Abstract
Although the bundle-forming pilus (BFP) of enteropathogenic Escherichia coli (EPEC) mediates microcolony formation on epithelial cells, the adherence of BFP-deficient mutants is significantly abrogated, but the mutants are still adherent due to the presence of intimin and possibly other adhesins. In this study we investigated the contribution of the recently described E. coli common pilus (ECP) to the overall adherence properties of EPEC. We found that ECP and BFP structures can be simultaneously observed in the course (between zero time and 7 h during infection) of formation of localized adherence on cultured epithelial cells. These two pilus types colocalized at different levels of the microcolony topology, tethering the adhering bacteria. No evidence of BFP disappearance was found after prolonged infection. When expressed from a plasmid present in nonadherent E. coli HB101, ECP rendered this organism highly adherent at levels comparable to those of HB101 expressing the BFP. Purified ECP bound in a dose-dependent manner to epithelial cells, and the binding was blocked with anti-ECP antibodies, confirming that the pili possess adhesin properties. An ECP mutant showed only a modest reduction in adherence to cultured cells due to background expression levels of BFP and intimin. However, isogenic mutants not expressing EspA or BFP were significantly less adherent when the ecpA gene was also deleted. Furthermore, a ΔespA ΔecpA double mutant (unable to translocate Tir and to establish intimate adhesion) was at least 10-fold less adherent than the ΔespA and ΔecpA single mutants, even in the presence of BFP. A Δbfp ΔespA ΔecpA triple mutant showed the least adherence compared to the wild type and all the isogenic mutant strains tested, suggesting that ECP plays a synergistic role in adherence. Our data indicate that ECP is an accessory factor that, in association with BFP and other adhesins, contributes to the multifactorial complex interaction of EPEC with host epithelial cells.
Colonization of host tissues by bacterial pathogens is a multifactorial event that often involves fimbrial and nonfimbrial adhesins, which may act at the same time or at different stages during the infectious process (33). Enteropathogenic Escherichia coli (EPEC), an important worldwide cause of pediatric diarrhea (32), adheres to small intestine enterocytes, forming tight three-dimensional microcolonies, an adherence pattern referred to as the localized adherence (LA) phenotype (38). A hallmark of EPEC pathogenicity is the production of attaching and effacing (AE) lesions, which are distinguished by intimate attachment and destruction of the intestinal brush border microvilli (31, 39).
Several EPEC adhesins leading to the colonization of cultured epithelial cells or the human gut have been well characterized (33). Intimin, the first EPEC outer membrane protein adhesin described (17), mediates intimate adhesion by binding to the cell membrane via its own translocated receptor (Tir) (19). The type IV bundle-forming pilus (BFP) is responsible for microcolony formation, promoting bacterium-bacterium interactions (10, 41), and was proven to be a virulence factor in volunteers (2). Some authors believe that the initial attachment to host cells is mediated by the BFP and the EspA filament; the latter protein is associated with the translocation into host cells of effectors via type 3 secretion (5, 24). New information regarding cellular receptors for BFP is emerging. Khursigara et al. showed previously that BFP mediates adherence to host cells via recognition of phosphoethanolamine on host cells (21). More recently, Hyland et al. (15) reported that alpha bundlins of EPEC strains possess lectin-like properties that could mediate the initial adherence of EPEC to N-acetyllactosamine-containing receptors on host cells (15). Knutton et al. (22) reported that after 6 h of infection, microcolonies were no longer present, although a complex three-dimensional network of thicker BFP bundles was seen, and they concluded that the BFP structures disappear, leading to microcolony dispersal. Tobe and Sasakawa (41) also reported that BFP, expressed by EPEC on epithelial cells, disappeared with the expansion of a microcolony 3 or 4 h postinfection. Flagella were shown to possess adhesive properties contributing to the adherence of motile strains to epithelial cells and to intestinal mucus in vitro (9, 11). Despite the importance of intimin and BFP in intimate adherence and microcolony formation, respectively, other unknown adhesins are likely to contribute to the efficient interaction of EPEC with epithelial cells during the course of an infection, at least in vitro. Analysis of the genome sequences of E. coli K-12 (3) and EPEC E2348/69 (www.sanger.ac.uk) suggests that these two organisms share several pilus-like operons; however, which of these operons are expressed or functional remains to be elucidated. Among these operons, a pilus gene cluster with 60% identity to the Salmonella enterica long polar fimbria cluster was identified in EPEC. However, the pilus was not demonstrated to be on the bacteria, and an EPEC lpfA mutant showed no defect in adherence (40).
Recently, we have shown that enterohemorrhagic E. coli O157:H7 strains are able to assemble an adhesive structure called the “E. coli common pilus” (ECP), which is composed of a major pilin subunit encoded by the ecpA gene (called yagZ in E. coli K-12 or matB in meningitis-associated E. coli) (34, 36). The ecpA gene appears to be widely distributed and highly conserved among commensal and pathogenic E. coli strains, and deletion of the ecpA gene in EHEC O157:H7 and in a fecal isolate of E. coli resulted in a reduction in adherence to cultured epithelial cells, suggesting that ECP are adherence factors of these E. coli (36). The aim of this study was to gain further understanding of the role of ECP produced by EPEC in the context of the complex multifactorial adherence mechanisms of this organism with host epithelial cells.
MATERIALS AND METHODS
Strains, plasmids, and antibodies.
Strains and plasmids employed in this study are listed in Table 1. Strains were propagated overnight in Luria-Bertani (LB) broth or in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 0.5% mannose at 37°C. When necessary, antibiotics were added at concentrations of 100 μg/ml (ampicillin) or 50 μg/ml (kanamycin). l-(+)-Arabinose (Sigma) was used at a final concentration of 100 mM to induce expression of the lambda Red system from plasmid pKD46. E. coli laboratory strain HB101 was used as a host of plasmids pCVD462 (29), pMAR7 (1), pMAT9 (34), and pSE380 (Invitrogen). Induction of ecp genes in recombinant E. coli strains was done with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Promega). For immunoassays, rabbit antibodies specific for BFP and EHEC O157:H7 ECP were obtained from previous studies (10, 11, 36). Chicken polyclonal antibodies were produced against purified EPEC ECP (Lampire Biological Laboratories).
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| EPEC strains | ||
| E2348/69 | O127:H6 (prototype wild type) | 28 |
| E2348/69ΔecpA | ecpA::km mutant | This study |
| E2348/69ΔecpA(pMR13) | ΔecpA complemented with pMR13 | This study |
| 31-6-1(1) | E2348/69 ΔbfpA | 7 |
| 31-6-1(1)ΔecpA | E2348/69 ΔbfpA ΔecpA::km | This study |
| UMD872 | E2348/69 ΔespA | 20 |
| UMD872ΔecpA | E2348/69 ΔespA ΔecpA::km | This study |
| JPN15.96 | EAF plasmid− intimin− | 17 |
| JPN15.96ΔecpA | JPN15.96 ΔecpA::km | This study |
| HB101 | E. coli B-K-12 hybrid | Lab collection |
| HB101(pCVD462) | HB101 carrying pCVD462 | This study |
| HB101(pMAR7) | HB101 carrying pMAR7 | This study |
| HB101(pMAT9) | HB101 carrying pMAT9 | This study |
| HB101(pSE380) | HB101 carrying pSE380 | This study |
| Plasmids | ||
| pKD46 | Red recombinase system plasmid | 6 |
| pKD4 | kan cassette template plasmid | 6 |
| pMR13 | ecpA cloned in pGEM-T | 36 |
| pCVD462 | LEE region of EPEC in pCVD551 | 29 |
| pMAR7 | EAF plasmid of EPEC E2348/69 | 1 |
| pMAT9 | ecpAB in pSE380 | 34 |
| pSE380 | Expression vector, trc promoter | Invitrogen |
Construction of nonpolar ecpA mutant.
To elucidate the role of ECP in EPEC adherence with respect to BFP- and intimin-Tir-mediated adherence, a nonpolar deletion of the ecpA gene of strains E2348/69, 31-6-1 (ΔbfpA), UMD872 (ΔespA), and JPN15.96 (Δbfp Δeae) was introduced by using the lambda Red recombinase method described previously (6), generating single, double, and triple isogenic mutants. Strain 31-6-1 (ΔbfpA) is unable to perform LA due to a lack of BFP production (7). UMD872 (ΔespA) is unable to translocate Tir due to a lack of the EspA fiber (20). JPN15.96 (Δbfp Δeae) lacks the EAF plasmid and hence does not produce BFP, and it carries a mutation in the eae gene (13). Primers G60 and G61 (Table 2) were employed to generate a PCR fragment containing ecpA sequences flanking a kanamycin cassette, using DNA of plasmid pKD4 as the template. The mutation was confirmed by PCR using primers flanking ecpA (G90 and G91), as well as primers inside the kanamycin cassette (K1 and K2). The loss of ECP was assessed by several immunoassays using antibodies against ECP. No growth defects were apparent in the mutants constructed.
TABLE 2.
Primers used in this study
| Primer | Sequence | Use |
|---|---|---|
| G60 | GTTCTGGCAATAGCTCTGGTAACGGTGTTTACCGGCGTGTAGGCTGGAGCTGCTTC | Mutagenesis |
| G61 | TTAACTGGTCCAGGTCGCGTCGAACTGTACGCTAACCATATGAATATCCTCCTTAG | Mutagenesis |
| G84 | CGCGGATCCATGAAAAAAAAGGTTCTGGC | Detection of ecpA |
| G85 | CGCGAATTCTAACTGGTCCAGGTCGCGTCG | Detection of ecpA |
| G90 | AACAGCAATATTAGGGGCGTG | Screening mutants |
| G91 | GGATAACAGCAGAGCGAGAAG | Screening mutants |
| G112 | ACTCAAATGAATTGACGGGGGC | Detection of 16S rRNA |
| G113 | AGGCCCGGGAACGTATTCAC | Detection of 16S rRNA |
| K1 | GCCCAGTCATAGCCGAATAGCCT | Screening mutants |
| K2 | CGGTGCCCTGAATGAACTGCAGG | Screening mutants |
| ecpA-F | ACTGAATGTGGGCGTGGA | Detection of ecpA |
| ecpA-R | GCCGCTGATGATGGAGAAAG | Detection of ecpA |
Ultrastructural studies.
Transmission electron microscopy was used to visualize pili on bacteria after negative staining with 1% phosphotungstic acid (pH 7.4) on 300-mesh carbon-Formvar copper grids. Immunoelectron microscopy studies were performed with rabbit anti-ECP antibody (diluted 1:10) in phosphate-buffered saline (PBS) containing 10% bovine serum albumin and goat anti-rabbit immunoglobulin G (IgG) conjugated to 10-nm gold particles diluted 1:10 (BB International) as previously described (11). For scanning electron microscopy (SEM) the preparations were first fixed with 0.1% glutaraldehyde (for immunostaining) or 3% glutaraldehyde (for standard SEM) as previously described (11). For immuno-SEM, anti-rabbit IgG conjugated to 30-nm gold particles was used. The specimens were examined with a high-resolution Hitachi S4500 scanning electron microscope (Hitachi, Japan).
Pilus purification and analysis.
E2348/69 growing in DMEM at 37°C with a 5% CO2 atmosphere was used to purify ECP. The pili were detached and purified by differential centrifugation, ammonium sulfate precipitation, and ultracentrifugation in a cesium chloride gradient as previously described (36). The final samples were analyzed for the presence of pili by electron microscopy and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (26). Before electrophoresis, the pili were denatured with HCl as previously described (30). Normalized bacterial whole-cell extracts were reacted with antibodies against ECP or DnaK (used as a loading control) (36). For mass spectrometry, a protein band at 21 kDa found in the pilus extract was excised from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and digested with trypsin at the Proteomics Core Facility at the College of Pharmacy, University of Arizona.
Interaction with eukaryotic cells.
Adherence assays were carried out between zero time and 7 h during infection employing human HeLa (cervix) and HT-29 (colonic) cell monolayers as previously described (11). Briefly, monolayers that were ∼80% confluent (∼5 × 105 cells) were infected with ∼107 bacteria grown overnight in LB medium or DMEM. After the desired incubation period, the monolayers were washed with PBS (pH 7.4). Adhering bacteria were quantitated by plating serial dilutions on LB agar plates with the appropriate antibiotics to determine the numbers of CFU. Replicate samples were fixed with 2% formaldehyde for Giemsa staining or for immunofluorescence microscopy (IFM) as previously described (11). Primary anti-ECP or anti-BFP antibodies were added to the fixed cells for 1 h in 10% horse serum in PBS, which was followed by addition of the appropriate secondary species-specific Alexa Fluor conjugates, and then the preparations were visualized using an Axio Imager1.0 Zeiss fluorescence or confocal microscope. For experiments examining the inhibition of adherence, 10 μl of a bacterial inoculum [E2348/69, E2348/69ΔecpA, or 31-6-1(1)] was preincubated for 30 min with 1:10, 1:50, and 1:100 dilutions of the anti-ECP or preimmune sera before addition to HeLa cells, and then the preparations were incubated for additional 2 h and adhering bacteria were quantitated as described above. The quantitative results shown below are the means of three experiments performed in triplicate on different days. The standard deviations are values for the averages for all the results obtained in the three experiments performed. A statistical analysis was done using the Student t test.
Binding of purified ECP to cell monolayers.
To determine if ECP has cell adherence properties, HeLa cells were incubated with different amounts (25, 50, and 100 μg/ml) of purified ECP for 1 h and then washed three times with PBS and fixed with 2% formalin. ECP binding was monitored by IFM using specific anti-ECP antibody. To confirm the results, a binding inhibition assay was performed, in which ECP was preincubated with a 1:20 dilution of anti-ECP antibodies for 30 min before addition to the HeLa cells. The samples were processed for IFM as described above.
Flow cytometry.
Flow cytometry was used to quantitatively evaluate the production of ECP on EPEC strains incubated for between 0 and 6 h with HT-29 cells. After gentle washing with PBS (pH 7.4), the adhering bacteria were detached with 0.2% Triton X-100 in PBS, and 60-μl aliquots were incubated with 25 μl of anti-ECP antibodies (1:500) for 1 h on ice. After three gentle washes with PBS, the bacteria were resuspended in 25 μl of a 1:500 dilution of goat anti-rabbit IgG(H+L)-Alexa Fluor conjugate (Invitrogen) and incubated for 1 h at 4°C, after which the bacteria were washed as described above and resuspended in 800 μl (final volume) of PBS. For analysis, the bacteria were labeled with 3 μl of a propidium iodide solution (Sigma Aldrich), and the emission was visualized through a 42-nm band-pass filter centered at 585 nm. These experiments were repeated in triplicate. The fluorescein isothiocyanate fluorescence emission was collected through a 30-nm band-pass filter centered at 530 nm, and 50,000 events were measured. The samples were read at the ARL Biotechnology/ACCC Cytometry Core Facility at the University of Arizona using a FACScan (Becton Dickinson, Franklin Lakes, NJ).
RESULTS
Identification of ECP in EPEC.
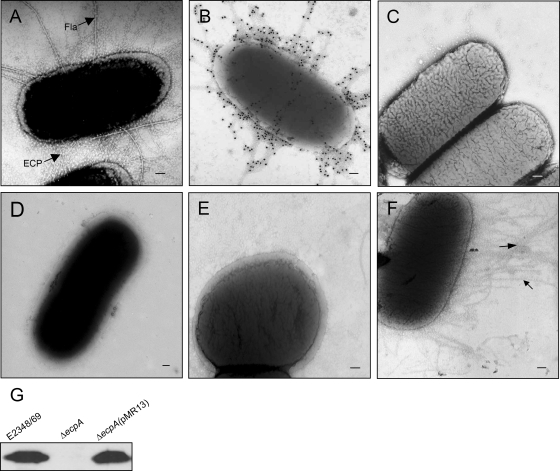
Expression of EPEC virulence factors (e.g., intimin, the BFP, the T3SS, and effectors) is induced when the bacteria are cultured in DMEM and are in contact with epithelial cells (7, 16, 35, 37). We set out to investigate the production of the recently described ECP under these growth conditions and to determine their role in adherence. Ultrastructural analysis of EPEC E2348/69 growing at 37°C in DMEM revealed the presence of peritrichous, thin (3-nm-wide), semiflexible pili (Fig. 1A), which were morphologically distinct from the rigid type I pili (diameter, 7 nm) (4) or the large BFP and flagellar structures (10, 11). These fibers bound anti-ECP antibodies as determined by immunogold labeling, indicating that they were ECP (Fig. 1B). As expected, no reactivity was seen with rabbit preimmune serum (Fig. 1C). No pili were observed on E2348/69 bacteria grown in LB broth at 37°C (Fig. 1D). Genetic proof of the identity of the pili was obtained by use of the E2348/69ΔecpA mutant, which showed neither production of pili as determined by transmission electron microscopy (Fig. 1E) nor synthesis of the pilin subunit as determined by immunoblotting of HCl-treated bacterial whole-cell extracts (Fig. 1G). Upon complementation of this mutant with pMR13, a plasmid that carries ecpA (pilus subunit gene), production of ECP was restored (Fig. 1F and G).
FIG. 1.
ECP on EPEC as shown by electron microscopy and immunogold labeling. (A) Electron micrograph of E2348/69 grown in DMEM displaying abundant peritrichous fine pili (ECP) and flagella (Fla). (B and C) Immunogold labeling of ECP on E2348/69 with anti-ECP antibodies and preimmune serum, respectively. (D) E2348/69 grown in LB medium showing no pili. (E) E2348/69ΔecpA mutant showing no ECP. (F) E2348/69ΔecpA(pMR13) with restored ECP production. Scale bars, 100 nm. (G) Detection of EcpA in normalized HCl-treated whole-cell extracts.
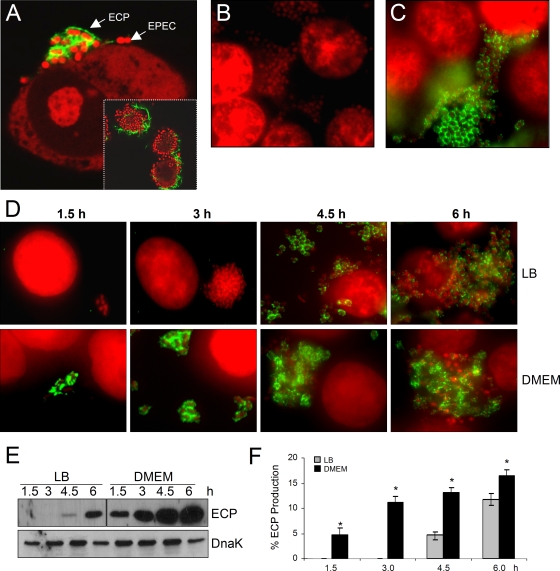
Next, the presence of ECP on the surface of bacteria adhering to human HT-29 epithelial cells was investigated by IFM and SEM (32). Upon incubation with anti-ECP antibodies, bacteria in microcolonies appeared to be tethered by peritrichous fluorescent fibers (Fig. 2A). The fluorescence pattern is different from that demonstrated for BFP (10, 12), which appear to be long thick structures, or for the EspA filaments, which are short thick spikes around the bacteria (24). No pilus-associated immunostaining was obtained with anti-ECP antibodies for the ecpA mutant adhering to cultured cells, confirming the specificity of the antiserum employed and the lack of pili in this mutant (Fig. 2B). As expected, E2348/69ΔecpA(pMR13) produced ECP when it was adhering to these cells (Fig. 2C).
FIG. 2.
ECP on EPEC strains adhering to cultured epithelial cells and kinetics of ECP production. (A) E2348/69 adhering to cultured epithelial cells after 6 h of incubation and reaction with anti-ECP antibodies and Alexa Fluor 488-conjugated secondary antibody (green). Cellular and bacterial DNA was stained with propidium iodide (red). The inset shows a lower magnification of an EPEC microcolony (red) with bacteria producing ECP (green). (B) E2348/69ΔecpA mutant. (C) E2348/69ΔecpA(pMR13). (D) Kinetics of ECP production by bacteria pregrown overnight in LB medium or DMEM. (E) Detection of EcpA in normalized HCl-treated whole-cell extracts. Detection of DnaK with anti-DnaK antibody was used as a loading control. (F) Level of ECP as determined by flow cytometry in bacteria adhering to epithelial cells. The data are representative data from two experiments performed in triplicate. The asterisk indicates that there was a statistically significant difference compared with LB medium-grown bacteria.
To monitor expression of ECP in space and for time of infection, we performed kinetics studies of EPEC adherence to HT-29 cells (between zero time and 7 h) using IFM, confocal microscopy, and immunoblotting. To study the effect of bacterial medium on growth, we infected cell monolayers with bacteria that were pregrown overnight in LB medium or in DMEM (ECP-inducing conditions). A striking difference in the time at which ECP was observed was evident depending on how the bacteria were precultured (Fig. 2D). When DMEM-grown bacteria were used as the inoculum, the presence of ECP was evident at early stages of infection (1.5 h), and ECP continued to be present for the duration of the experiment (Fig. 2D). In contrast, no ECP was evident during the first 3 h of infection (the typical LA assay) when LB medium-grown bacteria were used. However, after 4.5 h postinfection some of the bacteria in the LA cluster produced ECP, and pilus production continued for the duration of the experiment, while the bacteria multiplied and colonized almost the entire eukaryotic cell surface, as well as the glass surface (Fig. 2D). The time- and medium-dependent production of ECP was confirmed by immunoblotting of normalized whole-cell extracts of bacteria recovered from the adherence assays (Fig. 2E). Anti-DnaK antibody was used to ensure that equivalent concentrations of bacterial cell extracts were analyzed. Comparative analysis of ECP production by bacteria (LB medium or DMEM grown) adhering to host cells by flow cytometry produced results in agreement with the microscopy observations (Fig. 2F). Thus, it is clear that preculturing of the bacteria in DMEM allows detection of ECP at early stages of infection, suggesting that this pilus type could also be involved in the initial stage of adherence and microcolony formation, when the bacteria are programmed to express in synchronicity known virulence genes and ecpA.
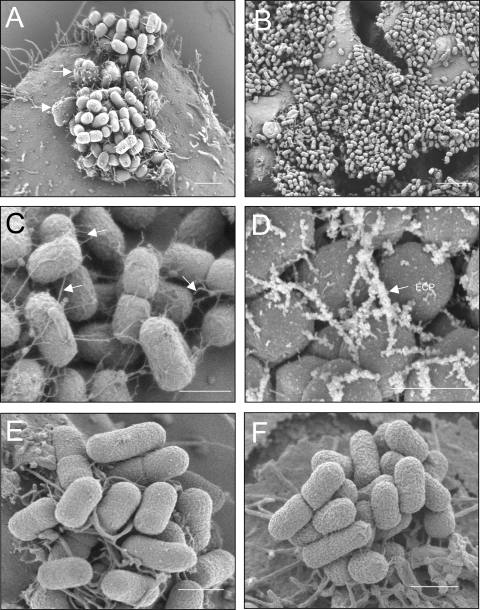
We then analyzed by using SEM the three-dimensional microcolonies formed by E2348/69 during the first 3 h of infection of cultured cells (Fig. 3A); this process, known as the LA, is proposed to be mediated by BFP, intimin, EspA fibers, and flagella (in the case of motile strains) (5, 10, 11, 17, 24). At this stage it is possible to observe the presence of pedestals, a manifestation of the AE lesion, with bacteria on top of them (Fig. 3A). At 6 h postinfection, the bacteria colonized almost the entire surface of the cell monolayer (Fig. 3B), and a high magnification at this infection time point showed the presence of a meshwork of abundant thin fibrillar structures protruding from the bacterial surface (Fig. 3C). Within this fibrillar meshwork, ECP were specifically identified by immuno-SEM using anti-ECP antibody and secondary antibody conjugated to 30-nm gold particles (Fig. 3D). No peritrichous pili were observed on the ecpA mutant analyzed in the same way, and no staining was observed with anti-ECP antibody (Fig. 3E and F).
FIG. 3.
High-resolution SEM analysis of EPEC E2348/69 adhering to HT-29 cells. (A) At 3 h postinfection EPEC forms a typical LA microcolony and AE lesions manifested by pedestals with bacteria on top (arrows). Scale bar, 2 μm. (B) At 6 h postinfection the microcolony has spread throughout the cell monolayer. Scale bar, 6 μm. (C) High magnification of panel B, showing abundant peritrichous thin fibers (arrows) that tether the bacteria together. Scale bar, 1 μm. (D) Immuno-SEM gold labeling of ECP protruding from bacteria with anti-ECP antibodies and secondary rabbit IgG conjugated to 30-nm gold particles. Scale bar, 1 μm (E) SEM analysis of E2348/69ΔecpA mutant. (F) Immuno-SEM gold labeling of E2348/69ΔecpA with anti-ECP antibodies. Scale bar, 1 μm.
ECP and BFP are simultaneously produced during formation of microcolonies on cultured cells.
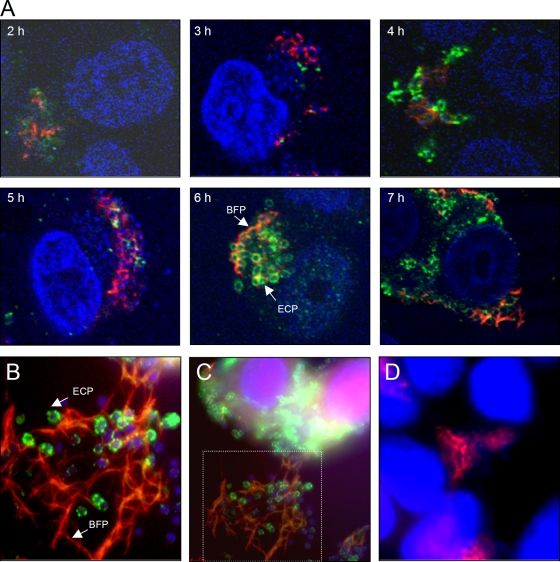
We were interested in investigating the topological location of ECP and BFP within the growing three-dimensional microcolony at different time points (between zero time and 7 h) during infection of HT-29 cells. For these experiments, bacteria precultured in DMEM were used. The fluorescence profiles obtained with specific antibodies against ECP and BFP are quite distinct. Due to the difference it was possible to clearly visualize the two pilus morphotypes simultaneously during formation of LA. The confocal microscopy images in Fig. 4 show that both ECP and BFP structures are produced during formation of LA. Of note, some bacteria in the cluster produced both ECP and BFP, while some of the bacteria produced only one type of pili or no pili. In general, ECP appeared to be tightly associated with the periphery of the individual bacteria, whereas the long BFP structures extended out and dispersed throughout the bacterial microcolony, generating a meshwork (Fig. 4). In addition, 1-μm sections were screened from bottom (epithelial cell basal surface) to top (tip of microcolony) by confocal microscopy (see Fig. S1 in the supplemental material). After the first hour of infection, ECP and BFP were present in each layer observed (Fig. 4; see Fig. S1 in the supplemental material). It is thought that the BFP mediates the initial bacterial attachment to host cells and that once the microcolony has formed at 3 h after infection, the bundles become shorter and disappear, leading to bacterial dispersal at 6 h after infection (25). In contrast to the previous studies, we found that under the experimental conditions tested here, long and thick BFP structures are still present in large EPEC microcolonies even more than 7 h after infection (Fig. 4B). The images shown in Fig. 4 support speculation that the BFP may serve as a substratum for bacterial binding. This scenario is consistent with the notion that BFP forms a filamentous lattice that embraces the three-dimensional microcolony where ECP-producing bacteria stick together. Together, our data indicate that both pilus types are produced (Fig. 4B and C) and function synergistically during LA. No peritrichous pili were observed on the ecpA mutant analyzed in the same way, and no staining was observed with anti-ECP antibody (Fig. 4D).
FIG. 4.
Simultaneous production of ECP and BFP during microcolony formation by E2348/69. (A) Kinetics (2 to 7 h) of ECP (green) and BFP (red) expression in the presence of HT-29 cells using confocal microscopy. Cellular and bacterial DNA was stained blue with DAPI. ECP are tightly associated with the bacterial surface, while the BFP extend out from the bacteria throughout the cluster. The confocal microscopy micrographs were taken at a magnification of ×60. (C) IFM detection of ECP and BFP after 6 h of infection. (B) Magnification of the framed area in panel C. Long and thick BFP structures are evident at this time point. (D) E2348/69ΔecpA mutant.
Role of ECP in EPEC adherence to cultured epithelial cells.
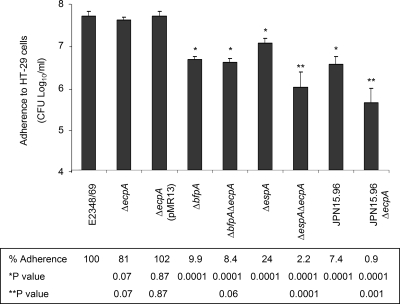
The presence of ECP interconnecting bacteria adhering to cultured epithelial cells is conceivably significant. To further judge the biological relevance of ECP in the interaction of EPEC with host cells, the adherence capabilities of ecpA single, double, and triple mutants were quantitatively compared to those of the parental strains. We observed only a modest quantitative difference (19% reduction) between the wild type and the ΔecpA single mutant (Fig. 5), and the most reasonable explanation for this result is that the adherence fitness of the ecpA mutant can be attributed to the production of wild-type levels of BFP, intimin-Tir, flagella, and likely other adhesins not yet characterized, which probably overshadow the role of ECP in adherence or, alternatively, play a redundant role in adherence. The ecpA gene was also mutated in UMD872 (E2348/69ΔespA) (Table 1), a strain that does not exhibit intimate attachment or produce AE lesions due to its inability to translocate Tir (19). The ΔespA ΔecpA double mutant adhered significantly less (P < 0.05) than the wild type and the ΔespA mutant (98% and 76% reductions, respectively) in spite of the presence of BFP in these strains (Fig. 5). It has been reported that EPEC ΔbfpA and intimin mutants are less adherent than the parental strains but otherwise are capable of interacting with host cells (7, 8, 17, 36). A ΔbfpA ΔecpA double mutant was not different than the ΔbfpA single mutant, but its adherence was significantly reduced compared to that of the wild-type strain or the ΔecpA mutant. This effect can probably be attributed to the lack of BFP in this double mutant. Cleary et al. (5) reported that a ΔbfpA Δeae ΔespA triple mutant of E2348/69 was not adherent to Caco-2 cells and concluded that there are no other adhesins in EPEC besides BFP, intimin, and EspA. We did not have the triple mutant used by these authors, and so in the present study we employed strain JPN15.96, a derivative of E2348/69, which lacks the EAF plasmid encoding BFP and contains a mutation in the eae intimin gene; thus, this strain does not form LA or AE lesions. In our hands, despite the lack of BFP- and intimin-Tir-mediated interactions, JPN15.96 continued to adhere, albeit poorly (92.6% reduction compared to the wild-type strain), to HT-29 cells after 6 h of incubation (Fig. 5), suggesting that the EspA fiber or other adherence factors in this strain still mediate adherence. Thus, we then introduced a mutation in ecpA into JPN15.96 to obtain a bfp Δeae ΔecpA triple mutant. We found that the adherence of the JPN15.96ΔecpA strain was significantly (P < 0.05) reduced (99.1%) compared to that of the E2348/69 control (100%) and that of JPN15.96 (Fig. 5). Together, these data indicate that in the absence of intimin-Tir-mediated intimate attachment, EPEC may use ECP to enhance BFP-mediated microcolony formation and adherence to host cells. Based on our data, we propose that ECP is an accessory adherence factor that in association with BFP and other adhesins contributes to the multifactorial complex interaction of EPEC with host epithelial cells.
FIG. 5.
Contribution of ECP to EPEC adherence compared with the contributions of other adhesins. The levels of adherence to HT-29 cells of wild-type and mutant strains were determined by plating serial dilutions of adhering bacteria. The adherence experiments were repeated at least three times in triplicate on separate days, and the bars indicate the means of the averages of the results obtained in the three experiments performed. The error bars indicate the standard deviations for the averages of all the results obtained in the three experiments performed. The levels of adherence and the P values are indicated below the graph. A statistical analysis was done using a paired Student t test. *, statistically significant compared with the wild-type strain; **, statistically significant compared with the wild-type strain and the parental strain.
Inhibition of adherence with anti-ECP antibodies.
In the first report on BFP, it was shown that EPEC adherence could be partially blocked using specific anti-BFP antibodies (10). Here we found that formation of EPEC LA by E2348/69 could be blocked only moderately with anti-ECP antibodies due to the overwhelming function of BFP (data not shown). However, the residual adherence in the EPEC ΔbfpA mutant was inhibited by these antibodies in a dose-dependent manner; in particular, a sixfold reduction was seen at a 1:10 dilution of the antibodies (see Fig. S2A to E in the supplemental material). Incubation with the preimmune serum did not have an inhibitory effect (see Fig. S2E in the supplemental material). In contrast, the production of LA by E2348/69ΔecpA was not inhibited with anti-ECP antibodies due to the presence of BFP in this strain (see Fig. S2F in the supplemental material). These results indicate that ECP assists in adherence when the BFP is absent.
ECP promotes adherence when it is expressed in nonadherent E. coli HB101.
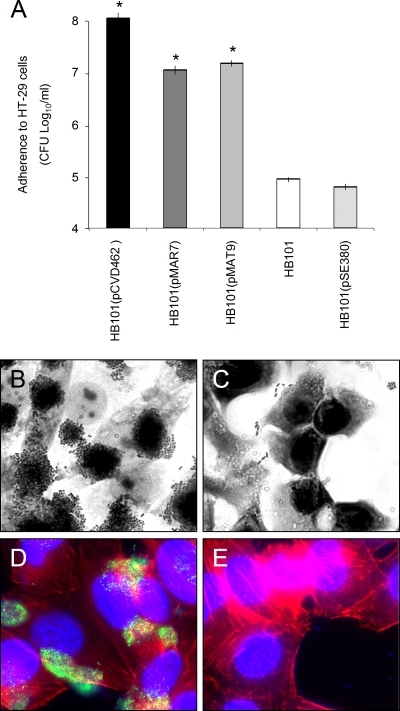
Next, we set out to investigate if ECP promotes adherence in a non-EPEC background. Thus, we employed laboratory strain E. coli HB101 because it is typically not adherent to most common human cell lines employed and because it does not express ECP under the experimental growth conditions used here. HB101 carrying plasmid pCVD462 (which contains the entire EPEC locus of enterocyte effacement [LEE]), pMAR7 (which is the E2348/69 EAF plasmid that carries the bfp operon), or pMAT9 (which contains matBC fimbrial genes, which are also called ecpAB) (Table 1) was analyzed using qualitative and quantitative adherence assays. HB101(pMAT9) adhered substantially to HT-29 epithelial cells, covering the entire cell monolayer, while the negative controls HB101 and HB101 transformed with expression vector pSE380 were poorly adherent (Fig. 6A to C). HB101 transformed with pMR13, which contains only the ecpA gene, was not able to produce ECP, suggesting that despite EcpA pilin subunit expression, other pilus biogenesis proteins, such as EcpB, which is predicted to be a pilin chaperone, are limiting factors. Most likely, the overproduction of ECP in HB101(pMAT9) was responsible for the hyperadherence phenotype observed. In fact, IFM experiments using anti-ECP antibody showed abundant reactive pili in HB101(pMAT9) (Fig. 6D), whereas no reactivity was found for the negative controls HB101 and HB101(pSE380) (Fig. 6E and data not shown). As shown by other workers, the presence of pCVD462 and pMAR7 also led to increased levels of adherence for HB101 (Fig. 6A) (1, 29). Overall, these results highlight the finding that overexpression of ECP leads to an increased-adherence phenotype.
FIG. 6.
Production of ECP confers a high level of adherence to nonadherent E. coli HB101. (A) Bacteria were pregrown in DMEM containing 0.1 mM IPTG to induce the expression of the ecpAB genes in HB101(pMAT9), and the adherence assay was performed for 6 h. *, P < 0.0001. (B and C) Light microscopy micrographs comparing the adherence of HB101(pMAT9) and of HB101, respectively, to HeLa cells (magnification, ×60). (D) Immunofluorescence showing high levels of ECP expression by HB101 carrying pMAT9. (E) Negative control strain HB101 showing no fluorescence (magnification, ×60). The adherence experiments were repeated at least three times in triplicate on separate days, and the bars indicate the means of the averages of the results obtained in the three experiments performed. The error bars indicate the standard deviations for the averages of all the results obtained in the three experiments performed.
Binding of ECP to cell monolayers.
To determine if ECP per se has any adhesive properties, purified pili were incubated with epithelial cell monolayers at different concentrations for 1 h, and after removal of unbound pili, the bound ECP were detected by IFM using anti-ECP antibodies. Dose-dependent binding of ECP to the mammalian cells that could be inhibited by anti-ECP antibodies but not by preimmune serum was observed (data not shown; see Fig. S3 in the supplemental material). These data suggest that ECP specifically recognizes a cellular receptor on the surface of epithelial cells and that the binding moieties are displayed along the pilus filaments, since no obvious minor pilin adhesin is predicted from the ecp operon sequence.
ECP is produced by a majority of EPEC strains.
For a collection of 30 EPEC strains (BFP+ and LEE+) of different serotypes that possess the ecpA gene, as determined by PCR (Table 3), it was found that 19 (63%) of them produced ECP after growth in DMEM and cultured epithelial cells at 37°C (Table 3). At this point, we cannot rule out the possibility that the remaining 11 flow cytometry-negative strains are able to produce ECP under other growth conditions.
TABLE 3.
Production of ECP by EPEC strains
| Serotype | No. of strains examined | No. ofa:
|
|
|---|---|---|---|
| ecpA+ strains | ECP+ strains | ||
| O55:H6 | 2 | 2 | 2 |
| O86:H2 | 2 | 2 | 1 |
| O86:H34 | 2 | 2 | 0 |
| O86:NM | 1 | 1 | 1 |
| O114:NM | 1 | 1 | 1 |
| O111:H2 | 2 | 2 | 2 |
| O111:NM | 7 | 7 | 5 |
| O119:H6 | 5 | 5 | 2 |
| O127:H6 | 3 | 3 | 2 |
| O127:H40 | 5 | 5 | 3 |
| Total | 30 | 30 (100)b | 19 (63) |
ecpA+, ecpA positive as determined by PCR; ECP+, ECP positive as determined by flow cytometry and IFM.
The numbers in parentheses are percentages.
DISCUSSION
The interaction of EPEC with host epithelial cells is a multifactorial and complex phenomenon that involves several adhesins, including intimin, BFP, EspA fiber, and flagella (in the case of motile strains). How these different adhesins are synchronized and differentially regulated during the interaction of EPEC with host cells remains to be defined. All of these adhesins are produced by bacteria adhering to host cells and seem to act in concert at different stages of infection (10, 11, 22, 24, 27, 41). Here we present compelling evidence arguing in favor of the presence and function of yet another adhesin, the newly described ECP, in the general scheme of EPEC's interaction with host cells.
First, EPEC is able to produce ECP when it is growing in DMEM at 37°C and in the presence of cultured epithelial cells, conditions that also induce the production of BFP, intimin, and LEE-encoded determinants (18, 35, 37). The fact that ECP is produced at 37°C by a large proportion of EPEC strains of different serotypes is conceivably biologically significant as it would be reasonable to expect that this pilus would be expressed in the human intestinal environment. Second, through the use of a repertoire of isogenic strains with mutations in one or several adhesin genes, we found that ECP participates in conjunction with other adhesins to promote cell adherence. For instance, a ΔespA ΔecpA double mutant showed a 10-fold reduction in adherence compared with the ΔespA single mutant (Fig. 5), even when both strains still produced BFP but were unable to translocate Tir into host cells. These results suggest that in the absence of intimin-Tir-mediated adherence, ECP may contribute, along with BFP, to cell binding. Consistent with this hypothesis, strain JPN15.96, which is unable to produce BFP and to mediate intimate attachment due to a mutation in the eae gene, still adheres to cultured cells after 6 h of infection, showing 7.5% adherence compared with wild-type strain E2348/69. Most likely, the residual adherence in this strain is due to yet another adhesin. In this regard, Cleary et al. (5) reported previously that in addition to BFP and intimin, EspA was also required for adherence to host cells, since a ΔbfpA ΔespA Δeae triple mutant was completely nonadherent compared with a ΔbfpA Δeae double mutant. We also constructed a ΔecpA mutant of JPN15.96, resulting in a bfp Δeae ΔecpA triple mutant, whose adherence turned out to be significantly reduced compared with that of wild-type strain JPN15.96 or any of the single mutants tested. This result argues in favor of a role for ECP in adherence. Cleary et al. reported that strain UMD880, which produces BFP but has mutations in espA and eae, shows the same level of adherence as E2348/69, whereas the Δeae (CVD206) and ΔespA (UMD872) single mutants showed only 72 and 59% adherence, respectively. It is intriguing that all three mutants behaved differently since they all produce BFP and are unable to mediate intimate attachment. In our study, we found that the ΔespA mutant was significantly less adherent (76% reduction) than the parent strain. Major differences between the experimental procedures used by Cleary's group and the experimental procedures used by our group may explain the discordant results. For example, Cleary et al. used LB medium-grown bacteria for their assays, while we used DMEM-grown bacteria, and as shown here, this results in a difference in expression of ECP at an early stage of infection; Cleary et al. used Caco-2 cells, whereas we used HT-29 cells; they incubated the bacteria for up to 6 h with the medium changed after 3 h, while we incubated the bacteria with HT-29 cells for 6 h with no interruption; they performed a semiquantitative assessment of adhesion by visual counting of bacteria by light microscopy, whereas we performed a quantitative determination by plating serial dilutions of adhering bacteria; and finally, the two groups employed genetically different triple mutants, although none of the triple mutants employed produced BFP or mediated intimate attachment. The fact that the adhesion of the ΔecpA single mutant was reduced by only 20% compared with that of the wild type is attributed to the presence of redundant multiple adhesins. A key conclusion from these results is that BFP-mediated adherence cannot be overshadowed by adherence due to any of the redundant EPEC adhesins.
Third, antibodies against ECP were able to block significantly the adherence of the bfpA mutant, an indication that ECP mediates adherence to some extent. Fourth, purified ECP was shown to bind specifically to cultured mammalian cells and antibodies against ECP could block this binding, suggesting that ECP per se has adhesive properties. No apparent minor pilin tip adhesin is predicted based on the nucleotide sequence of the ecp operon or has been observed in biochemical analyses of the purified pili. Thus, it is quite possible that the binding moieties that recognize specific host cell receptors reside in the major pilin itself, which is displayed along the pilus filaments. Fifth, our data showing that HB101 carrying ecpAB on plasmid pMAT9 produced ECP and exhibited high levels of adherence in the absence of BFP or intimin are consistent with our proposal that ECP are important for cell adherence. In fact, the levels of adherence observed for HB101(pMAT9) were comparable to those observed for HB101 expressing BFP from pMAR7 (Fig. 6).
How the different adhesins of EPEC are synchronized in space and time and how an LA microcolony is formed have been the subjects of several studies. While some authors suggested that the BFP mediate the initial adherence to host cells (5, 27, 41, 42), other authors support the notion that the BFP mediate interbacterial linkages that promote bacterial aggregation, leading subsequently to LA formation (10, 14). Our data indicate that ECP is produced when these events occur, as both ECP and BFP were found to colocalize within EPEC microcolonies. ECP appeared to emanate from the bacterial surface, whereas the BFP seemed to extend and be dispersed throughout the microcolony and to even serve as a substratum for bacterial binding. In line with the phenotypic and environmental regulation data, we found that the time at which the bacteria begin to produce ECP depends on whether the inoculum originates from LB medium or DMEM. When DMEM-grown bacteria were employed, ECP could be seen as early as 1.5 h postinfection, whereas LB medium-grown bacteria produced ECP after 4.5 h of infection (Fig. 2). Previous studies reported that the BFP was downregulated, was shortened, and even disappeared with the expansion of the microcolony 3 or 4 h postinfection and that after 6 h of infection the microcolonies were no longer present, although a complex three-dimensional network of thicker BFP bundles was seen (41). In contrast to these studies, we provide evidence that even after 7 h of incubation, large three-dimensional microcolonies and areas of dispersed bacteria with long thick BFP structures were present. BfpF has been proposed to be involved in bacterial dispersal from the microcolony as a result of retraction of BFP. Dynamic changes in the structure of BFP could explain the presence of short and long BFP structures seen by other authors (25, 41). However, we did not find indications that the BFP were disintegrating. This inconsistency could be explained by differences in the cell lines or the experimental conditions (e.g., time of incubation) employed by the different research groups. In agreement with our data, Leverton and Kaper (27) reported that expression of intimin and BFP occurs continuously between 3 and 5 h during infection (27), indicating that the BFP is not repressed as previously suggested (41).
Our data provokingly suggest that production of ECP may be necessary for stabilizing the adhering bacteria on the host cell membrane, favoring tissue colonization. In agreement with this, we found that purified ECP binds to host cells, and a recent report showed that EHEC and normal flora E. coli ΔecpA mutants are hampered in the ability to adhere to cultured epithelial cells compared to their parental strains (36). In summary, this study firmly established that EPEC produces ECP under conditions that are biologically relevant and highlighted a potential role of ECP as an accessory adherence factor in EPEC, which is engaged in promoting cell adherence and/or in bacterium-bacterium interactions, contributing to the multifactorial complex interaction of EPEC with host epithelial cells.
Supplementary Material
Acknowledgments
We thank Fabiola Avelino, Alejandra Vázquez, and María Rendón for technical assistance, Michael Donnenberg for providing strain UMD872, and Timo Korhonen for his kind donation of pMAT9.
This work was supported by NIH grant AI66012 to J.A.G., by NIH grants DK58957 and AI21657 to J.B.K., and by DGAPA grant IN224107 and CONACyT grant 60796 to J.L.P.
Footnotes
Published ahead of print on 13 February 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baldini, M. M., J. B. Kaper, M. M. Levine, D. C. Candy, and H. W. Moon. 1983. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J. Pediatr. Gastroenterol. Nutr. 2534-538. [DOI] [PubMed] [Google Scholar]
- 2.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 2802114-2118. [DOI] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Brinton, C. C., Jr. 1959. Non-flagellar appendages of bacteria. Nature 183782-786. [DOI] [PubMed] [Google Scholar]
- 5.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150527-538. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 63427-3437. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 594310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdem, A. L., F. Avelino, J. Xicohtencatl-Cortes, and J. A. Giron. 2007. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J. Bacteriol. 1897426-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254710-713. [DOI] [PubMed] [Google Scholar]
- 11.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44361-379. [DOI] [PubMed] [Google Scholar]
- 12.Gismero-Ordonez, J., M. Dall'Agnol, L. R. Trabulsi, and J. A. Girón. 2002. Expression of the bundle-forming pilus by enteropathogenic Escherichia coli strains of heterologous serotypes. J. Clin. Microbiol. 402291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 631767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 661570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyland, R. M., J. Sun, T. P. Griener, G. L. Mulvey, J. S. Klassen, M. S. Donnenberg, and G. D. Armstrong. 2008. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell. Microbiol. 10177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 927996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 877839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 652606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91511-520. [DOI] [PubMed] [Google Scholar]
- 20.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20313-323. [DOI] [PubMed] [Google Scholar]
- 21.Khursigara, C., M. Abul-Milh, B. Lau, J. A. Giron, C. A. Lingwood, and D. E. Barnett Foster. 2001. Enteropathogenic Escherichia coli virulence factor bundle-forming pilus has a binding specificity for phosphatidylethanolamine. Infect. Immun. 696573-6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutton, S., J. Adu-Bobie, C. Bain, A. D. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 651644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 172166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutton, S., R. K. Shaw, R. P. Anantha, M. S. Donnenberg, and A. A. Zorgani. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33499-509. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 27.Leverton, L. Q., and J. B. Kaper. 2005. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect. Immun. 731034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i1119-1122. [DOI] [PubMed] [Google Scholar]
- 29.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23399-407. [DOI] [PubMed] [Google Scholar]
- 30.McMichael, J. C., and J. T. Ou. 1979. Structure of common pili from Escherichia coli. J. Bacteriol. 138969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 411340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nougayrede, J. P., P. J. Fernandes, and M. S. Donnenberg. 2003. Adhesion of enteropathogenic Escherichia coli to host cells. Cell. Microbiol. 5359-372. [DOI] [PubMed] [Google Scholar]
- 34.Pouttu, R., B. Westerlund-Wikstrom, H. Lang, K. Alsti, R. Virkola, U. Saarela, A. Siitonen, N. Kalkkinen, and T. K. Korhonen. 2001. matB, a common fimbrillin gene of Escherichia coli, expressed in a genetically conserved, virulent clonal group. J. Bacteriol. 1834727-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puente, J. L., D. Bieber, S. W. Ramer, W. Murray, and G. K. Schoolnik. 1996. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol. Microbiol. 2087-100. [DOI] [PubMed] [Google Scholar]
- 36.Rendon, M. A., Z. Saldana, A. L. Erdem, V. Monteiro-Neto, A. Vazquez, J. B. Kaper, J. L. Puente, and J. A. Giron. 2007. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. USA 10410637-10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenshine, I., S. Ruschkowski, and B. B. Finlay. 1996. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect. Immun. 64966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaletsky, I. C., M. L. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staley, T. E., E. W. Jones, and L. D. Corley. 1969. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am. J. Pathol. 56371-392. [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsuno, I., R. Mundy, G. Frankel, Y. Chong, A. D. Phillips, A. G. Torres, and J. B. Kaper. 2006. The lpf gene cluster for long polar fimbriae is not involved in adherence of enteropathogenic Escherichia coli or virulence of Citrobacter rodentium. Infect. Immun. 74265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobe, T., and C. Sasakawa. 2001. Role of bundle-forming pilus of enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell. Microbiol. 3579-585. [DOI] [PubMed] [Google Scholar]
- 42.Tobe, T., and C. Sasakawa. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 429-42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.