Abstract
Among the different strains of Mycobacterium tuberculosis, Beijing has been identified as an emerging genotype. Enhanced transmissibility provides a potential mechanism for genotype selection. This study evaluated whether the Beijing genotype is more readily transmitted than other prevalent genotypes to children in contact with an adult tuberculosis (TB) index case in the child's household. We conducted a prospective, community-based study at two primary health care clinics in Cape Town, South Africa, from January 2003 through December 2004. Bacteriologically confirmed new adult pulmonary TB cases were genotyped by IS6110 DNA fingerprinting; household contacts less than 5 years were traced and screened for M. tuberculosis infection and/or disease. A total of 187 adult index cases were identified from 174 households with children aged less than 5 years. Of 261 child contacts aged 0 to 5 years, 219 (83.9%) were completely evaluated and the isolate from the index case was successfully genotyped. M. tuberculosis infection (induration of ≥10 mm by Mantoux tuberculin skin test) was documented in 118/219 (53.9%) children; 34 (15.5%) had radiographic signs suggestive of active TB. There was no significant difference in the ratio of infected children among those exposed to the Beijing genotype (51/89; 57.3%) and those exposed to non-Beijing genotypes (55/115; 47.8%) (odds ratio, 1.5; 95% confidence interval, 0.8 to 2.7). Genotyping was successful for six children diagnosed with active TB; the isolates from only two children had IS6110 fingerprints that were identical to the IS6110 fingerprint of the isolate from the presumed index case. We found no significant association between the M. tuberculosis genotype and transmissibility within the household. However, undocumented M. tuberculosis exposure may have been a major confounding factor in this setting with a high burden of TB.
From an evolutionary perspective, the global tuberculosis (TB) epidemic presents a dynamic picture. Mycobacterium tuberculosis generates significant genetic diversity through deletion, duplication, and recombination events; but unlike most other bacterial pathogens, gene exchange is rare (31, 33). The absence of horizontal gene transfer results in strict clonality with distinct genetic lineages that permit accurate phylogenetic reconstruction. Selection of the most successful genotypes is mediated by genotype-specific differences in host-pathogen interactions (15, 18), some of which have been well characterized in animal models (11, 24, 25). Pathogen-related factors that may contribute to M. tuberculosis genotype selection include variability in transmissibility (the ability to spread from person to person), pathogenicity (the ability to cause clinical disease), the level of protection afforded by Mycobacterium bovis Bacille Calmette-Guérin (BCG) vaccination, and the acquisition of drug resistance.
The Beijing genotype predominates in parts of East Asia (17, 23, 38, 41), northern Eurasia (12, 31), and southern Africa (8, 39). Beijing has been regarded as an emerging genotype on the basis of its global distribution, its association with young age (4), and its proportional increase in prevalence over time (8, 39). An increased ability to circumvent the protection afforded by BCG vaccination is suggested by the positive association (of the Beijing genotype) with the presence of a BCG scar in human populations (4) and has been observed in mice (24), although more recent findings challenge this observation (20). Multiple mechanisms have been explored to explain the potential link between the emergence of the Beijing genotype and low-level BCG protection (1), which may provide the Beijing genotype with a selective advantage in populations in which universal BCG vaccination is practiced.
The association between the Beijing genotype and drug-resistant TB is well documented in multiple settings (2, 9, 31, 32, 37). Although it has been demonstrated that the acquisition of drug resistance is usually associated with a fitness cost, this finding seems variable and strain dependent (14) and may be insufficient to prevent transmission (16). Some Beijing genotypes retain their fitness in vitro, despite the acquisition of drug resistance (36), while compensatory evolution may account for significantly higher levels of fitness in clinical strains than in their progenitors (16). The geographic clustering of drug-resistant cases with evidence of clonal expansion suggests the successful transmission of drug-resistant Beijing genotypes (37). This is supported by the frequency with which isolates of the Beijing genotype are identified among children with drug-resistant TB (30), which indicates successful transmission within the community (34).
Variable transmissibility, irrespective of drug resistance, represents a relatively unexplored potential mechanism for the emergence of an M. tuberculosis genotype. Conventional molecular tools are limited by an inability to distinguish factors related to transmissibility from those related to pathogenicity, since only patients with active disease can be evaluated. The value of experimental animal models is equally limited, since artificially induced infection does not allow simulation of the natural airborne transmission of M. tuberculosis. The household provides an appropriate setting in which variables related to recent M. tuberculosis transmission in the human host may be studied and allows the evaluation of young children likely to have been infected through contact with others at the household level.
The study described here aimed to determine whether the Beijing genotype is more readily transmitted than other prevalent genotypes to children in household contact with an adult TB index case.
MATERIALS AND METHODS
Study setting.
We conducted a prospective, community-based study over a 2-year period (January 2003 through December 2004) at two primary health care clinics in an urban community in Cape Town, South Africa. Ravensmead and Uitsig are two well-characterized poor urban communities whose populations are of predominantly mixed ethnicity. Residents live in formal and informal housing structures, often with multiple structures and families resident on the same property. High levels of M. tuberculosis transmission occur both inside and outside the household (42). In 2004, the total TB incidence recorded in the study setting and its immediate surroundings was 845/100,000 population/year among adults and 407/100,000 population/year among children (26). In 2004, the prevalence of human immunodeficiency virus (HIV) infection among TB patients in the study setting was less than 10% (Cape Town City Health Department). BCG (Danish strain 1331; Statens Serum Institute; Denmark) is routinely given to all children at birth; in 2005, the vaccination coverage rate reported for the Western Cape Province was 99% (7).
Study population.
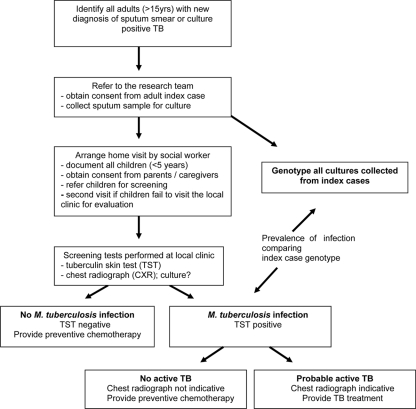
Clinic-based TB treatment registers were used to identify consecutive new adult patients (age, >15 years) diagnosed with pulmonary TB (by sputum smear and/or positivity by culture). Household contacts were defined as children living at the same residential address at the time of diagnosis. A social worker recorded the names and ages of all children identified as household contacts during a home visit. According to South African National TB Control Programme guidelines (5, 10a), all children less than 5 years old were invited for evaluation at the local primary health care clinic. Figure 1 provides a schematic overview of the study.
FIG. 1.
Summary of study design.
Genotype determination.
Adult patients provided a sputum specimen before the initiation of TB treatment. Specimens were cultured in MGIT or Bactec tubes by using standard automated detection systems (Becton Dickinson, Sparks, MD). Positive cultures were subcultured on Löwenstein-Jensen slants for genotyping by the standardized restriction fragment length polymorphism methodology based on the IS6110 transposable element (40). Standard protocols were in place to prevent cross contamination (6). Isolates were assigned to specific genotype families according to the specific IS6110 banding pattern (fingerprint).
The following internationally recognized genotype families were identified: Beijing, LAM (Latin American and Mediterranean family), X (an European clade of IS6110 low banders), and Haarlem (13). Genotype families with a low frequency (<10 cases) were categorized as “other.” If more than one new adult TB case was identified in the same household within a 2-month period or if multiple strains were isolated from a single index case (43), the genotype exposure was categorized as “multiple”.
Management of household contacts.
Children were evaluated within 1 month of the diagnosis of TB in the index case. A tuberculin skin test (TST) was performed on the volar aspect of the left forearm of all household contacts younger than 5 years of age by the Mantoux method (intradermal injection of 2 tuberculin units of M. tuberculosis purified protein derivative RT 23; Statens Serum Institute). In accordance with South African National TB Control Programme guidelines, an area of induration equal to or exceeding 10 mm (≥5 mm in HIV-infected children), determined by measuring the maximal transverse diameter, was regarded as a positive result. Children were offered a rapid HIV screening test (Determine rapid HIV test; Abbott), following the provision of standard pre- and posttest counseling and written informed consent from the parent and legal guardian, in the following instances: if the mother was known to be infected with HIV, if the child had symptoms or signs suggestive of HIV disease, or if the child was diagnosed with active TB.
Chest radiographs (anteroposterior and lateral views) were performed for all children and were reviewed in a standardized fashion by a single independent expert; a second independent expert confirmed the result for those with signs suggestive of active TB. Children diagnosed with active TB received supervised treatment consisting of isoniazid (INH), rifampin (rifampicin), and pyrazinamide for 2 months, followed by INH and rifampin for 4 months; the drugs were provided as water-dispersible fixed-dose combination tablets. Child contacts in whom active TB was excluded received 6 months of preventive chemotherapy with INH.
Data analysis.
Statistical analysis was done with SPSS software (version 16; SPSS Inc., Chicago, IL) and Epi-info (version 6.04) software. We compared the proportion of children with a positive TST result following documented household exposure to an index case with pulmonary TB caused by the Beijing genotype versus the proportion of children with a positive TST result following documented household exposure caused by the non-Beijing genotype using the χ2 test. Children with a documented exposure to multiple genotypes were excluded from the analysis. We repeated the same analysis with different variations: (i) an analysis restricted to child contacts less than 3 years of age, (ii) an analysis that excluded sputum smear-negative cases, (iii) an analysis that excluded drug-resistant index cases, and (iv) an analysis with a positive TST induration cutoff value of ≥15 mm instead of one of ≥10 mm.
The index cases and the parents or legal guardians of the children provided written informed consent for their participation in the study. Approval was obtained from the City of Cape Town Health Department, local community health committees, and the Committee for Human Research, Stellenbosch University.
RESULTS
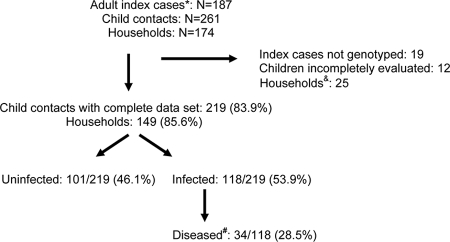
During the study period, 187 adult index cases with active pulmonary TB who had children less than 5 years old living at the same address were identified; these cases lived in 174 households. Of the adult index cases, 153 cases (81.8%) were sputum smear positive, and genotype identification was successful for 158 cases (84.5%). In total, 261 child (age, <5 years) contacts were identified (mean, 1.5 children per household). Two-thirds of the children (175/261 [67.0%]) were less than 3 years of age (i.e., 0 to 2 years of age); 60 were <1 year old, 54 were 1 year old, 61 were 2 years old, and 86 were 3 to 4 years old. Complete data, including successful genotyping of the adult index case together with the evaluation of all children 0 to 5 years of age in the household by TST and chest radiography, were collected from 149/174 (85.6%) households and included data for 157/187 (84.0%) adult index cases and 219/261 (83.9%) children. In 8/149 (5.4%) households, two adult index cases were identified within the specified 2-month time window. Figure 2 summarizes the patient recruitment data.
FIG. 2.
Summary of patient recruitment data. *, newly diagnosed sputum-positive TB cases (age, >15 years) with children less than 5 years of age living at the same address; #, children with disease constitute a subgroup of those infected; &, households were excluded if either the isolates from the index cases were not successfully genotyped or the child contacts were incompletely evaluated.
A positive TST result was recorded for 118/219 (53.8%) children. The mean induration size in those subjects with indurations greater than 0 mm was 17 mm (median, 18 mm); 5 readings fell in the range from 0 to 9 mm and were classified as negative. The Beijing genotype was the most common genotype identified and was present in 64/157 (40.8%) adult index cases. Seven Beijing genotype exposures were classified as multiple; five were due to the presence of multiple index cases within the same household and two were due to mixed infections (more than one genotype was present in a single sputum specimen), according to IS6110-based restriction fragment length polymorphism analysis. Data for children exposed to multiple genotypes and/or index cases were excluded from the comparative analysis. Table 1 reflects the number of children diagnosed with M. tuberculosis infection (positive TST result) and/or probable active TB in relation to the genotype isolated from the documented adult index case.
TABLE 1.
Proportion of children <5 years of age with M. tuberculosis infection and/or disease following genotype-specific household exposure to an adult pulmonary TB index case
| Genotype from index case | No. (%) of adult index cases | No. of exposed children
|
ORa (95% CI) | |||
|---|---|---|---|---|---|---|
| Total | Uninfected | Infected | Diseasedb | |||
| Beijing | 57 (36.3) | 89 | 38 | 51 | 15 | 1.5 (0.8-2.7) |
| LAM | 27 (17.2) | 35 | 16 | 19 | 7 | 1.0 (0.5-2.5) |
| Haarlem | 10 (6.4) | 11 | 5 | 6 | 1 | 1.0 (0.3-4.4) |
| Xc | 23 (14.6) | 36 | 18 | 18 | 5 | 0.9 (0.4-2.0) |
| Otherd | 22 (14.0) | 33 | 21 | 12 | 3 | 0.5 (0.2-1.1) |
| Multiplee | 18f (8.9) | 15 | 3 | 12 | 3 | 3.7 (0.9-17.0) |
| Total | 157 | 219 | 101 | 118 | 34 | |
The OR and 95% CI for the ratio of children infected versus children uninfected for each genotype family compared to the rest of the genotypes, excluding children exposed to multiple genotypes.
Diseased children constitute a subgroup of those infected.
X, European clade of IS6110 low banders, also referred to as the low-copy-number clade.
Other, any genotype family with a frequency of less than 10 cases.
Multiple, households with multiple index cases (n = 8) or in which more than one genotype was identified in a single index case (two cases had mixed infections).
These 18 adult cases were from 10 households.
There was no statistically significant association between the proportion of children with a positive TST result and the genotype of the isolate recovered from the index case. Beijing was the only genotype to exhibit a tendency toward increased transmissibility (odds ratio [OR], 1.5; 95% confidence interval [CI], 0.8 to 2.7), with genotypes that occurred at a low frequency tending to have reduced transmissibility (OR, 0.5; 95% CI, 0.5 to 1.1). Although they were excluded from the genotype-specific comparisons, overall, we observed more infections among children exposed to multiple genotypes and multiple index cases: 12/15 (80.0%) versus 106/204 (52.0%) for those exposed to a single genotype and index case (P < 0.05).
In total, 34/219 (15.5%) child contacts were diagnosed with probable active TB; these represented 28.8% (34/118) of the infected children. None of these children were infected with HIV. The majority of children diagnosed with disease (28/34 [82.4%]) had uncomplicated hilar adenopathy, 2 had cervical lymphadenitis, and 4 (11.8%) had radiographic signs of airway compression and/or parenchymal infiltrates. No differences in the numbers or types of diseases with which the children were diagnosed were observed by genotype exposure (data not shown). Specimens for culture were collected from 11/34 (32.3%) children, of whom 6 (54.5%) had culture-confirmed TB. The genotype of the isolate from the child was identical to the genotype of the isolate from the presumed index case in only two of six (33.3%) instances; both of these children were younger than 3 years of age. In an attempt to limit the potential confounding influence of previous infection and/or undocumented exposure outside of the household, we performed repeat analyses restricted to children less than 3 years of age; the results were similar (data not shown).
To assess additional factors that may potentially affect transmission, the relation between sputum smear grading and genotype was examined (Table 2); no statistically significant differences were observed. Table 2 also shows the number of children infected and/or diagnosed with disease according to the sputum smear status of the adult index case. Only 2/19 (10.5%) children exposed to a sputum smear-negative, culture-positive index case were infected, whereas 116/200 (58%) children exposed to a sputum smear-positive index case were infected (OR, 0.09; 95% CI, 0.01 to 0.40). Multidrug-resistant TB was recorded in four index cases: two were infected with the Beijing genotype, one was infected with the Haarlem genotype, and one was infected with the LAM genotype.
TABLE 2.
Index case sputum smear grading in relation to genotype, as well as number of child contacts infected and/or diseased, in relation to index case sputum smear grading
| Group | No. of cases with the following sputum smear gradinga:
|
||||
|---|---|---|---|---|---|
| Sm− | Sm1+ | Sm2+ | Sm3+ | Total | |
| Genotype | |||||
| Beijing | 4 | 24 | 12 | 17 | 57 |
| LAM | 6 | 14 | 2 | 5 | 27 |
| Haarlem | 0 | 3 | 4 | 3 | 10 |
| Xb | 5 | 10 | 2 | 6 | 23 |
| Otherc | 0 | 8 | 4 | 10 | 22 |
| Multipled | 0 | 6 | 4 | 8 | 18 |
| Total | 15 | 65 | 28 | 50 | 157 |
| Child contacts | |||||
| Uninfected | 17 | 33 | 18 | 33 | 101 |
| Infected | 2 | 49 | 23 | 44 | 118 |
| Diseasede | 0 | 16 | 6 | 12 | 34 |
| Total | 19 | 82 | 41 | 77 | 219 |
Smear grading reflects the highest grading recorded in any specimen collected from a particular individual or household. Sm−, smear negative; Sm1+ to Sm3+, smear-positive grades 1 to 3, respectively.
X, European clade of IS6110 low banders, as identified by spoligotyping.
Other, any genotype family with a frequency of less than 10 cases.
Multiple, households with multiple index cases or in which more than one genotype was identified in a single index case (mixed infection).
Children with probable active TB represent a subgroup of those infected.
DISCUSSION
In this household contact study, children exposed to an index case with TB caused by the Beijing genotype were not more frequently infected (TST positive) than children exposed to other prevalent genotypes. Although the sample size was limited, our findings support the conclusion reached by a recent study in The Gambia that progression to active TB, but not transmission, varies by M. tuberculosis genotype (10). The Gambian study mainly enrolled adults (median age, 17 years) and provided no preventive treatment to TB contacts. This allowed them to document the number of secondary cases that developed during a 2-year follow-up period. Our study was restricted to household contacts less than 5 years of age, all of whom received chemotherapy consisting of either chemoprophylaxis or treatment for active disease.
Due to the high infection pressure within the study community, it was considered beneficial to restrict our focus to young children in whom we postulated that infection is most likely to represent recent household exposure. Limiting selection bias in household contact studies involving children often presents a huge challenge, since parents are more likely to present symptomatic children for screening. A particular strength of the current study is confirmation that the vast majority of child contacts (children <5 years of age) were fully evaluated and included in the analysis. Evidence for high infection pressure within the study community is provided by a calculated annual risk of infection of 4.1% in 2005 (21) and molecular evidence indicating that the majority of cases of TB transmission occur outside of the household (35, 42). The observation that only two of six children with culture-confirmed disease were infected with an isolate with an IS6110 fingerprint identical to that of the isolate from the presumed index case provides further evidence supporting the important contribution of undocumented TB exposure within the study community.
A positive TST result fails to distinguish recent from past infection, which may act as a confounding factor in this setting with a high prevalence of TB. Since very young children are less likely to have experienced past infection and because of their limited social contact they are also less likely to become infected outside of the household, we performed additional analyses restricted to children less than 3 years of age. Observations from the prechemotherapy era demonstrated that a diagnosis of active TB in very young children usually indicates the presence of an adult index case at home (27, 28), which is supported by the fact that both children whose isolates had IS6110 fingerprints that matched those of the isolates from the presumed index case were less than 3 years of age. However, the absence of a significant association between the genotype from the index case and infection among household contacts persisted, despite a focus on the very young, in whom a positive TST result most likely reflects recent household transmission.
An additional potential confounder to be considered is the suboptimal specificity of the TST, especially in the presence of universal BCG vaccination. Novel gamma interferon release assays utilize antigens produced by region of difference 1 that do not cross-react with M. bovis BCG and should provide improved test specificity (29). These assays were not available at the time of this study, but we postulate that the positive TST results obtained in our study likely reflect true M. tuberculosis infection. Reactive TST values (all values greater than 0 mm of induration) had a unimodal distribution around a mean of 17 mm, and only five children registered a response of 1 to 9 mm, which indicates the limited influence of BCG vaccination and/or exposure to nontuberculous mycobacteria. The results also remained similar, despite the use of a positive TST cutoff value of ≥15 mm instead of one of ≥10 mm. Genotype-specific reductions in TST responses have been reported in the United Kingdom with a single outbreak strain (3), but they have not been associated with any of the prevalent strains identified in this study.
Sputum smear grading provides a crude surrogate of the organism load and possibly also the risk of transmission posed by the index case. We noted a significant reduction in the likelihood of infection following exposure to a sputum smear-negative index case, but the exclusion of those with smear-negative disease from the analysis did not alter the relation between the genotype of the isolate from the index case and the proportion of child contacts who were TST positive. Drug resistance is often related to a poor treatment response and prolonged transmission. In our study, two of four (50%) of the index cases with drug-resistant TB were infected with the Beijing genotype; but the number of cases was few, and exclusion of the data for those cases from the analysis did not influence the genotype-specific transmission ratios. Although the Beijing genotype has been associated with drug resistance in multiple cross-sectional studies (2, 9, 31, 32, 37), a recent prospective study that documented the emergence of the Beijing genotype within the study community failed to detect an association with drug resistance (39).
Variation in the ability to culture different M. tuberculosis genotypes (22) may theoretically introduce selection bias, but since isolates from the vast majority of adults with sputum smear-positive disease were successfully genotyped, this seems unlikely. It has recently been demonstrated that the Beijing genotype also predominates among children diagnosed with TB at major referral hospitals within Western Cape Province, reflecting successful transmission and emergence within a wider geographic context (8, 30). It is not always appreciated that the Beijing genotype exhibits a deep population structure; a total of seven independently evolving sublineages have been identified (19). The aggregation of strains into poorly differentiated genotypes may obscure underlying differences; however, within the study setting, the emergence of all seven sublineages has been documented (39).
We conclude that transmissibility of M. tuberculosis does not seem to be related to the genotype. However, sample size considerations and the fact that the results from settings with a high prevalence of TB are likely to be confounded by undocumented TB exposure should be taken into consideration. It would be highly informative to repeat similar studies in settings with a low prevalence of TB.
Acknowledgments
We thank the patients for their participation, the research nurses (Susan van Zyl and Danite Bester) and social worker (Charise Pedro) for assisting with data collection, Wendy Brittle for culturing of the isolates from the children, Rory Dunbar for assistance with data extraction, and the City of Cape Town Health Department for permission to conduct this study.
Financial assistance was provided by the Norwegian Research Council via the TB in the 21st Century Consortium and the College of Medicine of South Africa as part of the Phyllis Knocker Bradlow research award.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Abebe, F., and G. Bjune. 2006. The emergence of Beijing family of genotypes of Mycobacterium tuberculosis and low level protection by bacille Calmette-Guérin (BCG) vaccines: is there a link? Clin. Exp. Immunol. 145389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, D., C. Rodriques, T. F. Ashavid, A. Lalvani, Z. F. Udwadia, and A. Mehta. 2005. High incidence of the Beijing genotype among multi-drug resistant isolates of Mycobacterium tuberculosis in a tertiary care centre in Mumbai, India. Clin. Infect. Dis. 40881-886. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, S. T., A. J. Williams, J. R. Brown, S. M. Newton, M. Simsova, M. P. Nicol, P. Sebo, M. Levin, R. J. Wilkinson, and K. A. Wilkinson. 2006. Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing, Am. J. Resp. Crit. Care Med. 1731038-1042. [DOI] [PubMed] [Google Scholar]
- 4.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. N. Lan, van T. Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Carroll, N. M., M. Richardson, E. Engelke, C. Lombard, and P. van Helden. 2002. Reduction of the rate of false-positive cultures of Mycobacterium tuberculosis in a laboratory with a high culture positivity rate. Clin. Chem. Lab. Med. 40888-892. [DOI] [PubMed] [Google Scholar]
- 7.Corrigal, J. 2007. Western Cape provincial EPI vaccination survey 2005. University of the Western Cape, Cape Town, South Africa. www.capegateway.gov.za/Text/2007/6/cd_volume_7_childhood_diseases_overview.pdf.
- 8.Cowley, D., D. Govender, B. February, M. Wolfe, L. Steyn, J. Evans, R. J. Wilkinson, and M. P. Nicol. 2008. Recent and rapid emergence of W-Beijing strains of Mycobacterium tuberculosis in Cape Town, South Africa. Clin. Infect. Dis. 471252-1259. [DOI] [PubMed] [Google Scholar]
- 9.Cox, H. S., T. Kubica, D. Doshetov, Y. Kebede, S. Rusch-Gerdess, and S. Niemann. 2005. The Beijing genotype and drug resistant tuberculosis in the Aral Sea region of Central Asia. Respir. Res. 6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong, B. C., P. C. Hill, H. Aiken, T. Awine, M. Antonio, I. M. Adetifa, D. J. Jackson-Sillah, A. Fox, K. DeRiemer, S. Gagneux, M. W. Borgdorff, K. P. W. J. McAdam, T. Corrah, P. M. Small, and R. A. Adegbola. 2008. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J. Infect. Dis. 1981037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Department of Health, South Africa. 2000. National tuberculosis control programme: practical guidelines 2000, p. 32-37. Department of Health, Pretoria, South Africa.
- 11.Dormans, J., M. Burger, D. Aguilar, R. Hernandez-Pando, K. Kremer, P. Roholl, S. M. Arend, and D. van Soolingen. 2004. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after injection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin. Exp. Immunol. 137460-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drobniewski, F., Y. Balabanova, V. Nikolayevsky, M. Ruddy, S. Kuznetzov, S. Zakharova, A. Melentyev, and I. Fedorin. 2005. Drug-resistant tuberculosis, clinical virulence and the dominance of the Beijing strain family in Russia. JAMA 2932726-2731. [DOI] [PubMed] [Google Scholar]
- 13.Filiol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. D. Anh, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, H. M. Ly, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 411963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagneux, S., M. V. Burgos, K. DeRiemer, A. Enciso, S. Munoz, P. C. Hopewell, P. M. Small, and A. S. Pym. 2006. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 20603-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux, S., K. DeRiemer, T. Van, M. Kato-Maeda, B. C. de Jong, S. Narayanan, M. Nicol, S. Niemann, K. Kremer, M. C. Gutierrez, M. Hilty, P. C. Hopewell, and P. M. Small. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1032869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagneux, S., C. D. Long, P. M. Small, T. Van, G. K. Schoolnik, and B. J. M. Bohannan. 2006. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 3121944-1946. [DOI] [PubMed] [Google Scholar]
- 17.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 8843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanekom, M., G. D. van der Spuy, N. C. Gey van Pittius, C. R. E. McEvoy, S. L. Ndambambi, T. C. Victor, E. G. Hoal, P. D. van Helden, and R. M. Warren. 2007. Evidence that the spread of Mycobacterium tuberculosis strains with the Beijing genotype is human population dependent. J. Clin. Microbiol. 452263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanekom, M., G. D. Van der Spuy, E. Streicher, S. L. Ndabambi, C. R. E. McEvoy, M. Kidd, N. Beyers, T. C. Victor, P. D. van Helden, and R. M. Warren. 2007. A recently evolved sublineage of the Mycobacterium tuberculosis Beijing strain family is associated with an increased ability to spread and cause disease. J. Clin. Microbiol. 451483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon, B. Y., S. C. Derrick, J. Lim, K. Kolibab, V. Dheenadhayalan, A. L. Yang, B. Kreiswirth, and S. L. Morris. 2008. BCG immunization induces protective immunity in mice against nine different Mycobacterium tuberculosis strains. Infect. Immun. 765173-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kritzinger, F. E., S. den Boon, S. Verver, D. A. Enarson, C. J. Lombard, M. W. Borgdorff, R. P. Gie, and N. Beyers. 2008. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop. Med. Int. Health 14136-142. [DOI] [PubMed] [Google Scholar]
- 22.Li, Q., C. C. Whalen, J. M. Albert, R. Larkin, L. Zukowski, M. D. Cave, and R. F. Silver. 2002. Differences in rate and variability in intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 706489-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, W. M., S. M. Wang, C. Y. Li, Y. H. Liu, G. M. Shen, X. X. Zhang, T. G. Niu, Q. Gao, D. van Soolingen, K. Kremer, and H. J. Duanmu. 2000. Molecular epidemiology of Mycobacterium tuberculosis in China: a nationwide random survey in 2000. Int. J. Tuberc. Lung Dis. 91314-1319. [PubMed] [Google Scholar]
- 24.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espetia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 13330-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manabe, Y. C., A. M. Dannenberg, Jr., S. K. Tyagi, S. L. Hatem, M. Yoder, S. C. Woolwine, B. C. Zook, M. L. Pitt, and W. R. Bishai. 2003. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect. Immun. 716004-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marais, B. J., A. C. Hesseling, R. P. Gie, H. S. Schaaf, and N. Beyers. 2006. The burden of childhood tuberculosis and the accuracy of routine surveillance data in a high-burden setting. Int. J. Tuberc. Lung Dis. 10259-263. [PubMed] [Google Scholar]
- 27.Marais, B. J., R. P. Gie, H. S. Schaaf, A. C. Hesseling, C. Obihara, L. J. Nelson, D. A. Enarson, P. R. Donald, and N. Beyers. 2004. The clinical epidemiology of childhood pulmonary tuberculosis—a critical review of the pre-chemotherapy literature. Int. J. Tuberc. Lung Dis. 8278-285. [PubMed] [Google Scholar]
- 28.Marais, B. J., R. P. Gie, H. S. Schaaf, A. C. Hesseling, C. C. Obihara, J. J. Starke, D. A. Enarson, P. R. Donald, and N. Beyers. 2004. The natural history of disease of childhood intra-thoracic tuberculosis—a critical review of the pre-chemotherapy literature. Int. J. Tuberc. Lung Dis. 8392-402. [PubMed] [Google Scholar]
- 29.Marais, B. J., and M. Pai. 2007. New approaches and emerging technologies in the diagnosis of childhood tuberculosis. Paediatr. Respir. Rev. 8124-133. [DOI] [PubMed] [Google Scholar]
- 30.Marais, B. J., T. C. Victor, A. C. Hesseling, M. Barnard, A. Jordaan, W. Brittle, H. Reuter, N. Beyers, P. D. van Helden, R. M. Warren, and H. S. Schaaf. 2006. Beijing and Haarlem genotypes are over-represented among children with drug resistant tuberculosis in the Western Cape Province of South Africa. J. Clin. Microbiol. 443539-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokrousov, I. 2008. Genetic geography of Mycobacterium tuberculosis Beijing genotype: a multifacet mirror of human history? Infect. Genet. Evol. 8777-785. [DOI] [PubMed] [Google Scholar]
- 32.Moss, A. R., D. Alland, E. Telzak, D. Hewlett, V. Sharp, P. Chiliade, V. LaBombardi, D. Kabus, B. Hanna, L. Palumbo, K. Brudney, A. Weltman, K. Stoeckle, K. Chirgwin, M. Simberkoff, S. Moghazeh, W. Eisner, M. Lutfey, and B. Kreiswirth. 1997. A city-wide outbreak of a multiple-drug-resistant strain of Mycobacterium tuberculosis in New York. Int. J. Tuberc. Lung Dis. 1115-121. [PubMed] [Google Scholar]
- 33.Nicol, M. P., and R. J. Wilkinson. 2008. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 102955-965. [DOI] [PubMed] [Google Scholar]
- 34.Schaaf, H. S., B. J. Marais, A. C. Hesseling, R. P. Gie, N. Beyers, and P. R. Donald. 2006. Childhood drug-resistant tuberculosis in the Western Cape Province of South Africa. Acta Paediatr. 15523-528. [DOI] [PubMed] [Google Scholar]
- 35.Schaaf, H. S., I. A. Michaelis, M. Richardson, C. N. Booysen, R. P. Gie, R. W. Warren, P. D. van Helden, and N. Beyers. 2003. Adult-to-child transmission of tuberculosis: household or community contact. Int. J. Tuberc. Lung Dis. 7426-431. [PubMed] [Google Scholar]
- 36.Toungoussova, O. S., D. A. Caugant, P. Sandven, A. O. Mariandyshev, and G. Bjune. 2004. Impact of drug resistance on fitness of Mycobacterium tuberculosis strains of the W-Beijing genotype. FEMS Immunol. Med. Microbiol. 42281-290. [DOI] [PubMed] [Google Scholar]
- 37.Toungoussova, O. S., A. Mariandyshev, G. Bjune, P. Sandven, and D. A. Caugant. 2003. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates in the Archangel Prison in Russia: predominance of the W-Beijing clone family. Clin. Infect. Dis. 37665-672. [DOI] [PubMed] [Google Scholar]
- 38.Tsolaki, A. G., S. Gagneux, A. S. Pym, Y. O. Gogeut de la Salmoniere, B. N. Kreiswirth, D. van Soolingen, and P. M. Small. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 433185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Spuy, G. D., K. Kremer, S. Ndabambi, N. Beyers, B. J. Marais, P. D. van Helden, and R. M. Warren. 1 December 2008. Changing Mycobacterium tuberculosis population structure highlights clade-specific pathogenic characteristics. Tuberculosis [Epub ahead of print.]. [DOI] [PubMed]
- 40.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenbach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Soolingen, D., L. Qian, P. E. W. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 333234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verver, S., R. M. Warren, Z. Munch, M. Richardson, G. D. van der Spuy, M. W. Borgdorff, M. A. Behr, N. Beyers, and P. D. van Helden. 2004. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet 363212-214. [DOI] [PubMed] [Google Scholar]
- 43.Warren, R. M., T. C. Victor, E. M. Streicher, M. Richardson, N. Beyers, N. C. van Pittius, and P. D. van Helden. 2004. Patients with active tuberculosis often have different strains in the same sputum specimen. Am. J. Respir. Crit. Care Med. 169610-614. [DOI] [PubMed] [Google Scholar]