Abstract
Members of the viral subfamily Alphaherpesvirinae establish latency from which they can be reactivated. Bovine herpesvirus 1 causes infectious bovine rhinotracheitis and infectious pustular vulvovaginitis in cattle, as well as abortion and weak calves. Serological evidence of alphaherpesvirus infection has been reported for wild and semidomesticated reindeer (Rangifer tarandus tarandus) in Norway. To address the possibility that reindeer alphaherpesvirus (cervid herpesvirus 2 [CvHV-2]) infection might affect the respiratory system and in part explain the relatively high mortality of reindeer calves during their first year, tissue samples were obtained from reindeer and reindeer fetuses at slaughterhouses in Finnmark County, Norway. A nested pan-alphaherpesvirus PCR amplification targeting the highly conserved UL27 gene (encoding glycoprotein B) was used. Sequencing of amplicons revealed the presence of CvHV-2 DNA. The detection of CvHV-2 DNA in trigeminal ganglia (27 of 143 samples), nasal swabs (5 of 75 samples), and fetal tissues (12 of 48 samples) indicates that CvHV-2 infection is endemic in this reindeer population. Moreover, the virus is transmitted horizontally by the respiratory route, establishing latency in the trigeminal ganglion, and vertically to the fetus through the placenta. Further studies should focus on the reproductive impact of CvHV-2 infection in reindeer.
Herpesvirus infections occur in many groups of animals, including mammals, fish, bivalves, amphibians, and birds (24). Members of the subfamily Alphaherpesvirinae infect mammals and birds, have a fast replication cycle, and establish latency in neurons (24). Latency in neurons is the persistence of the viral genome in the nuclei of sensory neurons and ganglia by means of the expression of a multifunctional latency-associated transcript that, among other actions, represses the expression of viral lytic-phase gene products (12). The regulatory mechanisms of the latency-associated transcript are not yet totally understood. Reactivation and subsequent shedding of virus and occasional reappearance of disease may occur after stress, immune suppression, or concomitant infections (10).
Bovine herpesvirus type 1 (BoHV-1) causes the respiratory disease infectious bovine rhinotracheitis and the genital disease infectious pustular vulvovaginitis, as well as encephalitis and abortion, in bovines and is the most studied ruminant alphaherpesvirus (10, 20). Campaigns to eradicate infectious bovine rhinotracheitis and infectious pustular vulvovaginitis in bovines have long been conducted in many countries with differing success rates (1). Possible problems for the eradication of BoHV-1 infections may arise from cross-species infections and serological cross-reactions with antigenically related ruminant alphaherpesviruses, such as cervid herpesviruses 1 and 2 (CvHV-1 and CvHV-2), bubaline herpesvirus 1 (BuHV-1), elk herpesvirus 1 (ElkHV-1), and caprine herpesvirus 1 (CpHV-1). Experimentally it has been demonstrated that CvHV-2 can infect bovines and cause a mild rhinotracheitis, but no latency of CvHV-2 has been detected in bovines (18, 34). Serological evidence of CvHV-2 infection has been reported for both wild (13) and semidomesticated (31, 36) reindeer (Rangifer tarandus tarandus) in Norway, for barren-ground caribou (Rangifer tarandus groenlandicus) in Greenland (4), and for Grant's caribou (Rangifer tarandus granti) in Alaska (8).
CvHV-2 infection of reindeer has been described as asymptomatic, and like herpesviruses in general, CvHV-2 most likely establishes latency (9, 19, 33, 34). The latter possibility has been supported by the isolation of the virus from the vaginas and noses of reindeer after the induction of viral reactivation by dexamethasone treatment in Finland and Sweden, respectively, but latency was not verified by amplification of viral DNA from nerve ganglia (23).
Finnmark County in northern Norway (55,047 km2) is the largest reindeer-herding area in the country, with an estimated 168,779 animals in 2005 to 2006 (5). Reindeer are kept in a seminomadic manner, moving between summer and winter pastures, and are usually free-ranging within the borders of the geographically defined herding districts. The mortality of reindeer in Finnmark in 2006 was 37% during the reindeer herding year (1 April 2005 to 31 March 2006) (5). Among different causes of death, predators are considered by far the most important, but approximately 11% of the losses of animals are of unresolved etiology (5). Abortions might also contribute to the relatively high mortality; this hypothesis cannot be confirmed, since aborted materials normally would be consumed quickly by scavengers in the wild.
A serosurvey carried out between 2004 and 2006, which statistically represented approximately 70% of the reindeer population in Finnmark County, showed an overall alphaherpesvirus seroprevalence of 49%, with a great difference between animals with a low carcass mass (calves) and those with a high carcass mass (adults) (C. das Neves, J. Thiry, E. Skjerve, N. G. Yoccoz, E. Rimstad, E. Thiry, and M. Tryland, submitted for publication). A virus neutralization test showed that the antibodies were produced against CvHV-2 and not against BoHV-1. The high seroprevalence indicates that CvHV-2 is easily transmitted among semidomesticated reindeer in northern Norway. In view of the high mortality and the high prevalence of CvHV-2 infection in this region (31, 36), it was considered necessary to conduct a study of the possible impact of CvHV-2 infection on reindeer health.
In many ruminant species, alphaherpesvirus infections have an impact on reproductive success by causing abortions and increased offspring mortality (16, 21, 29, 30, 33, 34). However, there is no information on the impact of CvHV-2 infection in reindeer on respiratory disease or reproductive success. In this study, reindeer nasal swabs, trigeminal ganglia, and fetal samples were investigated by PCR for the presence of CvHV-2 DNA.
MATERIALS AND METHODS
Animal samples.
Reindeer heads were collected at an abattoir immediately after slaughter. The heads were bisected sagittally, and the entire trigeminal ganglion was dissected aseptically and placed in a cryotube (Nunc, Roskilde, Denmark) with 2 ml of Earle's minimal essential medium with 2% penicillin-streptomycin (10,000 U/ml penicillin and 10 mg/ml streptomycin; Sigma-Aldrich, Oslo, Norway). The nostrils were swabbed, and the swabs were put in Earle's minimal essential medium with 2% penicillin-streptomycin. Samples were immediately frozen at −20°C until further processing.
During the slaughter of reindeer in February 2005, a group of 270 adult females from three reindeer-herding districts was checked for pregnancy. Forty-eight fetuses approximately 5 months old, with calving expected in late April to early May, were removed aseptically from the uterus without disruption of the amniotic sac in order to ensure that there was no contact with maternal tissues apart from that naturally established via the placenta. Fetuses were placed in individual plastic bags and frozen at −20°C until further processing. Whenever possible, serum from the mother animal was sampled. After arrival in the laboratory, the fetuses were weighed and their sexes determined. The amniotic fluid, liver, lung, spleen, and blood from each fetus were sampled for DNA extraction.
DNA extraction and PCR.
DNA was extracted from 75 nasal swabs, 143 trigeminal ganglia, and 192 fetal tissue samples (from 48 fetuses) using DNeasy Blood & Tissue kits (Qiagen, Hilden, Germany). DNA concentrations were measured, and similar amounts were added to the reaction mixture for each sample. The animals from which trigeminal ganglion and nasal swab samples were collected represented 10 of the 14 reindeer-herding districts in Finnmark County that had been included in a previous serosurvey (das Neves et al., submitted).
Amplification by nested pan-alphaherpesvirus PCR was carried out as described previously (27). The primer sets used were primer pair CR30 (5′-TCGAARGCCGAGTACCTGCG-3′) and CR31 (5′-CCAGTCCCAGGCRACCGTCAC-3′) and primer pair CR32 (5′-TGGTGGCCTTYGACCGCGAC-3′) and CR33 (5′-GCTCCGGCGAGTAGCTGGTGTG-3′), but no mimic molecule, as designed by Ros and Belak (27), was used in this study. Cell culture-grown viruses (BoHV-1 and CvHV-2) were added as positive controls, while samples from a seal and a reindeer (both negative by a virus neutralization test and an enzyme-linked immunosorbent assay) were used as negative controls. Serial dilutions of controls and samples were tested to evaluate the sensitivity of PCR and to evaluate inhibition of amplification. The two primer sets amplified a region of UL27 (encoding glycoprotein B), a gene highly conserved among ruminant alphaherpesviruses, producing amplicons of 443 and 294 bp, respectively (26). The PCR products were separated by agarose gel electrophoresis and visualized with SYBR green (Roche Applied Biosciences, Basel, Switzerland). Amplicons of the expected size (294 bp) were sequenced either directly or after reamplification with CR32-CR33. Primers and deoxynucleoside triphosphates were removed from amplicons by using ExoSapIT reagent (Amersham Pharmacia, Uppsala, Sweden). Cycle sequencing was conducted in both directions using BigDye 3.1 reagents (ABI BigDye Terminator, version 3.1; Applied Biosystems, Carlsbad, CA). Two microliters of 125 M EDTA, 2 μl of 3 M sodium acetate, and 50 μl of ethanol were added to the 20-μl product. Electrophoresis of the cycle-sequencing extension products was conducted in an ABI Prism DNA analyzer, model 377 (Applied Biosystems).
Sequence alignment and phylogeny.
The sequences obtained were assembled and aligned with the DNAStar program package (DNAStar Inc., Madison, WI), which uses Clustal W (11).
For alignment and comparison, sequences from the UL27 genes of ruminant alphaherpesviruses BoHV-1 (accession number NC001847.1), CvHV-1 (AF078729.2), CvHV-2 (AF078727.2), BuHV-1 (EF624476.1), ElkHV-1 (EF624478.1), and CpHV-1 (AF078728.2) were retrieved from GenBank (National Center for Biotechnology Information). The phylogenetic distances between sequences were estimated, and a tree was drawn with MEGA, version 4 (32), using the maximum-parsimony method (7) with the close-neighbor-interchange algorithm (17). The reliability of the tree was assessed by bootstrapping (1,000 random data set repeats).
Serology.
It was possible to sample sera from 44 of the 48 slaughtered pregnant females whose fetuses were investigated. Sera were tested for antibodies against alphaherpesvirus by using a blocking enzyme-linked immunosorbent assay kit as described elsewhere (6a).
Nucleotide sequence accession numbers.
The sequences reported in this paper have been deposited in GenBank under accession numbers FJ409045 to FJ409051.
RESULTS
Necropsy of fetuses and serology of pregnant female reindeer.
Of the 48 fetuses, 30 were female and 18 male. Their mean weight was 0.53 kg (range, 0.07 to 1.03 kg). No gross pathological changes were observed in any of the fetuses. Of the 44 serum samples from the pregnant females, 36 were positive and 8 were negative for antibodies against alphaherpesviruses.
PCR.
The PCR conducted on nasal swabs, trigeminal ganglia, and fetal tissue samples produced amplicons of the predicted size. For some samples, two bands were visible, the 294-bp band produced by CR32-CR33 and the 443-bp band from the first primer set, CR30-CR31. In such cases, the 294-bp band was cut out from the gel and run in a new PCR with primer set CR32-CR33 before sequencing.
Twelve of the 48 fetuses had PCR-positive results, but only one type of tissue was positive for each. The 5 PCR-positive nasal swab samples originated from 4 different districts, while the 27 PCR-positive trigeminal ganglion samples originated from all 10 districts included in this study. Trigeminal ganglia were also available for two of the animals from which the five PCR-positive nasal swabs were collected, and both were found to be PCR positive. The PCR results are summarized in Table 1.
TABLE 1.
Results of a nested pan-alphaherpesvirus PCR for nasal swabs, trigeminal ganglion samples, and fetal tissue samples of semidomesticated reindeer from Finnmark County, Norwaya
| Sample type | No. of PCR-positive samples/ total samples |
|---|---|
| Trigeminal ganglia | 27/143 |
| Nasal swabs | 5/75 |
| Fetal samplesb | |
| Liver | 3/48 |
| Spleen | 3/48 |
| Lung | 2/48 |
| Blood | 4/48 |
PCR-positive trigeminal ganglion samples and nasal swabs originated from 10 and 4 reindeer-herding districts, respectively. Fetuses were sampled from three different reindeer districts, all of which had positive PCR results.
No fetus had positive results for more than one type of tissue.
Sequence alignment and phylogeny.
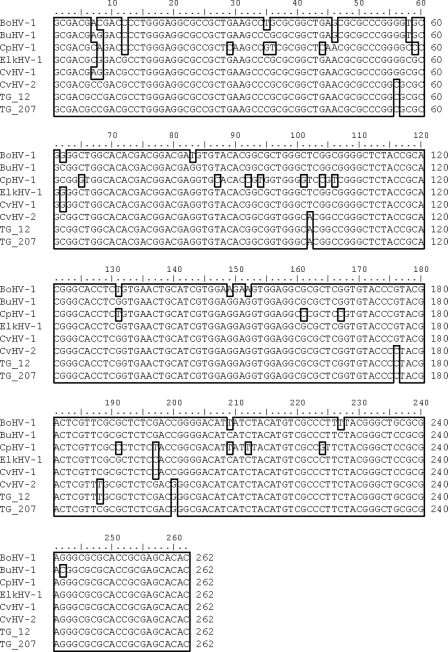
The nucleotide sequences of 15 PCR amplicons, originating from two nasal swabs and eight trigeminal ganglia from adult animals and from one blood, two liver, and two spleen samples from fetuses, were aligned with reference sequences. The 15 sequences were 99 to 100% homologous to the reference strain for CvHV-2, with only one nucleotide difference at position 188 of the consensus sequence (Fig. 1). The differing nucleotide (C versus T) was present in eight samples, of which seven came from trigeminal ganglion samples from different geographically separate districts. The mismatch was at a nonsynonymous site. All sequences obtained in this study were translated into an amino acid sequence similar to the sequence placed in GenBank for CvHV-2 (ID ADD46113.2).
FIG. 1.
Sequences from the UL27 gene obtained from two trigeminal ganglion samples, TG_12 and TG_207, aligned to corresponding sequences from other ruminant alphaherpesviruses. A consensus sequence of 262 nucleotides is displayed. The sequences retrieved from GenBank are identified by their taxonomic abbreviations. TG_207 represents eight identical sequences differing from CvHV-2 only at position 188, while TG_12 represents the remaining seven sequences, which are identical to CvHV-2. The sequences retrieved from GenBank (with accession numbers in parentheses) were BoHV-1 (NC001847.1), CvHV-1 (AF078729.2), CvHV-2 (AF078727.2), BuHV-1 (EF624476.1), ElkHV-1 (EF624478.1), and CpHV-1 (AF078728.2). The consensus is boxed.
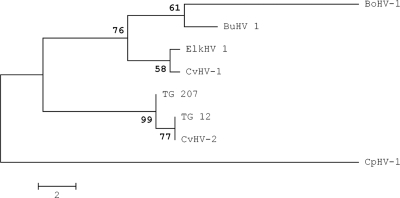
Phylogeny showed that the sequences obtained in this study clustered together with CvHV-2, while BoHV-1 and BuHV-1 clustered together, as did CvHV-1 and ElkHV-1, as found in previous studies (26, 35) (Fig. 2).
FIG. 2.
Phylogenetic relationships between the UL27 gene sequences of two samples obtained from trigeminal ganglia, TG_12 and TG_207, and those of other ruminant alphaherpesviruses. TG_207 was identical to eight of the sequences obtained in this study, while TG_12 was identical to the remaining seven sequences. The sequences retrieved from GenBank (with accession numbers in parentheses) were BoHV-1 (NC001847.1), CvHV-1 (AF078729.2), CvHV-2 (AF078727.2), BuHV-1 (EF624476.1), ElkHV-1 (EF624478.1), and CpHV-1 (AF078728.2). The maximum-parsimony method was used. Tree branches corresponding to partitions reproduced in <50% of bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to each branch. The maximum-parsimony tree was obtained by using the close-neighbor-interchange algorithm with a search level of 3, in which the initial trees were obtained by the random addition of sequences (10 replicates). The tree is drawn to scale. Branch lengths were calculated using the average pathway method; the unit is the number of changes over the whole sequence. There were 262 positions in the final data set, 18 of which were informative of parsimony.
DISCUSSION
The pan-alphaherpesvirus PCR primers used here successfully amplified CvHV-2 DNA from trigeminal ganglion, nasal, and fetal tissue samples from semidomesticated reindeer. The nucleotide sequences obtained, and their alignment with the sequences of other ruminant alphaherpesviruses, confirmed that the virus circulating in reindeer in Finnmark is CvHV-2. The sequenced amplicons either were identical to the only available CvHV-2 nucleotide sequence (strain Salla 82), which was obtained from a virus isolated from reindeer in Finland in 1982, or differed from it in just one nucleotide.
Several serological studies of CvHV-2 infection in reindeer that have been carried out in the past 10 to 20 years have confirmed a widespread distribution for alphaherpesvirus infections in semidomesticated reindeer (31, 36). Nonetheless, alphaherpesviruses have not been isolated from reindeer in Norway or detected by PCR. The presence of CvHV-2 DNA in the nasal, trigeminal ganglion, and fetal tissue samples indicated respiratory infection, latency in the trigeminal ganglion, and in utero infection, respectively.
CvHV-2 was found by PCR in the liver, lung, spleen, and blood samples of 12 of the 48 fetuses analyzed. This shows that CvHV-2 can cause infection in the fetus in utero. Curiously, only single samples from the individual fetuses were positive. The fetuses were obtained at slaughterhouses and did not represent aborted material. This could indicate that the infections in the fetuses were restricted or were in a primary phase, since fulminant infections were not observed. In utero transmission of alphaherpesviruses has been described for several other ruminant species (34) but has not been observed in reindeer. These CvHV-2 PCR-positive fetal tissues show that CvHV-2 can be transmitted from mother to fetus in reindeer and that it could thus be a causative factor of abortions and neonatal calf deaths in this species.
In reindeer husbandry systems such as that in Finnmark County, where close control of individual animals is not possible and where aborted material is swiftly removed by scavenger animals, the gestation status has to be assumed. It is therefore difficult to study the etiology of abortions and reproductive failure. In cattle, BoHV-1 is known to be a cause of abortion, depending on the virulence of the strain and the type of infection (primary versus activated latent infection), since reactivation of the virus normally does not induce abortion (16, 33). Transmission to the fetus is believed to occur via the umbilical vein (6, 29), since there is consistent evidence of BoHV-1 in the fetal liver, but the virus can also be recovered from, and cause lesions in, the spleen, where focal necrosis can be extreme, or in other organs, such as the lungs, kidneys, and brain (15, 16, 29). BoHV-1-induced abortions usually occur during the last trimester of gestation (30). Fetal death and abortion can occur as early as 48 h postinfection (29) but also as late as 64 days (16). Reindeer have a normal gestation period of 225 to 235 days, and delivery usually occurs toward the end of April or in early May (25, 28). The reindeer fetuses in this study were sampled in February, and the animals should then have been in the beginning of the last trimester of gestation. This would further explain the lack of lesions in organs where CvHV-2 was found, since the infection could have been in its early-onset stage. A low viral copy number or noninfective viral particles might explain the lack of lesions and disease. Attempts to isolate virus from the tissues in cell culture were not successful.
The presence of CvHV-2 in reindeer fetal tissues in utero indicates a preceding viremia in the adult mother. Bovine abortion due to BoHV-1 has been found to be a sequel to respiratory infection and viremia rather than a consequence of vaginal infection (16, 29). Therefore, a search for CvHV-2 on respiratory mucosal surfaces (on nasal swabs) and for latent viruses in nerves related to the respiratory system—the trigeminal ganglia—was conducted. The results indicated that respiratory CvHV-2 infection in reindeer is common; 27 of 143 trigeminal nerve ganglia sampled were found to be PCR positive for the virus. It is assumed that only a low number of target episomes are present in the ganglia during latency, so our findings might represent a minimum number. Furthermore, the finding of CvHV-2 in 5/75 nasal swabs investigated indicated that an upper respiratory tract infection was present at the time of sampling and that the virus was being shed, since it was present on the nasal mucosa. The trigeminal ganglia of two of these five nasally infected animals were also PCR positive, suggesting a possible reactivation of CvHV-2 and excretion via the respiratory tract. The higher seroprevalence in high-carcass-mass animals (adults) than in low-carcass-mass animals (calves) (das Neves et al., submitted) can be explained by the hypothesis that CvHV-2 is transmitted via the respiratory route rather than being restricted to the genital tract. The results presented in this study, together with the high seroprevalence of CvHV-2 in adult animals, indicates that CvHV-2 infection is endemic in the reindeer population in Finnmark County and might have a potential impact on reindeer health as well as on reproductive success.
The presence of CvHV-2 DNA in trigeminal ganglia is consistent with the well-known pathogenesis of other ruminant alphaherpesviruses, showing latency in the trigeminal ganglia after upper respiratory tract infections (2, 14, 22) and in the sacral ganglia after genital infections (3, 20). Previously, CvHV-2 was isolated from a genital swab after a reactivation protocol (9), indicating that most likely, the virus was reactivated after latency in the sacral ganglia.
These findings of CvHV-2 DNA in fetal tissues and in the trigeminal ganglia and nasal mucosae of adults make further studies of CvHV-2 infection in reindeer and its implications for reindeer health, including abortions and weak calves, important. Reindeer herds are key players in the arctic and subarctic ecosystems and are of the utmost economic and social importance for the indigenous peoples across Fenno-Scandinavia and other arctic regions. Serosurveys targeting alphaherpesviruses have pointed out the possible presence of alphaherpervirus infections. The study of diseases due to such infections, which might have an impact on sustainability and husbandry, should have a central place in management plans and future scientific research.
Acknowledgments
For excellent help in the lab, we acknowledge Eva M. Breines and Ellinor Hareide. For irreplaceable help in the field, we acknowledge Veronique Poulain, Ingebjørg Nymo, Mathieu Roger, and Gina Petrovich. Finally, we thank the staff at the Karasjok and Kautokeino slaughterhouses for their help and hospitality.
This project was partly funded by The Norwegian Reindeer Development Fund (RUF); the Foundation Calouste Gulbenkian, Portugal; and The Norwegian School of Veterinary Science.
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Ackermann, M., and. M. Engels. 2006. Pro and contra IBR-eradication. Vet. Microbiol. 113293-302. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, M., E. Peterhans, and R. Wyler. 1982. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am. J. Vet. Res. 4336-40. [PubMed] [Google Scholar]
- 3.Ackermann, M., and R. Wyler. 1984. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Vet. Microbiol. 953-63. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 1999. Report on the animal health situation in Greenland 1999. Ministry of Food, Agriculture and Fisheries, Danish Veterinary and Food Administration, Søborg, Denmark, and Ministry of Environment and Nature, Nuuk (Godthåb), Greenland. http://gl.foedevarestyrelsen.dk/FDir/Publications/2000640/Rapport.pdf.
- 5.Anonymous. June 2007. Ressursregnskap for Reindriftsnæringen. For Reindriftsåret 1 April 2005-31 Mars 2006. Reindriftsforvaltningen, Alta, Norway. http://www.reindrift.no/asset/893/1/893_1.pdf.
- 6.Chow, T. L., J. A. Molello, and N. V. Owen. 1964. Abortion experimentally induced in cattle by infectious bovine rhinotracheitis virus. J. Am. Vet. Med. Assoc. 1441005-1007. [PubMed] [Google Scholar]
- 6a.Das Neves, C. G., M. Roger, N. G. Yoccoz, E. Rimstad, and M. Tryland. 2009. Evaluation of three commercial bovine ELISA kits for detection of antibodies against Alphaherpesviruses in reindeer (Rangifer tarandus tarandus). Acta Vet. Scand. 519. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayhoff, M. O., and R. V. Eck. 1966. Atlas of protein sequence and structure. National Biomedical Research Foundation, Silver Spring, MD.
- 8.Dieterich, R. A. 1981. Alaskan wildlife diseases, p. 28-29. University of Alaska, Fairbanks.
- 9.Ek-Kommonen, C., S. Pelkonen, and P. F. Nettleton. 1986. Isolation of a herpesvirus serologically related to bovine herpesvirus 1 from a reindeer (Rangifer tarandus). Acta Vet. Scand. 27299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels, M., and M. Ackermann. 1996. Pathogenesis of ruminant herpesvirus infections. Vet. Microbiol. 533-15. [DOI] [PubMed] [Google Scholar]
- 11.Higgins, D. G., and P. M. Sharp. 1989. Fast and sensitive multiple sequence alignments on a microcomputer. Comput. Appl. Biol. Sci. 5151-153. [DOI] [PubMed] [Google Scholar]
- 12.Jones, C., V. Geiser, G. Henderson, Y. Jiang, F. Meyer, S. Perez, and Y. Zhang. 2006. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet. Microbiol. 113199-210. [DOI] [PubMed] [Google Scholar]
- 13.Lillehaug, A., T. Vikoren, I. L. Larsen, J. Akerstedt, J. Tharaldsen, and K. Handeland. 2003. Antibodies to ruminant alpha-herpesviruses and pestiviruses in Norwegian cervids. J. Wildl. Dis. 39779-786. [DOI] [PubMed] [Google Scholar]
- 14.Lovato, L., M. Inman, G. Henderson, A. Doster, and C. Jones. 2003. Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J. Virol. 774848-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell, D., and A. S. Greig. 1967. The incidence and significance of bovine herpesvirus (infectious bovine rhinotracheitis) antibodies in the sera of aborting cattle. Can. J. Comp. Med. Vet. Sci. 31234-238. [PMC free article] [PubMed] [Google Scholar]
- 16.Muylkens, B., J. Thiry, P. Kirten, F. Schynts, and E. Thiry. 2007. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 38181-209. [DOI] [PubMed] [Google Scholar]
- 17.Nei, M., and S. Kumar. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY.
- 18.Nettleton, P. F., C. Ek-Kommonen, R. Tanskanen, H. W. Reid, J. A. Sinclair, and J. A. Herring. 1988. Studies on the epidemiology and pathogenesis of alphaherpesvirus from red deer (Cervus elaphus) and reindeer (Rangifer tarandus), p. 143-148. In H. W. Reid (ed.), The management and health of farmed deer. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 19.Nettleton, P. F., E. Thiry, H. Reid, and P. P. Pastoret. 1988. Herpesvirus infection in cervidae. Rev. Sci. Tech. 7977-988. [DOI] [PubMed] [Google Scholar]
- 20.Pastoret, P. P., E. Thiry, B. Brochier, and G. Derboven. 1982. Bovid herpesvirus 1 infection of cattle: pathogenesis, latency, consequences of latency. Ann. Rech. Vet. 13221-235. [PubMed] [Google Scholar]
- 21.Penny, C. D., F. Howie, P. F. Nettleton, N. D. Sargison, and A. Schock. 2002. Upper respiratory disease and encephalitis in neonatal beef calves caused by bovine herpesvirus type 1. Vet. Rec. 15189-91. [DOI] [PubMed] [Google Scholar]
- 22.Rock, D. L., S. L. Beam, and J. E. Mayfield. 1987. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 613827-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockborn, G., C. Rehbinder, B. Klingeborn, M. Lefler, K. Klintevall, T. Nikkilä, A. Landèn, and M. Nordkvist. 1990. The demonstration of a herpesvirus, related to bovine herpesvirus 1, in reindeer with ulcerative and necrotizing lesions of the upper alimentary tract and nose. Rangifer special issue 3373-384. [Google Scholar]
- 24.Roizman, B., and P. E. Pellet. 2001. The family Herpesviridae: a brief introduction, p. 2381-2398. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 25.Ropstad, E. 2000. Reproduction in female reindeer. Anim. Reprod. Sci. 60561-570. [DOI] [PubMed] [Google Scholar]
- 26.Ros, C., and S. Belak. 2002. Characterization of the glycoprotein B gene from ruminant alphaherpesviruses. Virus Genes 2499-105. [DOI] [PubMed] [Google Scholar]
- 27.Ros, C., and S. Belak. 1999. Studies of genetic relationships between bovine, caprine, cervine, and rangiferine alphaherpesviruses and improved molecular methods for virus detection and identification. J. Clin. Microbiol. 371247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skjenneberg, S., and L. Slagsvold. 1968. Reindriften og dens naturgrunnlag. Universitetsforlaget, Oslo, Norway.
- 29.Smith, K. C. 1997. Herpesviral abortion in domestic animals. Vet. J. 153253-268. [DOI] [PubMed] [Google Scholar]
- 30.Straub, O. C. 1991. BHV1 infections—relevance and spread in Europe. Comp. Immunol. Microbiol. Infect. Dis. 14175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuen, S., J. Krogsrud, B. Hyllseth, and N. J. C. Tyler. 1993. Serosurvey of three virus infections in reindeer in northern Norway and Svalbard. Rangifer 13215-219. [Google Scholar]
- 32.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software, version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 33.Thiry, E. 2007. Clinical virology of ruminants, 2nd ed., p.19-31, 255-259. Editions du Point vétérinaire, Paris, France.
- 34.Thiry, J., V. Keuser, B. Muylkens, F. Meurens, S. Gogev, A. Vanderplasschen, and E. Thiry. 2006. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 37169-190. [DOI] [PubMed] [Google Scholar]
- 35.Thiry, J., F. Widen, F. Gregoire, A. Linden, S. Belak, and E. Thiry. 2007. Isolation and characterisation of a ruminant alphaherpesvirus closely related to bovine herpesvirus 1 in a free-ranging red deer. BMC Vet. Res. 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tryland, M., T. Mørk, K. A. Ryeng, and K. K. Sørensen. 2005. Evidence of parapox-, alphaherpes- and pestivirus infections in carcasses of semidomesticated reindeer (Rangifer tarandus tarandus) from Finnmark, Norway. Rangifer 2575-83. [Google Scholar]