Abstract
Sialidase activity is a putative virulence factor of the anaerobic periodontal pathogen Tannerella forsythia, but it is uncertain which genes encode this activity. Characterization of a putative sialidase, SiaHI, by others, indicated that this protein alone may not be responsible for all of the sialidase activity. We describe a second sialidase in T. forsythia (TF0035), an orthologue of Bacteroides fragilis NanH, and its expression in Escherichia coli. Sialidase activity of the expressed NanH was confirmed by using 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid as a substrate. Biochemical characterization of the recombinant T. forsythia NanH indicated that it was active over a broad pH range, with optimum activity at pH 5.5. This enzyme has high affinity for 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (Km of 32.9 ± 10.3 μM) and rapidly releases 4-methylumbelliferone (Vmax of 170.8 ± 11.8 nmol of 4-methylumbelliferone min−1 mg of protein−1). E. coli lysates containing recombinant T. forsythia NanH cleave sialic acid from a range of substrates, with a preference for α2-3 glycosidic linkages. The genes adjacent to nanH encode proteins apparently involved in the metabolism of sialic acid, indicating that the NanH sialidase is likely to be involved in nutrient acquisition.
The fastidious gram-negative, anaerobic, bacterium Tannerella forsythia is one of the main etiological agents of periodontitis—a multifactorial, destructive gum disease that causes tooth loss (44). Although a complete genome sequence is available for this bacterium (http://www.stdgen.lanl.gov/), relatively little is known about the biology of this species. It has been frequently isolated with Porphyromonas gingivalis from diseased sites in patients with chronic periodontitis, but its precise mode of action in periodontal disease has not been established (11, 13, 43). Nevertheless, a number of putative virulence factors have been identified, including a slime (S) layer (22, 34); an α-d-glucosidase and a N-acetyl-β-glucosaminidase (18); proteases (15, 35); and a cell surface-associated protein, BspA (39).
Another putative virulence factor of T. forsythia is its sialidase activity. Sialidases (neuraminidases, EC 3.2.1.18) are glycohydrolases, which release the terminal sialic acid residues from sialoglycoconjugates. Sialic acids are 9-carbon α-keto acids, which are common sugars at the terminal residue of glycoproteins and glycolipids. Such sialic acid-containing glycoconjugates are widely distributed on eukaryotic cells and secreted glycoproteins (3, 40). The sialic acid residues contribute to a range of important biological functions, including cellular interactions and stabilizing the conformation of glycoproteins and cellular membranes; these residues also expose or mask receptors for ligands, antibodies, or enzymes and contribute to the function and stability of glycoproteins in serum (3, 40). Sialidases are implicated in the pathogenicity of some bacteria, including Clostridium perfringens (33), Streptococcus pneumoniae (46), Pasteurella multocida (42), and Pseudomonas aeruginosa (41). They can modify the host's ability to respond to bacterial infection by increasing the susceptibility of immunoglobulin molecules to proteolytic degradation (28). Sialidases can also facilitate colonization by exposing cryptic receptors for bacterial adhesion (9). They may also provide bacteria with a nutritional carbon source (9, 38).
Several studies have demonstrated sialidase activity in T. forsythia isolates, and this is used as a diagnostic tool for identification of the species (5, 26). Although a T. forsythia sialidase gene, siaHI, has been reported (19), it is unclear whether this alone is responsible for sialidase activity in T. forsythia. A sialidase unrelated to T. forsythia SiaHI has been described in the related bacterium, Bacteroides fragilis (49), and it may be important for nutrient acquisition, supporting the growth of this bacterium in vivo (14). We report that an orthologue of B. fragilis NanH is the principal sialidase in T. forsythia. We describe the biochemical properties of this sialidase and discuss its association with genes encoding proteins that may affect sialic acid uptake and metabolism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in the present study are described in Table 1. T. forsythia ATCC 43037 was routinely grown on fastidious anaerobe agar (LabM, United Kingdom) containing 0.001% N-acetylmuramic acid and 5% defibrinated horse blood (TCS Biosciences, United Kingdom) at 37°C, under anaerobic conditions (80% N2, 10% H2, and 10% CO2). Escherichia coli strains were grown on Luria-Bertani (LB) agar or LB broth (Melford, United Kingdom) under aerobic conditions at 37°C. For transformant selection and plasmid maintenance in E. coli, media were supplemented with 30 or 50 μg of kanamycin/ml. For Topo cloning LB agar was also supplemented with 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml and 100 μg of IPTG (isopropyl-β-d-thiogalactopyranoside)/ml to select appropriate transformants.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| ATCC 43037 | T. forsythia | 45 |
| DH5α | E. coli K-12 cloning host | Invitrogen |
| α-Select | E. coli K-12 cloning host | Bioline |
| BL21(DE3) | E. coli B expression host | Novagen |
| KCL116 | BL21(DE3)/pET30 | This study |
| KCL117 | BL21(DE3)/pET30::nanH | This study |
| KCL120 | BL21(DE3)/pET30::siaHI | This study |
| Plasmids | ||
| pCR4-Topo | PCR cloning vector; Kmr Ampr | Invitrogen |
| pET30a, -b, and -c | pET30 expression vectors a, b, and c containing an inducible T7 promoter; Kmr | Novagen |
| pKCL175 | pCR4-Topo containing nanH from T. forsythia, amplified using primers P265 and P266 | This study |
| pKCL191 | pET30c containing nanH from pKCL175 cloned into the BamHI site | This study |
| pKCL202 | pET30b containing siaHI from T. forsythia, amplified using the primers P291 and P292 | This study |
Ampr, ampicillin resistance; Kmr, kanamycin resistance.
Genetic modification of bacterial strains.
The genetic manipulation of bacterial strains was carried out as described previously (36). Genomic DNA was isolated from T. forsythia cells by using Genelute (Sigma Aldrich, United Kingdom) according to the manufacturer's instructions.
PCR primers used to amplify nanH and siaHI (TF0035 and TF2207, respectively; see Fig. 1 for a representation of the binding sites)—P265 (GGATCCAAGGAGATATACATATGAAAAAGTTTTTTTGGAT), P266 (GGATCCAAAAGAAAAGACAAACGA), P291 (GGCTGATATCGGATCCAAGGAGATATACATATGACAAAAAAAAGCAGTAT), and P292 (GCTCGAATTCGGATCCGATACTCATGACTTTTTCTCTAA)—were designed by using the Vector NTI Suite (v10; Invitrogen, United Kingdom) and synthesized by MWG Biotech (Germany). Included on the 5′ end of selected primers were BamHI restriction enzyme recognition sites (underlined) and primers P265 and P291 also included a ribosome-binding site (boldfaced). To enable ligation-independent cloning, primers P291 and P292 were designed to include 15 nucleotides with identity at the 5′ end to the 15 nucleotides flanking the desired insertion point in pET30. The primers were used to amplify nanH and siaHI from T. forsythia genomic DNA using Bio-X-Act DNA polymerase (Bioline, United Kingdom). Amplifications were carried out using the following cycle parameters: 1 cycle at 95°C for 2 min; 30 cycles of 95°C for 0.5 min, 57°C for 0.5 min, and 72°C for 2.5 min; and a final extension cycle of 72°C for 10 min.
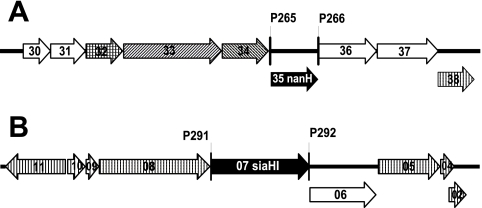
FIG. 1.
T. forsythia genetic loci containing genes encoding putative sialidases. The genes encoding the putative sialidases in T. forsythia are represented by the black arrows. Surrounding these are genes encoding putative outer membrane proteins (diagonal hatching, panel A only), a transport protein (cross-hatching, panel A only), enzymes (no hatching), or hypothetical proteins of unknown function (vertical hatching). The numbers correspond to gene numbers TF00xx (panel A, TF0030 to TF0038) or TF22xx (panel B, TF2211 to TF2202). The binding sites of the primers used to amplify nanH (A) and siaHI (B) are indicated by vertical lines.
Cloning was carried out as described in Table 1. Briefly, amplified nanH was initially cloned into pCR4-Topo (Invitrogen), creating pKCL175, and subsequently transferred into pET30c as a BamHI fragment to create pKCL191. Amplified siaHI DNA was cloned directly into the pET30b vector by using an In-Fusion Dry-Down PCR cloning kit (Clontech/Takara Bio, France) according to the manufacturer's instructions, to create pKCL202. The authenticity and orientation of the inserts was confirmed by DNA sequencing with T7 promoter and T7 terminator primers (TAATACGACTCACTATAGGG and CTAGTTATTGCTCAGCGGT, respectively).
Expression of putative sialidases from T. forsythia in E. coli.
Expression of the cloned proteins was induced in the exponential phase of growth (optical density at 600 nm of 0.5 to 0.8) by the addition of 1 mM IPTG and further incubation for 2 or 18 h at 37°C. Bacterial lysates were prepared by using a BugBuster (Novagen, United Kingdom) according to the manufacturer's protocol. The total protein concentration of the cell lysates was determined by using a bicinchoninic acid assay (Sigma). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5% stacking gel, 10% resolving gel) (21) and visualized by Coomassie brilliant blue staining.
Biochemical assays of sialidase activity.
The fluorogenic substrate, 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4-MU-NeuNAc; Sigma) was used to assay sialidase activity. Qualitative screening of sialidase activity in intact bacteria or lysates was done by using a rapid filter paper assay (5). Samples were applied to filter paper presoaked with 4-MU-NeuNAc (279 μM) in 0.1 M sodium acetate buffer (pH 4.6) and examined under long-wavelength UV light (365 nm) after 15 min at 37°C. Sialidase activity was indicated by blue-white fluorescence.
Quantitative sialidase assays involved measuring 4-methylumbelliferone (4-MU) fluorescence, released from 4-MU-NeuNAc by sialidase activity, in a Fluoroskan Ascent FL fluorimeter (Labsystems Thermo, United Kingdom) with an excitation wavelength of 380 nm and an emission wavelength of 460 nm (6, 7). To determine the optimal pH for sialidase activity, assays were carried out with 50 μM 4-MU-NeuNAc in 70 mM sodium citrate buffer (pH 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, or 6.0), 70 mM sodium phosphate buffer (pH 6.0, 6.5, 7.0, 7.5, or 8.0), 70 mM potassium phosphate buffer (pH 6.5, 7.0, or 7.5), or 70 mM Tris-HCl (pH 7.5, 8.0, 8.5, or 8.9) and appropriately diluted cell lysates, such that the rate of release of 4-MU from 4-MU-NeuNAc was linear with respect to time. Assays were stopped after 30 min of incubation at 37°C by the addition of an equal volume of 0.5 M sodium carbonate buffer (pH 10.5) (6). The kinetic characteristics of the sialidases were determined with assays carried out with 70 mM sodium citrate buffer (pH 5.5) and 0.05 to 2 mM 4-MU-NeuNAc (6).
The substrate specificity of the sialidases was determined using sialyl-α2,3-lactose, sialyl-α2,6-lactose, porcine gastric mucin, fetuin, bovine submaxillary mucin, and 4-MU-NeuNAc (Sigma) and colominic acid (containing α2,8 linked N-acetylneuraminic acid; MP Biomedicals, United Kingdom) in 50 mM sodium citrate buffer (pH 5.5). Sialic acid release was determined by using high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), essentially as described previously (6, 7). Briefly, HPAEC-PAD was performed by using a Dionex DX-500 chromatography system (Dionex, United Kingdom) and a Carbopac PA1 column (250 by 4 mm). Sialic acids were eluted from the column by using a linear gradient of sodium acetate (20 to 150 mM over 40 min) in 100 mM sodium hydroxide at a flow rate of 1 ml/min. N-Acetylneuraminic acid and N-glycolylneuraminic acid concentrations in assays were determined by comparison of peak areas with those of the authentic sialic acids (Sigma) as standards over a concentration range of 0 to 50 μM.
RESULTS
T. forsythia contains an orthologue of B. fragilis nanH.
Although a T. forsythia sialidase, SiaHI (TF2207; see Fig. 1B), has been described (19), it is not apparent whether this alone is sufficient to account for all of the sialidase activity in this bacterium. Sialidase activity not directly attributable to SiaHI was observed upon nondenaturing gel electrophoresis of T. forsythia proteins (19). Also, SiaHI is an unusual sialidase since it does not contain motifs associated with other characterized sialidases (31). For example, the SiaHI sequence does not include any Asp box motifs (29). It also has no significant similarity to sialidases from related species, such as Bacteroides fragilis (1). Therefore, further analysis of the available T. forsythia genome sequence was initiated.
The B. fragilis NanH sequence (accession number BAA05853) was used as a template to identify a putative orthologue in the T. forsythia genome (available in the OralGen database [http://www.stdgen.lanl.gov/]) by BLAST (2). This putative orthologue, encoded by TF0035, is 65% identical to B. fragilis NanH over the full length of the 540-amino-acid sequence. It also contains motifs typically associated with sialidases (31), including four perfect Asp boxes (SxDxGxTW; amino acids 236 to 243, 315 to 322, 375 to 382, and 482 to 489), a further imperfect Asp box (amino acids 422 to 429, TRDLGKSW), and the conserved FRIP region (amino acids 198 to 201, FRIP), which is typically located at the N terminus of sialidases (31). Therefore, we propose that gene TF0035 is renamed nanH. The original annotation of TF0035 included 13 amino acids (MPFFGLSCKNTCR) at the N terminus that had no significant similarity to B. fragilis NanH. An alternative start codon follows the sequence encoding these amino acids, in the same reading frame, and we propose that this is the true N terminus of the TF0035 preprotein. The N terminus of this “truncated” TF0035 protein contains a 20-amino-acid signal sequence (MKKFFWIIGLFASMQMTRAA), indicating that this putative NanH sialidase is most likely secreted and/or surface localized. There are approximately 170 amino acids between the signal sequence and the sialidase catalytic domain containing the Asp boxes and the FRIP region. This additional domain shares no similarity with known proteins; in particular, it is distinct from the lectin domain present in other large sialidases (48).
The genetic context of the T. forsythia nanH gene is illuminating (Fig. 1A). It is located in an apparent operon, which includes genes encoding an N-acetylneuraminate lyase (TF0030; NanA), an N-acetylglucosamine epimerase (TF0031; NanE), a transport protein of the major facilitator superfamily (TF0032; MFS), and two proteins with similarity to RagAB-like TonB-dependent receptors (TF0033 and TF0034). This operon also encodes a β-hexosaminidase (TF0036) and a putative sialate 9-O-acetylesterase (TF0037).
In contrast, the previously described T. forsythia sialidase, SiaHI, is encoded within a putative operon (Fig. 1B) that does not contain genes encoding proteins explicitly involved in glycoprotein degradation or sialic acid metabolism. Genome annotation (http://www.stdgen.lanl.gov/) and BLAST searches indicate that six genes in this putative siaHI operon encode hypothetical proteins of unknown function and one, TF2206, encodes a putative enzyme with some similarity to sugar phosphate isomerase/epimerases. Surprisingly, although SiaHI does not contain any motifs associated with typical sialidases, it does have a NAD-binding Rossman fold and C-terminal alpha/beta domains of the oxidoreductase family (pfam01408 and pfam02894 [24]) and is therefore more characteristic of a dehydrogenase (COG0673). This is not apparently consistent with SiaHI being a sialidase.
Sialidase activity of T. forsythia NanH and SiaHI.
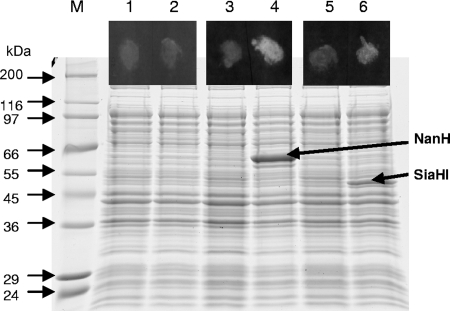
Since the genetic manipulation of T. forsythia is still in development (44), we selected a heterologous expression strategy to confirm that the T. forsythia nanH gene encodes a sialidase. TF0035 (nanH) and, for comparison, the previously described siaHI (TF2207) were cloned into pET30 and expressed in E. coli BL21(DE3). The protein profiles of the IPTG-induced KCL117 (NanH) and KCL120 (SiaHI) cultures contained an additional protein not apparent in the control cultures (Fig. 2). These proteins, approximately 60 and 50 kDa, respectively, for NanH and SiaHI, are consistent with the sizes predicted from the amino acid sequence: the predicted molecular mass of NanH is 59.7 kDa (57.4 kDa without the signal sequence) and that of SiaHI is 51.0 kDa (48.7 kDa without the signal sequence). The fluorescence observed in a qualitative assay of whole cells expressing recombinant sialidase, using the fluorogenic sialidase substrate 4-MU-NeuNAc, indicated that, when expressed in E. coli, both T. forsythia NanH and SiaHI have sialidase activity (Fig. 2).
FIG. 2.
Sialidase activity of T. forsythia NanH and SiaHI expressed in E. coli. Strains KCL116 (E. coli pET30; lanes 1 and 2), KCL117 [E. coli BL21(DE3)/pET30::nanH; lanes 3 and 4], and KCL120 [E. coli BL21(DE3)/pET30::siaHI; lanes 5 and 6] were grown in LB until mid-exponential phase and for a further 2 h with (lanes 2, 4, and 6) or without (lanes 1, 3, and 5) induction with 1 mM IPTG. Cells were assayed for sialidase activity in a filter paper spot assay using the fluorogenic substrate 4-MU-NeuNAc (spots in the black boxes above the corresponding lanes). Cell lysates were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, and proteins were visualized by Coomassie blue staining.
Biochemical characteristics of T. forsythia NanH.
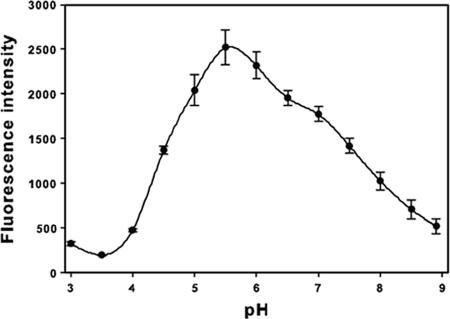
Quantitative 4-MU-NeuNAc sialidase assays of E. coli whole-cell lysates containing T. forsythia NanH (strain KCL117) indicated that this sialidase is active over a wide pH range (between pH 4.5 and 8.0 at least 40% of the maximum activity was observed), with an optimum pH of 5.5 (Fig. 3). Further assays carried out at pH 5.5 confirmed that NanH is a sialidase with a high affinity for 4-MU-NeuNAc (Km = 32.9 ± 10.3 μM) and a rapid reaction rate (Vmax = 170.8 ± 11.8 nmol of 4-methylumbelliferone min−1 mg of protein−1). In stark contrast, we were unable to detect any sialidase activity (<0.2 nmol of 4-methylumbelliferone min−1 mg of protein−1) in lysates containing SiaHI using such quantitative assays over the full pH range tested.
FIG. 3.
pH optimum of T. forsythia NanH. Strain KCL117 [E. coli BL21(DE3)/pET30::nanH] was grown in LB until mid-exponential phase and for a further 18 h after induction with 1 mM IPTG. Cell lysates were prepared as described in Materials and Methods and assayed using 4-MU-NeuNAc in sodium citrate buffer (pH 3.0 to 6.0), sodium phosphate buffer (pH 6.0 to 8.0), potassium phosphate buffer (pH 6.5 to 7.5), and Tris-HCl (pH 7.5 to 8.9). The release of 4-MU was determined as fluorescence intensity (excitation and emission wavelengths of 380 and 460 nm, respectively). The data shown are the means from at least three independent experiments. Error bars represent ± the standard error of the mean.
The specificity of sialidases is influenced by the nature of the glycosidic linkage to the next sugar. In sialylated oligosaccharides, sialic acid can be linked by its C-2-OH to the C-3, C-4, or C-6 (α2,3, α2,4, or α2,6) of the next sugar, which is typically galactose, N-acetylgalactosamine, or N-acetylglucosamine. If the linked sugar is another sialic acid, an α2,8-linkage is possible (40). Another factor that influences sialidase specificity is O acetylation of C-7 to C-9 of the sialic acid (9). Therefore, to determine the specificity of the T. forsythia NanH sialidase, we treated a range of commercially available substrates with KCL117 lysate and determined sialic acid release (Table 2). T. forsythia NanH favors substrates with α2,3 (sialyl-α2,3-lactose) linkages, and α2,6 (sialyl-α2,6-lactose) are preferred to α2,8 linkages (colominic acid). We observed no sialidase activity with more complex substrates (fetuin, bovine submaxillary mucin, and porcine gastric mucin), perhaps reflecting the extent of O acetylation of the sialic acids in these molecules. For example, 80% of the sialic acids in bovine submaxillary mucin are O acetylated (47). Consistent with the lack of release of 4-MU from 4-MU-NeuNAc in quantitative assays (see above), we were unable to detect any release of sialic acids when any of the other commercial substrates were treated with lysates containing SiaHI.
TABLE 2.
Substrate specificity of T. forsythia NanH
| Substrate | Total sialic acid concn (μM) | Sialyl linkage(s) | Relative rate of cleavage (%)a |
|---|---|---|---|
| 4-MU-NeuNAc | 10 | 100 | |
| Sialyl-α2,3-lactose | 20 | α2,3 | 35.6 |
| Sialyl-α2,6-lactose | 20 | α2,6 | 17.4 |
| Colominic acid (poly α2,8 Neu5Ac) | 100 | α2,8 | 3.3 |
| Bovine submaxillary mucin (NeuNAc) | 100 | α2,6 | 0 |
| Bovine submaxillary mucin (NeuNGc) | 100 | α2,6 | 0 |
| Pig gastric mucin | 100 | α2,3 | 0 |
| Fetuin | 100 | α2,3 and α2,6 | 0 |
The cleavage rates shown are relative to that obtained with 4-MU-NeuNAc as the substrate (121.7 nmol min−1 mg of protein−1).
DISCUSSION
The previously described SiaHI protein of T. forsythia (19) does not account for all of the sialidase activity of this putative periodontal pathogen. This bacterium has an orthologue of B. fragilis NanH, TF0035 (renamed NanH), which has sialidase activity when expressed in E. coli. Unlike T. forsythia SiaHI, T. forsythia NanH contains all of the attributes classically associated with sialidases. The NanH enzyme contains four or five Asp box motifs, which typically occur four to five times in bacterial sialidases (29, 30). The conserved FRIP motif, which may be involved in substrate binding (32), is also present in T. forsythia NanH. Although characterized sialidases from other bacterial species differ in size and structure, they each have Asp box and FRIP motifs. To our knowledge, the T. forsythia SiaHI enzyme is the only reported bacterial sialidase that does not. BLAST searches indicate that, in addition to B. fragilis, putative orthologues of T. forsythia NanH with >39% identity over the full length of the protein are present in other sequenced Bacteroides and Parabacteroides species, Capnocytophaga canimorsus (23), and Akkermansia muciniphilla (10; data not shown). Therefore, this subclass of sialidases is thus far only apparent in a small group of bacteria. These NanH orthologues each contain the novel 170-amino-acid region between the signal sequence and the sialidase region. However, the function of this additional domain in this group of sialidases is not apparent.
Most characterized bacterial sialidases are secreted or are cell bound (9). The signal sequence present in T. forsythia NanH indicates that this protein is also likely to be secreted, hydrolyzing the terminal sialic acid residues on glycoconjugates outside the cell. The habitat of T. forsythia, the periodontal pocket, contains an abundance of sialylated glycoproteins found in saliva and gingival crevicular fluid, such as fibronectin, transferrin, and immunoglobulins (27). A number of periodontal bacteria are known to exploit host sialylated glycoproteins as a nutrient source. For example, immunoglobulin G, which is abundant in gingival crevicular fluid, can support the growth of P. gingivalis, Prevotella oralis, and a number of other periodontal pathogens (20). The NanH of B. fragilis is important for growth in vivo: sialidase-deficient mutants of B. fragilis have impaired growth in a rat pouch model (14). Although it is likely that a primary role of the T. forsythia NanH is nutritional, bacterial sialidases have additional effects. Sialidase treatment of immunoglobulins makes them more susceptible to proteolytic degradation (28); sialidases may also reveal cryptic receptors and/or adhesion sites for bacteria (9). It is therefore likely that the NanH sialidase could contribute to the pathogenicity of T. forsythia in a number of different ways.
T. forsythia NanH has a preference for α2-3-linked sialyl oligosaccharides, like many sialidases from bacteria, including Salmonella enterica serovar Typhimurium NanH (17), Streptococcus oralis sialidase (6), and P. multocida NanH (25). Consistent with the properties reported for other bacterial sialidases (9), the T. forsythia NanH is active over a broad pH range, with optimum activity observed at pH 5.5. This activity at differing pHs may ensure that NanH remains active in vivo, withstanding fluctuations in pH. For example, NanH is active at pH 7.0, the average pH in periodontal pockets (12), with ca. 70% maximum sialidase activity. Our results also indicate that NanH should be active even at the higher pH detected by others in crevicular fluid at inflamed sites (up to pH 8.66) (4). Comparison of the kinetic properties of T. forsythia NanH to other bacterial sialidases is difficult, since a range of substrates and different assay conditions have been used in other studies. Nevertheless, the low Km indicates that the T. forsythia NanH has a high affinity for 4-MU-NeuNAc.
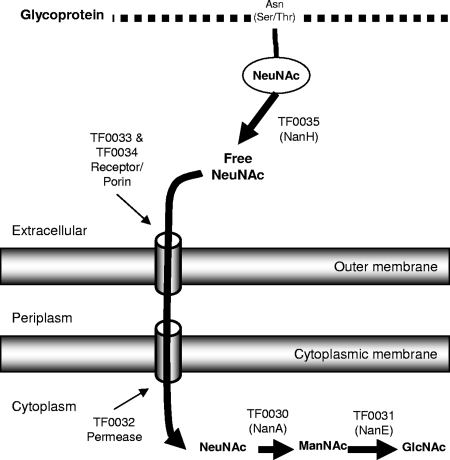
Although we observed no sialic acid release from mucin molecules, it is known that the sialic acids of such glycoproteins may be heavily O acetylated (16), which adversely affects bacterial sialidase activity (8). One of the enzymes encoded by the putative nanH operon is a sialate 9-O-acetylesterase (TF0037, EC 3.1.1.53), which may remove acetyl groups from sialic acids (8). Therefore, it is apparent that in vivo T. forsythia NanH and TF0037 may act in unison to facilitate removal of sialic acid from glycoconjugates in saliva or gingival crevice fluid. Another enzyme in the putative nanH operon (TF0036) is a putative N-acetyl-β-hexosaminidase (NahA, EC 3.2.1.52), which is distinct from a previously characterized T. forsythia N-acetyl-β-glucosaminidase (18), sharing only 18% amino acid identity. Such enzymes can be involved in the release of terminal N-acetylglucosamine or N-acetylgalactosamine from glycoproteins (37). Therefore, the proteins encoded by the nanH operon are not only likely to be involved in sialic acid metabolism but rather are dedicated to glycoprotein degradation in general. The remaining genes in the nanH operon encode proteins likely to be involved in the uptake and metabolism of sialic acid (Fig. 1 and 4).
FIG. 4.
Schematic representation of the proposed pathway for sialic acid metabolism in T. forsythia highlighting the initiating role of NanH. The removal of sialic acid from the terminus of glycoproteins by NanH allows it to pass through the outer membrane; we propose that this occurs with the help of the proteins encoded by TF0033 and TF0034. The free sialic acid is most likely further transported into the bacterium by the MFS permease encoded by TF0032. TF0030 and TF0031 encode the initial enzymes for sialic acid catabolism, NanA and NanE.
In contrast, the operon encoding the reported SiaHI sialidase does not obviously include genes encoding proteins which are required for sialic acid catabolism. Furthermore, although our qualitative screen for sialidase activity apparently confirmed the report of Ishikura et al. (19) since we observed fluorescence when E. coli expressing recombinant SiaHI was tested with 4-MU-NeuNAc, we were unable to detect any sialidase activity by quantitative assays of any sample containing SiaHI. Since SiaHI has none of the characteristics of typical sialidases but rather has similarity to NAD-dependent dehydrogenases, it is possible that the primary role of this enzyme is other than its supposed function as a sialidase.
The sialidase activity long associated with T. forsythia can now be attributed, at least in part, to the orthologue of B. fragilis NanH we describe here. Although the biological role of the alternative sialidase SiaHI remains unclear, it is probable that T. forsythia NanH is a sialidase involved in nutrient acquisition.
Acknowledgments
This study was supported by the Restorative Dentistry Research Group at King's College London Dental Institute and the Department of Health by a National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London.
We thank William G. Wade and Julie Downes (King's College London) for initial guidance in handling T. forsythia.
Footnotes
Published ahead of print on 20 March 2009.
REFERENCES
- 1.Akimoto, S., T. Ono, H. Tsutsui, T. Kinouchi, K. Kataoka, and Y. Ohnishi. 1994. Complete sequence of the Bacteroides fragilis Ych46 neuraminidase encoding gene. Biochem. Biophys. Res. Commun. 203914-921. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angata, T., and A. Varki. 2002. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102439-469. [DOI] [PubMed] [Google Scholar]
- 4.Bickel, M., and G. Cimasoni. 1985. The pH of human crevicular fluid measured by a new microanalytical technique. J. Periodont. Res. 2035-40. [DOI] [PubMed] [Google Scholar]
- 5.Braham, P. H., and B. J. Moncla. 1992. Rapid presumptive identification and further characterization of Bacteroides forsythus. J. Clin. Microbiol. 30649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers, H. L., E. Tarelli, K. A. Homer, and D. Beighton. 2000. Isolation and characterisation of sialidase from a strain of Streptococcus oralis. J. Med. Microbiol. 49235-244. [DOI] [PubMed] [Google Scholar]
- 7.Byers, H. L., E. Tarelli, K. A. Homer, and D. Beighton. 1999. Sequential deglycosylation and utilization of the N-linked, complex-type glycans of human α1-acid glycoprotein mediates growth of Streptococcus oralis. Glycobiology 9469-479. [DOI] [PubMed] [Google Scholar]
- 8.Corfield, A. P., S. A. Wagner, J. R. Clamp, M. S. Kriaris, and L. C. Hoskins. 1992. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 603971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corfield, T. 1992. Bacterial sialidases: roles in pathogenicity and nutrition. Glycobiology 2509-521. [DOI] [PubMed] [Google Scholar]
- 10.Derrien, M., E. E. Vaughan, C. M. Plugge, and W. M. de Vos. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 541469-1476. [DOI] [PubMed] [Google Scholar]
- 11.Dzink, J. L., A. C. R. Tanner, A. D. Haffajee, and S. S. Socransky. 1985. Gram-negative species associated with active destructive periodontal lesions. J. Clin. Periodontol. 12648-659. [DOI] [PubMed] [Google Scholar]
- 12.Forscher, B. K., A. G. Paulsen, and W. C. Hess. 1954. The pH of the periodontal pocket and the glycogen content of the adjacent tissue. J. Dent. Res. 33444-453. [DOI] [PubMed] [Google Scholar]
- 13.Gersdorf, H., A. Meissner, K. Pelz, G. Krekeler, and U. B. Gobel. 1993. Identification of Bacteroides forsythus in subgingival plaque from patients with advanced periodontitis. J. Clin. Microbiol. 31941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godoy, V., M. Dallas, T. Russo, and M. Malamy. 1993. A role for Bacteroides fragilis neuraminidase in bacterial growth in two model systems. Infect. Immun. 614415-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenier, D. 1995. Characterization of the trypsin-like activity of Bacteroides forsythus. Microbiology 141921-926. [Google Scholar]
- 16.Haverkamp, J., R. Schauer, M. Wember, and J. P. Farriaux. 1976. Neuraminic acid derivatives newly discovered in humans: N-acetyl-9-O-l-lactoylneuraminic acid, N,9-O-diacetylneuraminic acid and N-acetyl-2,3-dehydro-2-deoxyneuraminic acid. Hoppe-Seyler Z. Physiol. Chemie 3571699-1705. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer, L. L., P. Roggentin, R. Schauer, and E. R. Vimr. 1991. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus typical kinetic preference for sialyl α-2-3 linkages. J. Biochem. 110462-467. [DOI] [PubMed] [Google Scholar]
- 18.Hughes, C. V., G. Malki, C. Y. Loo, A. C. R. Tanner, and N. Ganeshkumar. 2003. Cloning and expression of α-d-glucosidase and N-acetyl-β-glucosaminidase from the periodontal pathogen, Tannerella forsythensis (Bacteroides forsythus). Oral Microbiol. Immunol. 18309-312. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura, H., S. Arakawa, T. Nakajima, N. Tsuchida, and I. Ishikawa. 2003. Cloning of the Tannerella forsythensis (Bacteroides forsythus) siaHI gene and purification of the sialidase enzyme. J. Med. Microbiol. 521101-1107. [DOI] [PubMed] [Google Scholar]
- 20.Jansen, H. J., J. S. van der Hoeven, C. W. A. Kieboom, J. H. C. Goertz, and P. J. M. Camp. 1994. Degradation of immunoglobulin-G by periodontal bacteria. Oral Microbiol. Immunol. 9345-351. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. W., M. Sabet, H. S. Um, J. Yang, H. C. Kim, and W. D. Zhu. 2006. Identification and characterization of the genes encoding a unique surface (S-) layer of Tannerella forsythia. Gene 371102-111. [DOI] [PubMed] [Google Scholar]
- 23.Mally, M., H. Shin, C. Paroz, R. Landmann, and G. R. Cornelis. 2008. Capnocytophaga canimorsus: a human pathogen feeding at the surface of epithelial cells and phagocytes. PLoS Pathogens 4e1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchler-Bauer, A., J. B. Anderson, M. K. Derbyshire, C. DeWeese-Scott, N. R. Gonzales, M. Gwadz, L. Hao, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, D. Krylov, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, S. Lu, G. H. Marchler, M. Mullokandov, J. S. Song, N. Thanki, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2007. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35D237-D240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizan, S., A. Henk, A. Stallings, M. Maier, and M. D. Lee. 2000. Cloning and characterization of sialidases with 2-6′ and 2-3′ sialyl lactose specificity from Pasteurella multocida. J. Bacteriol. 1826874-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moncla, B. J., P. Braham, and S. L. Hillier. 1990. Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J. Clin. Microbiol. 28422-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollanen, M. T., J. I. Salonen, and V. J. Uitto. 2003. Structure and function of the tooth-epithelial interface in health and disease. Periodontol. 2000 3112-31. [DOI] [PubMed] [Google Scholar]
- 28.Reinholdt, J., M. Tomana, S. B. Mortensen, and M. Kilian. 1990. Molecular aspects of immunogobulin-A1 degradation by oral streptococci. Infect. Immun. 581186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roggentin, P., B. Rothe, J. B. Kaper, J. Galen, L. Lawrisuk, E. R. Vimr, and R. Schauer. 1989. Conserved sequences in bacterial and viral sialidases. Glycoconj. J. 6349-353. [DOI] [PubMed] [Google Scholar]
- 30.Roggentin, P., and R. Schauer. 1993. Evolutionary studies on bacterial sialidases: common origin and irregular distribution. Glycoconj. J. 10234-235. [Google Scholar]
- 31.Roggentin, P., R. Schauer, L. L. Hoyer, and E. R. Vimr. 1993. The sialidase superfamily and its spread by horizontal gene transfer. Mol. Microbiol. 9915-921. [DOI] [PubMed] [Google Scholar]
- 32.Roggentin, T., R. G. Kleineidam, R. Schauer, and P. Roggentin. 1992. Effects of site-specific mutations on the enzymatic properties of a sialidase from Clostridium perfringens. Glycoconj. J. 9235-240. [DOI] [PubMed] [Google Scholar]
- 33.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52333-360. [DOI] [PubMed] [Google Scholar]
- 34.Sabet, M., S. W. Lee, R. K. Nauman, T. Sims, and H. S. Um. 2003. The surface (S-) layer is a virulence factor of Bacteroides forsythus. Microbiology 1493617-3627. [DOI] [PubMed] [Google Scholar]
- 35.Saito, T., K. Ishihara, T. Kato, and K. Okuda. 1997. Cloning, expression, and sequencing of a protease gene from Bacteroides forsythus ATCC 43037 in Escherichia coli. Infect. Immun. 654888-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Press, New York, NY.
- 37.Scigelova, M., and D. H. G. Crout. 1999. Microbial β-N-acetylhexosaminidases and their biotechnological applications. Enzyme Microb. Technol. 253-14. [Google Scholar]
- 38.Severi, E., D. W. Hood, and G. H. Thomas. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 1532817-2822. [DOI] [PubMed] [Google Scholar]
- 39.Sharma, A., H. T. Sojar, I. Glurich, K. Honma, H. K. Kuramitsu, and R. J. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 665703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sillanaukee, P., M. Ponnio, and I. P. Jaaskelainen. 1999. Occurrence of sialic acids in healthy humans and different disorders. Eur. J. Clin. Investig. 29413-425. [DOI] [PubMed] [Google Scholar]
- 41.Soong, G., A. Muir, M. I. Gomez, J. Waks, B. Reddy, P. Planet, P. K. Singh, Y. Kanetko, M. C. Wolfgang, Y. S. Hsiao, L. Tong, and A. Prince. 2006. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Investig. 1162297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steenbergen, S. M., C. A. Lichtensteiger, R. Caughlan, J. Garfinkle, T. E. Fuller, and E. R. Vimr. 2005. Sialic acid metabolism and systemic pasteurellosis. Infect. Immun. 731284-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanner, A. C. R., C. Haffer, G. T. Bratthall, R. A. Visconti, and S. S. Socransky. 1979. Study of the bacteria associated with advancing periodontitis in man. J. Clin. Periodontol. 6278-307. [DOI] [PubMed] [Google Scholar]
- 44.Tanner, A. C. R., and J. Izard. 2006. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontol. 2000 4288-113. [DOI] [PubMed] [Google Scholar]
- 45.Tanner, A. C. R., M. A. Listgarten, J. L. Ebersole, and M. N. Strezempko. 1986. Bacteroides forsythus sp. nov., a slow-growing, fusiform Bacteroides sp. from the human oral cavity. Int. J. Syst. Bacteriol. 36213-221. [Google Scholar]
- 46.Tong, H. H., D. Li, S. Chen, J. P. Long, and T. F. DeMaria. 2005. Immunization with recombinant Streptococcus pneumoniae neuraminidase NanA protects chinchillas against nasopharyngeal colonization. Infect. Immun. 737775-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varki, A., and S. Diaz. 1983. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J. Biol. Chem. 2582465-2471. [PubMed] [Google Scholar]
- 48.Vimr, E. R. 1994. Microbial sialidases: does bigger always mean better? Trends Microbiol. 2271-277. [DOI] [PubMed] [Google Scholar]
- 49.Vonnicolai, H., R. Hammann, H. Werner, and F. Zilliken. 1983. Isolation and characterization of sialidase from Bacteroides fragilis. FEMS Microbiol. Lett. 17217-220. [Google Scholar]