Abstract
Mutant influenza virus that lacks the transmembrane and cytoplasmic tail domains of M2 (M2 knockout [M2KO]) is attenuated in both cell culture and mice. Here, we examined the potency of M2KO influenza virus as a live attenuated influenza vaccine. M2KO virus grew as efficiently as the wild-type virus in cells stably expressing the wild-type M2, indicating the feasibility of efficient vaccine production. Mice intranasally vaccinated with M2KO virus developed protective immune responses and survived a lethal challenge with the wild-type virus, suggesting that the M2KO virus has potential as a live attenuated vaccine.
Influenza A viruses cause a highly contagious, acute respiratory disease responsible for human suffering and economic burden every winter. Vaccination is a primary means for prophylaxis against influenza infection, and inactivated and live attenuated influenza virus vaccines are currently available. Inactivated vaccines, administered parenterally, are generally 70% to 90% effective for reducing the incidence of clinical illness in healthy persons as long as the antigenicities of the circulating virus strains match those of the vaccine (5). However, because mucosal immunity and cytotoxic T-cell responses are limited, protective efficacy of inactivated vaccines lasts for only a short period, requiring annual vaccination. In contrast, live attenuated influenza virus vaccines are intranasally administered and elicit robust mucosal immunity and cellular responses; their protective efficacy, therefore, lasts for longer periods (6). Only two live attenuated vaccines are currently on the market, and use of these vaccines in the United States is limited to persons aged 2 to 49 years (4).
Several lines of evidence suggest that the M2 protein of influenza A virus is responsible for key steps in the viral life cycle (7, 8, 13, 14, 17, 23, 24). Indeed, we have previously shown that the influenza A virus that lacks the transmembrane and cytoplasmic tail domains (M2 knockout [M2KO]) of M2 is highly attenuated in cell culture and in mice compared to the wild-type virus (8, 26). Here, we examined the potential of M2KO influenza A virus as a live attenuated vaccine by immunizing mice and testing immune responses and protective efficacy in a mouse model experimentally infected with A/Puerto Rico/8/34 (PR8), a highly lethal virus in mice.
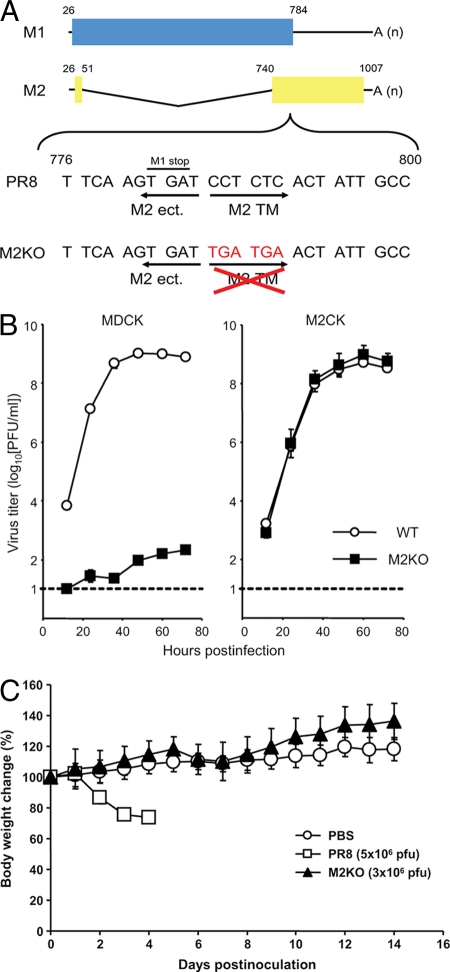
To generate the M2KO virus, we first constructed the mutated M segment into which we inserted two stop codons downstream of the open reading frame of the M1 protein to remove the transmembrane and cytoplasmic tail domains of the M2 protein (Fig. 1A). We then generated the wild-type PR8 and M2KO viruses by plasmid-based reverse genetics (15). To confirm attenuation of the M2KO virus we made in this study, we inoculated the PR8 and M2KO viruses into both MDCK and M2CK cells (8) at a multiplicity of infection of 0.001, and virus titers were determined at various times postinfection by using M2CK cells (Fig. 1B). M2KO virus was highly attenuated in MDCK cells (Fig. 1B, left) but replicated as well as the wild-type virus in M2CK cells (Fig. 1B, right). These data suggest that the M2KO virus is highly attenuated in normal cells but that high titer virus stocks can be produced in cells expressing the M2 protein.
FIG. 1.
Construction of the mutant M segment, growth kinetics of M2KO virus, and change in body weight of mice infected with M2KO virus. (A) Schematic diagram of the mutated M segment of the M2KO virus. Blue and yellow columns represent the open reading frame of the M1 and M2 proteins, respectively. Two stop codons (TGA TGA) were introduced downstream of the open reading frame of the M1 protein in the M segment to eliminate the transmembrane and cytoplasmic tail domains of the M2 protein. M2 ect., M2 TM, and A (n) denote the ectodomain domain of M2, the transmembrane domain of M2, and the poly(A) tail, respectively. Numbers refer to the nucleotide numbers from the 5′ end of the cRNA. (B) Growth properties of PR8 and M2KO viruses in MDCK and M2-expressing MDCK (M2CK) cells. MDCK and M2CK cells were infected with PR8 or M2KO virus at a multiplicity of infection of 0.001. Virus titers in the supernatant of MDCK (left) and M2CK (right) cells at various time points postinfection were determined by using M2CK cells. The dotted line indicates the detection limit of virus titer (10 PFU/ml). (C) Body weight changes in mice inoculated with PBS, PR8, or M2KO virus. The body weights of the control (PBS) or infected (PR8 or M2KO virus) mice were measured daily postinfection. Values are expressed as the mean change in body weight ± standard deviations (n = 8 for PBS and M2KO virus; n = 3 for PR8).
Attenuation of viruses in animals is essential for live vaccines. We therefore examined the pathogenicity of the M2KO virus in mice. We intranasally infected 4-week-old female BALB/c mice with different doses of M2KO virus and determined the virus titers in lungs and nasal turbinates. Body weights were also monitored. When mice were infected with even 3 × 106 or 3 × 105 PFU of virus, virus was recovered from the lungs, but titers were significantly lower than those in the lungs of mice infected with PR8 (P < 0.05) (Table 1). By day 8 postinfection, we no longer detected M2KO virus in the lungs (data not shown). Although virus was recovered from the lungs of one of the animals infected with 3 × 104 PFU of virus, we did not detect virus from any mice infected with lower titers (i.e., 3 × 102 or 3 × 103 PFU) of M2KO virus (Table 1 and data not shown). In nasal turbinates, no virus was recovered from any mice inoculated with the M2KO virus on days 3 and 6 postinfection (Table 1). The body weights of mice infected with 5 × 106 PFU of PR8 rapidly decreased, and these mice were euthanized by 4 days postinfection (Fig. 1C). On the other hand, mice infected with 3 × 106 PFU of M2KO virus showed no body weight loss (Fig. 1C). Taken together, these data indicate that the M2KO virus is highly attenuated in mice, satisfying its requirement for a live attenuated influenza vaccine.
TABLE 1.
Replication of M2KO virus in micea
| Virus | PFU of virus inoculated per mouse | Days postinfection | Virus titer (mean ± SD log10 [PFU/g]) from indicated sourceb
|
|
|---|---|---|---|---|
| Lungs | Nasal turbinates | |||
| PR8 | 1 × 103 | 3 | 8.5 ± 0.1 | 6.0, 5.8 |
| 1 × 103 | 6 | 7.0 ± 0.1 | 6.4 ± 0.2 | |
| M2KO | 3 × 106 | 3 | 5.7 ± 0.3 | ND |
| 3 × 106 | 6 | 5.5 ± 0.3 | ND | |
| 3 × 105 | 3 | 4.1 ± 0.2 | ND | |
| 3 × 105 | 6 | 5.3, 4.2 | ND | |
| 3 × 104 | 3 | 5.2 | ND | |
| 3 × 104 | 6 | ND | ND | |
Three BALB/c mice per group were intranasally infected with the indicated amounts of virus (50 μl per mouse) and sacrificed on days 3 and 6 postinfection for virus titration. When virus was not recovered from all three mice, individual titers were recorded. On day 3 postinfection, virus was not recovered from organs of mice infected with either 3 × 102 or 3 × 103 PFU of M2KO virus (data not shown).
ND, not detected (detection limit, 10 PFU/ml/lung).
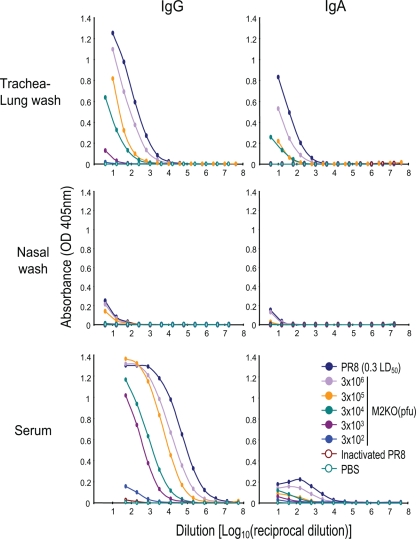
We next examined the level of antibody responses elicited by the M2KO virus. We intranasally inoculated 4-week-old female BALB/c mice with different doses of M2KO virus. As negative and positive controls, we also intranasally inoculated mice with phosphate-buffered saline (PBS) or a dose equivalent to 3 × 106 PFU of formalin-inactivated PR8 (32 hemagglutination units), and a 50% mouse lethal dose of PR8 of 0.3 (500 PFU), respectively. Four weeks after inoculation, titers of immunoglobulin G (IgG) and IgA antibodies against PR8 in sera, trachea/lungs, and nasal washes were determined by an enzyme-linked immunosorbent assay (Fig. 2). Neither the IgG nor the IgA response was appreciable in negative control mice (PBS and inactivated PR8). Although IgG and IgA titers in mice infected with a 50% mouse lethal dose of PR8 of 0.3 were higher, those in mice inoculated with 3 × 106 PFU of M2KO virus were also similarly increased. Moreover, antibody responses correlated with the doses of M2KO virus, although responses in mice inoculated with 3 × 102 PFU of M2KO virus were limited.
FIG. 2.
Virus-specific antibodies in trachea/lung and nasal washes and in sera of mice inoculated with M2KO virus. Samples from each group were obtained 4 weeks postimmunization. Samples were serially diluted, and IgG and IgA in samples from individual mice were detected by use of an enzyme-linked immunosorbent assay. Values are expressed as mean absorbances (n = 4).
To assess the protective efficacy of M2KO virus, mice intranasally inoculated with M2KO virus, formalin-inactivated PR8, or PBS were challenged with a lethal dose of PR8 at 4 and 12 weeks postimmunization. Virus titers in lungs and nasal turbinates of challenged mice were determined by using MDCK cells 3 days postchallenge. We could not detect virus in organs of mice inoculated either with 3 × 106 or 3 × 105 PFU of M2KO viruses, indicating that vaccination with these amounts of M2KO virus gave mice sterile immunity (Table 2). All mice immunized with lower doses (3 × 104 and 3 × 103 PFU), with the exception of one mouse inoculated with 3 × 103 PFU of virus, survived a lethal challenge, although we detected virus in both organs tested and the amounts of virus were not significantly lower than those in the control mice (Table 2). This is probably because virus clearance later than day 3 postchallenge (the time point at which organ virus titers were examined) was more efficient in these immunized mice than that in the control mice. Indeed, although these mice lost body weight until 7 days postchallenge, they ultimately regained their body weight (data not shown). Taken together, our data indicate that M2KO virus confers effective protection against challenge with a lethal dose of PR8.
TABLE 2.
Protection against challenge with lethal doses of PR8 in mice immunized with M2KO virusa
| Weeks postimmunization | Immunogen | PFU of virus inoculated per mouse | No. of survivors/no. of mice tested | Virus titer (mean ± SD log10 [PFU/g]) from indicated sourcec
|
|
|---|---|---|---|---|---|
| Lungs | Nasal turbinates | ||||
| 4 | M2KO | 3 × 106 | 8/8 | ND | ND |
| M2KO | 3 × 105 | 8/8 | ND | ND | |
| M2KO | 3 × 104 | 8/8 | 6.3 ± 1.5 | 5.1 ± 0.3 | |
| M2KO | 3 × 103 | 7/8 | 6.9, 6.6 | 6.2, 6.0 | |
| M2KO | 3 × 102 | 0/8 | 8.0 ± 0.1 | 6.3 ± 0.3 | |
| Inactivated PR8b | 0/8 | 7.6 ± 0.1 | 5.9 ± 0.2 | ||
| PBS | 0/8 | 7.5 ± 0.1 | 5.9 ± 0.1 | ||
| 12 | M2KO | 3 × 106 | 8/8 | ND | ND |
| M2KO | 3 × 105 | 8/8 | ND | ND | |
| M2KO | 3 × 104 | 8/8 | 7.2 ± 0.6 | 6.0 ± 0.3 | |
| M2KO | 3 × 103 | 7/8 | 7.4 ± 0.6 | 6.3 ± 0.2 | |
| M2KO | 3 × 102 | 0/8 | 8.5 ± 0.2 | 6.9 ± 0.2 | |
| Inactivated PR8 | 0/8 | 7.3 ± 0.2 | 6.7 ± 0.3 | ||
| PBS | 0/8 | 7.8 ± 0.3 | 6.2 ± 0.6 | ||
Twenty-two BALB/c mice per group were intranasally immunized with the indicated amounts of M2KO virus, inactivated PR8, or PBS (50 μl per mouse). Half of the mice were challenged with a 50% lethal dose of wild-type PR8 of 100, 4 weeks postimmunization, and the remaining mice were challenged 12 weeks postimmunization. Eight mice per group were monitored for survival for 14 days after challenge. Three mice per each group were sacrificed on day 3 postchallenge to measure virus titration. When virus was not recovered from all three mice, individual titers were recorded.
Virus particles equivalent to 3 × 106 PFU (32 hemagglutination units) were used.
ND, not detected (detection limit, 10 PFU/ml/lung).
Here, we demonstrated that M2KO influenza virus that lacks the transmembrane and cytoplasmic tail domains of the M2 protein can be used as a live attenuated influenza vaccine. It appears to be considerably safe and strongly immunogenic. Moreover, the growth profile of this virus is indistinguishable from that of the parent virus (PR8) in cells stably expressing the wild-type M2 protein, suggesting the feasibility of efficient vaccine production, although the cell line expressing the M2 protein will need to be validated for the lack of unwanted properties, such as the presence of adventitious agents and tumorigenicity, prior to its use in vaccine production for humans.
Embryonated hen eggs are currently used for the manufacture of the vaccine; however, they have potentially serious problems. One of the major problems is that cultivation of viruses in eggs can lead to the selection of variants that are antigenically distinct from viruses grown in mammalian cells (9, 19, 21). In addition, there is a risk of allergic sensitization and reactions to egg proteins present in vaccines made from embryonated eggs. However, isolation of human influenza viruses from mammalian cells allows one to obtain viruses that are closely related to those present in clinical specimens of influenza patients (10, 18, 20). In addition, it has been demonstrated that inactivated vaccines prepared from cell-grown viruses induce greater cross-reactive serum antibody and cellular responses or protect better than those made from egg-grown viruses compared in animal models (1, 3, 11, 28). The fact that M2KO virus amplification relies on cells stably expressing the wild-type M2 protein demonstrates its suitability for vaccine production and solves many of the problems associated with vaccines made from embryonated eggs.
There has always been a question regarding the safety of live influenza vaccines due to possible reassortment between field strains and attenuated vaccine strains during epidemics and pandemics. However, such concerns are unfounded because even if reassortment occurs, as long as the backbone virus for the live vaccines is less pathogenic than the field strains, the pathogenicity of the resultant reassortant would be the same or less than that of the field strain.
Recent outbreaks of highly pathogenic H5N1 avian influenza virus pose a serious threat to human health. Although several clinical trials using inactivated H5 vaccine have been conducted (2, 12, 16, 22, 25), the efficacy of inactivated vaccines against such highly pathogenic viruses in immunologically naïve populations remains unknown. It is important to explore live vaccines, which could provide better protective immunity than inactivated vaccines due to their ability to provide mucosal and cellular immune responses. Our M2KO vaccine presented here, together with the M2 cytoplasmic tail mutant vaccine that we recently reported (27), may set the stage for further development of live attenuated H5 influenza vaccines.
Acknowledgments
We thank Susan Watson for editing the manuscript and Krisna Wells and Martha McGregor for excellent technical assistance.
This work was supported by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases, by grants-in-aid for Scientific Research on Priority Areas Specially Promoted Research, by the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, and by a contract research fund from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Alymova, I. V., S. Kodihalli, E. A. Govorkova, B. Fanget, C. Gerdil, and R. G. Webster. 1998. Immunogenicity and protective efficacy in mice of influenza B virus vaccines grown in mammalian cells or embryonated chicken eggs. J. Virol. 724472-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 3671657-1664. [DOI] [PubMed] [Google Scholar]
- 3.Brühl, P., A. Kerschbaum, O. Kistner, N. Barrett, F. Dorner, and M. Gerencer. 2000. Humoral and cell-mediated immunity to Vero cell-derived influenza vaccine. Vaccine 191149-1158. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2007. Expansion of use of live attenuated influenza vaccine (FluMist) to children aged 2-4 years and other FluMist changes for the 2007-08 influenza season. Morb. Mortal. Wkly. Rep. 561217-1219. [Google Scholar]
- 5.Cox, N. J., and K. Subbarao. 1999. Influenza. Lancet 3541277-1282. [DOI] [PubMed] [Google Scholar]
- 6.Cox, R. J., K. A. Brokstad, and P. Ogra. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 591-15. [DOI] [PubMed] [Google Scholar]
- 7.Hay, A. J., A. J. Wolstenholme, J. J. Skehel, and M. H. Smith. 1985. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 43021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuki-Horimoto, K., T. Horimoto, T. Noda, M. Kiso, J. Maeda, S. Watanabe, Y. Muramoto, K. Fujii, and Y. Kawaoka. 2006. The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J. Virol. 805233-5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz, J. M., C. W. Naeve, and R. G. Webster. 1987. Host cell-mediated variation in H3N2 influenza viruses. Virology 156386-395. [DOI] [PubMed] [Google Scholar]
- 10.Katz, J. M., M. Wang, and R. G. Webster. 1990. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J. Virol. 641808-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz, J. M., and R. G. Webster. 1989. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J. Infect. Dis. 160191-198. [DOI] [PubMed] [Google Scholar]
- 12.Lin, J., J. Zhang, X. Dong, H. Fang, J. Chen, N. Su, Q. Gao, Z. Zhang, Y. Liu, Z. Wang, M. Yang, R. Sun, C. Li, S. Lin, M. Ji, Y. Liu, X. Wang, J. Wood, Z. Feng, Y. Wang, and W. Yin. 2006. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368991-997. [DOI] [PubMed] [Google Scholar]
- 13.Martin, K., and A. Helenius. 1991. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell 67117-130. [DOI] [PubMed] [Google Scholar]
- 14.McCown, M. F., and A. Pekosz. 2005. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J. Virol. 793595-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 969345-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 3571937-1943. [DOI] [PubMed] [Google Scholar]
- 17.Ohuchi, M., A. Cramer, M. Vey, R. Ohuchi, W. Garten, and H. D. Klenk. 1994. Rescue of vector-expressed fowl plague virus hemagglutinin in biologically active form by acidotropic agents and coexpressed M2 protein. J. Virol. 68920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson, J. S., J. S. Bootman, C. Nicolson, D. Major, E. W. Robertson, and J. M. Wood. 1990. The hemagglutinin of influenza B virus present in clinical material is a single species identical to that of mammalian cell-grown virus. Virology 17935-40. [DOI] [PubMed] [Google Scholar]
- 19.Robertson, J. S., C. W. Naeve, R. G. Webster, J. S. Bootman, R. Newman, and G. C. Schild. 1985. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology 143166-174. [DOI] [PubMed] [Google Scholar]
- 20.Robertson, J. S., C. Nicolson, J. S. Bootman, D. Major, E. W. Robertson, and J. M. Wood. 1991. Sequence analysis of the haemagglutinin (HA) of influenza A (H1N1) viruses present in clinical material and comparison with the HA of laboratory-derived virus. J. Gen. Virol. 722671-2677. [DOI] [PubMed] [Google Scholar]
- 21.Schild, G. C., J. S. Oxford, J. C. de Jong, and R. G. Webster. 1983. Evidence for host-cell selection of influenza virus antigenic variants. Nature 303706-709. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson, I., K. G. Nicholson, A. Colegate, A. Podda, J. Wood, E. Ypma, and M. Zambon. 2003. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 211687-1693. [DOI] [PubMed] [Google Scholar]
- 23.Sugrue, R. J., and A. J. Hay. 1991. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology 180617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi, K., and R. A. Lamb. 1994. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J. Virol. 68911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 3541343-1351. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe, T., S. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2001. Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity. J. Virol. 755656-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe, T., S. Watanabe, J. H. Kim, M. Hatta, and Y. Kawaoka. 2008. Novel approach to the development of effective H5N1 influenza A virus vaccine: use of M2 cytoplasmic tail mutants. J. Virol. 822486-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood, J. M., J. S. Oxford, U. Dunleavy, R. W. Newman, D. Major, and J. S. Robertson. 1989. Influenza A (H1N1) vaccine efficacy in animal models is influenced by two amino acid substitutions in the hemagglutinin molecule. Virology 171214-221. [DOI] [PubMed] [Google Scholar]