Abstract
In the treatment of serious bacterial infections, the rapid institution of appropriate antimicrobial chemotherapy may be lifesaving. Choosing the correct antibiotic or combination of antibiotics is becoming very important, as multidrug resistance is found in many pathogens. Using a collection of 75 well-characterized multidrug-resistant (MDR) Acinetobacter sp. isolates, we show that PCR followed by electrospray ionization mass spectrometry (PCR/ESI-MS) and base composition analysis of PCR amplification products can quickly and accurately identify quinolone resistance mediated by mutations in the quinolone resistance-determining regions of gyrA and parC, two essential housekeeping genes. Single point mutations detected by PCR/ESI-MS in parC (found in 55/75 of the isolates) and in gyrA (found in 66/75 of the isolates) correlated with susceptibility testing and sequencing. By targeting resistance determinants that are encoded by genes with highly conserved DNA sequences (e.g., gyrA and parC), we demonstrate that PCR/ESI-MS can provide critical information for resistance determinant identification and can inform therapeutic decision making in the treatment of Acinetobacter sp. infections.
Acinetobacter baumannii is a gram-negative, aerobic, non-lactose-fermenting bacterium of increasing clinical importance (10, 14, 19, 21, 23). Since the 1980s, the number of reports describing infections due to this organism has risen dramatically (5, 16, 20). Most A. baumannii infections occur within intensive care units in patients with serious underlying disease (2, 19). Sites of infection include lung, bloodstream, urinary tract, and skin (20). In addition, A. baumannii has emerged as an important pathogen in injured United States military personnel stationed in Afghanistan and Iraq as they return to major military medical treatment facilities (14, 24-26, 31).
An unwelcome feature of A. baumannii is the frequency in which isolates manifest resistance to the most commonly used antibiotics (12, 14). When resistance to three or more classes of antibiotics is demonstrated, A. baumannii is referred to as multidrug resistant (MDR) (21). Among MDR A. baumannii isolates, resistance to quinolones (e.g., ciprofloxacin and levofloxacin), aminoglycosides (e.g., gentamicin and amikacin), ampicillin-sulbactam, extended-spectrum cephalosporins (e.g., ceftazidime), and carbapenems (e.g., imipenem, meropenem, and doripenem) is escalating (21, 23). Concern exists that, if the incorrect antimicrobial agents are chosen to treat MDR A. baumannii infection, the outcome of patients may be poor (9, 22). Thus, a rapid assessment of antimicrobial susceptibility could have a significant impact on patient care.
In this report, we employ a rapid high-throughput method to identify unique DNA changes associated with quinolone resistance in a collection of MDR A. baumannii isolates. The method was originally developed to identify and genotype various species of bacteria from complex mixtures in respiratory samples (6). This process uses electrospray ionization mass spectrometry (ESI-MS) and base composition analysis of PCR amplification products derived from highly conserved genes (“housekeeping genes”). The primers that are designed for PCR/ESI-MS yield amplicons with unique mass signatures that can be measured by high-performance mass spectrometry and identified by base composition analysis (7, 13).
The best-described mechanism of resistance to quinolones in Acinetobacter spp. is mutations in the genes encoding DNA gyrase A (i.e., gyrA) and subunit A of topoisomerase IV (i.e., parC). The most important mutations, resulting in changes at codon 83 for gyrA and at codon 80 for parC, have been mapped to a unique location in each of these genes, the quinolone resistance-determining region (QRDR) (27, 28). Additional mutations in the QRDR of gyrA thought to enhance quinolone resistance include mutations at codons for amino acids Gly81, Ala84, and Glu87 (1, 11, 17, 27, 28, 30). Another previously described mutation (at the Val101 codon) does not appear to have an effect on the susceptibility profiles (1). Ancillary mutations in parC, at codons for amino acids Gly78 and Glu84, when combined with the Ser83 and Ser80 codon mutations, also contribute to high-level quinolone resistance (11, 27, 30).
Appreciating that mutations in the housekeeping genes gyrA and parC also define the quinolone resistance phenotype, we reasoned that PCR/ESI-MS and base composition analysis can be applied to determine the sequence variability of the QRDRs of Acinetobacter spp. and rapidly predict a quinolone-resistant phenotype. Therefore, we chose to study 73 well-characterized clinical isolates of A. baumannii, one Acinetobacter genome species strain 3 isolate, and one Acinetobacter johnsonii isolate. This paper describes the novel application of this highly sensitive and specific method to the analysis of resistance phenotypes in this clinically important pathogen.
MATERIALS AND METHODS
Bacterial isolates.
The Acinetobacter sp. isolates were obtained from 75 civilian and military patients treated at the Walter Reed Army Medical Center in Washington, DC. All isolates were collected from March 2003 through April 2005 and represent 16 unique Acinetobacter sp. clone types (14).
Determination of susceptibility to quinolones.
MICs of ciprofloxacin and levofloxacin were determined by the broth microdilution method using cation-adjusted Mueller-Hinton medium according to the Clinical and Laboratory Standards Institute (CLSI) standard criteria (3). Specific panels were “custom-made” by Trek Diagnostics (Cleveland, OH) to better measure the level of quinolone resistance. Ciprofloxacin was tested in the range of 0.06 to 64 mg/liter, whereas levofloxacin was tested in the range of 0.06 to 32 mg/liter. ATCC control strains, including A. baumannii isolates 9955 and 17961, Escherichia coli 25922, Pseudomonas aeruginosa 27853, and Klebsiella pneumoniae 700603, were used. Susceptibility results were interpreted according to the guidelines recommended by CLSI (4). In this paper, A. baumannii isolates were defined as quinolone resistant when the ciprofloxacin or levofloxacin MIC or both MICs were in the nonsusceptible range (MIC ≥ 1 mg/liter).
PCR amplification for RE digestion and DNA sequencing.
All primers used for PCR amplification along with the product sizes are listed in Table 1. The restriction endonuclease (RE) digestions with HinfI of the gyrA and parC QRDRs have been previously described (14).
TABLE 1.
Primers used to amplify the gyrA and parC genes for sequencing and PCR/ESI-MS
| Gene | ESI-MS primer pair | Primer
|
Product size (bp) | ||
|---|---|---|---|---|---|
| Purpose or name | Directiona | Sequence (5′-3′) | |||
| gyrA | PCR | For | ATGAGCGTATCGGAAATCC | 620 | |
| Rev | ATCAATCCTTCAATCGAGATATTC | ||||
| Sequencing | For | GGAAATCCGACCGATTGCC | |||
| Rev | CGAGATATTCGGATTGTCAGC | ||||
| parC | PCR | For | ATGGAAGATAAGCTGACTATG | 680 | |
| Rev | GTTGGTAAATCCGGAGC | ||||
| Sequencing | For | GAAGATAAGCTGACTATGACCAG | |||
| Rev | GAGCCGGAATATATTCAGC | ||||
| gyrA | BCT2852 | BCT6608 | For | TAAATCTGCCCGTGTCGTTGGTGAC | 101 |
| BCT6609 | Rev | TGCTAAAGTCTTGAGCCATACGAACAATGG | |||
| gyrA | BCT2853 | BCT6610 | For | TAATCGGTAAATATCACCCGCATGGTGAC | 121 |
| BCT6611 | Rev | TCGATCGAACCGAAGTTACCCTGACC | |||
| gyrA | BCT2854 | BCT6610 | For | TAATCGGTAAATATCACCCGCATGGTGAC | 64 |
| BCT6612 | Rev | TGAGCCATACGAACAATGGTTTCATAAACAGC | |||
| parC | BCT2846 | BCT6597 | For | TCCAAAAAAATCAGCGCGTACAGTGG | 121 |
| BCT6598 | Rev | TAAAGGATAGCGGTAACTAAATGGCTGAGCCAT | |||
| parC | BCT2847 | BCT6599 | For | TACTTGGTAAATACCACCCACATGGTGA | 114 |
| BCT6600 | Rev | TACCCCAGTTCCCCTGACCTTC | |||
| parC | BCT2848 | BCT6601 | For | TGGTAAATACCACCCACATGGTGAC | 60 |
| BCT6602 | Rev | TGAGCCATGAGTACCATGGCTTCATAACATGC | |||
For, forward; Rev, reverse.
Amplification of the gyrA and parC genes for direct sequencing was performed using the forward and reverse primers listed in Table 1. A 1:10 dilution of an overnight lysogeny broth culture was boiled for 15 min. High-fidelity amplification using rTth polymerase (GeneAmp XL kit; Applied Biosystems, Foster City, CA) was performed with 5 μl of this dilution as the DNA template. PCR conditions included an initial denaturation at 95°C for 1 min followed by 30 cycles of 95°C for 30 s and annealing for 45 s at 54°C for both gyrA and parC, with an extension at 72°C for 30 s. Cycling was followed by a final extension at 72°C for 5 min. The amplified products (25 μl) were purified with 5 U exonuclease I and 1 U shrimp alkaline phosphatase (USB Corporation, Cleveland, OH) and were incubated for 30 min at 37°C, 15 min at 45°C, and 80°C for 15 min to degrade the unincorporated primers and nucleotides. Sequencing reactions were performed using nested primers (Table 1) and employing Applied Biosystems BigDye v1.1 sequencing kits. Sequence data were obtained on an Applied Biosystems 3730xl DNA analyzer. Trace files were analyzed by Vector NTI (Invitrogen, Carlsbad, CA), and the resulting consensus sequences were compared to GenBank (www.ncbi.nlm.nih.gov/GenBank) reference sequences X82165 for gyrA and X95819 for parC of A. baumannii.
DNA preparation and PCR amplification for PCR/ESI-MS.
Template DNA was prepared by making a 1:100 dilution of a lysogeny broth overnight culture in TE buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) and boiling for 15 min. All PCRs were performed in 96-well plates. The PCR mixture consisted of 3 U of AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA); 20 mM Tris (pH 8.3); 75 mM KCl; 1.5 mM MgCl2; 0.4 M betaine; 800 μM equal mixture of dCTP, dTTP, dGTP, and dATP; and 250 nM of each primer. PCR cycling under the following conditions was performed on an MJ Dyad 96-well thermocycler (Bio-Rad Inc., Hercules, CA): 95°C for 10 min, followed by 8 cycles of 95°C for 30 s, 48°C for 30 s, and 72°C 30 s, with the 48°C annealing temperature increasing 0.9°C each cycle. PCR was then continued for 37 additional cycles of 95°C for 15 s, 56°C for 20 s, and 72°C for 20 s. The PCR cycle ended with a final extension of 2 min at 72°C followed by a 4°C hold. Five microliters of the template DNA was used in each PCR.
Primer design for PCR/ESI-MS and base composition analysis.
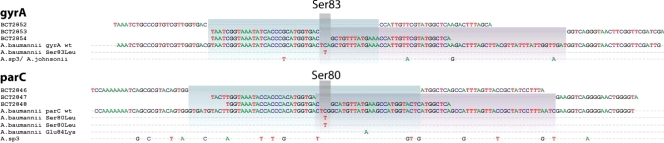
The primer pairs used for mass spectrometry and base composition analysis of the QRDRs of gyrA and parC in Acinetobacter spp. are also listed in Table 1. To completely cover the QRDR, three sets of overlapping primers were constructed for both topoisomerase genes. Two sets of primers encompass the flanking regions as well as the nucleotides in parC that encode amino acid Ser80 or those in gyrA that encode Ser83 (Fig. 1). The third set of primers specifically targeted only the nucleotides that encode the Ser83 or Ser80 amino acid. The length of each amplified region is listed in Table 1.
FIG. 1.
Priming strategy used for PCR/ESI-MS of the QRDRs of gyrA and parC in Acinetobacter baumannii. Six primer pairs (BCT2852 to BCT2854 and BCT2846 to BCT2848) were developed to cover the entire length of the gyrA and parC QRDRs, respectively. The principal mutations captured by these primer pairs are indicated below the wild-type sequences. Colored boxes indicate the scope of these primer pairs, all of them covering the structurally homologous gyrA codon for Ser83 and parC codon for Ser80.
ESI-MS.
The study was performed using the T5000 Biosensor (Ibis Biosciences, Carlsbad, CA). After amplification, 15-μl aliquots of each PCR mixture were desalted and purified using a weak-anion-exchange protocol as previously described (15). Accurate-mass (±1 ppm), high-resolution (M/ΔM > 100,000 full-width half-maximal) mass spectra were acquired for each sample by using high-throughput ESI-MS protocols described previously (13). For each sample, 1.5 μl of analyte solution was consumed during the 74-s spectral acquisition. Raw mass spectra were converted to monoisotopic molecular masses. Unambiguous base compositions were derived from the exact mass measurements of the complementary single-stranded oligonucleotides (18). Quantitative results were obtained by comparing the peak heights with an internal PCR calibration standard present in every PCR well at 100 molecules (13). The ESI-MS measurements required approximately 45 s per PCR, so analysis of each Acinetobacter sp. isolate required less than 5 min of mass spectrometry time (338 min for 75 samples).
RESULTS
Susceptibility testing.
The results of quinolone susceptibility testing for all 75 Acinetobacter sp. isolates are summarized in Table 2. Overall, 89.3% (67/75) of the isolates in this collection demonstrated MICs to quinolones in the resistance range (all of these isolates had ciprofloxacin MICs of ≥2 mg/liter). High-level ciprofloxacin resistance (i.e., ≥64 mg/liter) was seen in 74.7% of the isolates. Similarly, 84.9% (62/73) of the isolates were resistant (i.e., MIC ≥ 4 mg/liter) to levofloxacin.
TABLE 2.
Quinolone MICs of the 75 Acinetobacter sp. isolates in this study
| Isolate | MIC (mg/liter) of:
|
|
|---|---|---|
| CIPa | LVXb | |
| AB001 | >64 | 8 |
| AB002 | >64 | 8 |
| AB003 | >64 | 8 |
| AB004 | >64 | 8 |
| AB005 | 64 | 16 |
| AB006 | >64 | 8 |
| AB007 | >64 | 8 |
| AB008 | 64 | 8 |
| AB009 | >64 | 8 |
| AB0010 | 16 | 4 |
| AB0011 | >64 | 8 |
| AB0012 | 64 | 16 |
| AB0013 | >64 | 8 |
| AB0014 | 64 | 8 |
| AB0015 | 32 | 8 |
| AB0016 | >64 | 8 |
| AB0017 | 64 | 8 |
| AB0018 | >64 | 8 |
| AB0019 | >64 | 8 |
| AB0020 | 64 | 4 |
| AB0021 | 64 | 4 |
| AB0022 | 64 | 8 |
| AB0023 | 64 | 8 |
| AB0024 | 64 | 16 |
| AB0025 | >64 | 8 |
| AB0026 | >64 | 16 |
| AB0027 | 64 | 8 |
| AB0028 | 64 | 8 |
| AB0029 | 64 | 8 |
| AB0030 | 64 | 8 |
| AB0031 | 64 | 8 |
| AB0032 | 64 | 8 |
| AB0033 | >64 | 8 |
| AB0034 | >64 | 8 |
| AB0035 | 64 | 8 |
| AB0036 | 64 | 4 |
| AB0037 | 64 | 8 |
| AB0038 | 64 | 8 |
| AB0039 | 64 | 8 |
| AB0040 | >64 | 8 |
| AB0041 | 4 | 2 |
| AB0042 | 64 | 8 |
| AB0043 | 16 | 8 |
| AB0044 | 32 | 8 |
| AB0045 | >64 | NDf |
| AB0046 | 32 | 4 |
| AB0047 | 32 | 4 |
| AB0048 | >64 | 8 |
| AB0049 | 8 | 4 |
| AB0050 | 16 | 4 |
| AB0051 | 0.5 | 0.5 |
| AB0052 | >64 | 16 |
| AB0053 | >64 | 16 |
| AB0054 | 32 | 4 |
| AB0055 | >64 | 16 |
| AB0056 | 64 | 8 |
| AB0057 | 64 | 8 |
| AB0058 | 64 | 4 |
| AB0059 | 64 | 8 |
| AB0060 | >64 | 4 |
| AB0061 | >64 | 8 |
| AB0062 | 64 | 4 |
| AB0063 | 64 | 2 |
| AB0064 | 0.12 | 0.06 |
| AB0065 | >64 | 8 |
| AB0066 | 64 | 8 |
| AB0067 | >64 | ND |
| AB0068 | 0.5 | 0.5 |
| AB0069 | 2 | 2 |
| AB0070 | >64 | 16 |
| AB0071 | 0.5 | 0.25 |
| AB0072 | 0.12 | 0.12 |
| AG0073c | ≤0.06 | ≤0.06 |
| AB0074 | 0.12 | 0.25 |
| AJ0075d | 0.12 | 0.06 |
| A. baumannii 9955e | 0.12 | 0.12 |
| A. baumannii 17961e | 0.12 | 0.12 |
| E. coli 25922e | 0.06 | 0.06 |
| P. aeruginosa 27853e | 0.25 | 1 |
| K. pneumoniae 700603e | 0.25 | 1 |
CLSI ciprofloxacin (CIP) MIC criteria: susceptible, ≤1 mg/liter; intermediate, 2 mg/liter; resistant, ≥4 mg/liter.
CLSI levofloxacin (LVX) MIC criteria: susceptible, ≤2 mg/liter; intermediate, 4 mg/liter; resistant, ≥8 mg/liter.
Acinetobacter genome sp. strain 3.
Acinetobacter johnsonii.
ATCC control.
ND, not determined.
PCR amplification, RE digestion, and DNA sequencing of gyrA and parC.
To determine if RE digestion patterns using HinfI obtained in our laboratory correctly identified mutations in the QRDR loci, we sequenced the QRDR and flanking regions of each topoisomerase gene. Analysis of the QRDRs of gyrA and parC revealed mutations in this area previously described as resulting in ciprofloxacin resistance. In addition, DNA sequencing of the parC QRDR amplification products showed a Glu84Lys mutation in three isolates (i.e., AB0042, AB0048, and AB0058).
We further determined that isolates AB0056, AB0057, and AB0059 possess silent mutations in the codons of parC encoding Pro98, Leu99, Glu101, Gly102, and Gln103 (the importance of these mutations is still unknown). Comparing our results with the reference sequence, we discovered that silent mutations were found in gyrA for isolate AG0073 (Acinetobacter genome sp. strain 3) and AJ0075 (A. johnsonii) at codons for amino acids Ala67, Val69, Pro79, Val90, Gln94, and Val103.
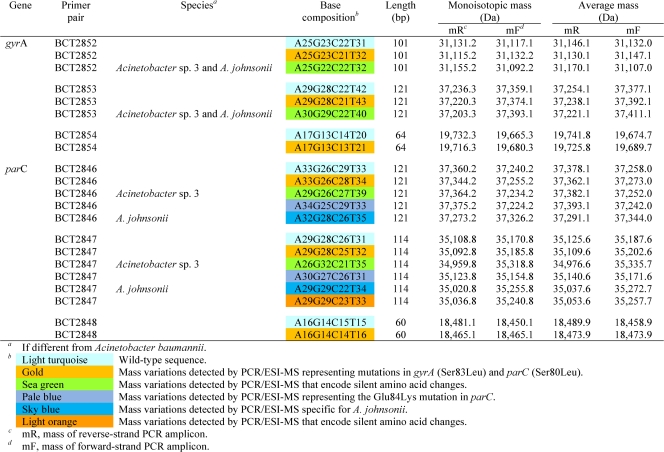
PCR/ESI-MS and base composition analysis.
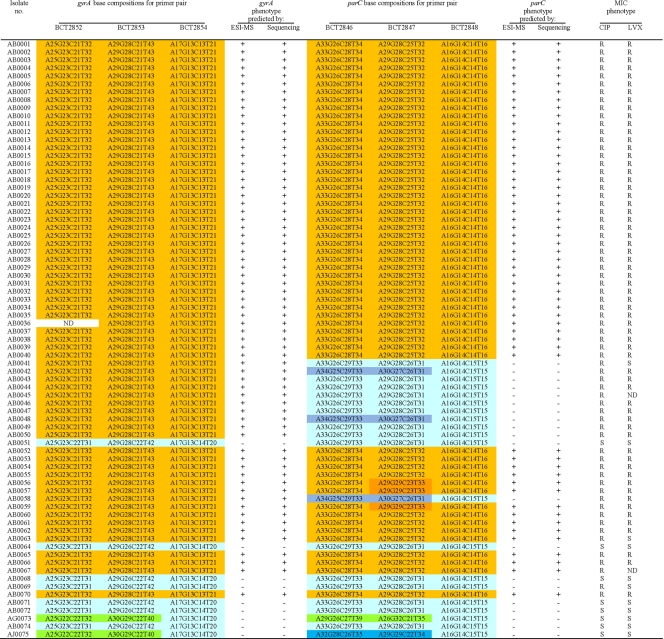
In Table 3, we present the target base composition analysis for all PCR/ESI-MS primer sets resulting from the generated monoisotopic masses for each forward and reverse PCR amplicon strand. Table 4 lists the ESI-MS data for both the gyrA and parC primer sets for each Acinetobacter sp. isolate analyzed. For ease of interpretation, the various base compositions detected by ESI-MS are color coded and correspond to substitutions from the wild-type sequence (Table 3). In every case, when base substitutions were detected using PCR/ESI-MS, standard DNA sequencing confirmed these findings (100% correlation). In addition, MIC determination identified 67 out of 75 (89.3%) strains as quinolone resistant. By PCR/ESI-MS 88% of the isolates are predicted to be quinolone resistant, and overall the accurate prediction of quinolone susceptibility or resistance was 98.7% (74/75 isolates).
TABLE 3.
Target base composition analysis for all PCR/ESI-MS primer sets based on the generated monoisotopic mass for each forward and reverse PCR amplicon strand
TABLE 4.
Summary for PCR/ESI-MS base composition analysis for gyrA and parC QRDR ampliconsa
+, predicted resistant phenotype; −, predicted susceptible phenotype; R, resistant phenotype determined from MICs; S, susceptible phenotype determined from MICs; ND, not determined. Base compositions are defined in Table 3. Within each column, base compositions that are common to multiple types are similarly colored.
By using three sets of overlapping primers, we were able to discriminate the Ser83 and Ser80 point mutations as well as detect mutations in the remaining nucleotides spanning the QRDRs of both gyrA and parC. The deduced overall net base changes (addition or deletion of A, G, C, or T) from the expected wild-type sequence are listed in Table 4. As stated above, three overlapping primer sets were used to target the regions of interest in gyrA. Primer set BCT2852, which corresponds to the 5′ flanking region through the Ser83 codon position in gyrA (Fig. 1 and Table 4), yielded three distinct mass signature profiles. The wild-type sequence (i.e., A25G23C22T31; turquoise) was found in strains AB0051, AB0064, AB0068, AB0069, AB0071, AB0072, and AB0074. The second base composition (i.e., A25G23C21T32; gold) indicates a base change from C to T. The third mass signature (i.e., A25G22C22T32; green) reflects sequence variations or polymorphisms observed in the gyrA genes from different Acinetobacter spp. (Acinetobacter genome sp. strain 3 and A. johnsonii).
Primer set BCT2853 amplifies the nucleotide sequence of the gyrA QRDR that encodes amino acids 83 to 104. This primer set also yielded three distinct mass signature profiles. As with primer set BCT2852, the wild-type signature (turquoise), the second distinct signature representing the Ser83Leu mutation (gold), and the third signature (green) represent sequence variations that correspond to changes in codons for amino acids Val90, Gln94, and Val103 in Acinetobacter genome sp. strain 3 and A. johnsonii.
Primer set BCT2854 specifically targets the gyrA-encoded Ser83 mutation and allows us to discern with confidence that there is a C→T change at the codon for amino acid 83, not in the surrounding sequence, and that this encodes an amino acid change from Ser to Leu (i.e., from TCA→TTA).
Like the complementary primer set in gyrA, primer set BCT2846 corresponds to the 5′ flanking region, including the Ser80 codon in parC (Fig. 1). Five distinct mass signature profiles were determined and confirmed by DNA sequencing. As explained for gyrA, the first profile is the expected wild-type sequence (i.e., A33G26C29T33; turquoise) in Tables 3 and 4. The second base composition (i.e., A33G26C28T34; gold) indicates a base change from C to T at the codon for amino acid 80. The third (i.e., A29G26C27T39; green) and fifth (i.e., A32G28C26T35; sky blue) mass signatures reflect sequence variations or polymorphisms in the parC gene of Acinetobacter genome sp. strain 3 and A. johnsonii, respectively (Fig. 1). The fourth unique mass signature (pale blue) (Table 3) reflects the Glu84Lys mutation in isolates AB0042, AB0048, and AB0058. This G→A nucleotide change was uncovered by the base composition analysis and confirmed by DNA sequencing (i.e., A34G25C29T33; Table 4).
Isolates AB0056, AB0057, and AB0059 have silent mutations in the nucleotides encoding amino acids Pro98, Leu99, Glu101, Gly102, and Gln103 in parC, which were also identified by PCR/ESI-MS using primer set BCT2847 (i.e., A29G29C23T33; orange in Tables 3 and 4). This primer set encompasses the 3′ flanking region of the codon for the Ser80 amino acid (Table 4). Six unique mass profiles were obtained for the amplicons generated by primer set BCT2847. As with gyrA, a specific primer set for parC, BCT2848, targets only the nucleotides that encode amino acid Ser80, and therefore the wild-type and mutated genes can be identified.
DISCUSSION
PCR/ESI-MS is emerging as a very important method to accurately type A. baumannii and other Acinetobacter spp. (8). PCR/ESI-MS has also been employed to track epidemics of A. baumannii associated with war trauma (31). In a multiclonal collection of Acinetobacter spp., PCR/ESI-MS analysis revealed that there was significant genetic diversity among clinical isolates (14). The increasing application of PCR/ESI-MS to outbreaks of MDR Acinetobacter spp. is providing important information regarding clonal relatedness, hospital transmission dynamics, correct bacterial species identification, and microorganism quantification (6, 14, 29). We demonstrate herein for the first time that PCR/ESI-MS can also be used to correctly identify antibiotic resistance genes that impact clinical decision making. Our study also shows for the first time that PCR/ESI-MS can determine the genetic basis of the quinolone resistance phenotype.
PCR/ESI-MS accurately identified quinolone-resistant isolates. PCR/ESI-MS predicted that 88% of the isolates would be resistant to one or both of the quinolones, whereas MIC determinations identified 89.3% of the isolates as quinolone resistant. Overall, the PCR/ESI-MS method identified the correct phenotype in 74/75 (98.7%) isolates. Although it is possible that upregulation of efflux pumps such as AdeABC or AbeM may also contribute to quinolone resistance in these isolates, when mutations were found in both the gyrA and parC QRDRs by PCR/ESI-MS, high-level ciprofloxacin resistance was also detected by traditional susceptibility testing.
In the case of A. baumannii isolates possessing the GyrA Ser83 mutation but not the corresponding ParC Ser80 mutation (i.e., isolates AB0041, AB0042, AB0043, AB0044, AB0045, AB0046, AB0047, AB0048, AB0049, AB0050, and AB0058), the MICs show that the gyrA-encoded Ser83 mutation is enough to confer quinolone resistance in these isolates. Although levofloxacin is thought to better target ParC than ciprofloxacin, only one isolate in this group (i.e., AB0041) remained susceptible to levofloxacin when the primary Ser83Leu mutation was encoded by gyrA.
We also demonstrate that PCR/ESI-MS accurately identified the correct phenotype in the quinolone-susceptible isolates (i.e., isolates AB0051, AB0064, AB0068, AB0071, AB0072, AG0073, AB0074, and AJ075). For only one isolate, AB0069, PCR/ESI-MS and phenotypic tests were not in agreement. The MICs show a reduced susceptibility to quinolones (intermediate for ciprofloxacin, susceptible to levofloxacin), although, by DNA sequencing, RE digestion, and PCR/ESI-MS, AB0069 was predicted to have a susceptible phenotype.
In conclusion, we report the discriminating ability of PCR/ESI-MS for detecting quinolone resistance by targeting the QRDRs in 75 isolates of Acinetobacter spp. Furthermore, our results show that the observed variations in base composition of gyrA and parC can easily be detected even among isolates of related species. Although the informational content provided by base composition is less than that generated by sequencing large regions or entire genes, PCR/ESI-MS has the same practical utility in detecting resistance mutations when the amplified target sites are “information rich” and properly chosen (7).
Two important limitations of PCR/ESI-MS must be recognized. First, it remains uncertain if detecting a resistance gene always indicates that a resistance phenotype will be present. Second, the interpretation of mutations in resistance genes that exist in multiple copies is potentially problematic. The levels of clinical accuracy and predictability remain a significant challenge, as gene dosage, level of transcription, and expression are not yet measured by these methods. This further refinement will require other types of studies. Nevertheless, assessment of quinolone susceptibility by a rapid molecular method (in this case, less than 6 h) could have a significant impact on clinical outcome by aiding in the proper selection of antibiotics to treat infections.
This series of experiments served as a “proof of principle” for the application of PCR/ESI-MS to detect and evaluate resistance genes not only in A. baumannii but also in other gram-negative pathogens. Using this platform for the rapid determination of resistance phenotypes in hospital-associated infections, biodefense, and molecular epidemiology can have a profound impact on clinical microbiology.
Acknowledgments
The Veterans Affairs Merit Review Program and Geriatric Research Education and Clinical Center VISN 10 supported R.A.B. The National Institutes of Health (RO1 AI072219) supported both R.A.B. and P.N.R. J.M.T. was supported in part by NIH grant T32 GM07250 and the Case Medical Scientist Training Program.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Adams-Haduch, J. M., D. L. Paterson, H. E. Sidjabat, A. W. Pasculle, B. A. Potoski, C. A. Muto, L. H. Harrison, and Y. Doi. 2008. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob. Agents Chemother. 523837-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chastre, J. 2003. Infections due to Acinetobacter baumannii in the ICU. Semin. Respir. Crit. Care Med. 2469-78. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. CLSI, Wayne, Pa.
- 4.CLSI. 2007. Performance standards for antimicrobial testing; 17th informational supplement. M100-S17. CLSI, Wayne, PA.
- 5.Dijkshoorn, L., A. Nemec, and H. Seifert. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5939-951. [DOI] [PubMed] [Google Scholar]
- 6.Ecker, D. J., R. Sampath, L. B. Blyn, M. W. Eshoo, C. Ivy, J. A. Ecker, B. Libby, V. Samant, K. A. Sannes-Lowery, R. E. Melton, K. Russell, N. Freed, C. Barrozo, J. Wu, K. Rudnick, A. Desai, E. Moradi, D. J. Knize, D. W. Robbins, J. C. Hannis, P. M. Harrell, C. Massire, T. A. Hall, Y. Jiang, R. Ranken, J. J. Drader, N. White, J. A. McNeil, S. T. Crooke, and S. A. Hofstadler. 2005. Rapid identification and strain-typing of respiratory pathogens for epidemic surveillance. Proc. Natl. Acad. Sci. USA 1028012-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecker, D. J., R. Sampath, C. Massire, L. B. Blyn, T. A. Hall, M. W. Eshoo, and S. A. Hofstadler. 2008. Ibis T5000: a universal biosensor approach for microbiology. Nat. Rev. Microbiol. 6553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecker, J. A., C. Massire, T. A. Hall, R. Ranken, T. T. Pennella, C. Agasino Ivy, L. B. Blyn, S. A. Hofstadler, T. P. Endy, P. T. Scott, L. Lindler, T. Hamilton, C. Gaddy, K. Snow, M. Pe, J. Fishbain, D. Craft, G. Deye, S. Riddell, E. Milstrey, B. Petruccelli, S. Brisse, V. Harpin, A. Schink, D. J. Ecker, R. Sampath, and M. W. Eshoo. 2006. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J. Clin. Microbiol. 442921-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas, M. E., S. K. Kasiakou, P. I. Rafailidis, G. Zouglakis, and P. Morfou. 2006. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J. Antimicrob. Chemother. 571251-1254. [DOI] [PubMed] [Google Scholar]
- 10.Gootz, T. D., and A. Marra. 2008. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev. Anti Infect. Ther. 6309-325. [DOI] [PubMed] [Google Scholar]
- 11.Hamouda, A., and S. G. Amyes. 2004. Novel gyrA and parC point mutations in two strains of Acinetobacter baumannii resistant to ciprofloxacin. J. Antimicrob. Chemother. 54695-696. [DOI] [PubMed] [Google Scholar]
- 12.Higgins, P. G., H. Wisplinghoff, D. Stefanik, and H. Seifert. 2004. Selection of topoisomerase mutations and overexpression of adeB mRNA transcripts during an outbreak of Acinetobacter baumannii. J. Antimicrob. Chemother. 54821-823. [DOI] [PubMed] [Google Scholar]
- 13.Hofstadler, S. A., R. Sampath, L. B. Blyn, M. W. Eshoo, T. A. Hall, Y. Jiang, J. J. Drader, J. C. Hannis, K. A. Sannes-Lowery, L. L. Cummins, B. Libby, D. J. Walcott, A. Schink, C. Massire, R. Ranken, J. Gutierrez, S. Manalili, C. Ivy, R. Melton, H. Levene, G. Barrett-Wilt, F. Li, V. Zapp, N. White, V. Samant, J. A. McNeil, D. Knize, D. Robbins, K. Rudnick, A. Desai, E. Moradi, and D. J. Ecker. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 24223-41. [Google Scholar]
- 14.Hujer, K. M., A. M. Hujer, E. A. Hulten, S. Bajaksouzian, J. M. Adams, C. J. Donskey, D. J. Ecker, C. Massire, M. W. Eshoo, R. Sampath, J. M. Thomson, P. N. Rather, D. W. Craft, J. T. Fishbain, A. J. Ewell, M. R. Jacobs, D. L. Paterson, and R. A. Bonomo. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 504114-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, Y., and S. A. Hofstadler. 2003. A highly efficient and automated method of purifying and desalting PCR products for analysis by electrospray ionization mass spectrometry. Anal. Biochem. 31650-57. [DOI] [PubMed] [Google Scholar]
- 16.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11868-873. [DOI] [PubMed] [Google Scholar]
- 17.Lee, J. K., Y. S. Lee, Y. K. Park, and B. S. Kim. 2005. Mutations in the gyrA and parC genes in ciprofloxacin-resistant clinical isolates of Acinetobacter baumannii in Korea. Microbiol. Immunol. 49647-653. [DOI] [PubMed] [Google Scholar]
- 18.Muddiman, D. C., G. A. Anderson, S. A. Hofstadler, and R. D. Smith. 1997. Length and base composition of PCR-amplified nucleic acids using mass measurements from electrospray ionization mass spectrometry. Anal. Chem. 691543-1549. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Price, L. S., and R. A. Weinstein. 2008. Acinetobacter infection. N. Engl. J. Med. 3581271-1281. [DOI] [PubMed] [Google Scholar]
- 20.Murray, C. K., and D. R. Hospenthal. 2005. Treatment of multidrug resistant Acinetobacter. Curr. Opin. Infect. Dis. 18502-506. [DOI] [PubMed] [Google Scholar]
- 21.Peleg, A. Y., H. Seifert, and D. L. Paterson. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21538-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peralta, G., M. B. Sanchez, J. C. Garrido, I. De Benito, M. E. Cano, L. Martinez-Martinez, and M. P. Roiz. 2007. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J. Antimicrob. Chemother. 60855-863. [DOI] [PubMed] [Google Scholar]
- 23.Perez, F., A. M. Hujer, K. M. Hujer, B. K. Decker, P. N. Rather, and R. A. Bonomo. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 513471-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott, P., G. Deye, A. Srinivasan, C. Murray, K. Moran, E. Hulten, J. Fishbain, D. Craft, S. Riddell, L. Lindler, J. Mancuso, E. Milstrey, C. T. Bautista, J. Patel, A. Ewell, T. Hamilton, C. Gaddy, M. Tenney, G. Christopher, K. Petersen, T. Endy, and B. Petruccelli. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 441577-1584. [DOI] [PubMed] [Google Scholar]
- 25.Sebeny, P. J., M. S. Riddle, and K. Petersen. 2008. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 47444-449. [DOI] [PubMed] [Google Scholar]
- 26.Turton, J. F., M. E. Kaufmann, M. J. Gill, R. Pike, P. T. Scott, J. Fishbain, D. Craft, G. Deye, S. Riddell, L. E. Lindler, and T. L. Pitt. 2006. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J. Clin. Microbiol. 442630-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila, J., J. Ruiz, P. Goni, and T. Jimenez de Anta. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J. Antimicrob. Chemother. 39757-762. [DOI] [PubMed] [Google Scholar]
- 28.Vila, J., J. Ruiz, P. Goni, A. Marcos, and T. Jimenez de Anta. 1995. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 391201-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitman, T. J., S. S. Qasba, J. G. Timpone, B. S. Babel, M. R. Kasper, J. F. English, J. W. Sanders, K. M. Hujer, A. M. Hujer, A. Endimiani, M. W. Eshoo, and R. A. Bonomo. 2008. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin. Infect. Dis. 47439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisplinghoff, H., M. Decker, C. Haefs, O. Krut, G. Plum, and H. Seifert. 2003. Mutations in gyrA and parC associated with resistance to fluoroquinolones in epidemiologically defined clinical strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 51177-180. [DOI] [PubMed] [Google Scholar]
- 31.Wortmann, G., A. Weintrob, M. Barber, P. Scott, S. T. Zoll, M. W. Eshoo, R. Sampath, D. J. Ecker, and C. Massire. 2008. Genotypic evolution of Acinetobacter baumannii strains in an outbreak associated with war trauma. Infect. Control Hosp. Epidemiol. 29553-555. [DOI] [PubMed] [Google Scholar]