Abstract
Opisthorchiasis is a major public health problem in Southeast Asia. Affected individuals often have mixed infections with the liver fluke Opisthorchis viverrini and minute intestinal flukes such as Haplorchis taichui. The usual methods of diagnosing these infections involve the demonstration of fluke eggs in stool samples under light microscopy, but sensitivity and specificity are low. We developed two PCR tests that detect and discriminate between O. viverrini and H. taichui infections. PCR tests were validated by stool samples from purged individuals. We then applied the PCR tests to estimate the prevalence of O. viverrini and H. taichui infections from a random sample of individuals selected from a community in an area of endemicity in Khong District, Laos. PCR results were compared with those from the Kato-Katz (KK) method and the formalin-ether concentration technique (FECT). When validated with purge results, PCR tests of O. viverrini and H. taichui had sensitivities of 93.7% (95% confidence interval [CI], 85.8 to 97.9%) and 73.3% (95% CI, 60.3 to 83.9%) and could detect as little as 0.75 pg DNA and 1.32 ng DNA, respectively. The PCR-determined community prevalences of O. viverrini and H. taichui infections were 63.9% (95% CI, 54.1 to 72.9%) and 30.6% (95% CI, 22.1 to 40.2%), respectively. Using PCR as the gold standard to detect O. viverrini, three KK thick smears performed comparably well, whereas one KK smear and FECT were poorer (sensitivities of 91.4% [95% CI, 81.0 to 97.1%,], 62.3% [95% CI, 49.8 to 73.7%], and 49.3% [95% CI, 37.0 to 61.6%], respectively). PCR may be a valuable and sensitive diagnostic tool, particularly for low-intensity O. viverrini and H. taichui infections.
Food-borne trematodiases are an emerging but neglected public health issue (12). One of the food-borne trematodes, the liver fluke Opisthorchis viverrini is endemic in Cambodia, Lao People's Democratic Republic (PDR), Thailand and Viet Nam (8). It has been estimated that the number of infections caused by O. viverrini amounts to 9 million cases, and more than 67 million people are at risk of infection (11). Chronic O. viverrini infection is an important risk factor (class 1 carcinogen) for developing cholangiocarcinoma, or biliary duct cancer, which is associated with a poor prognosis (17, 24-26).
O. viverrini infection often coexists with other food-borne trematodes, including several species of flukes called the minute intestinal flukes (MIF) owing to their small size (families Heterophyidae and Lecithodendriidae). In recent studies in Lao PDR (2, 4, 23), different species of MIF were detected after purging individuals infected with O. viverrini. Of these MIF, Haplorchis taichui was the predominant species in concurrent infection with O. viverrini. The coexistence of O. viverrini with H. taichui infections has been described previously (7, 21, 29).
Fecal examination under light microscopy is currently the standard method to diagnose O. viverrini infections. The Kato-Katz technique (KK) (10) is the recommended field method (34). This technique permits estimation of infection intensity expressed in eggs per gram feces (epg). Unfortunately, a single stool examination read under light microscopy has low sensitivity (24), particularly for low-intensity infections, and examination of repeated specimens per individual may be needed to improve detection rates. Furthermore, KK does not permit the differentiation of O. viverrini eggs from MIF eggs such as those of H. taichui, because the eggs are similar in size and are both oval and operculated (30). Detection by light microscopy is also operator dependent, and infection may simply be missed.
When screening for O. viverrini by KK under light microscopy, identified eggs can be characterized only as “Opisthorchis-like” (21, 29). Distinction of O. viverrini eggs from MIF eggs can be achieved if the specimen is preserved and examined by the formalin-ether concentration technique (FECT) and if the eggs are later stained with iodine (9), potassium permanganate (28), or methylene blue (20). One method of confirming O. viverrini infections in the presence or absence of MIF with certainty is by identification of adult flukes, which may be obtained by treating infected individuals with praziquantel, the current drug of choice, offering them bowel purging with an oral laxative and then examining their diarrhetic stools.
As an alternative to microscopy and its limitation of potential diagnostic misclassification, molecular methods have recently been developed. A PCR method for the detection of O. viverrini eggs in human stool specimens was developed (37) and then evaluated as a diagnostic tool with human stool samples collected in Lao PDR (27). A rapid cleanup procedure for human stool samples was developed using inexpensive chemicals (19). This test recognizes all opisthorchiids by a single assay and differentiates them from MIF. Most recently, PCR tests were developed to discriminate between O. viverrini, H. taichui, and another food-borne trematode, Clonorchis sinensis (22, 31, 32). Some studies using PCR tests on human stool samples were validated with light microscopy (27, 32, 33). A more accurate method such as the identification of adult flukes would be valuable to validate PCR tests.
In this paper, we present the development of PCR diagnostic tests using sensitive and specific primers to differentiate O. viverrini from H. taichui with PCR tests validated by stool samples from purged individuals. We then used these PCR methods as the gold standard to estimate the infection prevalences of O. viverrini and H. taichui in a random sample drawn from a cross-sectional community survey in Khong District, Lao PDR. Finally, we compared the results of our PCR method with those from two parasitological diagnostic tests, the KK and FECT.
MATERIALS AND METHODS
Laboratory procedures to develop the PCR tests. (i) DNA extraction from stool samples.
Total genomic DNA was extracted from stool using the commercial QIAamp DNA stool mini kit (Qiagen, Basel, Switzerland) according to the manufacturer's instructions or with modifications specified below. For each stool sample, 1.4 ml of a lysis solution (ASL buffer [Qiagen]) was added to 180 to 240 mg feces. After homogenization, the suspension was heated at 95°C for 4 min and then frozen in dry ice for 8 min. The freeze-thaw cycle was repeated twice before incubation at 95°C for 10 min. The contents were then mixed by pulse-vortexing for 15 s and centrifuged at 14,000 rpm for 1 min to pellet stool particles. A 1.2-ml portion of the supernatant was incubated with one InihbitEX tablet (Qiagen) and processed according to the manufacturer's instructions. The final filtrate containing the eluted DNA was stored at 4°C, or at −20°C for long-term storage, of which 5 μl DNA template was used in the PCR amplification.
(ii) Test for PCR inhibitors.
Samples that were negative after O. viverrini PCR amplification were tested for inhibitors. Samples were artificially inoculated with 0.15 pg of O. viverrini genomic DNA, and the PCR test was repeated. For samples not showing PCR inhibition, PCR was repeated using 10 μl template. Samples showing inhibition were subjected to a second DNA purification by applying a DNA extraction protocol, similar to that described above, but with the following modifications: 120 μl of ASL buffer and 20 μl of 2% polyvinylpolypyrolidone were added to 100 μl of extracted DNA, and the mixture was incubated for 5 min at 70°C and added to 15 μl proteinase K. The remaining steps were identical, with the exception that 100 μl AE buffer was added instead of 200 μl. Five microliters was used as the template in the PCR test.
(iii) Primer design and PCR amplification.
A nested PCR (nPCR) protocol was developed to optimize sensitivity. Specific primers for primary PCR (pPCR) and nPCR were designed from mitochondrial DNA sequences available in GenBank (release 161.0) for O. viverrini (GenBank accession number DQ119551) and from a mitochondrial cytochrome c oxidase subunit I gene for H. taichui (GenBank accession number EF055885). The forward and reverse primers designed for O. viverrini were 5′-AGGATGTGAGCTTTGGGAGTTCGT-3′ and 5′-ACCCAACAGCCAGGATACAAAGGA-3′, respectively, for the pPCR and 5′-TTGTGGGATTAAGTCTCCGTCGGT-3′ and 5′-TCCTCTTACCCAAGAACGAAACCC-3′, respectively, for the nPCR. The sizes of the pPCR and nPCR products were 508 bp and 226 bp, respectively. The forward and reverse primers designed for H. taichui were 5′-GGGGGTTTAGTTCTTGCTATGTTTTC-3′ and 5′-CTTGTTTGGTTATGGGGGTTTAGTTC-3′, respectively, for the pPCR and 5′-GACAATAAAACCCAATATCCACCAC-3′ and 5′-GCCCCGACTTCCCGCCAA-3′, respectively, for the nPCR. The sizes of the pPCR and nPCR products were 245 bp and 186 bp, respectively.
PCR identifications of O. viverrini and H. taichui were performed separately. PCR tests were carried out in a final volume of 50 μl containing 5 μl 10× buffer, 200 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, 0.3 μl Taq polymerase, and 0.25 mM of primer for each species. For nPCR, 2 μl of primary product was used. PCRs were performed in a DNA thermal cycler (Eppendorf Mastercycler gradient thermocycler) with initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 56°C (O. viverrini) or 53°C (H. taichui) for 1 min, and extension at 72°C for 1 min and a final elongation at 72°C for 7 min. The nPCR products were detected on 1.5% (O. viverrini) and 3% (H. taichui) agarose gels stained with ethidium bromide by UV light using a white/UV transilluminator (Alpha Innotech).
(iv) Positive controls.
For positive controls, O. viverrini DNA was extracted by the usual phenol-chloroform protocol from adult worms obtained from O. viverrini-infected hamsters in our laboratories. H. taichui DNA was extracted from adult worms collected during previous purging studies in Saravane province, Lao PDR. For H. taichui DNA extraction, the protocol for DNA extraction from stool samples was used, with the exception that no InhibitEX tablet was added. DNA concentrations were estimated by spectrophotometry (ND-1000 spectrophotometer).
(v) Sensitivity and specificity of the primers.
Serial dilutions of O. viverrini and H. taichui DNAs from adults were prepared from 150 ng/μl to 1.5 × 10−6 ng/μl for O. viverrini and from 132 ng/μl to 1.32 × 10−2 ng/μl for H. taichui. Five microliters from each dilution was used to measure the sensitivity of PCR detection. The detection limit was determined as the last of the serial dilutions that consistently resulted in a PCR product.
To assess primers specificity, O. viverrini and H. taichui primers were used together with human DNA and with total genomic DNAs of the following helminth species, which were sequentially tested: H. taichui or O. viverrini, Schistosoma japonicum, Necator americanus, Ancylostoma duodenale, Strongyloides stercoralis, Trichuris trichiura, Ascaris lumbricoides, and Strongyloides ratti. Adult worms were obtained from our collaborators and from infected rodents in our laboratories.
(vi) Validation of the PCR tests.
The PCR tests were validated using 80 frozen stool samples available from previous studies conducted in 2005 to 2006 in rural villages in areas of endemicity in Lao PDR (23). Briefly, in those studies stool samples were collected on three consecutive days from patients aged ≥15 years, and a single KK was performed on each stool specimen. Opisthorchis-like egg intensity was expressed in epg, and O. viverrini-positive individuals were grouped into three categories, i.e., light infections (1 to 999 epg), moderate infections (1,000 to 9,999 epg), and heavy infections (≥10,000 epg), as described by Maleewong et al. (15). Eighty adults harboring Opisthorchis-like eggs in their stools, as diagnosed by KK (19 heavy, 57 moderate, and 4 light infections), were nonrandomly selected. All patients were treated with a single dose of oral praziquantel (40 mg/kg), and their stools were collected following an oral purgative (sodium phosphate). Morphological identification of adult worms in the purged stools showed that all 80 individuals had O. viverrini infections, of whom 61 (76%) had confirmed mixed infections with H. taichui.
Estimating the prevalence of O. viverrini and H. taichui infection by PCR, KK, and FECT in a cross-sectional community study, Khong District, Lao PDR.
Following validation, we applied the above-described PCR tests on 114 frozen stool specimens, corresponding to a 10% random sample of specimens collected in a cross-sectional, community-based survey that we conducted in March 2007 in Long Song and Hang Long, two neighboring villages in an area of endemicity and having an estimated total population of 1,095 villagers, on Don Long island, Khong District, Champasack Province, southern Lao PDR (coordinates 14°02′34″N, 105°47′58″E [Google Earth 4.3.7284 beta version]) (Fig. 1). A sample of the first stool specimen of each participant was frozen, kept in a 2-ml cryotube, and transported to the laboratory facilities in Switzerland with the cold chain maintained. Samples were kept at −20°C for long-term storage for PCR analysis.
FIG. 1.
Map of Lao PDR. The arrow indicates Khong District, Champasack Province, where Long Song and Hang Long villages were selected for the cross-sectional community study.
In order to compare the performance of our PCR tests with that of frequently used parasitological diagnostic methods, the KK and FECT were also performed in the Khong community study. For the 114 participants, one KK thick smear was performed from each of three stool specimens during the field survey. FECT and PCR were performed on samples taken from the first fecal specimen. The KK was performed in accordance with the kit instructions (Vestergaard Frandsen, Lausanne, Switzerland). KK slides were read under light microscopy within 1 h following slide preparation. Opisthorchis-like eggs intensities were expressed in epg and grouped into light, moderate, and heavy infections as described by Maleewong et al. (15). For the FECT, 0.3 g of stool of the first specimen was fixed in 10 ml sodium acetate-acetic acid-formalin solution and centrifuged, and the concentration was reviewed under light microscopy for egg counts (the method is fully described by Marti and Escher (16). For PCR analysis, samples were processed as described above.
Statistical analyses.
The sensitivities and specificities of the O. viverrini PCR and H. taichui PCR tests were established using purge results for validation. Coprological diagnosis by PCR was compared with purge results of the same individuals using McNemar's χ2 test for discordant pairs. O. viverrini infection prevalences in the community as assessed by PCR, one KK thick smear (1 KK), three KK thick smears (3 KK), and FECT were also tested by McNemar's χ2 test. The strength of the agreement between methods was assessed with the kappa statistical test.
Ethical considerations.
For field investigations, ethical clearance was obtained from the National Ethics Committee, Ministry of Health, in Vientiane, Lao PDR (reference number 027/NECHR), and by the Ethics Committee of the University and the Canton of Basel, Switzerland (EKBB) (reference number 255/06). Signed informed consent was obtained from each participant.
RESULTS
Laboratory procedures to develop the PCR tests. (i) Sensitivity of O. viverrini and H. taichui primers.
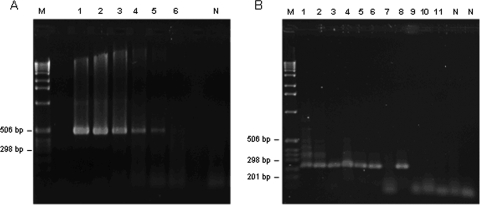
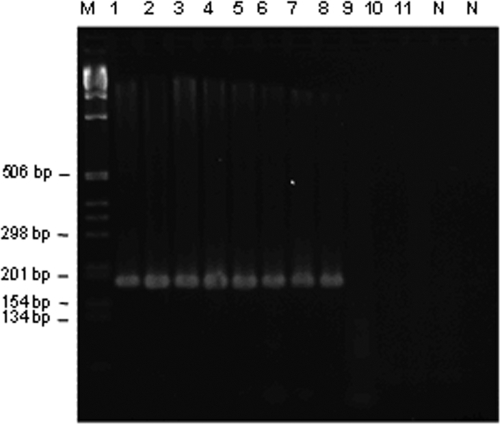
Amplification of DNA from adult worms by the outer primer sets (pPCR) and the inner primer sets (nPCR) yielded products of the expected sizes for O. viverrini (508 bp and 226 bp, respectively) (Fig. 2A and B) and H. taichui (245 bp and 186 bp, respectively) (Fig. 3). Primary primers specific to O. viverrini detected as little as 7.5 pg DNA (Fig. 2A). Nested primers specific to O. viverrini and H. taichui consistently detected levels as low as 0.75 pg DNA (Fig. 2B) and 1.32 ng DNA (Fig. 3), respectively.
FIG. 2.
Limit of detection of Opisthorchis viverrini DNA by pPCR (A) and nPCR (B) on agarose gels (1.5%) stained with ethidium bromide. Lane M, DNA size marker (1-kb DNA ladder; Merck); lane N: negative controls containing no DNA. (A) Primary primers tested in 10-fold serial dilutions with O. viverrini DNA at 7.5 × 104 pg (lane 1), 7.5 × 103 pg (lane 2), 750 pg (lane 3), 75 pg (lane 4), 7.5 pg (lane 5), and 0.75 pg (lane 6). The size of the amplicon was 508 bp. (B) Nested primers amplifying the pPCR product of serial dilutions of O. viverrini DNA at 75 pg (lane 1), 7.5 pg (lane 2), 0.75 pg (lanes 3 to 5), 7.5 × 10−2 pg (lanes 6 to 8), and 3.75 × 10−2 pg (lanes 9 to 11). The size of the amplicon was 226 bp.
FIG. 3.
Limit of detection of H. taichui DNA by nPCR on agarose gels (3%) stained with ethidium bromide. Lane M, DNA size marker (1-kb DNA ladder; Merck); lane N, negative controls containing no DNA. Primary and nested primers sets were tested in serial dilutions with H. taichui DNA at 660 ng (lane 1), 66 ng (lanes 2 and 3), 6.6 ng (lanes 4 and 5), 1.32 ng (lanes 6 and 7), 0.66 ng (lanes 8 and 9), and 6.6 × 10−2 ng (lanes 10 and 11). The size of the amplicon was 186 bp.
(ii) Specificity of O. viverrini and H. taichui primers.
O. viverrini primers did not amplify H. taichui genomic DNA and vice versa. O. viverrini and H. taichui primers also did not amplify S. japonicum, N. americanus, A. duodenale, S. stercoralis, T. trichiura, A. lumbricoides, S. ratti, or human genomic DNA.
(iii) Validation of O. viverrini PCR and H. taichui PCR.
O. viverrini and H. taichui PCR assays were validated using frozen stool samples collected from 80 Lao adults in previous purging studies as described above. Inhibitory controls of the O. viverrini PCR revealed a 12.5% inhibition rate (10/80) for stool samples from O. viverrini-infected patients. One sample (1.3%) was removed from the analysis due to persistent inhibition. Repeating DNA purification and increasing amounts of DNA resulted in 74/79 confirmed O. viverrini-positive patients. H. taichui PCR detected 51/79 H. taichui-positive patients. Hence, purging detected more O. viverrini infections (79) and H. taichui infections (60) than O. viverrini PCR (74) and H. taichui PCR (51) among the 79 uninhibited samples. The determined O. viverrini PCR sensitivity was 93.7% (74/79; 95% confidence interval [CI], 85.8 to 97.9%). H. taichui PCR detected seven additional cases of H. taichui infection that were negative by purging. H. taichui PCR yielded a sensitivity of 73.3% (44/60; 95% CI, 60.3 to 83.9%) and a specificity of 63.2% (12/19; 95% CI, 38.4 to 83.7%). These results are presented in Table 1.
TABLE 1.
O. viverrini PCR and H. taichui PCR validation based on O. viverrini and H. taichui infections diagnosed by purging in 79 PCR-uninhibited stool samples
| Organism | No. of samples positive by:
|
PCR sensitivity, % (95% CI) | PCR specificity, % (95% CI) | |
|---|---|---|---|---|
| Purging | PCR | |||
| O. viverrini positive | 79 | 74 | 93.7 (85.8-97.9) | NDa |
| H. taichui positive | 60 | 51 | 73.3 (60.3-83.9) | 63.2 (38.4-83.7) |
ND, not defined.
Estimating the prevalence of O. viverrini and H. taichui infection by PCR, KK, and FECT in a cross-sectional community study, Khong District, Lao PDR.
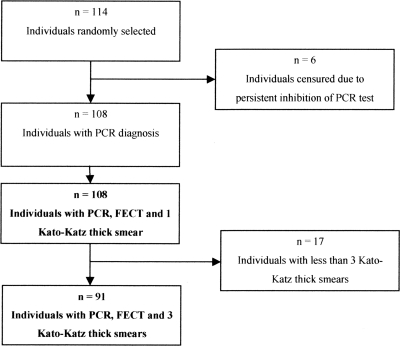
We applied the PCR tests to determine the prevalence of O. viverrini and/or H. taichui infections in 114 stool samples randomly selected from a cross-sectional community-based survey in Khong District, Lao PDR. The DNA inhibition rate in O. viverrini PCR before the second purification was 12.3% (14/114), of which six samples were finally removed due to persistent inhibition (5.3%) (Fig. 4). A second purification and increased amounts of DNA template allowed detection of an additional 14 positive samples, for a total of 69/108.
FIG. 4.
Individuals randomly selected from a cross-sectional community study in Khong District, Lao PDR. Analyses were performed separately on those having PCR, FECT, and 1 KK results (n = 108) and those having PCR, FECT, and 3 KK results (n = 91).
Table 2 presents PCR results for the detection of O. viverrini and H. taichui infections. The estimated community prevalence of O. viverrini infection confirmed by PCR was 63.9% (69/108; 95% CI, 54.1 to 72.9%), and the H. taichui prevalence was 30.6% (33/108; 95% CI, 22.1 to 40.2%). Of 108 villagers, 26.9% (95% CI, 18.8 to 36.2%) had neither infection, 9.3% (95% CI, 4.5 to 16.4%) had only H. taichui infection, 42.6% (95% CI, 33.1 to 52.5%) had only O. viverrini infection, and 21.3% (95% CI, 14.0 to 30.2%) had both infections.
TABLE 2.
O. viverrini and/or H. taichui infections diagnosed by PCR in 108 stool samples from a cross-sectional community study, Khong District, Lao PDR
| H. taichui PCR result | No. of samples with O. viverrini PCR result
|
||
|---|---|---|---|
| Positive | Negative | Total (%) | |
| Positive | 23 | 10 | 33 (30.6) |
| Negative | 46 | 29 | 75 (69.4) |
| Total (%) | 69 (63.9) | 39 (36.1) | 108 |
(i) Comparison of methods to estimate prevalence of O. viverrini infections: KK, FECT, and PCR.
Figure 4 summarizes the number of individuals available for analyses. Table 3 summarizes the diagnostic performances of 3 KK, 1 KK, and FECT in comparison with PCR as the gold standard.
TABLE 3.
Diagnostic performance of the KK method and FECT to detect O. viverrini infection in stool samples from a cross-sectional community study, Khong District, Laos
| Method | Total no. of samples | No. of samples with O. viverrini diagnosis by PCRa
|
% (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Opisthorchis-like egg prevalence | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Kappa | ||
| 3 KK slides positive | 91 | 53 (58) | 19 (33) | 79.1 (69.8-86.0) | 91.4 (81.0-97.1) | 42.4 (25.5-60.8) | 73.6 (61.9-83.3) | 73.7 (48.8-90.9) | 0.37 (0.18-0.57) |
| 1 KK slide positive | 108 | 43 (69) | 11 (39) | 50.0 (39.9-61.2) | 62.3 (49.8-73.7) | 71.8 (55.1-85.0) | 79.6 (66.5-89.4) | 51.9 (37.8-65.7) | 0.31 (0.14-0.49) |
| 1 FECT positive | 108 | 34 (69) | 3 (39) | 34.3 (24.5-44.7) | 49.3 (37.0-61.6) | 92.3 (79.1-98.4) | 91.9 (78.1-98.3) | 50.7 (38.6-62.8) | 0.35 (0.21-0.49) |
O. viverrini PCR prevalence of 63.9% (69/108; 95% CI, 54.1 to 72.9%). Values in parentheses are total numbers of the indicated type of samples.
(ii) 3 KK.
Analyses were restricted to the samples from the 91/108 villagers who provided three stool specimens (3 KK) and for whom PCR testing could be performed. As shown in Table 3, the prevalence of Opisthorchis-like eggs measured by 3 KK was 79.1% (72/91; 95% CI, 69.8 to 86.0%), which showed moderate agreement with the prevalence of 63.9% measured by PCR (McNemar's χ2 = 8.17, P = 0.004; κ = 0.37). The sensitivity and specificity of 3 KK were 91.4% (53/58; 95% CI, 81.0 to 97.1%) and 42.4% (14/33; 95% CI, 25.5 to 60.8%), respectively. All 19 samples that were positive by 3 KK but negative by O. viverrini PCR were light-intensity infections; four of these samples were found to be positive by H. taichui PCR.
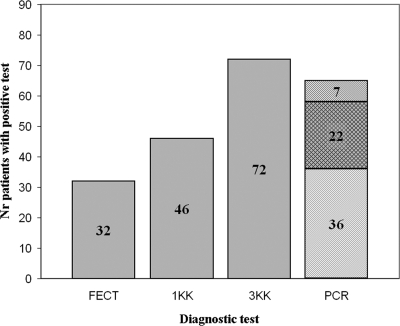
Figure 5 compares detection of O. viverrini and H. taichui infection by FECT, 1 KK, 3 KK, and PCR for the 91 individuals who provided three stool specimens and indicates the number of O. viverrini and H. taichui infections differentiated by PCR.
FIG. 5.
Comparison of diagnostic methods for O. viverrini and H. taichui infection within the same 91 individuals. ░⃞, O. viverrini and/or H. taichui infection (nondifferentiated); ▒, O. viverrini infection only; ▧, H. taichui infection only; ▩, O. viverrini and H. taichui coinfections.
(iii) 1 KK.
Analyses included the samples from all 108 villagers who provided at least one stool specimen and which could be examined by PCR. The prevalence of Opisthorchis-like eggs assessed by 1 KK was 50.0% (95% CI, 39.9 to 61.2%). This was in moderate agreement with the prevalence assessed by O. viverrini PCR of 63.9% (McNemar's χ2 = 6.08, P = 0.014; κ = 0.31) or by 3 KK (exact McNemar's χ2 = 26.0, P < 0.001; κ = 0.42). The sensitivity and specificity of 1 KK were 62.3% (43/69; 95% CI, 49.8 to 73.7%) and 71.8% (28/39; 95% CI, 55.1 to 85.0%), respectively. All 11 samples which detected Opisthorchis-like eggs by 1 KK but which were negative by O. viverrini PCR had light infections; two of these samples were found to be positive by H. taichui PCR.
(iv) FECT.
Analyses were based on a single stool sample obtained from all 108 villagers and which could be analyzed by PCR. The sensitivity of FECT was 49.3% (34/69; 95% CI, 37.0 to 61.6%), whereas its specificity was 92.3% (36/39; 95% CI, 79.1 to 98.4%). The prevalence of O. viverrini infection assessed by FECT was 34.3% (37/108; 95% CI, 24.5 to 44.7%), which showed moderate agreement with the prevalence measured by PCR (McNemar's χ2 = 26.95, P < 0.0001; κ = 0.35). There was also moderate agreement between FECT and 1 KK regarding O. viverrini infection prevalence (McNemar's χ2 = 9.32, P = 0.002; κ = 0.43).
Of 108 samples, FECT diagnosed three MIF infections (2.8%), whereas H. taichui PCR detected 30.6%.
DISCUSSION
Diagnosis of O. viverrini infections is usually performed by microscopic detection of eggs in stool samples. KK is likely the most frequently used method, but it does not permit differentiation of O. viverrini eggs from MIF eggs such as those from H. taichui. A more sensitive and specific diagnostic method is still needed.
To our knowledge, this is the first study to validate O. viverrini PCR and H. taichui PCR sensitivity by testing stool samples obtained by purging infected individuals and then to apply these PCR tests in combination with two coprological diagnostic tests to estimate infection prevalence in a 10% random sample of stool specimens from a community in an area of endemicity in a cross-sectional field survey. This approach facilitated a comparison of the PCR results with light microscopy and provided precise estimates of O. viverrini and H. taichui community prevalence in field settings. Other O. viverrini PCR tests have been evaluated using artificially inoculated feces without comparing the sensitivity with another method (19, 36, 37), while other studies used O. viverrini DNA extracted directly from the eggs (14, 31). Stool samples from infected individuals have been used previously, but the PCR tests were validated with FECT microscopy (27, 33). Some PCR tests discriminating O. viverrini from H. taichui were also previously developed, but their sensitivity and specificity were not compared with those of another diagnostic method (31) or were validated only with light microscopy (22, 32).
The primers in the O. viverrini PCR and H. taichui PCR tests developed in this study did not cross-react with DNAs from several other helminths that commonly cause coinfections in settings of endemicity, showing high specificity for O. viverrini and H. taichui. O. viverrini primers were able to detect as little as 0.75 pg of DNA and H. taichui primers as little as 1.32 ng of DNA. These levels of detection are in a range comparable with those for other PCR tests (14, 31, 32, 36, 37).
It is well-known that feces contain various substances that strongly inhibit amplification by PCR (35). Our study showed the importance of a second DNA purification step to decrease inhibition rates. An inhibitory control is ideally performed for each sample in order to avoid false-negative results. Adding more template may also increase DNA inhibition, and the volume should not be maximized in general. The inhibitory control could be performed systematically on all samples or, alternatively, could be performed only on an initially negative sample. Systematic inhibitory controls should be performed when prevalence is low and most results by PCR are expected to be negative. In our study, a second purification and use of an increased amount of template led to an increase in sensitivity of the O. viverrini PCR from 81.3% to 93.7%. Duenngai et al. (5) reported a new fecal DNA extraction protocol for the detection of O. viverrini DNA to remove PCR inhibitors, with a sensitivity of 79.3%, representing an improvement from their previous study using the commercial stool kit method, where the sensitivity was only 44.8% (27).
In the present study, purging results were used to validate the molecular tests in order to avoid inclusion of false-positive cases. The infections detected by purging but not by PCR may be explained by the facts that eggs are shed irregularly in feces and that PCR was tested on a single stool specimen. We found a much higher O. viverrini PCR sensitivity than Stensvold et al. (27) (45%), who validated their test by FECT on samples with high egg counts (>1,000 epg). The low sensitivity of their O. viverrini PCR test may possibly be explained by false positives from FECT due to misclassification of some eggs as O. viverrini.
The relatively low H. taichui PCR specificity obtained when validated with stools from purged infected individuals may be a result of false-negative purging results. Indeed, H. taichui adult collection after purging is challenging due to the worm's size of less than 0.5 mm (6). Adult MIF may be missed in the purged stools, particularly when infection intensities are low. This raises the question of whether purging is the most appropriate in vivo method to validate a PCR-based test for MIF. A better validation for H. taichui PCR may be to use stools artificially inoculated with H. taichui eggs (31).
We found high and moderate PCR-estimated prevalences of O. viverrini and H. taichui in Khong communities along the Mekong River, which contrasts with infection rates found by purging by Radomyos et al. (21) in northern Thailand (11.6% O. viverrini and 63.1% H. taichui). Although H. taichui is expected to be the predominant MIF in our study setting, the presence of other MIF, as well as other helminths, is common in Lao PDR. Chai et al. (4) conducted a study in Saravane Province, Lao PDR, which showed through purging results that the relative proportions of O. viverrini and MIF adults vary by location along the Mekong River Basin. H. taichui was the predominant species present in concurrent infection with O. viverrini. Other MIF detected were Haplorchis pumilio, Haplorchis yokogawai, Prosthodendrium molenkampi, and Phaneropsolus bonnei (2, 23).
Because we had selected a random sample of stool specimens from about 10% of the population in our Khong community study, we could compare actual prevalence estimates of infections determined by the different diagnostic methods under field conditions. The kappa statistical tests indicated that the level of agreement between PCR and 3 KK, 1 KK, or FECT results was only moderate (Table 3).
Examination of three specimens (one thick smear per stool specimen) increased KK sensitivity, stressing the importance of testing consecutive stool specimens. However, we found a substantial number of false-positive O. viverrini infections by 3KK, compared with the number of true positives by PCR as the gold standard. Thus, whereas 1 KK will likely underestimate prevalence, 3 KK may overestimate it in areas where both O. viverrini infection and MIF infection are endemic. 3 KK is a preferable method for field studies, rather than 1 KK, when sensitivity is emphasized over specificity and detection of low-intensity infections is important.
In this study, one FECT yielded a higher specificity to detect O. viverrini infections than 1 KK or 3 KK, while FECT sensitivity was considerably lower than that of 1 KK or 3 KK. Detection of MIF infections by FECT was considerably lower. Based on these findings, FECT should be repeated on multiple stool samples and would not be the recommended method in field studies, where the aim is to determine infection prevalence in communities in areas of endemicity and where methods with efficient and maximal sensitivity are required (23, 24).
O. viverrini and H. taichui diagnoses by PCR present several advantages compared with microscopic methods. First, PCR has fairly high sensitivity with the first stool specimen. Second, PCR tests avoid visual diagnoses and potential misclassification of O. viverrini with MIF. This avoids having to collect several stool specimens on consecutive days to achieve a comparable and reasonable sensitivity, as required by the KK method or FECT. Third, PCR may be a preferred sensitive method to diagnose symptomatic returning travelers or immigrants to countries where the infections are not endemic, where PCR technicians and resources may be more readily available than microscopists with regular experience in screening stools for trematode eggs. However, the requirements for molecular diagnostic methods also present some constraints. In addition to cost and technician skills, PCR also requires laboratory facilities which are unlikely to be available in many rural underdeveloped settings. If appropriate laboratory facilities are not proximal, the stool specimens require immediate freezing and cold-chain maintenance during transport, adding to feasibility issues.
Praziquantel, an effective and broad-spectrum trematocidal agent, is used in community-based deworming programs in Southeast Asia to reduce the burden of food-borne trematodiases and (of particular concern in areas of endemicity, including Lao PDR) to reduce schistosomiasis mekongi (18). A sensitive diagnostic tool is needed to screen communities following repeated rounds of chemotherapy, when the overall infection intensity has decreased. Assessment of prevalence after treatment by microscopic methods may underestimate the infection rate and overestimate the cure rate. A sensitive test is also important to monitor for emerging drug resistance and in efficacy trials comparing different anthelmintic drugs or regimens. Finally, as highlighted for schistosomiasis, low-intensity infection in low-transmission areas may be responsible for persistence of transmission of the disease, hamper its eradication (1), and continue to cause chronic diseases.
Distinguishing O. viverrini infections from MIF infections has important research implications. Identifying the infective species can improve our knowledge of clinical manifestations of these chronic infections in regions of endemicity. Clinical manifestations of opisthorchiasis can vary from asymptomatic carriage or nonspecific abdominal complaints to serious biliary tract disease. The clinical symptoms due to infections from H. taichui and other heterophyids are generally mild and transient (3). Infections with lecithodendriids are not known to produce significant clinical disease in humans (13), but clinical information is sparse. Hence, MIF infections, such as H. taichui infection, are not considered to have a serious public health impact, whereas O. viverrini chronic infection is known to be an important risk factor for developing cholangiocarcinoma. PCR is a useful tool in distinguishing these species and establishing their geographical distributions.
In conclusion, this study has shown that PCR is a valuable tool offering reasonable sensitivity to diagnose O. viverrini and H. taichui infections from a single stool specimen. PCR testing may be of particular interest for detecting low-intensity infections and monitoring low-transmission areas. Its use may be limited by cost and technical requirements.
Acknowledgments
We thank all the study participants, the laboratory technicians for stool sample analysis, Dalouny Bouakhasith and Youthanavanh Vonghachack for assistance in the fieldwork, and Penelope Vounatsou for reviewing the statistical analyses.
This study received financial support from the Swiss National Science Foundation and the Swiss Agency for Development and Cooperation (project no. NF3270B0-110020). Jennifer Keiser is grateful to the Swiss National Science Foundation for financial support (PPOOA-114941). Khampheng Phongluxa is supported by the Rudolf Geigy Foundation, and Phonepasong Soukhathammavong is supported by the City of Basel.
Author contributions were as follows: study concept and design, I.F., P.O., T.K.M., L.L., and J.K.; overall supervision of the study in Switzerland, I.F.; overall supervision of the study in Lao PDR, K.A.; acquisition of laboratory data, analysis, and interpretation, L.L. and I.F.; supervision of the field survey in Lao PDR, T.K.M., L.L., K.P., and P.S.; acquisition of field survey data in Lao PDR, L.L., T.K.M., K.P., and P.S.; analysis and interpretation of field survey data, L.L., T.K.M., and J.K.; acquisition of purging data (for PCR validation) in Lao PDR, S.S.; statistical analysis, L.L. and T.K.M.; first draft of the manuscript, L.L.; critical revision of the manuscript and substantial contribution to intellectual content, L.L., T.K.M., J.K., and I.F.; obtaining of funding, P.O., K.A., and J.K. The final version was approved by all authors.
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Alarcon de Noya, B., R. Ruiz, S. Losada, C. Colmenares, R. Contreras, I. M. Cesari, and O. Noya. 2007. Detection of schistosomiasis cases in low-transmission areas based on coprologic and serologic criteria: the Venezuelan experience. Acta Trop. 10341-49. [DOI] [PubMed] [Google Scholar]
- 2.Chai, J. Y., E. T. Han, S. M. Guk, E. H. Shin, W. M. Sohn, T. S. Yong, K. S. Eom, K. H. Lee, H. G. Jeong, Y. S. Ryang, E. H. Hoang, B. Phommasack, B. Insisiengmay, S. H. Lee, and H. J. Rim. 2007. High prevalence of liver and intestinal fluke infections among residents of Savannakhet Province in Laos. Korean J. Parasitol. 45213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai, J. Y., and S. H. Lee. 2002. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol. Int. 51129-154. [DOI] [PubMed] [Google Scholar]
- 4.Chai, J. Y., J. H. Park, E. T. Han, S. M. Guk, E. H. Shin, A. Lin, J. L. Kim, W. M. Sohn, T. S. Yong, K. S. Eom, D. Y. Min, E. H. Hwang, B. Phommmasack, B. Insisiengmay, and H. J. Rim. 2005. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J. Helminthol. 79283-289. [DOI] [PubMed] [Google Scholar]
- 5.Duenngai, K., P. Sithithaworn, U. K. Rudrappa, K. Iddya, T. Laha, C. R. Stensvold, H. Strandgaard, and M. V. Johansen. 2008. Improvement of PCR for detection of Opisthorchis viverrini DNA in human stool samples. J. Clin. Microbiol. 46366-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried, B., T. K. Graczyk, and L. Tamang. 2004. Food-borne intestinal trematodiases in humans. Parasitol. Res. 93159-170. [DOI] [PubMed] [Google Scholar]
- 7.Giboda, M., O. Ditrich, T. Scholz, T. Viengsay, and S. Bouaphanh. 1991. Human Opisthorchis and Haplorchis infections in Laos. Trans. R. Soc. Trop. Med. Hyg. 85538-540. [DOI] [PubMed] [Google Scholar]
- 8.Kaewkes, S. 2003. Taxonomy and biology of liver flukes. Acta Trop. 88177-186. [DOI] [PubMed] [Google Scholar]
- 9.Kaewkes, S., D. B. Elkins, P. Sithithaworn, and M. R. Haswell-Elkins. 1991. Comparative studies on the morphology of the eggs of Opisthorchis viverrini and lecithodendriid trematodes. Southeast Asian J. Trop. Med. Public Health 22623-630. [PubMed] [Google Scholar]
- 10.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14397-400. [PubMed] [Google Scholar]
- 11.Keiser, J., and J. Utzinger. 2007. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 23555-562. [DOI] [PubMed] [Google Scholar]
- 12.Keiser, J., and J. Utzinger. 2005. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 111507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, V. 1999. Trematode infections and diseases of man and animals, p. 343-344. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 14.Le, T. H., N. Van De, D. Blair, P. Sithithaworn, and D. P. McManus. 2006. Clonorchis sinensis and Opisthorchis viverrini: development of a mitochondrial-based multiplex PCR for their identification and discrimination. Exp. Parasitol. 112109-114. [DOI] [PubMed] [Google Scholar]
- 15.Maleewong, W., P. Intapan, S. Wongwajana, P. Sitthithaworn, V. Pipitgool, C. Wongkham, and W. Daenseegaew. 1992. Prevalence and intensity of Opisthorchis viverrini in rural community near the Mekong River on the Thai-Laos border in northeast Thailand. J. Med. Assoc. Thai. 75231-235. [PubMed] [Google Scholar]
- 16.Marti, H., and E. Escher. 1990. SAF—an alternative fixation solution for parasitological stool specimens. Schweiz. Med. Wochenschr. 1201473-1476. [PubMed] [Google Scholar]
- 17.Mayer, D. A., and B. Fried. 2007. The role of helminth infections in carcinogenesis. Adv. Parasitol. 65239-296. [DOI] [PubMed] [Google Scholar]
- 18.Montresor, A., D. T. Cong, M. Sinuon, R. Tsuyuoka, C. Chanthavisouk, H. Strandgaard, R. Velayudhan, C. M. Capuano, T. L. Anh, and A. S. Tee Dató. 2008. Large-scale preventive chemotherapy for the control of helminth infection in western Pacific countries: six years later. PLoS Negl. Trop. Dis. 2e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller, B., J. Schmidt, and H. Mehlhorn. 2007. PCR diagnosis of infections with different species of Opisthorchiidae using a rapid clean-up procedure for stool samples and specific primers. Parasitol. Res. 100905-909. [DOI] [PubMed] [Google Scholar]
- 20.Pasuralertsakul, S., W. Ngrenngarmlert, S. Sripochang, N. Khantiyanan, and O. Akkara-Ngamsiri. 2005. Methylene blue staining method for identification of Opisthorchis viverrini egg. Southeast Asian J. Trop. Med. Public Health 36(Suppl. 4)107-109. [PubMed] [Google Scholar]
- 21.Radomyos, B., T. Wongsaroj, P. Wilairatana, P. Radomyos, R. Praevanich, V. Meesomboon, and P. Jongsuksuntikul. 1998. Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J. Trop. Med. Public Health 29123-127. [PubMed] [Google Scholar]
- 22.Sato, M., U. Thaenkham, P. Dekumyoy, and J. Waikagul. 2009. Discrimination of O. viverrini, C. sinensis, H. pumilio and H. taichui using nuclear DNA-based PCR targeting ribosomal DNA ITS regions. Acta Trop. 10981-83. [DOI] [PubMed] [Google Scholar]
- 23.Sayasone, S., Y. Vonghajack, M. Vanmany, O. Rasphone, S. Tesana, J. Utzinger, K. Akkhavong, and P. Odermatt. 2009. Diversity of human intestinal helminthiasis in Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 103247-254. [DOI] [PubMed] [Google Scholar]
- 24.Sithithaworn, P., P. Yongvanit, S. Tesana, and C. Pairojkul. 2007. Liver flukes, p. 3-52. In K. Darwin Murrell and Bernard Fried (ed.), Food-borne parasitic zoonoses. Fish and plant-borne parasites. Springer, New York, NY.
- 25.Sripa, B., S. Kaewkes, P. Sithithaworn, E. Mairiang, T. Laha, M. Smout, C. Pairojkul, V. Bhudhisawasdi, S. Tesana, B. Thinkamrop, J. M. Bethony, A. Loukas, and P. J. Brindley. 2007. Liver fluke induces cholangiocarcinoma. PLoS. Med. 4e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sripa, B., and C. Pairojkul. 2008. Cholangiocarcinoma: lessons from Thailand. Curr. Opin. Gastroenterol. 24349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stensvold, C. R., W. Saijuntha, P. Sithithaworn, S. Wongratanacheewin, H. Strandgaard, N. Ornbjerg, and M. V. Johansen. 2006. Evaluation of PCR based coprodiagnosis of human opisthorchiasis. Acta Trop. 9726-30. [DOI] [PubMed] [Google Scholar]
- 28.Sukontason, K., S. Piangjai, K. Sukontason, and U. Chaithong. 1999. Potassium permanganate staining for differentiation the surface morphology of Opisthorchis viverrini, Haplorchis taichui and Phaneropsolus bonnei eggs. Southeast Asian J. Trop. Med. Public Health 30371-374. [PubMed] [Google Scholar]
- 29.Sukontason, K. L., K. Sukontason, S. Piangjai, S. Pungpak, and P. Radomyos. 2001. Prevalence of Opisthorchis viverrini infection among villagers harboring Opisthorchis-like eggs. Southeast Asian J. Trop. Med. Public Health 32(Suppl. 2)23-26. [PubMed] [Google Scholar]
- 30.Tesana, S., T. Srisawangwonk, S. Kaewkes, P. Sithithaworn, P. Kanla, and C. Arunyanart. 1991. Eggshell morphology of the small eggs of human trematodes in Thailand. Southeast Asian J. Trop. Med. Public Health 22631-636. [PubMed] [Google Scholar]
- 31.Thaenkham, U., K. Visetsuk, D. T. Dung, and J. Waikagul. 2007. Discrimination of Opisthorchis viverrini from Haplorchis taichui using COI sequence marker. Acta Trop. 10326-32. [DOI] [PubMed] [Google Scholar]
- 32.Traub, R. J., J. Macaranas, M. Mungthin, S. Leelayoova, T. Cribb, K. D. Murrell, and R. C. Thompson. 2009. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to central Thailand. PLoS. Negl. Trop. Dis. 3e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesha, K. R., S. Kumar, A. Parvathi, K. Duenngai, P. Sithithaworn, I. Karunasagar, and I. Karunasagar. 2008. Opisthorchis viverrini: detection by polymerase chain reaction (PCR) in human stool samples. Exp. Parasitol. 120353-356. [DOI] [PubMed] [Google Scholar]
- 34.WHO. 1991. Basic laboratory methods in medical parasitology. WHO, Geneva, Switzerland.
- 35.Wilde, J., J. Eiden, and R. Yolken. 1990. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J. Clin. Microbiol. 281300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wongratanacheewin, S., W. Pumidonming, R. W. Sermswan, and W. Maleewong. 2001. Development of a PCR-based method for the detection of Opisthorchis viverrini in experimentally infected hamsters. Parasitology 122175-180. [DOI] [PubMed] [Google Scholar]
- 37.Wongratanacheewin, S., W. Pumidonming, R. W. Sermswan, V. Pipitgool, and W. Maleewong. 2002. Detection of Opisthorchis viverrini in human stool specimens by PCR. J. Clin. Microbiol. 403879-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]