Abstract
The genus Legionella contains a diverse group of motile, asaccharolytic, nutritionally fastidious gram-negative rods. Legionella pneumophila is the most important human pathogen, followed by L. micdadei, L. longbeachae, L. dumoffii, and other rare species. Accurate identification of Legionella spp. other than L. pneumophila is difficult because of biochemical inertness and phenotypic identity of different species. The feasibility of using an oligonucleotide array for identification of 18 species of Legionella was evaluated in this study. The method consisted of PCR amplification of the macrophage infectivity potentiator mip gene, followed by hybridization of the digoxigenin-labeled PCR products to a panel of 30 oligonucleotide probes (16- to 24-mers) immobilized on a nylon membrane. A collection of 144 target strains (strains we aimed to identify) and 50 nontarget strains (44 species) were analyzed by the array. Both test sensitivity (144/144 strains) and specificity (50/50 strains) of the array were 100%. The whole procedure for identification of Legionella species by the array can be finished within a working day, starting from isolated colonies. It was concluded that species identification of clinically relevant Legionella spp. by the array method is very reliable and can be used as an accurate alternative to conventional or other molecular methods for identification of Legionella spp.
The genus Legionella currently contains 50 validly named species (http://www.dsmz.de/bactnom/bactname.htm), and among them, 20 have been found to be human pathogens (6, 10). Legionnaires' disease (LD) is caused mainly by inhalation of aerosols generated from water sources contaminated with Legionella spp. (6, 40). While most species of Legionella are normal environmental flora, many are implicated in opportunistic infections in immunocompromised patients (14). Pulmonary infections caused by Legionella may be subclinical or severe (27), and the fatality rate can approach 50% in immunocompromised patients (49).
Legionella pneumophila accounts for about 85 to 90% of cases of LD (6, 26, 49). Other Legionella spp. implicated in human infections include L. micdadei, L. longbeachae, L. dumoffii, and some less encountered species, such as L. anisa, L. bozemanae, L. feeleii, and L. wadsworthii (49). L. pneumophila is normally identified by immunofluorescent-antibody assay. A specific FDA-cleared fluorescein isothiocyanate-labeled monoclonal antibody (Bio-Rad, Hercules, CA) for all serogroups of L. pneumophila and fluorescein isothiocyanate-labeled polyclonal antisera specific for L. pneumophila serogroup 1 (m-TECH, Atlanta, GA) are commercially available (6). Accurate identification of Legionella spp. other than L. pneumophila and L. pneumophila serogroup 1 can be quite difficult due to serological cross-reactivities between serogroups and species, biochemical inertness, and phenotypic identity of different species (6). Legionella isolates which fail to react with L. pneumophila antibodies are recommended to be identified by public health or reference laboratories (6). Antigen detection in urine specimens is also commonly used in hospitals for diagnosing infection caused by L. pneumophila (46).
Molecular approaches have been developed to provide more rapid and accurate identification of Legionella spp. These methods include PCR (20, 25, 34), gene probe hybridization (24, 41), restriction fragment length polymorphism analysis (21, 38), and sequence analysis of the rRNA gene (47) and the macrophage infectivity potentiator gene mip (35, 41). Since diagnostic delay may result in increased mortality for patients with LD (15), real-time PCR assay has been a focus of many studies in recent years (5, 13, 14, 17, 19, 34, 36, 41, 48). However, with real-time PCR assay, only L. pneumophila and a very limited number of Legionella spp. can be detected or identified.
Recently, DNA array technology has been applied to identify a wide variety of bacteria that are difficult to be differentiated by phenotypic traits or whose identification may take a long time (12, 31, 43). This study aimed to develop an oligonucleotide array based on mip gene sequences to identify 18 species of Legionella that have been found to cause human infections in the literature (10).
MATERIALS AND METHODS
Bacterial strains.
A collection of 52 reference strains and 92 clinical isolates were used as target strains (species we aimed to identify) of the array (Table 1). Reference strains were obtained from the American Type Culture Collection (ATCC), Manassas, VA, the Bioresources Collection and Research Center (Hsichu, Taiwan), and Culture Collection, University of Göteborg, Sweden. Clinical isolates were obtained from the Centers for Disease Control (Taipei, Taiwan) and the Super Laboratory (Taipei, Taiwan). Clinical isolates were isolated from respiratory specimens and identified as L. pneumophila (82 strains) by standard techniques (42) or as other Legionella species (10 strains) by mip gene sequence analysis (35). A total of 50 nontarget strains (44 species) were used for specificity testing of the array (see Table S1 in the supplemental material). Buffered charcoal yeast extract medium supplemented with 0.1% α-ketoglutaric acid was used for culture of Legionella spp., while sheep blood agar was used to cultivate non-Legionella strains. All plates were incubated at 35°C for 24 to 72 h.
TABLE 1.
Reference strains and clinical isolates used in this study
| Species | Reference strain(s)a | No. of clinical isolatesc | Total no. of strains |
|---|---|---|---|
| L. anisa | ATCC 35292T, ATCC 35290, ATCC 35291 | 7 | 10 |
| L. birminghamensis | ATCC 43702T, ATCC 700709 | 0 | 2 |
| L. bozemanii | ATCC 33217T, ATCC 35545, CCUG 31569, CCUG 48836 | 2 | 6 |
| L. cincinnatiensis | ATCC 43753T | 0 | 1 |
| L. dumoffii | ATCC 33279T, CCUG 47789 | 0 | 2 |
| L. feeleii | ATCC 35072T, ATCC 35849, ATCC 700513, ATCC 700514 | 0 | 4 |
| L. gormanii | ATCC 33297T, ATCC 43769 | 0 | 2 |
| L. hackeliae | ATCC 35250T, ATCC 35999 | 0 | 2 |
| L. jordanis | ATCC 33623T, ATCC 700762 | 0 | 2 |
| L. lansingensis | ATCC 49751T | 0 | 1 |
| L. longbeachae | ATCC 33462T, ATCC 33484, CCUG 28612 | 1 | 4 |
| L. maceachernii | ATCC 35300T | 0 | 1 |
| L. micdadei | ATCC 33218T | 0 | 1 |
| L. oakridgensis | ATCC 33761T, ATCC 700515, ATCC 700516 | 0 | 3 |
| L. pneumophilab | ATCC 33152T, ATCC 33154, ATCC 33155, ATCC 33156T, ATCC 33215, ATCC 33216, ATCC 33823, ATCC 35096, ATCC 35251, ATCC 35289, ATCC 43109, ATCC 43130, ATCC 43283, ATCC 43290, ATCC 43703, ATCC 43736 | 82 | 98 |
| L. sainthelensi | ATCC 35248T, ATCC 49322, ATCC 700517 | 0 | 3 |
| L. tucsonensis | ATCC 49180T | 0 | 1 |
| L. wadsworthii | ATCC 33877T | 0 | 1 |
| Total | 52 | 92 | 144 |
ATCC, American Type Culture Collection, Manassas, VA; CCUG, Culture Collection, University of Göteborg, Sweden.
ATCC 33156T, ATCC 33216, and ATCC 35251 are strains of L. pneumophila subsp. fraseri. Others are strains of L. pneumophila subsp. pneumophila comprising 12 serogroups.
Non-L. pneumophila clinical strains were identified by mip gene sequence analysis.
DNA preparation.
One to several colonies of pure cultures were suspended in an aliquot (50 μl) of sterilized water, heated at 100°C for 15 min in a heating block, and centrifuged in a microcentrifuge (6,000 × g, 10 min) (28). The supernatant containing bacterial DNA was stored at −20°C for further use.
Design of species-specific oligonucleotide probes.
Oligonucleotide probes (16- to 24-mers) were designed from the mip gene to identify the Legionella spp. listed in Table 1. One or multiple probes were designed to identify a single Legionella species, depending on the availability of divergent sequences in the mip regions (Table 2). Multiple sequence alignment of the mip fragments was performed by using the software Vector NTI (Invitrogen Corporation, Carlsbad, CA), and areas displaying low intraspecies and high interspecies sequence divergences were used for probe synthesis. The designed probes were checked for self-annealing, secondary structure, internal repeats, and GC content by using the software Vector NTI (Invitrogen Corporation). In addition, the designed probes were screened against the databases of the National Center for Biotechnology Information for homology with other bacterial sequences, using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Fifteen, 10, or 5 thymine bases were added to probes that had a melting temperature (Tm) of ≤40°C, ≤45°C, or >45°C, respectively (3) (Table 2). The positive control probe was designed from a conserved region in the mip region for all Legionella species. An irrelevant 20-mer oligonucleotide labeled with a digoxigenin (DIG) molecule at its 5′ end (5′-DIG-GGGTTYCCCCRTTCRGAAAT-3′; Y = C or T and R = A or G) was used as a position marker of hybridization (Fig. 1 and 2).
TABLE 2.
Oligonucleotide probes used in this study
| Species | Probe | Sequence (5′-3′)a | Probe length (mer) | Tm (°C) | Locationb | GenBank accession no. |
|---|---|---|---|---|---|---|
| L. anisa | Lagtwc | CCTTCAGACTTAGCTTAT(T) | 18 | 35.1 | 1072-1089 | U91607 |
| Lan | GGCGTAACGGTACTGCC(T) | 17 | 48.5 | 832-848 | U91607 | |
| L. birminghamensis | Lbi1 | CAGCGGACTCCAGTACA(T) | 17 | 43.6 | 412-428 | U91608 |
| Lbi2 | AACTGGCAGCGGTGCAA(T) | 17 | 53.1 | 439-455 | U91608 | |
| L. bozemanii | Lbo | (T)CCTTCAGATTTAGCGTA | 18 | 39.6 | 1070-1087 | U91609 |
| L. cincinnatiensis | Lci | AGGTAAGTCAGACACTGTAA(T) | 20 | 37.9 | 855-874 | U91636 |
| L. dumoffii | Ldu1 | AAGAGAATAAAGCAAAAGGC(T) | 20 | 45.5 | 747-766 | U91637 |
| Ldu2 | CAGGTTCAGGCGCTAAGCC(T) | 19 | 54.9 | 846-864 | U91637 | |
| L. feeleii | Lfe1d | AAAATAATCCAGGCTGCTA(T) | 19 | 44.1 | 778-796 | U92205 |
| Lfe2 | AAGCGTTCATGAGCCAGA(T) | 18 | 48.6 | 713-730 | U92205 | |
| L. gormanii | Lagtwc | CCTTCAGACTTAGCTTAT(T) | 18 | 35.1 | 1072-1089 | U91607 |
| Lgo1 | CAGCGCAGAGTTTAACAAGA(T) | 20 | 48.4 | 613-632 | U91638 | |
| Lgo2 | GTACAGGCAGTAAACCAG(T) | 20 | 46.5 | 739-758 | U91638 | |
| L. hackeliae | Lha | AGGGTGACGGCGCTAA(T) | 16 | 49.3 | 690-705 | U92207 |
| L. jordanis | Ljo | AAGCATTCTTAAACGCAAAC(T) | 20 | 46.9 | 767-786 | U92209 |
| L. lansingensis | Lla1 | GTTACTCACAGAGCAGCAAA(T) | 20 | 45.3 | 630-649 | U92210 |
| Lla2 | AAACGCAGCAACGCCTACT(T) | 19 | 52.6 | 447-465 | U92210 | |
| L. longbeachae | Ll | ACTGGTACCTTGATTGATG(T) | 19 | 41.5 | 1004-1022 | X83036 |
| L. maceachernii | Lma1 | ACAATAAGGCAAAAGGAG(T) | 18 | 41.6 | 680-697 | U92211 |
| Lma2 | TCATTGAGCGCGGTGAT(T) | 17 | 50.1 | 767-783 | U92211 | |
| L. micdadei | Lmi1 | AGCTTTCCTTAACGAAAA(T) | 18 | 41.9 | 1039-1056 | S62141 |
| Lmi2 | CACCGGCAAGCTGATTG(T) | 17 | 50.3 | 1165-1181 | S62141 | |
| L. oakridgensis | Loa1 | AATGGTTCAAGGGTTGCA(T) | 18 | 48.2 | 284-301 | U92214 |
| Loa2 | CTGGCTCCGTTTGGGA(T) | 18 | 49.4 | 685-700 | U92214 | |
| L. pneumophila | Lp1 | AAACAAGCCAGGCGTT(T) | 16 | 45.6 | 329-344 | AF022334 |
| Lp2 | CAATTGGCTTTAACCGAACAA(T) | 21 | 52.2 | 189-208 | AF022334 | |
| L. sainthelensi | Lsai | ACTGGTGCGAAACCCG(T) | 16 | 49.3 | 853-868 | U91219 |
| L. tucsonensis | Lagtwc | CCTTCAGACTTAGCTTAT(T) | 18 | 35.1 | 1072-1089 | U91607 |
| Ltu | (T)TCAAAATCCGGCGTAGT | 17 | 45.8 | 804-820 | U92224 | |
| L. wadsworthii | Lagtwc | CCTTCAGACTTAGCTTAT(T) | 18 | 35.1 | 1072-1089 | U91607 |
| Lwad1 | CAGTAAGACAGATACTGTTACTGT(T) | 24 | 42.2 | 740-756 | U92225 | |
| Lwad2 | AGGCGATTCATTCTTA(T) | 16 | 36.6 | 638-653 | U92225 | |
| Positive control | PC | CARGTNATHCCNGGNTGGACHGA(T) | 23 | 60.5 | 510-532 | AF022334 |
(T), additional bases of thymine (T) were added to the 5′ or 3′ end of the probe. Fifteen, 10, or 5 thymine bases were added to probes that had a Tm of ≤40°C, ≤45°C, or >45°C, respectively. The positive control probe contained 15 thymine bases at the 3′ end.
The location of the probe is indicated by the nucleotide numbers of the mip gene, unless otherwise indicated.
Group-specific probe.
A single mismatch base was intentionally incorporated into the probe to avoid cross-hybridization by other Legionella spp.
FIG. 1.
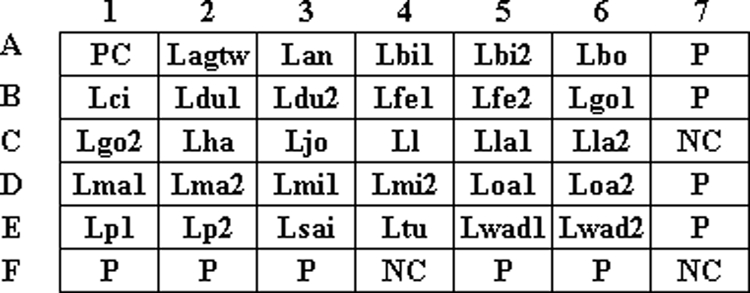
Layout of oligonucleotide probes on the array. Probes coded “NC” were negative controls (tracking dye only). The probe coded “P” was an irrelevant 20-mer oligonucleotide labeled with a DIG molecule at its 5′ end and was used as a position marker. The corresponding sequences of all probes are listed in Table 2.
FIG. 2.
Hybridization results for type strains of 18 Legionella species. The corresponding probes hybridized on the arrays are indicated in Fig. 1, and the corresponding sequences of the hybridized probes are shown in Table 2.
Preparation of oligonucleotide arrays.
The arrays contained 42 dots (6 by 7 dots), including 29 dots for species identification, 3 dots for the negative controls (probe code NC; tracking dye only), 1 dot for the positive control (probe code PC), and 9 dots (probe code P) for the position markers (Fig. 1). The oligonucleotide probes were diluted 1:1 (final concentration, 10 μM) with a tracking dye solution, drawn into wells of 96-well microtiter plates, and spotted onto positively charged nylon membranes (Roche, Mannheim, Germany) as described previously (43). The arrays were fabricated with an automatic arrayer (model SR-A300; Ezspot, Taipei, Taiwan) by use of a solid pin (400 μm in diameter).
Amplification of the mip gene.
The mip gene was amplified by PCR with a pair of degenerate primers, MIPF (5′-GGGRATTVTTTATGAAGATGARAYTGG-3′) and MIPR (5′-DIG-GGGTTYCCCCRTTCRGAAAT-3′) (R = A or G, V = A, C, or G). The reverse primer MIPR was labeled with a DIG molecule at the 5′ end. The conditions used for PCR were the same as those described previously (35). The presence of the PCR product was checked by 2% agarose gel electrophoresis.
Array hybridization.
All reagents except for buffers were included in each DIG nucleic acid detection kit (Roche). Unless indicated otherwise, the hybridization procedures were carried out at room temperature in an oven with a shaking speed of 60 rpm. Each array was prehybridized for 2 h with 1 ml of hybridization solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1% [wt/vol] blocking reagent, 0.1% N-laurylsarcosine, 0.02% sodium dodecyl sulfate) in an individual well of a 24-well cell culture plate. The DIG-labeled PCR product amplified from a strain was heated in a 100°C heating block for 5 min and immediately cooled on an ice bath. Ten microliters of the denatured PCR product of the test organism was diluted with 0.3 ml of hybridization solution and added to each well. Hybridization was carried out at 45°C for 90 min. After removal of the nonhybridized PCR products, the array was washed four times (3 min each) in 1 ml of 0.25× SSC-0.1% sodium dodecyl sulfate, followed by incubation for 1 h with 1 ml of blocking solution (1% [wt/vol] blocking reagent dissolved in maleic acid buffer [0.1 M maleic acid, 0.15 M NaCl, pH 7.5]). After removal of the blocking solution, 0.3 ml of alkaline phosphatase-conjugated anti-DIG antibodies (diluted 1:2,500 in blocking solution) was added to each well and incubated for 1 h. The array was washed three times (15 min each) in 1 ml of washing solution (0.3% [vol/vol] Tween 20 in maleic acid buffer), followed by washing in 1 ml of detection buffer (0.1 M Tris-HCl, 0.15 M NaCl, pH 9.5) for 5 min. Finally, 0.2 ml of alkaline phosphatase substrate (stock solution of nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate diluted 1:50 in detection buffer) was placed on each array and incubated at 37°C without shaking. Color development was visible between 30 min and 1 h after the start of the reaction. The hybridized spots (400 μm in diameter) could be read by the naked eye. The images of the hybridization patterns were captured and processed by a scanner (PowerLook 3000; UMAX, Taipei, Taiwan). A strain was identified as one of the 18 Legionella species listed in Table 1 when both the positive control probe and the probe (or all probes) specified for that species were hybridized (Table 2).
Detection limit of L. pneumophila in spiked urine samples.
L. pneumophila ATCC 33152 was used to determine the detection limit of the oligonucleotide array. A clean-catch urine sample obtained from a healthy person was spiked with L. pneumophila ATCC 33152 to a concentration of 108 CFU/ml. The DNA of the spiked urine sample was extracted (28) and serially diluted 10-fold with a carrier DNA (10 ng/μl) extracted from Escherichia coli TW1 by the boiling method (28). After PCR amplification of the diluted DNA, the amplicon was hybridized to the oligonucleotide array.
RESULTS
Probe development.
In the beginning of this study, one to six probes (data not shown) were designed for identification of each species, and a total of 70 probes were synthesized to identify the 18 Legionella species listed in Table 1. Through extensive hybridization screening, many probes cross-reacted with heterologous species or produced no hybridization signals with homologous species. Finally, 30 probes were selected for fabrication of the array (Fig. 1). Among the 30 probes, 28 were species specific, 1 was group specific, and 1 was a positive control that could hybridize with all Legionella spp. L. anisa, L. gormanii, L. tucsonensis, and L. wadsworthii shared a group-specific probe (code Lagtw) due to high mip sequence similarities among these species. However, each of the four species had its own species-specific probe(s) and could be differentiated from the others (Table 2). An individual species was identified by one, two, or three probes, depending on the availability of divergent sequences in the mip gene. For example, L. bozemanii was identified by a single probe (code Lbo), while L. birminghamensis and L. gormanii were identified by two (codes Lbi1 and Lbi2) and three probes (codes Lagtw, Lgo1, and Lgo2), respectively (Table 2).
Identification of Legionella strains.
Of 52 target reference strains representing 18 Legionella species, all strains hybridized to the positive control probe and their species-specific probes. In other words, all strains were correctly identified by the array. The hybridized spots (400 μm in diameter), appearing blue on a white nylon membrane, could easily be read by the naked eye. The hybridization patterns of 18 type strains representing the 18 species are shown in Fig. 2. In addition, a total of 92 clinical isolates, including 82 strains of L. pneumophila and 10 strains of other Legionella species, were analyzed, and all strains were correctly identified by array hybridization. Therefore, the test sensitivity of the array was 100% (144/144 strains). All reference strains and clinical isolates were also tested in a blinded fashion, and the same hybridization results were obtained. In addition, reproducible results were obtained for all target strains after repeat testing.
Hybridization of nontarget strains.
Of 50 nontarget strains (44 species), including five Legionella species (see Table S1 in the supplemental material), no strain hybridized to any probe on the array, resulting in a test specificity of 100% (50/50 strains). Pseudomonas aeruginosa, which is a common mimic of Legionella colonies on buffered charcoal yeast extract plates (6), produced a negative hybridization reaction, although the bacterium exhibits no growth dependence on l-cysteine. In addition, Staphylococcus aureus, which can cross-react with L. pneumophila-specific monoclonal antibody (6), also produced a negative hybridization reaction (see Table S1 in the supplemental material).
Detection limit of the array.
Serial 10-fold dilutions of the DNA of L. pneumophila ATCC 33152 were used to determine the detection limit of the microorganism in urine. The present method was able to detect the microorganism at a concentration of 25 CFU per assay (data not shown). No hybridization signal was observed for the nonspiked urine sample.
DISCUSSION
The biochemical data for legionellae other than L. pneumophila are very limited (10), although some laboratories have described methods for identifying putative Legionella isolates to the genus or species level by using phenotypic characteristics (11, 44). Currently, immunological methods are the most widely used techniques for identification of L. pneumophila and L. pneumophila serogroup 1 (6, 42). Antibodies to L. pneumophila serogroup 1 are quite specific, but otherwise cross-reactions exist, making immunological identification only presumptive (6). Cross-reactions have been experienced between different species and serogroups even when monovalent antisera were used (1, 6, 8, 32, 37). Adsorbed antisera may have the ability to avoid cross-reactivity, but these antisera are only available in some research laboratories and are not commercially available (2, 6).
At present, there is still a low level of clinical awareness regarding LD 30 years after it was first reported. Large, focal outbreaks of LD continue to occur worldwide (10). Most diagnostic tests currently used are directed at the species that causes most human cases of legionellosis, L. pneumophila serogroup 1. For this reason, information on the incidence of human respiratory disease attributable to other Legionella species is lacking (10). An accurate identification method, such as the array proposed in this study, is a prelude to revealing the epidemiology of non-L. pneumophila Legionella species that may cause human infections.
In this study, an individual species was identified by either one, two, or three probes, depending on the availability of divergent sequences in the mip region. The advantage of using multiple probes is the increase in specificity, since the chance of an irrelevant strain hybridizing to all probes designed for a species is very low. However, the use of multiple probes to identify a species may potentially decrease sensitivity due to the possibility of a single nucleotide polymorphism that occurs in strains at the region used for probe design. The successful design of species-specific probes was based on the known mip sequences in public databases. Multiple sequence alignment (interspecies and intraspecies) plays an essential role in finding regions that can be used for probe design.
The two probes (Lp1 and Lp2) used to identify L. pneumophila were species specific rather than serogroup or subspecies specific. Among the 16 strains of L. pneumophila obtained from the ATCC (Table 1), 3 strains (ATCC 33156, ATCC 33216, and ATCC 35251) were L. pneumophila subsp. fraseri, while the remaining 13 strains were L. pneumophila subsp. pneumophila. The 13 strains of L. pneumophila subsp. pneumophila covered serogroups 1, 2, 3, 6, 7, 8, 9, 10, 11, 12, 13, and 14 (http://www.atcc.org/). Regardless of serogroup and subspecies, all 16 strains were identified as L. pneumophila by the array.
Due to the inadequateness of conventional identification methods, molecular techniques have been developed to provide more rapid and accurate alternatives for identification of Legionella spp. Some PCR tests that have been developed for legionellae target random DNA sequences (39), the 16S rRNA gene (20, 22, 29), the 5S rRNA gene (23, 24), and mip genes (4, 14, 24, 35, 41, 48). Legionella DNA has been detected in a variety of clinical specimens, including respiratory secretions, pharyngeal swabs, nasopharyngeal swabs, urine, serum, and peripheral blood mononuclear cells (10, 16, 18, 20, 30, 33). Sequence analysis of the mip gene was successfully used to differentiate Legionella spp. (35, 41), and identification through sequence data comparison is available on the Internet (http://www.ewgli.org/). After analyzing the mip genes of 17 clinical and environmental Legionella isolates, Bumbaugh et al. (4) found that the mip gene is highly conserved in each species of Legionella and is a good target for molecular diagnosis. Stølhaug et al. (41) observed that the mip gene sequence discriminates more reliably between Legionella spp. than does the partial (386 bp) 16S rRNA gene sequence. For this reason, the mip gene was selected as a target for identification of Legionella species in this study. For several Legionella species, a single reference strain was used in this study (Table 1); this was due to the limited availability of strains of these species in public culture collections.
The identification of legionellae to the species level is usually not of clinical significance, but it may have significant public health and scientific importance (6). LD caused by Legionella spp. other than L. pneumophila may have less of a response to erythromycin treatment (9), but whether accurate identification of the Legionella spp. causing infection would have a positive impact on patient management is debatable, especially since erythromycin is currently not used for treating severe LD (7).
The oligonucleotide probes (16- to 24-mers) used in this study were relatively short, and the Tm values of these probes varied to a large degree (35.1 to 54.9°C). Many probes even had Tm values lower than the hybridization temperature (45°C) (Table 2). Although some species produced relatively weak hybridization, clear signals were obtained for all 18 species tested (Fig. 2). Volokhov et al. (45) also reported the successful use of probes having Tm values significantly lower than the hybridization temperature for identification of Listeria species. The addition of multiple thymine bases to the 3′ end (or 5′ end) of an oligonucleotide can improve the hybridization signal of a probe, probably due to the increased binding of the probe to the nylon membrane, and thus an increased hybridization intensity (3).
In conclusion, an oligonucleotide array based on mip sequences was developed to identify 18 species of Legionella that have been reported to cause human infections (6, 10). With a high sensitivity and specificity, the array technique is an accurate tool for differentiating Legionella species that may be encountered in clinical settings. The current array utilizes a standardized protocol encompassing DNA extraction, PCR amplification, and hybridization of PCR products to probes on the array.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Science Council (96-2320-B-006-024-MY3) and from the Ministry of Education, Taiwan, Republic of China, under the NCKU project (D97-2720), National Cheng Kung University, Taiwan.
Footnotes
Published ahead of print on 4 March 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Adeleke, A. A., B. S. Fields, R. F. Benson, M. I. Daneshvar, J. M. Pruckler, R. M. Ratcliff, T. G. Harrison, R. S. Weyant, R. J. Birtles, D. Raoult, and M. A. Halablab. 2001. Legionella drozanskii sp. nov., Legionella rowbothamii sp. nov. and Legionella fallonii sp. nov.: three unusual new Legionella species. Int. J. Syst. Evol. Microbiol. 511151-1160. [DOI] [PubMed] [Google Scholar]
- 2.Benson, R. F., W. L. Thacker, J. A. Lanser, N. Sangster, W. R. Mayberry, and D. J. Brenner. 1991. Legionella adelaidensis, a new species isolated from cooling tower water. J. Clin. Microbiol. 291004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, T. J., and R. M. Anthony. 2000. The addition of low numbers of 3′ thymine bases can be used to improve the hybridization signal of oligonucleotides for use within arrays on nylon supports. J. Microbiol. Methods 42203-207. [DOI] [PubMed] [Google Scholar]
- 4.Bumbaugh, A., E. A. McGraw, K. L. Page, R. K. Selander, and T. S. Whittam. 2002. Sequence polymorphism of dotA and mip alleles mediating invasion and intracellular replication of Legionella pneumophila. Curr. Microbiol. 44314-322. [DOI] [PubMed] [Google Scholar]
- 5.Diederen, B. M. W., C. M. A. de Jong, F. Marmouk, J. A. J. W. Kluytmans, M. F. Peeters, and A. Van der Zee. 2007. Evaluation of real-time PCR for the early detection of Legionella pneumophila DNA in serum samples. J. Med. Microbiol. 5694-101. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein, P. H. 2007. Legionella, p. 835-849. In P. R Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 7.Edelstein, P. H., and N. P. Cianciotto. 2005. Legionella, p. 2711-2724. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Elsevier Churchill Livingstone, Philadelphia, PA.
- 8.Edelstein, P. H., R. M. McKinney, R. D. Meyer, M. A. Edelstein, C. J. Krause, and S. M. Finegold. 1980. Immunologic diagnosis of Legionnaires' disease: cross-reactions with anaerobic and microaerophilic organisms and infections caused by them. J. Infect. Dis. 141652-655. [DOI] [PubMed] [Google Scholar]
- 9.Fang, G.-D., V. L. Yu, and R. M. Vickers. 1989. Disease due to the Legionellaceae (other than Legionella pneumophila): historical, microbiological, clinical, and epidemiological review. Medicine 68116-132. [DOI] [PubMed] [Google Scholar]
- 10.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, K. F., and A. Brown. 1989. Application of numerical systematics to the phenotypic differentiation of legionellae. J. Clin. Microbiol. 271952-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima, M., K. Kakinuma, H. Hayashi, H. Nagai, K. Ito, and R. Kawaguchi. 2003. Detection and identification of Mycobacterium species isolates by DNA microarray. J. Clin. Microbiol. 412605-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giglio, S., P. T. Monis, and C. P. Saint. 2005. Legionella confirmation using real-time PCR and SYTO9 is an alternative to current methodology. Appl. Environ. Microbiol. 718944-8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden, R. T., J. R. Uhl, X. Qian, M. K. Hopkins, M. C. Aubry, A. H. Limper, R. V. Lloyd, and F. R. Cockerill. 2001. Direct detection of Legionella species from bronchoalveolar lavage and open lung biopsy specimens: comparison of LightCycler PCR, in situ hybridization, direct fluorescence antigen detection, and culture. J. Clin. Microbiol. 392618-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, C. H., D. I. Grove, and D. F. Looke. 1996. Delay in appropriate therapy of Legionella pneumonia associated with increased mortality. Eur. J. Clin. Microbiol. Infect. Dis. 15286-290. [DOI] [PubMed] [Google Scholar]
- 16.Helbig, J. H., T. Engelstadter, M. Maiwald, S. A. Uldum, W. Witzleb, and P. C. Luck. 1999. Diagnostic relevance of the detection of Legionella DNA in urine samples by the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 18716-722. [DOI] [PubMed] [Google Scholar]
- 17.Herpers, B. L., B. M. de Jongh, K. van der Zwaluw, and E. J. van Hannen. 2003. Real-time PCR assay targets the 23S-5S spacer for direct detection and differentiation of Legionella spp. and Legionella pneumophila. J. Clin. Microbiol. 414815-4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaulhac, B., M. Nowicki, N. Bornstein, O. Meunier, G. Prevost, Y. Piemont, J. Fleurette, and H. Monteil. 1992. Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J. Clin. Microbiol. 30920-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joly, P., P.-A. Falconnet, J. Andre, N. Weill, M. Reyrolle, F. Vandenesch, M. Maurin, J. Etienne, and S. Jarraud. 2006. Quantitative real-time Legionella PCR for environmental water samples: data interpretation. Appl. Environ. Microbiol. 722801-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas, D., A. Rosenbaum. S. Weyrich, and S. Bhakdi. 1995. Enzyme-linked immunoassay for detection of PCR-amplified DNA of legionellae in bronchoalveolar fluid. J. Clin. Microbiol. 331247-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko, K. S., S. K. Hong, K. H. Lee, H. K. Lee, M. Y. Park, H. Miyamoto, and Y. H. Kook. 2003. Detection and identification of Legionella pneumophila by PCR-restriction fragment length polymorphism analysis of the RNA polymerase gene (rpoB). J. Microbiol. Methods 54325-337. [DOI] [PubMed] [Google Scholar]
- 22.Lisby, G., and R. Dessau. 1994. Construction of a DNA amplification assay for detection of Legionella species in clinical samples. Eur. J. Clin. Microbiol. Infect. Dis. 13225-231. [DOI] [PubMed] [Google Scholar]
- 23.MacDonell, M. T., and R. R. Colwell. 1987. The nucleotide sequence of the 5S rRNA from Legionella pneumophila. Nucleic Acids Res. 151335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahbubani, M. H., A. K. Bej, R. Miller, L. Haff, J. DiCesare, and R. M. Atlas. 1990. Detection of Legionella with polymerase chain reaction and gene probe methods. Mol. Cell. Probes 4175-187. [DOI] [PubMed] [Google Scholar]
- 25.Maiwald, M., M. Schill, C. Stockinger, J. H. Helbig, P. C. Luck, W. Witzleb, and H. G. Sonntag. 1995. Detection of Legionella DNA in human and guinea pig urine samples by the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 1425-33. [DOI] [PubMed] [Google Scholar]
- 26.Marston, B. J., H. B. Lipman, and R. F. Breiman. 1994. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch. Intern. Med. 1542417-2422. [PubMed] [Google Scholar]
- 27.McDonough, E. A., D. Metzgar, C. J. Hansen, C. A. Myers, and K. L. Russell. 2007. A cluster of Legionella-associated pneumonia cases in a population of military recruits. J. Clin. Microbiol. 452075-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar, B. C., X. Jiru, J. E. Moore, and J. A. Earle. 2000. A simple and sensitive method to extract bacterial, yeast and fungal DNA from blood culture material. J. Microbiol. Methods 42139-147. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto, H., H. Yamamoto, K. Arima, J. Fujii, K. Maruta, K. Izu, T. Shiomori, and S. Yoshida. 1997. Development of a new seminested PCR method for detection of Legionella species and its application to surveillance of legionellae in hospital cooling tower water. Appl. Environ. Microbiol. 632489-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdoch, D. R., and S. T. Chambers. 2000. Detection of Legionella DNA in peripheral leukocytes, serum, and urine from a patient with pneumonia caused by Legionella dumoffii. Clin. Infect. Dis. 30382-383. [DOI] [PubMed] [Google Scholar]
- 31.Park, H., H. Jang, E. Song, C. L. Chang, M. Lee, S. Jeong, J. Park, B. Kang, and C. Kim. 2005. Detection and genotyping of Mycobacterium species from clinical isolates and specimens by oligonucleotide array. J. Clin. Microbiol. 431782-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelaz, C., L. García Albert, and C. M. Bourgon. 1987. Cross-reactivity among Legionella species and serogroups. Epidemiol. Infect. 99641-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez, J. A., S. Ahkee, A. Tolentino, R. D. Miller, and J. T. Summersgill. 1996. Diagnosis of Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae lower respiratory-infection using the polymerase chain reaction on a single throat swab specimen. Diagn. Microbiol. Infect. Dis. 247-14. [DOI] [PubMed] [Google Scholar]
- 34.Rantakokko-Jalava, K., and J. Jalava. 2001. Development of conventional and real-time PCR assay for detection of Legionella DNA in respiratory specimens. J. Clin. Microbiol. 392904-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratcliff, R. M., J. A. Lanser, P. A. Manning, and M. W. Heuzenroeder. 1998. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 361560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reischl, U., H.-J. Linde, N. Lehn, O. Landt, K. Barrat, and N. Wellinghausen. 2002. Direct detection and differentiation of Legionella spp. and Legionella pneumophila in clinical specimens by dual-color real-time PCR and melting curve analysis. J. Clin. Microbiol. 403814-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson, I. R., and N. F. Lightfoot. 1992. Legionella pneumophila species identification using a commercial latex agglutination kit: a potential cross-reaction problem with serogroup 12. Med. Lab. Sci. 49144-146. [PubMed] [Google Scholar]
- 38.Riffard, S., F. Lo Presti, P. Normand, F. Forey, M. Reyrolle, J. Etienne, and F. Vandenesch. 1998. Species identification of Legionella via intergenic 16S-23S ribosomal spacer PCR analysis. Int. J. Syst. Bacteriol. 48723-730. [DOI] [PubMed] [Google Scholar]
- 39.Starnbach, M. N., S. Falkow, and L. S. Tompkins. 1989. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J. Clin. Microbiol. 271257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinert, M., U. Hentschel, and J. Hacker. 2002. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26149-162. [DOI] [PubMed] [Google Scholar]
- 41.Stølhaug, A., and K. Bergh. 2006. Identification and differentiation of Legionella pneumophila and Legionella spp. with real-time PCR targeting the 16S rRNA gene and species identification by mip sequencing. Appl. Environ. Microbiol. 726394-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stout, J. E., J. D. Rihs, and V. L. Yu. 2003. Legionella, p. 809-823. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 43.Tung, S. K., L. J. Teng, M. Vaneechoutte, H. M. Chen, and T. C. Chang. 2006. Array-based identification of species of the genera Abiotrophia, Enterococcus, Granulicatella, and Streptococcus. J. Clin. Microbiol. 444414-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vesey, G., P. J. Dennis, J. V. Lee, and A. A. West. 1988. Further development of simple tests to differentiate the legionellas. J. Appl. Bacteriol. 65339-345. [DOI] [PubMed] [Google Scholar]
- 45.Volokhov, D., A. Rasooly, K. Chumakov, and V. Chizhikov. 2002. Identification of Listeria species by microarray-based assay. J. Clin. Microbiol. 404720-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Baum, H., S. Ewig, R. Marre, N. Suttorp, S. Gonschior, T. Welte, C. Lück, et al. 2008. Community-acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. Clin. Infect. Dis. 461356-1364. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, D. A., U. Reischl, G. S. Hall, and G. W. Procop. 2007. Use of partial 16S rRNA gene sequencing for identification of Legionella pneumophila and non-pneumophila Legionella spp. J. Clin. Microbiol. 45257-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson, D. A., B. Yen-Lieberman, U. Reischl, S. M. Gordon, and G. W. Procop. 2003. Detection of Legionella pneumophila by real-time PCR for the mip gene. J. Clin. Microbiol. 413327-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousboe, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186127-128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



