Abstract
Balancing metal uptake is essential for maintaining a proper intracellular metal concentration. Here, we report the transcriptional control exerted by the two metal-responsive regulators of Helicobacter pylori, Fur (iron-dependent ferric uptake regulator) and NikR (nickel-responsive regulator), on the three copies of the fecA genes present in this species. By monitoring the patterns of transcription throughout growth and in response to nickel, iron, and a metal chelator, we found that the expression of the three fecA genes is temporally regulated, responds to metals in different ways, and is selectively controlled by either one of the two regulators. fecA1 is expressed at a constant level throughout growth, and its expression is iron sensitive; the expression of fecA2 is mainly off, with minor expression coming up in late exponential phase. In contrast, the expression of fecA3 is maximal in early exponential phase, gradually decreases with time, and is repressed by nickel. The direct roles of Fur and NikR were studied both in vitro, by mapping the binding sites of each regulator on the promoter regions via DNase I footprinting analysis, and in vivo, by using primer extension analyses of the fecA transcripts in fur and nikR deletion strains. Overall, the results show that the expression of each fecA gene is finely tuned in response to metal availability, as well as during the bacterial growth phase, suggesting specific and dedicated functions for the three distinct FecA homologues.
Metals are important for many biological functions as cofactors of essential metalloproteins and enzymes. At the same time, metal overload may be lethal. Therefore, several mechanisms control the intracellular metal concentration so that uptake, availability, and storage are tuned to the physiological needs and possible toxic effects are limited (16, 17). In the human pathogen Helicobacter pylori, the causative agent of several gastric pathologies (2), metal homeostasis is maintained principally through the transcriptional regulation of genes coding for metal uptake and storage proteins (5, 6, 13, 25). The ability to acquire iron as well as nickel plays a central role in the successful colonization of the gastric niche and has been shown to be a prerequisite for infection in a mouse model (27, 31). To date, two pleiotropic regulators are known to be involved in the concerted control of iron- and nickel-responsive gene expression: a member of the ferric uptake regulator family (Fur) (9) and a homologue of the NikR protein (5, 33). Several open reading frames, annotated as putative metal transport systems, appear to be under the control of either one or both regulators, as revealed by analyses of transcript abundance in wild-type and mutant strains cultivated in medium enriched with or depleted of metal (12, 14, 15, 30). However, few such metal transport systems have been studied in some detail.
Herein, we report the study of the transcriptional regulation of three fecA genes of H. pylori encoding putative outer membrane proteins that in Escherichia coli are involved in ferric dicitrate transport (19). In gram-negative bacteria, the FecA system appears to be regulated at multiple levels: by metal-dependent repressors such as Fur or by iron starvation sigma factors (fecR-fecI system) whose expression is in turn regulated by a feedback loop involving the N-terminal domain of TonB (22). The genome of H. pylori (1, 3, 29) includes three genes annotated as fecA homologues. Two, namely, fecA1 and fecA2, have been shown previously via chromatin immunoprecipitation-on-chip enrichment (6) and/or transcriptional studies (12, 30) to be part of the Fur regulon. The regulation of fecA3 is less straightforward. Although it appears to belong to the Fur regulon, its transcription appears to be affected by fur mutation in the advanced growth phase only, when intracellular Fur protein concentrations increase in the wild-type strain (6). Others have reported it to be iron regulated but in a Fur-independent fashion (12). The fecA3 gene was also proposed to be indirectly repressed by NikR (5). In contrast, Ernst and coworkers (15) showed that the transcription of the fecA3 gene is repressed by NikR only in the presence of nickel and that in vitro a recombinant NikR protein appears to bind a specific region of the fecA3 promoter (PfecA3) in a nickel-dependent manner, substantiating the hypothesis of its Ni2+-dependent regulation (15).
To gain a better understanding of the regulation of the fecA genes in H. pylori, we studied their transcriptional regulation during growth and after enrichment with metal or chelation and analyzed the binding of Fur and NikR to their promoter regions. We demonstrated that the three fecA genes are differentially transcribed during the growth phase, that Fur directly controls the iron-dependent transcriptional responses of fecA1 and fecA2, and that NikR brings about the metal-dependent regulation of fecA3 by binding cooperatively to two adjacent operator sites.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The H. pylori strains used in this study are listed in Table 1. All strains were recovered from −80°C glycerol stocks and grown on Columbia agar plates containing 5% horse blood (Oxoid), 0.2% cyclodextrin, and Dent's or Skirrow's antibiotic supplement or on Brucella agar plates with 5% fetal calf serum (Oxoid) and Dent's or Skirrow's antibiotic supplement at 37°C under microaerophilic conditions (9% CO2, 91% air atmosphere, and 95% humidity) in a water-jacketed thermal incubator. Liquid cultures were grown in modified Brucella broth supplemented with 5% fetal calf serum (Oxoid) and Dent's or Skirrow's antibiotic supplement at 37°C with constant agitation (125 rpm). To measure the metal-dependent transcriptional response, master cultures (40 ml) of the wild-type and mutant strains were grown to mid-log phase (optical density at 600 nm [OD600], 0.5 to 0.6), divided into four equal-volume subcultures, and treated for 15 min with freshly made 1 mM FeSO4, 1 mM NiSO4, or 100 μM 2,2-dipyridyl prior to RNA extraction. Similarly, to monitor the expression of the fecA genes over time, aliquots of 10 ml from a 100-ml culture were obtained at different time points, harvested by centrifugation in the presence of phenol-ethanol stop solution, and frozen for RNA extraction. E. coli strains DH5α and BL21(DE3) were grown in Luria-Bertani broth. Ampicillin (100 μg/ml), kanamycin (25 μg/ml), and chloramphenicol (30 μg/ml) were added when required.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1β | 18 |
| BL21(DE3) | hsdS gal (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1) | 28 |
| H. pylori strains | ||
| G27 | Clinical isolate; wild type | 32 |
| G27 fur (Δfur::Km) | G27 derivative with fur gene mutation; Kmr | 8 |
| G27 nikR (ΔnikR::Km) | G27 derivative with nikR gene mutation; Kmr | 23 |
| G27 fur nikR (Δfur::Km ΔnikR::cat) | G27 derivative with fur and nikR gene mutations; Kmr Cpr | 23 |
| G27 vac::PfecA3-lacZ | G27 derivative containing the wild-type PfecA3 promoter-lacZ fusion in the vacA locus; Kmr | This study |
| G27 vac::OP-I mutant PfecA3-lacZ | G27 derivative containing the OP-I mutant PfecA3 promoter-lacZ fusion in the vacA locus; Kmr | This study |
| G27 vac::OP-II mutant PfecA3-lacZ | G27 derivative containing the OP-II mutant PfecA3 promoter-lacZ fusion in the vacA locus; Kmr | This study |
| Plasmids | ||
| pGEM-T Easy | General cloning vector; Ampr | Promega |
| pGEMT-PfecA1 | pGEM-T Easy derivative containing 402 bp of fecA1 promoter region, amplified by PCR with primers A1F and A1R | This study |
| pGEMT-PfecA2 | pGEM-T Easy derivative containing 380 bp of fecA2 promoter region, amplified by PCR with primers A2F and A2R | This study |
| pGEMT-PfecA3 | pGEM-T Easy derivative containing 265 bp of fecA3 promoter region, amplified by PCR with primers A3F new and A3R new | This study |
| pBlueScript (pBS) | General cloning vector; Ampr | Stratagene |
| pBS-PfecA3Rx1 | pBlueScript derivative containing a 130-bp fragment of fecA3 promoter region, amplified by PCR with primers A3.1-A3.4 | This study |
| pBS-PfecA3Rx2 | pBlueScript derivative containing a 60-bp fragment of fecA3 promoter region, amplified by PCR with primers A3.1-A3.2 | This study |
| pBS-PfecA3Rx3 | pBlueScript derivative containing a 60-bp fragment of fecA3 promoter region, amplified by PCR with primers A3.3-A3.4 | This study |
| pBS-PA3MuOpI | pBlueScript derivative containing the 265-bp fecA3 promoter region with a SmaI site introduced into OP-I by PCR with the primers A3Fnew-A3.9 and A3Rnew-A3.8 | This study |
| pBS-PA3MuOpII | pBlueScript derivative containing the 265-bp fecA3 promoter region with a SmaI site introduced into OP-II by PCR with the primers A3Fnew-A3.7 and A3Rnew-A3.6 | This study |
| pVac::Km | pGEMZ derivative containing a kanamycin cassette | 10 |
| pVac::PA3lacZ | pVac::Km derivative containing the transcriptional fusion PfecA3-lacZ; Kmr | This study |
| pVac::PA3MuOpIlacZ | pVac::Km derivative containing the transcriptional fusion OP-I mutant PfecA3-lacZ; Kmr | This study |
| pVac::PA3MuOpIIlacZ | pVac::Km derivative containing the transcriptional fusion OP-II mutant PfecA3-lacZ; Kmr | This study |
| pET15b | IPTG-inducible vector overexpressing N-terminally His6-tagged recombinant protein; Ampr | Novagen |
| pET15b-nikR | pET15b derivative containing the nikR coding sequence cloned in frame within the NdeI/BamHI restriction sites | 7 |
| pET15b-fur | pET15b derivative containing the fur coding sequence cloned in frame within the NdeI/XhoI restriction sites | 8 |
IPTG, isopropyl-β-d-thiogalactopyranoside.
DNA manipulation and cloning of the promoter regions.
Standard molecular biology techniques were carried out for DNA purification, PCR analyses, restriction digestion, and cloning (24). All restriction and modification enzymes were used according to the instructions of the manufacturer (New England Biolabs). The promoter regions of interest were amplified from genomic DNA by PCR using the primer pairs listed in Table 2 (A1F-A1R for fecA1, A2F-A2R for fecA2, A3F-A3R for fecA3, A3.1-A3.4 for fecA3 promoter fragment Rx1, A3.1-A3.2 for fecA3 promoter fragment Rx2, and A3.3-A3.4 for fecA3 promoter fragment Rx3) and cloned into the pGEM-T Easy (Promega) or pBlueScript (Stratagene) vector to generate plasmids pGemT-PfecA1, pGemT-PfecA2, pGemT-PfecA3, pBS-PfecA3Rx1, pBS-PfecA3Rx2, and pBS-PfecA3Rx3. Appropriate restriction sites were introduced at the primer 5′ end to facilitate the subsequent cloning. Qiagen gel extraction and PCR purification kits were used according to the manufacturer's instructions.
TABLE 2.
Primers used for PCR amplification of the promoter regions and for primer extension reactions
| Oligonucleotide name | Sequence (5′→3′)a |
|---|---|
| A1F | CCTTGGATCCATTATGAGTTTTGACAGCAT |
| A1R | GGGAGAATTCAAGTTTGTAGCGTATTTATT |
| A2F | TTTAGGATCCTTTTGCTCAGTGGTTGTCAC |
| A2R | TATAGAATTCGACGCTGGTTTTGCGATAGC |
| A3Fnew | ATTTGGATCCAGCGTCAAAGAATGTCTTGT |
| A3Rnew | TAATGAATTCTTTCAAGTAGAATCACG |
| A3.1 | GGAATTCACGAACGCCTAT |
| A3.2 | CGGGATCCTATGATAAAATT |
| A3.3 | GGAATTCAATTCGCAGAAT |
| A3.4 | CGGGATCCAAAAGATTTTCA |
| A3.6 | TCCCCCGGGAATAAAATTTTA |
| A3.7 | TCCCCCGGGCCCATGAAAATC |
| A3.8 | TCCCCCGGGTTAATAGGCGTT |
| A3.9 | TCCCCCGGGAAAATAAAAAAA |
| PEfecA1 | CCAAAACAGCCAAAGAGACTA |
| PEfecA2 | ATTTTTGCTCAGTGGTTGTCA |
| 5PEfecA3 | ATTCTTTCAAGTAGAATCACGC |
| A3Z1 | GTATCGATAAGCTTGATATC |
| 5′kat | CACATCTTTATTAACCAT |
Bases in italics correspond to exogenous restriction sites.
Mutagenesis of the promoter of fecA3.
The promoter region of fecA3 was mutagenized by introducing an SmaI site within the putative NikR consensus sites identified by sequence analysis in order to replace the hemioperators partially or completely. The primer pairs A3Fnew-A3.7 and A3Rnew-A3.6 and the primer pairs A3Fnew-A3.9 and A3Rnew-A3.8 were used to mutate the operator II (OP-II) and operator I (OP-I) sites, respectively. The regions of interest were amplified from pGemT-PfecA3, subcloned into pBlueScript, and subsequently ligated via the SmaI site to generate the plasmids pBS-PA3MuOpII and pBS-PA3MuOpI. The mutations were confirmed by sequence analyses.
Construction of lacZ transcriptional fusions and integration into the vacA locus of H. pylori.
The wild-type and mutant fecA3 promoters were cloned via the restriction sites BamHI and EcoRI into pBlueScript SK so as to have in-frame transcriptional fusions with the lacZ 3′ region occurring on the vector. These constructs were recovered by PvuII-BamHI double digestion, blunted, and cloned into the pVac::Km transformation vector (10) by exploiting a HincII site. The transcriptional fusions were inserted into the vacA locus on the chromosome of H. pylori by homologous recombination; positive colonies on agar plates were selected according to the antibiotic resistance phenotype. The integrations were confirmed by PCR amplification with primers A3Z1 and A3Fnew.
RNA isolation and primer extension analyses.
Total RNA was extracted by a hot-phenol procedure as described previously (6); RNA integrity and purity were ensured by electrophoresis on 1% agarose gels. Primer extension analyses were performed with 15 μg of total RNA and 10 pmol of 5′-end-labeled primers as described previously (11). The oligonucleotides used for primer extension reactions are listed in Table 2. The same set of primers was utilized to map the transcription start site of the corresponding cloned promoter region in a sequencing reaction using a T7 sequencing kit (USB Corp.). The quantification of the fecA mRNA extension products was performed by using ImageQuant software, after image acquisition by a Storm PhosphorImager (Molecular Dynamics).
Overexpression and purification of recombinant His6-Fur and His6-NikR.
Recombinant His6-Fur (11) and His6-NikR (7) were overexpressed and purified under native conditions as described previously. Thrombin protease (10 U/mg) was used to remove the N-terminal histidine tag according to the instructions of the manufacturer (Amersham GE Healthcare). The purified, untagged proteins are hereinafter referred to as Fur and NikR. Proteins were stored in phosphate-buffered saline at −80°C and dialyzed overnight against the footprinting buffer (50 mM Tris-Cl, pH 7.85, 50 mM KCl, 10 mM MgCl2, 0.01% Igepal CA-630, 10% glycerol, 1 mM dithiothreitol) or 20 mM HEPES buffer (pH 7.85) prior to the DNA binding experiments. A Bradford colorimetric assay kit (Bio-Rad) was used to quantify the protein fractions with bovine serum albumin as the standard.
Probe preparation and DNase I footprinting.
The vectors pGEMT-PfecA1, pGEMT-PfecA2, and pGEMT-PfecA3 were linearized with BamHI (for pGEMT-PfecA1 and pGEMT-PfecA2) or EcoRI (for pGEMT-PfecA3), dephosphorylated with calf intestinal phosphatase, and labeled at the 5′ ends with [γ-32P]ATP (5,000 Ci/mmol; Amersham GE Healthcare) by using T4 polynucleotide kinase. The labeled DNA probes were further digested with EcoRI (for pGEMT-PfecA1 and pGEMT-PfecA2) or BamHI (for pGEMT-PfecA3), and the products were separated by native polyacrylamide gel electrophoresis and purified as described previously (7). The binding reactions between approximately 20 fmol of labeled probe and increasing concentrations of NikR (expressed per NikR tetramer) and Fur (expressed per Fur dimer) were carried out in the footprinting buffer (50 mM Tris-Cl, pH 7.85, 50 mM KCl, 10 mM MgCl2, 0.01% Igepal CA-630, 10% glycerol) at room temperature for 15 min using 1 μg of salmon sperm DNA (Invitrogen) as a nonspecific competitor in a final volume of 50 μl. An excess of NiSO4 (100 μM) or FeSO4 (100 μM) was added where indicated. Afterwards, DNase I (0.04 U), diluted in footprinting buffer containing 5 mM CaCl2, was added to the reaction mixture and digestion was allowed to occur for 90 s. The reaction was then stopped, and the samples were treated as described previously (11). Samples were resuspended in 6 μl of formamide loading buffer, denatured at 95°C for 3 min, separated on 8 M urea-6% acrylamide sequencing gels, and autoradiographed. A modified G+A sequencing ladder protocol (21) was employed to map the binding sites.
DNA electrophoretic mobility shift assay.
A DNA gel retardation assay of discrete regions of the fecA3 promoter was performed. Three fragments of approximately 130, 60, and 60 bp were amplified from pGEMT-PfecA3 by using the primer pairs listed in Table 2 to generate the plasmids pBS-PfecA3Rx1, pBS-PfecA3Rx2, and pBS-PfecA3Rx3, respectively. Following double restriction digestion to isolate the fragment of interest, the DNA was dephosphorylated with calf intestinal phosphatase and labeled at both ends with T4 polynucleotide kinase and [γ-32P]ATP; unincorporated radiolabeled nucleotide was removed with a G-50 microspin column (GE Healthcare). The binding reaction was carried out for 15 min in a mixture of 20 mM HEPES-OH (pH 7.85), 50 mM KCl, 10% glycerol, 0.02% Igepal CA-630, and 0.1 mM dithiothreitol with 200 ng of salmon sperm DNA as a nonspecific competitor. Twenty femtomoles of radiolabeled target DNA and increasing concentrations of NikR were used in a final volume of 15 μl in the presence of 100 μM NiSO4. Reaction mixtures were resolved on a native gel (6% polyacrylamide [19:1], 20 mM MOPS [morpholinepropanesulfonic acid], 5 mM sodium acetate, pH 7.0), and the gels were prerun at 50 V for 30 min prior to loading and then run at 170 V for 2 h at room temperature.
RESULTS
Genome and promoter organization of the genes encoding the outer membrane FecA homologue proteins in H. pylori.
In the sequenced H. pylori genomes, three homologues of the E. coli fecA gene, which encodes an iron dicitrate transporter belonging to the family of TonB-dependent transporters, are annotated (1, 3, 29). In strain G27, the fecA1 gene maps 248 bp downstream of the iron transporter gene feoB, the fecA2 gene maps 194 bp downstream of a hypothetical polycistronic group of genes involved in fatty acid and phospholipid metabolism, and the fecA3 gene maps 360 bp downstream of the rocF gene, encoding an arginase.
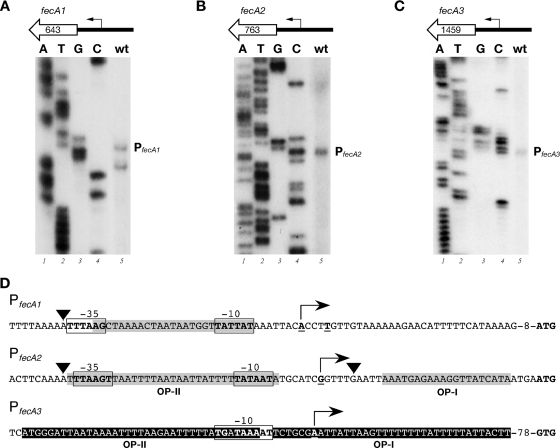
To map the fecA transcriptional initiation sites, primer extension experiments were performed with total RNAs extracted from H. pylori G27 cultures, and results are shown in Fig. 1. The fecA1 start site showed as two bands mapping 36 and 40 bases upstream of the annotated start codon (Fig. 1A). The transcriptional start site of fecA2 mapped 33 bp upstream of the translational start site (Fig. 1B), while the fecA3 transcriptional start site mapped 112 bp upstream of the annotated GTG start codon (Fig. 1C). All upstream sequences showed −10 regions with homology to the canonical TATAAT E. coli promoter consensus sequence and less conserved −35 regions (Fig. 1D). Notably, the −10 sequence of PfecA3 is preceded by a TG motif (20), indicative of a −10 extended promoter. Based on the presence of conserved sequence elements upstream of the transcriptional start sites, we conclude that the fecA genes of H. pylori are transcribed by the vegetative σ80-containing RNA polymerase.
FIG. 1.
Identification of the H. pylori fecA promoter regions. (A to C) The 5′ ends of the fecA1 (A), fecA2 (B), and fecA3 (C) transcripts were identified by primer extension analyses. A schematic representation of the fecA gene and its promoter in H. pylori strain G27 is shown at the top of each panel. fecA1, fecA2, and fecA3 are preceded by intergenic regions of 248, 194, and 360 bp, respectively. Fifteen micrograms of total RNA extracted from cultures of the wild-type (wt) strain were hybridized with specific primers (Table 2) and elongated with reverse transcriptase. The elongated primer bands mapping the 5′ ends of the fecA transcripts are indicated. Sequence reactions (A, T, G, and C) were performed with the same primers and with plasmids pGEMT-PfecA1 (A), pGEMT-PfecA2 (B), and pGEMT-PfecA3 (C) as templates. (D) Summary of relevant features within the nucleotide sequences of the fecA promoter regions. The mapped transcriptional start site (+1) of each promoter is represented by a bent arrow; hexamers corresponding to the putative −10 and −35 regions are boxed and shown in boldface; gray boxes indicate the regions protected by Fur in DNase I footprinting assays; black boxes indicate NikR binding regions; arrowheads identify nucleotides hypersensitive to DNase I digestion. The bases in the start codons for the FecA1, FecA2, and FecA3 proteins are indicated in bold. The three H. pylori fecA genes encode polypeptides with less than 30% amino acid sequence identity and approximately 50% sequence similarity to E. coli FecA.
Growth phase-dependent activity of the fecA promoters.
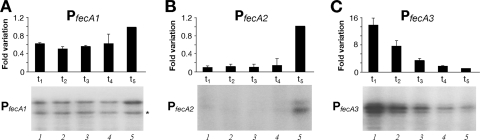
To begin the functional characterization of the fecA genes, we monitored their transcription during bacterial growth. Aliquots of bacterial cultures were sampled at different time points of growth, and the extracted RNAs were assayed by quantitative primer extension analyses, with the results shown in Fig. 2. Transcription from the PfecA1 promoter showed no significant variations in the amount of mRNA throughout growth (Fig. 2A). In contrast, transcription from the PfecA2 promoter was detectable only at the end of the time course experiment (when the cultures had an OD of 1.0) (Fig. 2B), while transcription from the PfecA3 promoter decreased over time (Fig. 2C). We conclude that while transcription from the PfecA1 promoter remains unchanged during growth, transcription from the PfecA2 and PfecA3 promoters appears to be inversely regulated, with the maximum expression of fecA2 and fecA3 in late and early log phase, respectively. It is likely that this temporal transcriptional regulation reflects distinct roles for FecA2 and FecA3 during growth.
FIG. 2.
Growth phase-dependent transcription of PfecA1 (A), PfecA2 (B), and PfecA3 (C) promoters. The wild-type strain was grown to an OD600 of 1.0, starting from an overnight stationary-phase preinoculum freshly diluted to an OD600 of 0.1. Total RNAs were extracted from equal volumes of culture at time points t1, t2, t3, t4, and t5, corresponding to OD600s of 0.2, 0.4, 0.6, 0.8, and 1.0. Primer extensions were performed in triplicate with promoter-specific primers and 15-μg RNA samples extracted from independent cultures. Results from representative time course experiments are shown in the bottom panels. The fast-migrating band in panel A (*) was taken as an internal control for the RNA samples. The intensity of the bands at each time point for the PfecA1 transcript (A), the PfecA2 transcript (B), and the PfecA3 transcript (C) is reported as the change (n-fold) from the signal obtained at time t5. Error bars indicate standard deviations of results for three independent replicates.
PfecA1 and PfecA2 promoters are repressed by Fur.
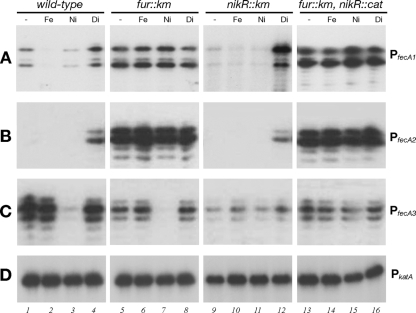
To study the transcriptional regulation of the fecA genes, primer extension analyses of RNAs extracted from the wild-type, ΔnikR, Δfur, and double mutant ΔnikR Δfur strains, grown to mid-log phase and treated in parallel for 15 min with iron, nickel, or iron chelator, were carried out. Representative results are shown in Fig. 3.
FIG. 3.
Fur- and NikR-mediated metal regulation of the PfecA promoters. (A to C) Quantitative primer extension analyses of the responses of PfecA1 (A), PfecA2 (B), and PfecA3 (C) promoters to metal addition or chelation. Total RNAs were extracted from cultures of the H. pylori G27 wild type, H. pylori G27 nikR::Km (ΔnikR) and G27 fur::Km (Δfur), and the G27 double mutant (nikR::cat fur::Km) grown to exponential phase and treated for 15 min with 1 mM FeSO4 (Fe), 1 mM NiSO4 (Ni), or 100 μM 2,2-dipyridyl (Di). A control reaction (−, no treatment) is also shown. (D) As a negative control, primer extension of the catalase gene (PkatA [8]) transcript was carried out.
As expected, transcription from the PfecA1 promoter was detected under conditions of no treatment, whereas no transcription from PfecA2 was detected (Fig. 3A and B, lanes 1). PfecA1 was repressed after the addition of 1 mM FeSO4 to the medium (Fig. 3A, lane 2) and slightly repressed by 1 mM NiSO4 (Fig. 3A, lane 3), and both PfecA1 and PfecA2 were induced by the addition of the iron chelator (Fig. 3A and B, lanes 4). Transcription levels for both promoters in the Δfur mutant were high under all conditions tested (Fig. 3A and B, lanes 5 to 8), suggesting that PfecA1 and PfecA2 are Fur and iron repressed, and the moderate response to nickel at the PfecA1 promoter also appears to be Fur mediated. In the ΔnikR mutant, while transcription from PfecA1 was completely derepressed by iron chelation, it appeared to be repressed under all other conditions. The general trend of PfecA1 and PfecA2 transcription observed in the nikR mutant was comparable to that in the wild-type strain (Fig. 3A and B, lanes 9 to 12). As expected, both promoters were derepressed in the double mutant (Fig. 3A and B, lanes 13 to 16). We conclude that transcription from the PfecA1 and PfecA2 promoters is iron regulated in a Fur-dependent manner.
Nickel-dependent repression of PfecA3 is mediated by NikR.
The levels of fecA3 transcripts in RNA samples from wild-type cultures after the addition of iron or iron chelator were comparable to those from the untreated cultures (Fig. 3C, lanes 1, 2, and 4), indicating that fecA3 is not iron regulated. In contrast, upon the addition of 1 mM NiSO4 to growing cells, a significant reduction in the transcript level was detected (Fig. 3C, lane 3), suggesting that the addition of nickel strongly downregulates the fecA3 promoter. The negative effect exerted by nickel was lost in the ΔnikR mutant strain (Fig. 3C, lanes 9 to 12), suggesting that NikR is responsible for the nickel-dependent repression of PfecA3. It is worth noting that there was a general reduction in the level of transcription from PfecA3 in the NikR mutant (Fig. 3C, lanes 9 to 12) and also, to a lesser extent, in the Δfur mutant (Fig. 3C, lanes 5 to 8) and in the Δfur ΔnikR double mutant (Fig. 3C, lanes 13 to 16) compared to that in the wild-type strain. However, the downregulation in response to Ni2+ was not affected in the Δfur mutant strain (Fig. 3C, lane 7), suggesting that Fur is not directly involved in the transcriptional regulation of PfecA3. As expected, the response to nickel was lost in the double mutant (Fig. 3C, lane 15). These data suggest that the repressive effect of Ni2+ on PfecA3 is mediated by NikR and that the deletion of fur and/or nikR may affect the steady-state level of transcription.
Differential patterns of Fur and NikR binding to the PfecA1, PfecA2, and PfecA3 promoter regions.
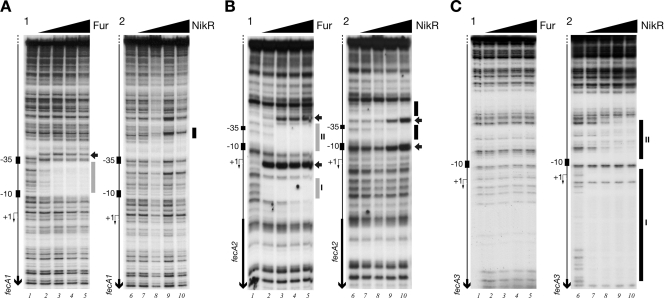
In order to understand whether the transcriptional responses revealed by RNA analyses were due to the direct binding of the metal-responsive transcriptional regulators Fur and NikR to specific operator sites, we performed footprinting assays of recombinant purified proteins with PfecA1, PfecA2, and PfecA3 promoter probes.
Figure 4A, B, and C show the patterns of protection of Fur and NikR on the PfecA1, PfecA2, and PfecA3 promoter probes. Fur binds to the PfecA1 promoter, protecting a region of 27 bp spanning positions −8 to −34, overlapping with the −10 and partially with the −35 promoter elements, and at a concentration of 18 nM, fully preventing DNase I digestion (Fig. 4A1, lane 3). A DNase I-hypersensitive site is apparent upstream of the binding region. The addition of NikR at high concentrations (74 to 148 nM) to the same promoter probe resulted in a faint area of protection spanning positions −54 to −65 of the PfecA1 promoter (Fig. 4A2, lanes 9 and 10).
FIG. 4.
In vitro binding of Fur and NikR to the PfecA1 (A), PfecA2 (B), and PfecA3 (C) promoter regions of H. pylori G27. Divalent iron and nickel (100 μM) were added as cofactors in the binding buffers of Fur and NikR, respectively. Samples of approximately 20 fmol of 5′-end-labeled probes were incubated with increasing concentrations of Fur dimer, 0 nM (lanes 1), 6 nM (lanes 2), 18 nM (lanes 3), 60 nM (lanes 4), and 180 nM (lanes 5), or NikR tetramer, 0 nM (lanes 6), 14.8 nM (lanes 7), 29.6 nM (lanes 8), 74 nM (lanes 9), and 148 nM (lanes 10). A G+A sequence reaction ladder for each promoter probe was run in parallel (data not shown) to map the binding sites with respect to the transcriptional start site (+1), indicated by a bent arrow to the left of each gel. The relative positions of the putative −10 and −35 elements are symbolized by small rectangles, and vertical black arrows indicate open reading frames. The vertical bar(s) to the right of each gel indicates the region(s) protected from DNase I digestion at increasing concentrations of protein (gray, Fur; black, NikR); horizontal black arrows represent bands of hypersensitivity.
The PfecA2 promoter in the presence of Fur presented two regions of altered DNase I digestion (Fig. 4B1, lanes 2 to 5), one extending from nucleotide +10 to nucleotide +29 (region I) and the other spanning nucleotides −7 to −39 (region II). In addition, Fur appears to have differential affinities for the two operators, binding at 6 nM (Fig. 4B1, lane 2) to OP-I and at 18 nM to OP-II (Fig. 4B1, lane 3). NikR, at high concentrations (Fig. 4B2), also protected a region of approximately 60 bp of the PfecA2 promoter, from nucleotide −7 to nucleotide −66, resulting in a DNase I-hypersensitive site at position −40 and overlapping with the lower-affinity OP-II binding site for Fur (Fig. 4B2, lanes 8 to 10).
No Fur-dependent protection on PfecA3 was observed (Fig. 4C, lanes 1 to 5). However, at high Fur concentrations, weak protection spanning positions −6 to −35 could be detected (data not shown). NikR demonstrates a high-affinity binding site, OP-I (Fig. 4C), completely saturated at 14.8 nM NikR (Fig. 4C, lanes 7 to 10) and corresponding to the previously reported operator region (15). Surprisingly, a lower-affinity site, OP-II (Fig. 4C, lanes 8 to 10), spanning nucleotides −8 to −44 and comprising the −10 region, was detectable at 74 nM protein. This may indicate that effective regulation of the PfecA3 promoter by NikR in response to nickel requires two adjacent binding sites.
The high-affinity binding of Fur to PfecA1 and PfecA2 and the high-affinity binding of NikR to PfecA3 are consistent with the transcriptional analyses in Fig. 3 and support the idea of the repressive role of Fur at the PfecA1 and PfecA2 promoters and the repressive role of NikR at the PfecA3 promoter in response to iron and nickel, respectively.
Cooperative binding of NikR to the OP-I and OP-II sites of the PfecA3 operator.
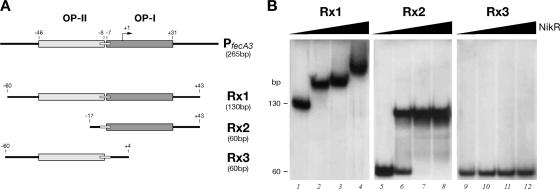
The presence of two adjacent binding sites for NikR on the PfecA3 promoter raised the question of their interdependence. Therefore, we decided to subclone discrete fragments of this promoter: Rx1, containing the whole region recognized by NikR in the footprinting assay, and Rx2 and Rx3, containing the proximal (OP-I) and distal (OP-II) binding sites, respectively (Fig. 5A). We tested these distinct regions in gel shift assays with increasing amounts of NikR in the presence of 100 μM NiSO4 (Fig. 5B).
FIG. 5.
Schematic representation of key features of the NikR operators on the PfecA3 promoter and DNA segments used as probes (A) in DNA binding assays with the NikR protein (B). DNA retardation was induced by the addition of NikR (100 μM NiSO4 was present in the binding buffer). NikR was added at the following concentrations: lanes 1, 5, and 9, 0 nM; lanes 2, 6, and 10, 9.8 nM; lanes 3, 7, and 11, 98 nM; and lanes 4, 8, and 12, 490 nM.
The addition of 9.8 nM NikR to the Rx1 probe (Fig. 5B, lane 2) resulted in the complete sequestration of the free probe to a slow-migrating band, indicative of high-affinity NikR-DNA probe complex formation. Interestingly, upon the addition of 98 and 490 nM NikR (Fig. 5B, lanes 3 and 4, respectively), the migration of the complex was further retarded, indicative of additional NikR bindings. In contrast, similar amounts of NikR led to the formation of a unique complex with OP-I (Fig. 5B, lanes 6 to 8) and no complexes with OP-II (Fig. 5B, lanes 10 to 12). This finding suggests that the distal site (OP-II) requires the occupancy of the proximal one to bind NikR in a cooperative manner.
In vivo role of the two NikR operators at PfecA3.
In order to better understand the roles of the two NikR binding sites in the transcriptional regulation of PfecA3, we constructed two mutant promoters, with mutations in either the OP-I or the OP-II site, fused to a truncated lacZ reporter gene. Based on the proposed NikR consensus motif (7), a SmaI site was inserted in order to disrupt one NikR hemioperator site, that of OP-I or OP-II, by the replacement of the nucleotides likely to be important for protein-DNA interaction (Fig. 6A). The wild-type and mutated transcriptional fusions were inserted into the vacA locus of the H. pylori genome, and their activities were monitored by primer extension analyses (Fig. 6B). The wild-type construct gave a product whose transcription was nickel sensitive (Fig. 6B, lanes 1 and 2). The OP-I-mutated fusion resulted in nickel-insensitive transcription (Fig. 6B, lanes 3 and 4). The OP-II-mutated fusion produced a more intense band and maintained the nickel-dependent repression (Fig. 6B, lanes 5 and 6). These results suggest that the wild-type levels of the fecA3 transcript result from the balanced activities of the two operators, with OP-I controlling the response to nickel while OP-II controls the total level of transcripts.
FIG. 6.
(A) Alignment of the wild-type (wt) and mutated operators of the PfecA3 promoter. The inverted repeats within the operator sites corresponding to the NikR consensus region are highlighted, and the SmaI restriction site is underlined and represented in italics. (B) Primer extension with mid-log-phase total RNA extracted from a recombinant G27 strain harboring the PfecA3-lacZ transcriptional fusions with wild-type and mutant OP-I and OP-II sites in response to a pulse of 100 μM NiSO4. +, present; −, absent.
DISCUSSION
Nickel and iron are essential cofactors for the activity of several enzymes. In H. pylori, they are important determinants for the colonization of the stomach epithelium, survival in the gastric mucosa, and virulence. H. pylori codes for two metal-responsive transcriptional regulators, Fur and NikR, which control the expression of many genes important for infection and are indispensable for pathogenesis, as both fur and nikR deletion strains are attenuated in the mouse model (4). Several lines of experimental evidence show that their regulons are overlapping and interconnected (4-7). In addition, their expression is interdependent, as mutual downregulation of the promoters of fur and nikR has been shown to occur (7). Moreover, both regulators have been implicated in the control of transcription of the fecA genes.
In H. pylori, the three fecA genes are independent monocistronic genes, transcribed by the vegetative sigma factor and selectively regulated by Fur and NikR. Based on results from transcriptional analyses (Fig. 3) and in vitro studies (Fig. 4), we can conclude that Fur represses fecA1 and fecA2 in response to iron. Fur shows high affinity for the PfecA2 promoter in vitro, and in vivo transcription from PfecA2 is repressed fully. The Fur-dependent transcription from PfecA1, for which Fur shows lower affinity in vitro, can be stimulated by the addition of iron to the cell and, to a lesser extent, also by the addition of nickel. The iron-dependent transcription of fecA1 and fecA2 is in accordance with previously reported analyses (12, 30). The expression patterns of these two genes throughout growth are different, with fecA1 showing a basal, constitutive level of expression over time, whereas fecA2 is repressed in the early phase of growth (Fig. 2). Although no study to date has examined the actual physiological roles of FecA1 and FecA2 in H. pylori or their binding activities toward siderophores or iron-associated molecules, it is legitimate to envisage that the different patterns of expression may reflect distinct roles for the two iron transporters. For instance, FecA1 may be a low-affinity iron transporter, ensuring the iron supply under unstimulated conditions, whereas FecA2 may be a high-affinity iron transporter whose expression needs to be tightly controlled. The low-affinity nickel-dependent binding of NikR to PfecA1 and PfecA2 in vitro seems to have a minor physiological role under the conditions tested, as there is no clear NikR-dependent response. We speculate that the observed lower level of transcription of PfecA1 in the NikR mutant than in the wild type may be an indirect effect of Fur-mediated repression, as Fur is overexpressed in the NikR mutant (7). However, we cannot exclude the possibility that the low-affinity binding of NikR on PfecA1, upstream of the putative −35 promoter element, observed in vitro may exert a positive influence on transcription. In contrast, an explanation of NikR binding to PfecA2 could not be assigned, since there is no apparent nickel-sensitive response or nikR-dependent modulation of this promoter under the conditions tested. In view of the NikR- and nickel-responsive regulation of fecA3, we tentatively speculate that FecA3 plays a role in Ni2+ uptake, as has recently been shown for another, similarly regulated protein, FrpB4, which was annotated as an iron transporter (26), although this role has not been addressed to date.
Recently, Ernst and coworkers (15) showed that NikR represses the transcription of the fecA3 gene by binding to a specific region of PfecA3 in a nickel-dependent manner. Herein, we have shown that the expression of fecA3 is temporally regulated during growth (Fig. 2) and that NikR binds cooperatively, with different affinities, to two distinct sites within the PfecA3 promoter (Fig. 4 and 5). Our data indicate that the nickel-dependent NikR regulation of fecA3 seems to be less straightforward than expected. In fact, transcriptional analyses suggest a direct and an indirect role for NikR in the regulation of fecA3 (Fig. 3). PfecA3 in the wild-type strain seems to have a high level of transcription, under conditions of no treatment as well as in the presence of iron or an iron chelator. The addition of NiSO4 results in NikR-dependent downregulation of fecA3, as indicated by the loss of the response to nickel in the ΔnikR strain. However, the constitutive level of transcription of fecA3 in the ΔnikR mutant and, to a lesser extent, also that in the Δfur mutant are considerably lower than that in the wild type, implying indirect effects of the nikR and fur regulatory genes on the basal transcription of PfecA3. These results appear to be in contradiction with the finding of Ernst and coworkers (15), who reported that Northern blot analysis showed apparently similar amounts of fecA3 mRNA in wild-type and nikR mutant strains. A possible explanation may lie in the fact that while Northern blots show all mRNA species accumulated in the cell, quantitative primer extension analyses reveal specific variations in the amounts of 5′ ends of the fecA3 mRNA, as substantiated by the results of the control experiment shown in Fig. 3 (panels C and D, lanes 9 to 12 versus 1 to 4). The repression of PfecA3 transcription in response to nickel occurs by the binding of NikR to a high-affinity primary binding site (OP-I) (Fig. 5) corresponding to a previously reported operator site (15), followed by cooperative binding to a repressing upstream site (OP-II) (Fig. 5).
PfecA3 transcription was shown to be substantially unaffected by iron treatment, in agreement with the data in previous reports (30). Nonetheless, transcriptome analyses conducted in exponential and advanced growth phases suggested that fecA3 transcript levels are derepressed in the Δfur strain only in the advanced growth phase, while chromatin immunoprecipitation-on-chip analysis identified fecA3 as a direct target of Fur in vivo (6). In vitro, Fur binds to PfecA3 with very low affinity (data not shown). These observations may tentatively point to a role of Fur in modulating the basal levels of fecA3 transcription in advanced growth phase, when the levels of Fur increase sufficiently to compete with NikR for binding to PfecA3.
In conclusion, our analyses suggest that the transcription of the fecA genes is regulated in response to Fe2+ and Ni2+ concentrations via the specific interactions of the Fur and NikR regulatory proteins with the fecA gene promoters and possibly through direct and/or indirect feedback regulation of the Fur-NikR regulatory circuit. The degree of complexity of the overlapping regulatory circuits of these two proteins, required to guarantee metal homeostasis, is becoming more evident.
Acknowledgments
We thank G. Corsi for artwork.
This work was supported in part by grants from the University of Bologna (ex60% and Strategic project) to V.S. and in part by Novartis Vaccines & Diagnostics.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397176-180. [DOI] [PubMed] [Google Scholar]
- 2.Atherton, J. C. 2006. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 163-96. [DOI] [PubMed] [Google Scholar]
- 3.Baltrus, D. A., M. R. Amieva, A. Covacci, T. M. Lowe, D. S. Merrell, K. M. Ottemann, M. Stein, N. R. Salama, and K. Guillemin. 2008. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. [DOI] [PMC free article] [PubMed]
- 4.Bury-Mone, S., J. M. Thiberge, M. Contreras, A. Maitournam, A. Labigne, and H. De Reuse. 2004. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53623-638. [DOI] [PubMed] [Google Scholar]
- 5.Contreras, M., J. M. Thiberge, M. A. Mandrand-Berthelot, and A. Labigne. 2003. Characterization of the roles of NikR, a nickel-responsive pleiotropic autoregulator of Helicobacter pylori. Mol. Microbiol. 49947-963. [DOI] [PubMed] [Google Scholar]
- 6.Danielli, A., D. Roncarati, I. Delany, V. Chiarini, R. Rappuoli, and V. Scarlato. 2006. In vivo dissection of the Helicobacter pylori Fur regulatory circuit by genome-wide location analysis. J. Bacteriol. 1884654-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delany, I., R. Ieva, A. Soragni, M. Hilleringmann, R. Rappuoli, and V. Scarlato. 2005. In vitro analysis of protein-operator interactions of the NikR and Fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J. Bacteriol. 1877703-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delany, I., A. B. Pacheco, G. Spohn, R. Rappuoli, and V. Scarlato. 2001. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J. Bacteriol. 1834932-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2003. An anti-repression Fur operator upstream of the promoter is required for iron-mediated transcriptional autoregulation in Helicobacter pylori. Mol. Microbiol. 501329-1338. [DOI] [PubMed] [Google Scholar]
- 10.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2002. Growth phase-dependent regulation of target gene promoters for binding of the essential orphan response regulator HP1043 of Helicobacter pylori. J. Bacteriol. 1844800-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 421297-1309. [DOI] [PubMed] [Google Scholar]
- 12.Ernst, F. D., S. Bereswill, B. Waidner, J. Stoof, U. Mader, J. G. Kusters, E. J. Kuipers, M. Kist, A. H. van Vliet, and G. Homuth. 2005. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology 151533-546. [DOI] [PubMed] [Google Scholar]
- 13.Ernst, F. D., G. Homuth, J. Stoof, U. Mader, B. Waidner, E. J. Kuipers, M. Kist, J. G. Kusters, S. Bereswill, and A. H. van Vliet. 2005. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J. Bacteriol. 1873687-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst, F. D., E. J. Kuipers, A. Heijens, R. Sarwari, J. Stoof, C. W. Penn, J. G. Kusters, and A. H. van Vliet. 2005. The nickel-responsive regulator NikR controls activation and repression of gene transcription in Helicobacter pylori. Infect. Immun. 737252-7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst, F. D., J. Stoof, W. M. Horrevoets, E. J. Kuipers, J. G. Kusters, and A. H. van Vliet. 2006. NikR mediates nickel-responsive transcriptional repression of the Helicobacter pylori outer membrane proteins FecA3 (HP1400) and FrpB4 (HP1512). Infect. Immun. 746821-6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300931-936. [DOI] [PubMed] [Google Scholar]
- 17.Giedroc, D. P., and A. I. Arunkumar. 2007. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 20073107-3120. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 19.Hussein, S., K. Hantke, and V. Braun. 1981. Citrate-dependent iron transport system in Escherichia coli K-12. Eur. J. Biochem. 117431-437. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, A., R. A. Malloch, N. Fujita, D. A. Smillie, A. Ishihama, and R. S. Hayward. 1993. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J. Mol. Biol. 232406-418. [DOI] [PubMed] [Google Scholar]
- 21.Liu, S. T., and G. F. Hong. 1998. Three-minute G + A specific reaction for DNA sequencing. Anal. Biochem. 255158-159. [DOI] [PubMed] [Google Scholar]
- 22.Mahren, S., H. Schnell, and V. Braun. 2005. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch. Microbiol. 184175-186. [DOI] [PubMed] [Google Scholar]
- 23.Pflock, M., S. Kennard, I. Delany, V. Scarlato, and D. Beier. 2005. Acid-induced activation of the urease promoters is mediated directly by the ArsRS two-component system of Helicobacter pylori. Infect. Immun. 736437-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 25.Scarlato, V., I. Delany, G. Spohn, and D. Beier. 2001. Regulation of transcription in Helicobacter pylori: simple systems or complex circuits? Int. J. Med. Microbiol. 291107-117. [DOI] [PubMed] [Google Scholar]
- 26.Schauer, K., B. Gouget, M. Carriere, A. Labigne, and H. de Reuse. 2007. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol. Microbiol. 631054-1068. [DOI] [PubMed] [Google Scholar]
- 27.Stahler, F. N., S. Odenbreit, R. Haas, J. Wilrich, A. H. Van Vliet, J. G. Kusters, M. Kist, and S. Bereswill. 2006. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 743845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189113-130. [DOI] [PubMed] [Google Scholar]
- 29.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388539-547. [DOI] [PubMed] [Google Scholar]
- 30.van Vliet, A. H., J. Stoof, R. Vlasblom, S. A. Wainwright, N. J. Hughes, D. J. Kelly, S. Bereswill, J. J. Bijlsma, T. Hoogenboezem, C. M. Vandenbroucke-Grauls, M. Kist, E. J. Kuipers, and J. G. Kusters. 2002. The role of the ferric uptake regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter 7237-244. [DOI] [PubMed] [Google Scholar]
- 31.Velayudhan, J., N. J. Hughes, A. A. McColm, J. Bagshaw, C. L. Clayton, S. C. Andrews, and D. J. Kelly. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37274-286. [DOI] [PubMed] [Google Scholar]
- 32.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 6394-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambelli, B., A. Danielli, S. Romagnoli, P. Neyroz, S. Ciurli, and V. Scarlato. 2008. High-affinity Ni2+ binding selectively promotes binding of Helicobacter pylori NikR to its target urease promoter. J. Mol. Biol. 3831129-1143. [DOI] [PubMed] [Google Scholar]