Abstract
The examination of rectoanal mucosa-associated lymphoid tissue (RAMALT) biopsy specimens for the diagnosis of transmissible spongiform encephalopathies has been described in sheep, elk, and small numbers of mule and white-tailed deer. Previous sample numbers have been too small to validate examination of this type of tissue as a viable antemortem diagnostic test. In this study, we examined RAMALT collected postmortem from 76 white-tailed deer removed from a farm in Wisconsin known to be affected by chronic wasting disease (CWD) and from 210 free-ranging white-tailed deer harvested from an area in Wisconsin where the overall prevalence of CWD among the deer was approximately 4 to 6%. The results of immunohistochemical (IHC) staining of the RAMALT sections were compared to the results of IHC staining of sections from the brain stem at the convergence of the dorsal motor nucleus of the vagus nerve, sections of the medial retropharyngeal lymph nodes (RLNs), and sections of tonsil (sections of tonsil only from captive animals were tested). The sensitivities of the IHC staining test with RAMALT sections were 81% for the captive animals and 91% for the free-ranging animals. False-negative results were usually associated with early infection, indicated by a low intensity of immunostaining in the obex and/or a polymorphism at PRNP codon 96. While the RLN remains the tissue of choice for use for the diagnosis of CWD in white-tailed deer, the results of the present study further support the use of RAMALTs collected antemortem as an adjunct to testing of tonsil biopsy specimens and surveillance by necropsy for the screening of farmed deer which have been put at risk through environmental exposure or exposure to deer with CWD.
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy that has been reported in free-ranging and captive Rocky Mountain elk (Cervus elaphus nelsoni), mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and moose (Alces alces shirasi) in North America (1, 23, 27, 28). Clinical signs of CWD do not appear until very late in the infection process (11), and as the successful management of CWD appears to be dependent on the early detection and elimination of infected individuals (8), a reliable diagnostic test for the identification of infected animals during the early preclinical stage is of paramount importance. Currently, diagnosis is generally dependent on the finding of PrPCWD, the abnormal isoform of the cellular prion protein (PrPC), in obex and/or lymphoid tissues of animals collected postmortem. Antemortem evaluation of tonsil biopsy specimens has been shown to detect preclinical CWD in deer (17, 26, 29), but the collection of tonsil tissue is not a universally practical procedure for use in the field, in that it requires general anesthesia and highly trained personnel to collect adequate samples. On the basis of the findings of recent studies that have shown that scrapie-associated PrP (PrPSc) is deposited in the rectoanal mucosa-associated lymphoid tissues (RAMALTs) of infected sheep relatively early in the course of infection and that samples from this site can be used to diagnose preclinical scrapie in sheep (5-7), studies have been conducted with elk and deer to evaluate the use of RAMALTs for the diagnosis of CWD (11, 21, 24, 30). This study was undertaken to further evaluate the use of RAMALTs for the diagnosis of CWD in white-tailed deer from a farm known to be positive for CWD and also in free-ranging white-tailed deer harvested in an area of Wisconsin known to have a 4 to 6% prevalence of CWD (10) and to investigate the factors contributing to the false-negative findings with RAMALTs from some deer with CWD.
MATERIALS AND METHODS
Samples from a white-tailed deer farm known to be positive for CWD.
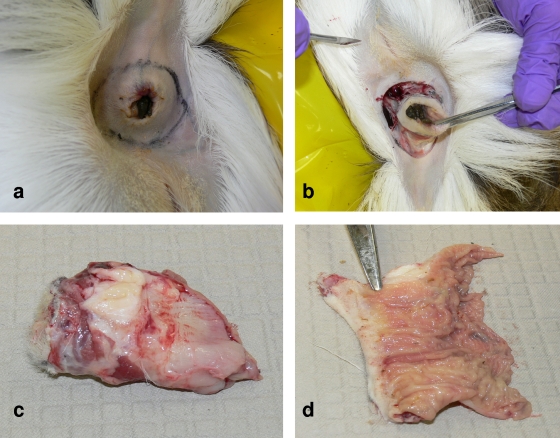
Seventy-six white-tailed deer were culled from a deer farm in Portage County, WI, in January 2006 after detection of the first animal with CWD in September 2002 (11). The animals were euthanized by gunshot, and samples were taken as follows: brain tissue was frozen for prion protein gene (PRNP) sequence analysis, and the obex at the level of the convergence of the dorsal motor nuclei of the vagus nerve (DMNV) where the spinal canal begins was placed in 10% neutral buffered formalin. Both medial retropharyngeal lymph nodes (RLNs) and both tonsils were also dissected and placed in formalin. The rectum of each animal was removed with a scalpel by dissecting a circular area through the skin approximately 1 cm lateral to the anus and cutting progressively deeper while maintaining traction on the anus (Fig. 1). The rectum was transected 4 to 5 cm cranial to the anus, and the tissue sample was removed. A longitudinal cut was made through the anus and rectal lumen with scissors, and the entire tissue was laid flat, exposing the mucosal surface. The RAMALT is located immediately beneath the last 1 cm of rectal mucosa proximal to the squamous epithelial tissue of the rectoanal junction (5-7), and the lymphoid follicles are found in aggregates underneath the crypts of the rectal mucosal folds. The mucosa between the rectal folds was lifted with fine-tip forceps, and the small “tent” of mucosa was removed with scissors. This tissue was placed mucosa surface down on a sponge in the base of a labeled cassette, with care being taken to ensure that all of the tissue was flattened. A second sponge was placed on top of the tissue to keep it flat, a lid was fixed to the cassette, and the sample was placed in 10% neutral buffered formalin. The scalpels, forceps, and scissors were discarded after the tissue sample was obtained from each animal.
FIG. 1.
Collection of RAMALT from white-tailed deer postmortem. (a) Anus with circular line of demarcation drawn to indicate the line of incision. (b) The rectum and anus are removed by using a scalpel and dissecting a circular area through the skin approximately 1 cm lateral to the anus while maintaining traction on the anus. (c) A 4- to 5-cm portion of the rectum and anus is removed. (d) A longitudinal cut has been made through the anus and rectal lumen, and the entire tissue has been laid flat, thus exposing the mucosal surface. The tip of the scissors indicates the area in which follicles are found just proximal to the rectoanal junction.
Samples from free-ranging white-tailed deer.
Wisconsin Department of Natural Resources biologists collected obex, both RLNs, and RAMALTs from 210 free-ranging white-tailed deer in a manner similar to that described above. The animals had been culled by agency staff in an area of Wisconsin in which CWD is known to be enzootic (10).
IHC staining and histology.
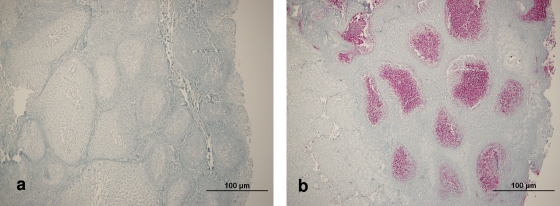
The obex, RLNs, and RAMALTs were fixed for a minimum of 5 days before they were processed. The obex was transected at the level of the convergence of the DMNV, and samples immediately cranial and caudal were taken with the facing surfaces placed down in a labeled cassette. One section of RLN and one section of tonsil from captive deer (two samples of RLNs for free-ranging animals) were placed in a second cassette; and these and the cassettes containing the RAMALT biopsy specimens were processed, embedded in paraffin, sectioned at 5 μm, mounted on positively charged glass slides (Superfrost/plus; Fisher Scientific, Pittsburgh, PA), deparaffinized, and dehydrated in preparation for immunohistochemistry (IHC) staining, as described previously (21). Briefly, tissue treatment prior to IHC staining consisted of immersion of the slide in 98% formic acid solution for 5 min, followed by several sequential rinses in an acetic acid wash solution buffer and water. Tissue sections were then autoclaved at 120°C in a proprietary acetic acid solution (Target Retrieval solution; Dako North America, Inc., Carpinteria, CA) for 20 min with a 25-min cooling period. The prepared slides were immunolabeled by using an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) and anti-PrP murine monoclonal antibody F99/97.6.1, a biotinylated secondary antibody, an alkaline phosphatase-streptavidin conjugate, a substrate chromogen (fast red A, naphthol, fast red B), and a hematoxylin and bluing counterstain (Ventana Medical Systems, Inc.) (21, 22). The testing was conducted in accordance with approved practices for the diagnosis of CWD in the United States: a positive control section was included in each run, and each slide was read by a pathologist trained in the diagnosis of CWD by IHC staining in a federally approved veterinary diagnostic laboratory. Tissues were classified as positive if they contained the typical coarse, bright red, granular immunolabeling in the DMNV or in the follicles of the RLN, tonsil, or RAMALT (Fig. 2b). No deposits were detected for samples considered negative (Fig. 2a). An animal was considered positive for CWD if any of the tissues contained positive immunolabeling. The presence of the DMNV at the level where the spinal canal begins was required for a section of obex to be acceptable; samples for which this anatomic region could not be identified were considered unacceptable. The number of lymphoid follicles per field examined ranged from 0 to >50. A minimum of six follicles was required to be present in a section of RLN, tonsil, or RAMALT for the pathologist to designate that section acceptable. For the farmed animals, if the section was designated unacceptable, another section was taken from the original block and stained as described above. This was repeated up to four times for the RAMALT. The tissues from the free-ranging animals were sectioned only once. Positive sections were scored for IHC staining intensity and distribution, as described previously (11). Briefly, immunolabeling in the medulla at the level of the obex where the fourth ventricle enters the spinal canal were scored as follows: 0, no PrPCWD detected; 1, scant PrPCWD present in <50% of the DMNV; 2, PrPCWD present in >50% of the DMNV but no immunolabeling of the surrounding tissue; 3, DMNV totally filled with PrPCWD with some immunolabeling of surrounding tissue; and 4, heavy PrPCWD deposits within the DMNV and the surrounding tissue. Lymphoid tissue (RLN, tonsil, and RAMALT) was considered to have a score of 0 if no PrPCWD was detected in lymphoid follicles, a score of 1 if less than six PrPCWD-positive follicles were detected in the entire section examined, a score of 2 if more than six positive follicles were detected in a field examined in a section in which some fields had no stain in any follicles, and a score of 3 if PrPCWD was detected in follicles in all fields examined.
FIG. 2.
IHC staining of RAMALT with monoclonal antibody F99/97.6.1. (a) Photomicrograph of several lymphoid follicles in RAMALT of a white-tailed deer free of PrPCWD; (b) photomicrograph of several lymphoid follicles with heavy immunolabeling of PrPCWD in RAMALT of a white-tailed deer with chronic wasting disease.
Prion protein genotyping.
The allelic sequence of the prion protein gene (PRNP) was determined for the farmed animals by PCR and DNA sequence analysis (15). Genotypes with the sequence encoding glutamine (Q) at codon 95, glycine (G) at codon 96, alanine at codon 116, and glutamine at codon 226 were considered to be homozygous for the wild-type (wt) allele and are indicated wt/wt. Alleles with polymorphisms at codon 96, G to serine (G96S), and codon 226, Q to lysine (Q226K), were observed; and deer heterozygous for these alleles and the wt allele are designated wt/G96S and wt/Q226K, respectively. Homozygous G96S (G96S/G96S) deer were rare in the population, and homozygous Q226K/Q226K deer were not observed. Genotyping was not performed on all the samples from free-ranging deer; state and federally supported surveillance programs test large numbers of animals during the annual U.S. and Canadian hunting season, and genotype analysis of those samples is not cost-effective.
Data analysis.
Deer were considered CWD positive if PrPCWD was detected in any of the tissue samples examined (obex, tonsils from captive animals, or RLNs). The tissue-specific sensitivity for PrPCWD detection by IHC staining was expressed as the percentage with positive detection compared to the total number evaluated from CWD-positive deer. The statistical and graphing software used were JMP for Windows (version 8.0; SAS Institute Inc., Cary, NC) and Origin (version 6.0; Microcal Software Inc., Northampton, MA), respectively.
RESULTS
Detection of PrPCWD in RAMALTs compared with that in RLNs and tonsils.
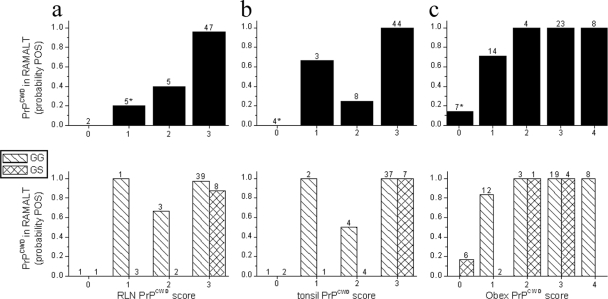
CWD surveillance in deer is currently based on examination of the RLN, the tissue in which PrPCWD is most likely to be detected in infected animals. Tonsil biopsy specimens from live animals are used because the tissue is accessible to trained personnel. The frequency of detection of PrPCWD in the RAMALTs of CWD-positive captive deer was related to the PrPCWD scores in RLNs (Fig. 3a, upper panels) and tonsils (Fig. 3b, upper panels).
FIG. 3.
Relationship of PrPCWD detection in the RAMALT with PrPCWD IHC staining scores for the medial RLN, tonsil, and obex from a captive herd of CWD-positive white-tailed deer. Bars depict the probability of PrPCWD detection (probability POS) in the RAMALTs for all CWD-positive deer (upper panels) or by PrP genotype at codon 96 (lower panels; GG = wt/wt, GS = wt/G96S). The number above each bar indicates the number of deer; *, inclusion of one homozygous G96S deer.
PRNP genotype and RAMALT test sensitivity.
The PRNP genotype is related to the prevalence of CWD in free-ranging deer (9, 11, 15) and the sensitivity of tests with RAMALTs from experimentally infected white-tailed deer (30). We therefore evaluated the effect of genotype on the results obtained with RAMALTs for the CWD-positive captive deer in this study (Fig. 3a and b, lower panel). Note that PrPCWD was detected in the RAMALTs of 41 of 43 (95%) CWD-positive wt/wt deer in which PrPCWD was also detected in the RLNs and/or tonsils (Table 1). In CWD-positive wt/G96S deer, however, PrPCWD was detected in the RAMALTs of only 7 of 14 (50%) deer in which PrPCWD was also detected in the RLNs and/or tonsils and was detected only in the RAMALTs of deer in which the RLN and/or tonsil PrPCWD scores were 3. In this study, the odds for the detection of PrPCWD in the RAMALTs in wt/G96S deer with CWD was 0.073 times lower than that in wt/wt deer with CWD (95% confidence interval, 0.015 to 0.352; two-tailed Fisher's exact test, P = 0.0009).
TABLE 1.
Number of animals of different genotypes positive for PrPCWD in obex, medial RLN, tonsil, and RAMALT
| Genotype | Total no. of animals | No. (%) of animals positive by evaluation of the following:
|
||||
|---|---|---|---|---|---|---|
| Total | Obex | RLNs | Tonsils | RAMALTs | ||
| wt/wt | 51 | 44 (86.3) | 42a (95.5) | 43 (97.7) | 43 (97.7) | 41 (93.2) |
| wt/G96S | 21 | 14 (66.7) | 7b (50) | 13 (92.9) | 12 (85.7) | 7 (50) |
| G96S/G96S | 2 | 1 (50) | 0 | 1 (50) | 0 | 0 |
| wt/Q226K | 1 | 1 (100) | 0c | 1 (100) | 1 (100) | 0c |
| G96S/Q226K | 1 | 0 | 0 | 0 | 0 | 0 |
Two samples were not suitable for evaluation.
One sample was not suitable for evaluation.
Samples were not suitable for evaluation.
Infection stage and sensitivity of test with RAMALTs.
To define the potential sources of the false-negative findings with RAMALTs, the infection status of each animal was estimated from the obex score. As shown in the upper panel of Fig. 3c, the frequency of detection of PrPCWD in the RAMALTs of CWD-positive captive deer was related to the obex PrPCWD score. This relationship is subcategorized by the codon 96 genotype (Fig. 3c, lower panel) because G96S has been significantly associated with disease progression (11, 30). Note that during early infection (obex scores, 0 and 1), PrPCWD was detected in the RAMALTs from only 1 of 8 (13%) wt/G96S deer, whereas PrPCWD was detected in 10 of 12 (83%) wt/wt deer. PrPCWD was detected in the RAMALTs of 100% of the wt/wt and wt/G96S deer in this study that were further progressed in disease (obex scores, 2 to 4).
For the 11 CWD-positive captive deer in which no PrPCWD was detected within the RAMALTs (potential false-negative results), the PrPCWD scores for the RLNs and tonsils were 0 or 1 (absent or minimal) in five deer (including one deer in which PrPCWD was detected only in the obex) and 2 or 3 (moderate or strong) in at least one of these tissues in six deer. Ten of the 11 RAMALT-negative deer had an obex score of less than 2; the obex sample from one deer was considered unacceptable for scoring. The 11 RAMALT-negative CWD-positive captive deer included two fawns, four yearlings, four deer that were 2 years of age, and a 3-year-old animal.
Of the 210 free-ranging white-tailed deer sampled, PrPCWD was detected in the RAMALTs of 10 of 11 (91%) CWD-positive deer; the RAMALT samples from two additional CWD-positive deer were unacceptable for evaluation. All 10 of the RAMALT-positive free-ranging deer had a PrPCWD RLN score of 3 and 3 had obex scores of less than 2. The single RAMALT-negative, CWD-positive deer had an RLN score of 1 and an obex score of 0.
Acceptability of samples from captive and wild populations.
Four sections of obex (5.3%) and three sections of RAMALT (3.9%) from the farmed deer were considered unacceptable for diagnostic evaluation. Seven sections of obex (3.3%) and 44 sections of RAMALT (21%) from the 210 free-ranging white-tailed deer were considered unacceptable.
DISCUSSION
On the basis of the results of our study, the detection of PrPCWD in RAMALTs by IHC staining should be considered a useful tool for the preclinical diagnosis of CWD in white-tailed deer. It was previously shown that in white-tailed deer, the accumulation of PrPCWD occurs in the RLNs or tonsils prior to accumulation in the obex (11) and the presence of PrPCWD in the RLNs and tonsils is a reliable marker for the antemortem and preclinical postmortem diagnosis of CWD (26, 29, 30). The collection of lymphatic tissues from the head and neck of live animals is not an easy task; tonsil biopsy procedures require general anesthesia, the tonsil is difficult to visualize, the collection of a tonsil biopsy specimen requires experienced personnel, and the samples tend to be small (4 or 6 mm). As a tool for the screening of free-ranging or captive populations, this technique is not as efficient or as economical as rectal tissue biopsy. The latter procedure can be performed without general anesthesia, visualization of the rectal mucosa is much easier than that of the tonsil, and larger samples can be obtained without the need for specialized equipment. However, the diagnosis of CWD by the use of RAMALTs, as with tonsil tissues, is dependent on the collection of adequate samples.
An adequate lymphoid tissue sample in our study was defined as one with at least six follicles in any one section examined. A recent study (21) demonstrated that similar testing of elk required 10 follicles, and higher rates of false-negative results for sheep were observed when the samples had less than 14 follicles (7). Previous studies with tonsil biopsy specimens have defined adequate sections as having just one follicle (29) or less than three follicles (26) per section, so a direct comparison of the number of adequate samples between rectal and tonsil biopsy specimens is difficult. We were unable to obtain adequate RAMALT samples from 3 of the captive animals (3.9%) and 44 (21%) of the free-ranging animals, even though the samples were collected postmortem. Some of this difference may be explained by the collection technique used; the samples from the captive animals were collected by one experienced veterinarian, and the samples from the free-ranging animals were collected by a number of wildlife biologists with limited training. However, the major difference is likely explained by the number of sections of RAMALT examined for individuals in each population. The RAMALT samples in this study were embedded with the mucosal surface down, which requires the use of a precise sectioning technique to ensure that the sections are not taken too shallow, in which case there will be few lymphoid follicles and large numbers of crypts, or too deep, in which case a large percentage of lymphoid follicles will have been lost and submucosal tissue and muscle will be present. In the farmed deer, several sections from various depths of the mucosa were examined in an attempt to have at least six follicles present in a single section. This procedure was not done for animals in the wild population, in which only one section from each animal was examined, which more closely approximates the procedure that would be used for high-volume sampling schemes. The number of unsuitable samples from the free-ranging population could likely have been decreased considerably by collecting sections from various depths of the block. This is an important aspect to keep in mind when laboratory personnel are being trained.
In this study, the sensitivity of IHC staining of RAMALTs was approximately 80%, which is slightly lower than the estimated sensitivity of the testing of RAMALTs for the diagnosis of scrapie in sheep (5-7). As with sheep, the sensitivity of the test is apparently improved by the exclusion of animals with genotypes associated with reduced PrPSc or PrPCWD accumulation, very young animals, or animals with very early disease. In white-tailed deer, the wt/G96S genotype is associated with a delay in the progression of CWD infection (11, 30), and the confounding effects of a prolonged early infection stage and the PRNP genotype itself cannot be resolved for this sample set. The mechanism by which the PRNP genotype influences the kinetics of PrPCWD deposition in the tissues of white-tailed deer (9, 11, 15, 30) is not known. In the current study, 41/44 (93%) of the CWD-positive wt/wt deer were RAMALT positive but only 7/14 (50%) of the CWD-positive wt/G96S deer were RAMALT positive. This finding is consistent with that of an experimental pathogenesis trial (30) in which 90% (9/10) of the experimentally infected PRNP wt/wt deer were RAMALT positive at 342 days postinfection, although only 50% (4/8) of the wt/G96S deer were RAMALT positive at 381 days postinfection. The use of RAMALTs for the testing of captive herds may benefit from supplementary PRNP genotype testing to identify deer likely to have false-negative RAMALT findings. The G96S homozygous population and Q226K homozygous or heterozygous deer were relatively rare in the populations examined; and there were insufficient numbers of animals of the G96S/G96S, wt/Q226K, and G96S/Q226K genotypes in our study to determine the role of those PRNP genotypes in the testing of RAMALTs.
With the exception of one fawn in the current study, there was no evidence of a nonlymphocytic scrapie strain responsible for CWD comparable to the ovine Nor98 scrapie strain (2) or of a lymphoid-sparing deposition pattern, as has been observed in some studies of classical sheep scrapie (13, 16, 25) and in up to 15% of elk with CWD (20). However, the deer in this study represented only a single captive population and a sample of free-ranging animals originating from a relatively small geographic area. The findings of this study may therefore be limited to a single strain of CWD. The presence of multiple strains responsible for CWD is suggested by studies with a transgenic mouse model (3). Although additional strains responsible for CWD may be discovered as our understanding of prion strains (4) and strain typing methodologies are refined and developed, this and previous studies (9, 11, 12, 15) suggest that the predominant U.S. strains result in the early deposition of PrPCWD in lymphoid tissue in most white-tailed deer.
This study was performed by using an IHC detection method validated for use in approved veterinary diagnostic laboratories in the United States. Adaptation and validation of laboratory methods such as protein misfolding cyclic amplification assay (19) or paraffin-embedded tissue immunoblotting (18) may increase the sensitivity of diagnostic testing of RAMALTs from deer. Those methods may also be useful adjuncts to bioassay methods (14) for estimation of the potential of fecal shedding of prions from the gut of deer with PrPCWD-positive tissues in the gastrointestinal tract.
The results of this study are remarkably similar to those reported from a similar study describing the IHC detection of PrPSc in the RAMALTs of sheep with classical scrapie (6). The sensitivity of the assay in that study was 86% with sheep with preclinical CWD. False-negative readings were observed for young sheep, many with scant accumulations of PrPSc in other lymphoid tissues and older sheep with genotypes associated with prolonged incubation times and scant lymphoid accumulation of PrPSc at all stages of infection.
The RLN is the most sensitive indicator of CWD but is suitable for use only for analysis by necropsy. Because the sensitivity of the PrPCWD IHC assay with RAMALTs does not appear to be as high as it is with tonsil tissues, the use of RAMALT biopsy specimens may not be as reliable a tool as the use of tonsil biopsy specimens for the testing of individual animals for the purpose of movement. However, the advantages of cost and ease of sampling make testing of RAMALTs a reasonable alternative in situations in which large numbers of live white-tailed deer are to be tested. This study further supports the use of RAMALTs as an alternative or adjunct to tonsil biopsy specimens, particularly for farmed deer with potential environmental exposure or exposure to cervids with CWD.
Acknowledgments
We thank all the Wisconsin Department of Natural Resources staff and those from the U.S. Department of Agriculture, the Wisconsin Department of Agriculture, Trade, and Consumer Protection, and the Wisconsin Veterinary Diagnostic Laboratory who were involved in the organization and implementation of the culling and sampling of the deer used in this study.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Baeten, L. A., B. E. Powers, J. E. Jewell, T. R. Spraker, and M. W. Miller. 2007. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J. Wildl. Dis. 43309-314. [DOI] [PubMed] [Google Scholar]
- 2.Benestad, S. L., P. Sarradin, B. Thu, J. Schönheit, M. A. Tranulis, and B. Bratberg. 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 153202-208. [DOI] [PubMed] [Google Scholar]
- 3.Browning, S. R., G. L. Mason, T. Seward, M. Green, G. A. J. Eliason, C. Mathiason, M. W. Miller, E. S. Williams, E. Hoover, and G. C. Telling. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 7813345-13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collinge, J., and A. R. Clarke. 2007. A general model of prion strains and their pathogenicity. Science 318930-936. [DOI] [PubMed] [Google Scholar]
- 5.Espenes, A., C. M. Press, T. Landsverk, M. A. Tranulis, M. Aleksandersen, G. Gunnes, S. L. Benestad, R. Fuglestveit, and M. J. Ulvund. 2006. Detection of PrPSc in rectal biopsy and necropsy samples from sheep with experimental scrapie. J. Comp. Pathol. 134115-125. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez, L., M. Jeffrey, S. Siso, S. Martin, S. J. Bellworthy, M. J. Stack, M. J. Chaplin, L. Davis, M. P. Dagleish, and H. W. Reid. 2005. Diagnosis of pre-clinical scrapie in post-mortem and biopsy samples of rectal mucosa. Vet. Rec. 156846-847. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, L., M. P. Dagleish, S. J. Bellworthy, S. Siso, M. J. Stack, M. J. Chaplin, L. A. Davis, S. A. C. Hawkins, J. Hughes, and M. Jeffrey. 2006. Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrPSc in their rectal tissue. Vet. Rec. 158325-331. [DOI] [PubMed] [Google Scholar]
- 8.Gross, J. E., and M. W. Miller. 2001. Chronic wasting disease in mule deer: disease dynamics and control. J. Wildl. Manage. 65205-215. [Google Scholar]
- 9.Johnson, C., J. Johnson, J. P. Vanderloo, D. Keane, J. M. Aiken, and D. McKenzie. 2006. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J. Gen. Virol. 872109-2114. [DOI] [PubMed] [Google Scholar]
- 10.Joly, D. O., M. D. Samuel, J. A. Langenberg, J. A. Blanchong, C. A. Batha, R. E. Rolley, D. P. Keane, and C. A. Ribic. 2006. Spatial epidemiology of chronic wasting disease in Wisconsin white-tailed deer. J. Wildl. Dis. 42578-588. [DOI] [PubMed] [Google Scholar]
- 11.Keane, D. P., D. J. Barr, P. N. Bochsler, S. M. Hall, T. Gidlewski, K. I. O'Rourke, T. R. Spraker, and M. D. Samuel. 2008. Chronic wasting disease in a Wisconsin captive white-tailed deer farm. J. Vet. Diagn. Investig. 20698-703. [DOI] [PubMed] [Google Scholar]
- 12.Keane, D. P., D. J. Barr, J. E. Keller, S. M. Hall, J. A. Langenberg, and P. N. Bochsler. 2008. Comparison of retropharyngeal lymph node and obex region of the brainstem in detection of chronic wasting disease in white-tailed deer (Odocoileus virginianus). J. Vet. Diagn. Investig. 2058-60. [DOI] [PubMed] [Google Scholar]
- 13.Ligios, C., M. G. Cancedda, L. Madau, C. Santucciu, C. Maestrale, U. Agrimi, G. Ru, and G. di Guardo. 2006. PrP-Sc deposition in nervous tissues without lymphoid tissue involvement is frequently found in ARQ. ARQ Sarda breed sheep preclinically affected with natural scrapie. Arch. Virol. 1512007-2020. [DOI] [PubMed] [Google Scholar]
- 14.Mathiason, C. K., J. G. Powers, S. J. Dahmes, D. A. Osborn, K. V. Miller, R. J. Warren, G. L. Mason, S. A. Hays, J. Hayes-Klug, D. M. Seelig, M. A. Wild, L. L. Wolfe, T. R. Spraker, M. W. Miller, C. J. Sigurdson, G. C. Telling, and E. A. Hoover. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314133-136. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke, K. I., T. R. Spraker, L. K. Hamburg, T. E. Besser, K. A. Brayton, and D. P. Knowles. 2004. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J. Gen. Virol. 851339-1346. [DOI] [PubMed] [Google Scholar]
- 16.Schreuder, B. E., L. J. van Keulen, M. E. Vromans, J. P. Langeveld, and M. A. Smits. 1998. Tonsillar biopsy and PrPSc detection in the preclinical diagnosis of scrapie. Vet. Rec. 23564-568. [DOI] [PubMed] [Google Scholar]
- 17.Schuler, K. L., J. A. Jenks, C. S. DePerno, W. A. Wild, and C. C. Swanson. 2005. Tonsillar biopsy test for chronic wasting disease: Two sampling approaches in mule deer and white-tailed deer. J. Wildl. Dis. 41820-824. [DOI] [PubMed] [Google Scholar]
- 18.Schulz-Schaeffer, W. J., R. Fatzer, M. Vandevelde, and H. A. Kretzschmar. 2000. Detection of PrPSc in subclinical BSE with the paraffin-embedded tissue (PET) blot. Arch. Virol. Suppl., p.173-180. [DOI] [PubMed]
- 19.Soto, C., G. P. Saborio, and L. Anderes. 2002. Cyclic amplification of protein misfolding: application to prion-related disorders and beyond. Trends Neurosci. 25390-394. [DOI] [PubMed] [Google Scholar]
- 20.Spraker, T. R., A. Balachandran, D. Zhuang, and K. I. O'Rourke. 2004. Variable patterns of PrPCWD distribution in obex and cranial lymphoid tissues of Rocky Mountain elk with non-clinical chronic wasting disease. Vet. Rec. 155295-302. [DOI] [PubMed] [Google Scholar]
- 21.Spraker, T. R., K. C. Vercauteren, T. Gidlewski, D. A. Schneider, R. Munger, A. Balachandran, and K. I. O'Rourke. 2009. Antemortem detection of PrP-CWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa. J. Vet. Diagn. Invest. 2115-24. [DOI] [PubMed] [Google Scholar]
- 22.Spraker, T. R., K. I. O'Rourke, A. Balachandran, R. R. Zink, B. A. Cummings, M. W. Miller, and B. E. Powers. 2002. Validation of monoclonal antibody F99/97.6 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J. Vet. Diagn. Investig. 143-7. [DOI] [PubMed] [Google Scholar]
- 23.Spraker, T. R., M. W. Miller, E. S. Williams, D. M. Getzy, W. J. Adrian, G. G. Schoonveld, R. A. Spowart, K. I. O'Rourke, J. M. Miller, and P. A. Merz. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (Cervus elaphus nelsoni) in north central Colorado. J. Wildl. Dis. 331-6. [DOI] [PubMed] [Google Scholar]
- 24.Spraker, T. R., T. L. Gidlewski, A. Balachandran, K. C. VerCauteren, L. Creekmore, and R. D. Munger. 2006. Detection of PrP-CWD in postmortem rectal lymphoid tissues in Rocky mountain elk (Cervus elaphus nelsoni) infected with chronic wasting disease. J. Vet. Diagn. Investig. 18553-557. [DOI] [PubMed] [Google Scholar]
- 25.van Keulen, L. J., B. E. Schreuder, R. H. Meloen, G. Mooij-Harkes, M. E. Vromans, and J. Langeveld. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie J. Clin. Microbiol. 341228-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild, M. A., T. R. Spraker, C. J. Sigurdson, K. I. O'Rourke, and M. W. Miller. 2002. Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J. Gen. Virol. 832629-2634. [DOI] [PubMed] [Google Scholar]
- 27.Williams, E. S., and S. Young. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis. 1689-98. [DOI] [PubMed] [Google Scholar]
- 28.Williams, E. S., and S. Young. 1982. Spongiform encephalopathy of Rocky Mountain elk. J. Wildl. Dis. 18465-471. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe, L. L., M. M. Conner, T. H. Baker, V. J. Dreitz, K. P. Burnham, E. S. Williams, N. T. Hobbs, and M. W. Miller. 2002. Evaluation of antemortem sampling to estimate chronic wasting disease prevalence in free-ranging mule deer. J. Wildl. Manage. 66564-573. [Google Scholar]
- 30.Wolfe, L. L., T. R. Spraker, L. Gonzalez, M. L. Dagleish, T. M. Sirochman, J. C. Brown, M. Jeffrey, and M. W. Miller. 2007. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.). J. Gen. Virol. 882078-2082. [DOI] [PubMed] [Google Scholar]