical resection for NTM lymphadenitis (12). In these examples, the elimination of a member of the MTC as the causative organism and the identification of the etiologic NTM would drastically change the therapeutic approach and the type of clinical management used.
At our institution, 93% of the pathogenic mycobacterial isolates consist of MAC (47%), MCAG (22%), MTC (15%), MFG (5%), and M. mucogenicum (4%). This epidemiology seems to slightly differ from that reported for other geographical locations where M. kansasii is more common (24). At present, there is no single commercially available molecular test in the United States that can rapidly and easily identify these isolates. To address the diagnostic deficiency, we developed a simple multiplex, real-time PCR assay that can identify all of these mycobacteria in two parallel reactions in less than 2 h. This assay was designed to detect MTC, MAC, and the genus Mycobacterium in one reaction and MCAG, MFG, M. mucogenicum, and the genus Mycobacterium in a second reaction. The reactions were divided to identify the slowly growing MTC and MAC and the rapidly growing MCAG, MFG, and M. mucogenicum. While the growth rate on solid medium would suffice to assign the isolates between these broad groups, it was essential to run both reactions in parallel to determine the Tm for the Mycobacterium genus control amplicon. The primers specific for the genus Mycobacterium served two purposes in our assay: first, to serve as a positive internal control to establish amplification of mycobacterial genomic DNA in the absence of a complex-specific target versus a failed reaction; second, to correlate the species identification with another value, since some mycobacteria have a characteristic genus control Tm (Table 1). This was crucial for the identification of M. mucogenicum, which amplifies with the MFG-specific primers but which can be differentiated from MFG on the basis of the higher Tm of its Mycobacterium genus amplicon (82.4 and 81.5°C, respectively).
The PCR targets in our assay included the rRNA genomic sequences, which are highly stable and which are unlikely to undergo deletions, as is known to occur with insertion sequences (27). The multiplex, real-time PCR assay was shown to be highly sensitive and specific when it was used to blindly test the organisms in 314 clinical cultures (Table 3). In addition to evaluating ribosomal RNA gene-specific primers, we also tested multiplexed primers targeting IS1311 and DT1 for the identification of M. avium and M. intracellulare, respectively (9, 31). These sequences are thought to be specific for M. avium and M. intracellulare, respectively, and DT1 has previously been used to identify M. intracellulare by PCR (10). However, unlike ribosomal primers, which were 100% sensitive, the IS1311- and DT1-specific primers were 76% sensitive for the identification of the MAC isolates. Furthermore, the DT1-specific primers failed to identify isolates shown to be M. intracellulare with our 16S rRNA-specific primers. Thus, these findings suggest that DT1 may not be a stable target for the identification of M. intracellulare.
The multiplex, real-time PCR assay that we have developed has distinct advantages over existing commercial assays for the identification of mycobacteria. Currently, mycobacteria are commonly identified by biochemical means (6, 33); HPLC analysis of the mycolic acids (13); gene sequencing (17); singleplex, real-time PCR, such as PCR with the RealArt Mycobact Diff kit (Qiagen, Hamburg, Germany) (3); or assays with commercially available hybridization probes, such as the AccuProbe assay (Gen-Probe) (37), the INNO-LiPA line probe assay (Innogenetics, Ghent, Belgium) (20), or the GenoType assay (Hain Lifescience, Nehren, Germany) (28). All methods have advantages and limitations. Biochemical tests require live organisms, which pose a hazard to laboratory staff and which require examination of the cultures for several days to weeks, which can delay identification (33). HPLC is relatively simple and diagnostic, but it requires expertise and dedicated instrumentation, which may not be available in a basic clinical microbiology laboratory (13). DNA sequencing is robust and accurate (17), but it is time-consuming; it requires one round of PCR and one round of cycle sequencing, followed by analysis, as opposed to the single round of PCR and concurrent analysis afforded by real-time PCR. Although the assay performed with the RealArt Mycobact Diff kit is a real-time PCR assay, it is limited to the identification of MTC and MAC and it is not commercially available in the United States. Finally, hybridization probes are highly sensitive and specific and are easy to use, but they are limited in scope; the commercially available AccuProbe assay in the United States can detect only MTC, MAC and its members, M. kansasii, and M. gordonae (37) but not any of the rapidly growing mycobacteria. The INNO-LiPA and GenoType assays are broader in their identification scopes (20), but they are not commercially available in the United States.
A number of studies have previously described the application of in-house PCR assays to the identification of mycobacteria from culture. Methods commonly employed include monoplex and multiplex, conventional PCR for the identification of MTC and MAC (18, 30) and singleplex and multiplex, real-time PCR for the identification of MTC and NTM (3, 7, 19, 21, 25, 29, 32, 35). PCR-restriction analysis has also been used for the identification of rapidly growing mycobacteria (36). The gel-based PCR assays are laborious and are prone to error and contamination due to postamplification handling. The real-time PCR assay that we describe here is simple, rapid, and appropriate in scope for the routine mycobacteriology laboratory. It facilitates the accurate identification of commonly encountered slowly growing and rapidly growing mycobacteria and guides reflex testing for the definitive identification of mycobacteria not identified by this assay. A limitation of this assay is that it does not meet the needs of laboratories in geographical regions with high rates of occurrence of M. kansasii infections (4, 38). However, given the simple design of this assay, it would be possible to replace or add primer sets to meet the individual needs of those laboratories. Similarly, in laboratories with high rates of drug-resistant M. tuberculosis, it is also possible to perform genotypic drug susceptibility testing on the same real-time PCR platform (22). When indicated, laboratories can also determine the species of mycobacterial complexes on the same real-time PCR instrument (5, 26). Given that real-time PCR instruments are becoming a common item in clinical microbiology laboratories, PCR-based assays will be useful in meeting the diagnostic needs of mycobacteriology.
Acknowledgments
We thank Edward Desmond and Grace Lin for providing the reference strains and the M. kansasii isolates. We also thank Ben Pinsky and Edward Desmond for commenting on the manuscript and Indre Budvytiene for performing sequencing.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Adékambi, T., A. Stein, J. Carvajal, D. Raoult, and M. Drancourt. 2006. Description of Mycobacterium conceptionense sp. nov., a Mycobacterium fortuitum group organism isolated from a posttraumatic osteitis inflammation. J. Clin. Microbiol. 441268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, K., and R. Medzon. 2007. Scrofula as a presentation of tuberculosis and HIV. Can. J. Emerg. Med. Care 9176-179. [DOI] [PubMed] [Google Scholar]
- 3.Beqaj, S. H., R. Flesher, G. R. Walker, and S. A. Smith. 2007. Use of the real-time PCR assay in conjunction with MagNA Pure for the detection of mycobacterial DNA from fixed specimens. Diagn. Mol. Pathol. 16169-173. [DOI] [PubMed] [Google Scholar]
- 4.Bittner, M. J., E. A. Horowitz, T. J. Safranek, and L. C. Preheim. 1996. Emergence of Mycobacterium kansasii as the leading mycobacterial pathogen isolated over a 20-year period at a midwestern Veterans Affairs hospital. Clin. Infect. Dis. 221109-1110. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2007. Mycobacterium: clinical and laboratory characteristics of rapidly growing mycobacteria, p. 589-600. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, vol. 1, 9th ed. ASM Press, Washington, DC. [Google Scholar]
- 7.Bruijnesteijn van Coppenraet, E. S., J. A. Lindeboom, J. M. Prins, M. F. Peeters, E. C. Claas, and E. J. Kuijper. 2004. Real-time PCR assay using fine-needle aspirates and tissue biopsy specimens for rapid diagnosis of mycobacterial lymphadenitis in children. J. Clin. Microbiol. 422644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimara, E., L. Ferrazoli, S. Y. Ueky, M. C. Martins, A. M. Durham, R. D. Arbeit, and S. C. Leão. 2008. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol. 848. http://www.biomedcentral.com/1471-2180/8/48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, D. M., S. Cavaignac, and G. W. de Lisle. 1997. Use of four DNA insertion sequences to characterize strains of the Mycobacterium avium complex isolated from animals. Mol. Cell. Probes 11373-380. [DOI] [PubMed] [Google Scholar]
- 10.Devallois, A., M. Picardeau, C. N. Paramasivan, V. Vincent, and N. Rastogi. 1997. Molecular characterization of Mycobacterium avium complex isolates giving discordant results in AccuProbe tests by PCR-restriction enzyme analysis, 16S rRNA gene sequencing, and DT1-DT6 PCR. J. Clin. Microbiol. 352767-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devulder, G., M. Pérouse de Montclos, and J. P. Flandrois. 2005. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 55(Pt 1)293-302. [DOI] [PubMed] [Google Scholar]
- 12.Flint, D., M. Mahadevan, C. Barber, D. Grayson, and R. Small. 2000. Cervical lymphadenitis due to non-tuberculous mycobacteria: surgical treatment and review. Int. J. Pediatr. Otorhinolaryngol. 53187-194. [DOI] [PubMed] [Google Scholar]
- 13.Garza-Gonzalez, E., M. Guerrero-Olazaran, R. Tijerina-Menchaca, and J. M. Viader-Salvado. 1998. Identification of mycobacteria by mycolic acid pattern. Arch. Med. Res. 29303-306. [PubMed] [Google Scholar]
- 14.Glassroth, J. 2008. Pulmonary disease due to nontuberculous mycobacteria. Chest 133243-251. [DOI] [PubMed] [Google Scholar]
- 15.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzaro, C. Daley, F. Gordin, S. M. Holland, R. Horsburgh, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Ruoss, C. F. von Reyn, R. J. Wallace, Jr., K. Winthrop, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, and Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175367-416. [DOI] [PubMed] [Google Scholar]
- 16.Jensen, P. A., L. A. Lambert, M. F. Iademarco, and R. Ridzon. 2005. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recommend. Rep. 54(RR-17)1-141. [PubMed] [Google Scholar]
- 17.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F. C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 312882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulski, J. K., and T. Pryce. 1996. Preparation of mycobacterial DNA from blood culture fluids by simple alkali wash and heat lysis method for PCR detection. J. Clin. Microbiol. 341985-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachnik, J., B. Ackermann, A. Bohrssen, S. Maass, C. Diephaus, A. Puncken, M. Stermann, and F.-C. Bange. 2002. Rapid-cycle PCR and fluorimetry for detection of mycobacteria. J. Clin. Microbiol. 403364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebrun, L., F. X. Weill, L. Lafendi, F. Houriez, F. Casanova, M. C. Gutierrez, D. Ingrand, P. Lagrange, V. Vincent, and J. L. Herrmann. 2005. Use of the INNO-LiPA-MYCOBACTERIA assay (version 2) for identification of Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex isolates. J. Clin. Microbiol. 432567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim, S. Y., B. J. Kim, M. K. Lee, and K. Kim. 2008. Development of a real-time PCR-based method for rapid differential identification of Mycobacterium species. Lett. Appl. Microbiol. 46101-106. [DOI] [PubMed] [Google Scholar]
- 22.Lin, S. Y., W. Probert, M. Lo, and E. Desmond. 2004. Rapid detection of isoniazid and rifampin resistance mutations in Mycobacterium tuberculosis complex from cultures or smear-positive sputa by use of molecular beacons. J. Clin. Microbiol. 424204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maartens, G., and R. J. Wilkinson. 2007. Tuberculosis. Lancet 3702030-2043. [DOI] [PubMed] [Google Scholar]
- 24.Marras, T. K., and C. L. Daley. 2002. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin. Chest Med. 23553-567. [DOI] [PubMed] [Google Scholar]
- 25.Miller, N., T. Cleary, G. Kraus, A. K. Young, G. Spruill, and H. J. Hnatyszyn. 2002. Rapid and specific detection of Mycobacterium tuberculosis from acid-fast bacillus smear-positive respiratory specimens and BacT/ALERT MP culture bottles by using fluorogenic probes and real-time PCR. J. Clin. Microbiol. 404143-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinsky, B. A., and N. Banaei. 2008. Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J. Clin. Microbiol. 462241-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radhakrishnan, I., Y. K. Manju, R. A. Kumar, and S. Mundayoor. 2001. Implications of low frequency of IS6110 in fingerprinting field isolates of Mycobacterium tuberculosis from Kerala, India. J. Clin. Microbiol. 391683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter, E., M. Weizenegger, A. M. Fahr, and S. Rüsch-Gerdes. 2004. Usefulness of the GenoType MTBC assay for differentiating species of the Mycobacterium tuberculosis complex in cultures obtained from clinical specimens. J. Clin. Microbiol. 424303-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrestha, N. K., M. J. Tuohy, G. S. Hall, U. Reischl, S. M. Gordon, and G. W. Procop. 2003. Detection and differentiation of Mycobacterium tuberculosis and nontuberculous mycobacterial isolates by real-time PCR. J. Clin. Microbiol. 415121-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka, I. I., I. S. Anno, S. R. Leite, R. C. Cooksey, and C. Q. Leite. 2003. Comparison of a multiplex-PCR assay with mycolic acids analysis and conventional methods for the identification of mycobacteria. Microbiol. Immunol. 47307-312. [DOI] [PubMed] [Google Scholar]
- 31.Thierry, D., V. Vincent, F. Clément, and J. L. Guesdon. 1993. Isolation of specific DNA fragments of Mycobacterium avium and their possible use in diagnosis. J. Clin. Microbiol. 311048-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Coppenraet, L. S., V. T. Smit, K. E. Templeton, E. C. Claas, and E. J. Kuijper. 2007. Application of real-time PCR to recognize atypical mycobacteria in archival skin biopsies: high prevalence of Mycobacterium haemophilum. Diagn. Mol. Pathol. 1681-86. [DOI] [PubMed] [Google Scholar]
- 33.Vincent, V., and M. C. Gutíerrez. 2007. Mycobacterium: laboratory characteristics of slowly growing mycobacteria, p. 573-588. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, vol. 1, 9th ed. ASM Press, Washington, DC. [Google Scholar]
- 34.Wayne, L. G., and H. A. Sramek. 1992. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin. Microbiol. Rev. 51-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, K. J., C. L. Ling, C. Jenkins, S. H. Gillespie, and T. D. McHugh. 2007. A paradigm for the molecular identification of Mycobacterium species in a routine diagnostic laboratory. J. Med. Microbiol. 56(Pt 5)598-602. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, R. W., V. A. Steingrube, B. A. Brown, and R. J. Wallace, Jr. 1998. Clinical application of PCR-restriction enzyme pattern analysis for rapid identification of aerobic actinomycete isolates. J. Clin. Microbiol. 36148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yam, W. C., K. Y. Yuen, S. Y. Kam, L. S. Yiu, K. S. Chan, C. C. Leung, C. M. Tam, P. O. Ho, W. W. Yew, W. H. Seto, and P. L. Ho. 2006. Diagnostic application of genotypic identification of mycobacteria. J. Med. Microbiol. 55(Pt 5)529-536. [DOI] [PubMed] [Google Scholar]
- 38.Yates, M. D., J. M. Grange, and C. H. Collins. 1986. The nature of mycobacterial disease in south east England, 1977-84. J. Epidemiol. Community Health 40295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]