Abstract
The p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) play important roles in the host innate immune response. The protein kinase regulated by RNA (PKR) is implicated in p38 MAPK activation in response to proinflammatory signals in mouse embryonic fibroblasts. To test the role of PKR in the activation of p38 and JNK MAPKs in human cells following viral infection, HeLa cells made stably deficient in PKR by using an RNA interference strategy were compared to cells with sufficient PKR. The phosphorylation of both p38 and JNK in cells with sufficient PKR was activated following either infection with an E3L deletion (ΔE3L) mutant of vaccinia virus or transfection with double-stranded RNA (dsRNA) in the absence of infection with wild-type vaccinia virus. The depletion of PKR by stable knockdown impaired the phosphorylation of both p38 and JNK induced by either the ΔE3L mutant virus or dsRNA but not that induced by tumor necrosis factor alpha. The PKR-dependent activation of MAPKs in ΔE3L mutant-infected cells was abolished by treatment with cytosine β-d-arabinoside. The complementation of PKR-deficient cells with the human PKR wild-type protein, but not with the PKR catalytic mutant (K296R) protein, restored p38 and JNK phosphorylation following ΔE3L mutant virus infection. Transient small interfering RNA knockdown established that the p38 and JNK kinase activation following ΔE3L infection was dependent upon RIG-I-like receptor signal transduction pathway components, including the mitochondrial adapter IPS-1 protein.
The importance of PKR, the protein kinase regulated by double-stranded RNA (dsRNA), in the antiviral actions of interferon (IFN) is well established (33, 42, 46). The Pkr gene, constitutively expressed at low but variable levels in untreated and uninfected cells, is transcriptionally activated by IFN treatment and virus infection (33, 38). The PKR protein includes two distinct functional regions, regulatory and catalytic. The RNA binding activity of PKR that mediates kinase autoactivation is conferred by a repeated domain within the N-terminal region of PKR; the C-terminal region of PKR possesses the kinase catalytic subdomains required for enzymatic activity (12, 27, 31, 33). Among the substrates phosphorylated by PKR are the PKR protein itself through autophosphorylation (41), the α subunit of eukaryotic protein synthesis initiation factor 2 (eIF-2α) (32), and protein phosphatase 2A (49). The PKR protein, in addition to its role in translational control through eIF-2α phosphorylation, has been found to interact with components of signal transduction and transcriptional activation pathways, including STAT1, IκBα, and p53 (27, 31, 33). As a consequence of protein phosphorylation events and protein-protein interaction events, PKR is implicated as a modulator of signaling pathways in response to cellular stresses, including viral infection and treatment with dsRNA, bacterial lipopolysaccharide (LPS), and the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), hence playing important roles in a variety of physiologic processes, including cell proliferation and apoptosis (27, 31, 33, 37, 42, 46). Among the stress-associated kinases, p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) are activated by stresses including pathogen infection, UV irradiation, and treatment with dsRNA, LPS, and TNF-α (3, 47). p38 and JNK MAPKs are also key components in the host innate immune response, in part through the phosphorylation of the AP-1 family transcription factors c-Jun and ATF-2, required for robust transcriptional activation of some cytokine genes (14, 21). The activities of p38 and JNK MAPKs are also implicated as facilitators of apoptosis in some cell types in response to certain stimuli (3).
The PKR protein has been proposed previously to be directly involved in the p38 and JNK kinase signaling, based on the results of studies using cells derived from Pkr genetic knockout mice, including mouse embryonic fibroblasts (MEFs) and bone marrow macrophages, that have shown that PKR and the stress-activated MAPKs both contribute to stress-activated responses (2, 11, 13, 35, 37). But the role of PKR in the activation process of p38 MAPK implied from the findings of these studies remains controversial. For example, the PKR-dependent activation of p38 MAPK in mouse cells is described as occurring in response to bacterial endotoxin (13) and during skeletal muscle cell differentiation (2), but studies with Pkr null mouse cells have yielded conflicting results for a possible role of PKR in dsRNA-dependent signaling to activate p38 MAPK (13). Furthermore, while two groups found that PKR is required for p38 MAPK activation by LPS, poly(I:C), or TNF-α in both primary and immortalized MEFs and that PKR is also required for JNK activation but only in primary MEFs (2, 11, 13), another group reported an apparently contradictory finding that the depletion of PKR in the Pkr−/− MEF cells potentiates p38 MAPK activation but inhibits JNK activation in response to TNF-α (37).
The vaccinia virus E3L gene encodes two proteins synthesized early during infection which possess Z-DNA binding and dsRNA binding activities, conferred by the N-terminal and C-terminal domains, respectively, of E3L (17). The E3L proteins are important modulators of virus-host interactions. First identified as an IFN resistance gene, E3L is now recognized as a determinant of host range and viral pathogenesis and a modulator of cellular apoptotic and signal transduction pathways (18, 19, 22, 51). Furthermore, the vaccinia virus E3L proteins are known to suppress proinflammatory signal transduction responses, including those mediated by p38 MAPK (19). However, the role of the PKR kinase in the E3L-mediated modulation of p38 activation is unknown. Curiously, both in primary mouse keratinocytes (9) and in established human cell lines (53), the activation of transcription factor IRF-3 is impaired in cells infected with wild-type (WT) vaccinia virus but activated in those infected with an E3L deletion mutant virus through a pathway involving cytoplasmic RNA sensors that signal through the mitochondrial adapter protein IPS-1 (9, 53).
As an approach to clarify the contradictory observations gained from studies of mouse cells and to gain insight into the importance of the PKR protein in MAPK activation in human cells, we utilized our previously established PKR-deficient human cell lines (52) to examine the PKR dependency of p38 and JNK MAPK activation in response to different stimuli. Our results reveal that in human HeLa and human amnion U cell lines, the stable knockdown of PKR by short hairpin RNA (shRNA) interference impaired both p38 and JNK MAPK activation induced by infection with vaccinia virus E3L mutants or by transfection with dsRNA. The complementation of knockdown cells with WT human PKR (WT hPKR), but not with the catalytically inactive K296R PKR mutant, restored the activation of p38 and JNK MAPKs by vaccinia virus infection and dsRNA transfection. Furthermore, MAPK activation induced by vaccinia virus required components of the RIG-I-like receptor (RLR) pathway, including the dsRNA cytosolic sensor mda-5 and the mitochondrial adapter IPS-1.
MATERIALS AND METHODS
Cells, reagents, and viruses.
HeLa and U cells were maintained in Dulbecco's modified Eagle's medium complemented with 5 or 10% (vol/vol) fetal bovine serum (HyClone), 100 μg/ml of penicillin, and 100 U/ml streptomycin (Invitrogen). HeLa cells with the stable knockdown of PKR (PKRkd cells) and PKR knockdown control HeLa cells with sufficient PKR (PKRkd-con cells) were described previously (52); they were maintained in the above-described medium with the addition of 1 μg/ml puromycin (Sigma) (52). Stable PKR knockdown lines of human amnion U cells were also generated using the strategy and targeting construct described earlier for the generation of the HeLa PKRkd cells (52). Briefly, U cells were transfected with the hPKR shRNA pSUPER.retro.puro construct by using Lipofectamine 2000 (Invitrogen). Transfected U cells were trypsinized at 24 h, seeded at various dilutions, and then maintained in the presence of 1 μg/μl puromycin (Sigma) for 4 weeks. Puromycin-resistant U cell clones were isolated and screened by Western immunoblot analysis for PKR protein knockdown. TNF-α and LPS from Salmonella enterica serotype Typhimurium were purchased from Sigma (St. Louis, MO). The Copenhagen strain (VC-2) of WT vaccinia virus and virus mutants with the deletion of the E3L gene (ΔE3L), the 83 N-terminal amino acids of E3L (Δ83N), or the 26 C-terminal amino acids of E3L (Δ26C) were described previously (5, 6, 51). Treatment with cytosine β-d-arabinofuranoside (Ara C [Sigma]; 10 μg/ml) was initiated 2 h before virus infection and was maintained throughout the 6-h incubation period. Vaccinia viruses were grown in baby hamster kidney (BHK21) cells, and their titers were determined on rabbit kidney (RK13) cells. Virus infections of HeLa and U cell lines were carried out at a multiplicity of infection of 5.
The mammalian expression plasmid expressing WT hPKR (pSG5-WTRSC) was generously provided by S. Rothenburg (NIH, MD), and the plasmid expressing the catalytic activity-deficient mutant hPKR (pcDNA6-K296RRSC) was generated from pcDNA6-WTRSC by mutagenesis to encode the K296R substitution in kinase catalytic subdomain II (39, 40). These two modified knockdown-resistant PKR expression plasmids were mutated at synonymous sites in the shRNA-targeted sequence (GCAGGGAGTAGTACTTAAATA) present in the hPKR open reading frame (52) in order to circumvent knockdown by the stably expressed silencing shRNA.
Transient siRNA knockdown.
The sequences targeted by chemically synthesized small interfering RNAs (siRNAs; Dharmacon) in transient knockdown experiments were as follows (53): firefly luciferase gene, ACTTACGCTGAGTACTTCGA; TRIF gene, GACCAGACGCCACTCCAAC; IPS-1 gene, TAGTTGATCTCGCGGACGA; RIG-I gene, GGAAGAGGTGCAGTATATT; mda-5 gene, GGTGAAGGAGCAGATTCAG; and PKR gene, GCAGGGAGTAGTACTTAAATA. A double-transfection approach was used to achieve maximal knockdown of the target proteins. Briefly, HeLa cells at 30 to 50% confluence were transfected with 50 pmol of siRNA per well (in a 12-well plate) by using Lipofectamine 2000 (Invitrogen). At 48 h after the first transfection, cells were reseeded into 12-well plates at 1.5 × 105 cells per well. After overnight incubation, the second transfection with siRNA was performed, and 48 h after the second transfection, cells were infected with vaccinia virus at a multiplicity of infection of 5.
Western immunoblot analysis.
Whole-cell extracts were prepared in the presence of 1 mM phenylmethylsulfonyl fluoride, 1% (vol/vol) protease inhibitor cocktail (Sigma), and 1% (vol/vol) phosphatase inhibitor cocktail (Sigma). Sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and Western immunoblot analyses were performed as described previously (51-53). Rabbit polyclonal antibodies were used to detect p38 (Santa Cruz Biotechnology Inc.), phospho-p38 (Cell Signaling Technology), JNK (Santa Cruz Biotechnology Inc.), phospho-JNK (Cell Signaling Technology), human IPS-1 (Bethyl Laboratories Inc), human TRIF (Alexis Biochemicals Inc.), hPKR (Santa Cruz Biotechnology Inc.), PKR phosphorylated at Thr446 [phospho(Thr446)-PKR; Santa Cruz Biotechnology Inc.], eIF-2α (Cell Signaling Technology), and eIF-2α phosphorylated at Ser51 (Cell Signaling Technology). Mouse monoclonal antibodies were used to detect poly(ADP-ribose) polymerase (BD Pharmingen), β-actin (Sigma), and α-tubulin (Sigma). The monospecific antibody against vaccinia virus I3 protein was generously provided by P. Tracktman (Medical College of Wisconsin, Milwaukee), and that against E3L was as described previously (44). Western blot detection was performed with IRDye 800CW-conjugated anti-rabbit immunoglobulin G or IRDye 680-conjugated anti-mouse immunoglobulin G secondary antibody according to the protocol of the manufacturer (LI-COR). Immunoreactive bands were visualized using an Odyssey infrared imaging system.
RESULTS
The activation of p38 and JNK MAPKs is enhanced by PKR and antagonized by E3L.
The vaccinia virus E3L gene products are known to suppress proinflammatory signal transduction responses and to affect the activation of p38 MAPK in human HeLa cells (19). To test whether PKR plays a role in MAPK activation following vaccinia virus infection, WT and E3L mutant viruses in HeLa cells with sufficient PKR and in PKRkd cells generated by an shRNA interference silencing strategy were examined. The PKRkd cells possess <5% of the PKR protein present in parental (PKR+) or puromycin-resistant PKRkd-con cells (51, 52).
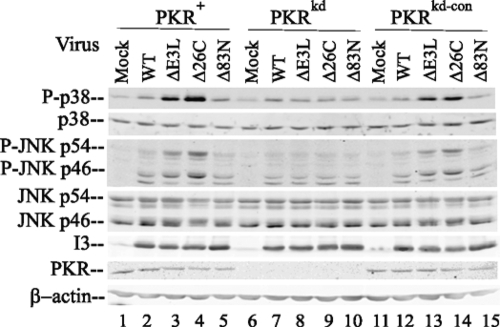
As shown in Fig. 1, the phosphorylation of both p38 and JNK in cells with sufficient PKR following infection with mutant viruses either lacking E3L completely (the ΔE3L mutant) or lacking only the C-terminal 26-amino-acid (aa) region of E3L including the dsRNA binding domain (the Δ26C mutant) was substantially increased over that in mock-infected cells. Following infection with ΔE3L and Δ26C viruses, the large increases in the phosphorylation of p38 and JNK, 7- to 12-fold and 5- to 10-fold, respectively (Fig. 1, lanes 3 and 4 and lanes 13 and 14), seen in the PKR+ and PKRkd-con cells, which have sufficient PKR, were not seen in PKRkd cells (in which the increases were 1.5- to 2-fold) (Fig. 1, lanes 8 and 9). Likewise, the levels of phosphorylation of both p38 and JNK in cells with sufficient PKR following infection with WT vaccinia virus or the Δ83N E3L mutant virus lacking the N-terminal 83-aa region that includes the Z-DNA binding domain of E3L remained low and similar to those in uninfected cells (Fig. 1). Compared to the levels of phosphorylation in uninfected cells, the increase in p38 and JNK phosphorylation in cells infected with these viruses was only ∼1.5-fold (Fig. 1, lanes 2, 5, 7, and 10 and lanes 12 and 16). Previously published results have demonstrated p38 and JNK phosphorylation in HeLa cells infected with Δ83N E3L mutant virus, but at a later time postinfection than in this experiment (19). As a control for infection, the levels of expression of vaccinia virus I3 protein, a 34-kDa single-stranded DNA binding protein expressed at early times after infection (28), were comparable in cells infected with the WT and all three E3L mutant viruses, both PKR-deficient (PKRkd) cells and cells with sufficient PKR (PKR+ and PKRkd-con cells). Furthermore, the levels of p38 MAPK and the p46 JNK and p54 JNK proteins were similar in WT- and mutant virus-infected cells and comparable to those in uninfected cells. The level of PKR protein in the PKRkd cells, as measured by a Western immunoblot assay, remained low after infection relative to that in cells with sufficient PKR (Fig. 1) as described previously for even IFN-treated cells (51). These results suggest that PKR plays a role in the process of vaccinia virus-induced phosphorylation of p38 and JNK proteins and that the C-terminal region of E3L likely impairs these PKR-dependent modifications of p38 and JNK MAPKs.
FIG. 1.
PKR-dependent MAPK activation is antagonized by the vaccinia virus E3L protein. Whole-cell extracts were prepared from uninfected HeLa cells (mock) or HeLa cells infected with either WT vaccinia virus or one of the following E3L mutant viruses at 6 h postinfection: the ΔE3L deletion mutant; the Δ26C mutant, lacking the RNA binding domain; and the Δ83N mutant, lacking the Z-DNA binding domain. The cells included PKR+ and PKRkd-con cells, which had sufficient PKR, and the PKR-deficient PKRkd cells as indicated. Western immunoblot analyses (with 30 μg of protein per lane) were performed with antibodies against phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, vaccinia virus I3, PKR, and β-actin as a loading control.
Vaccinia virus induction of p38 and JNK phosphorylation is blocked by Ara C treatment.
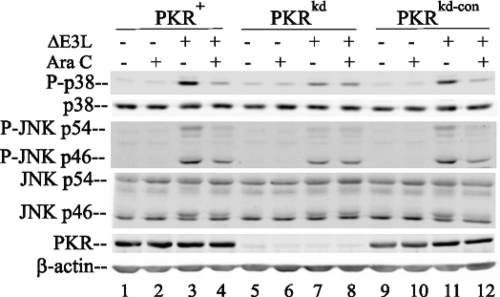
Because PKR knockdown in HeLa cells inhibited the phosphorylation of p38 and JNK induced by infection with mutant viruses with the dsRNA binding region of E3L deleted (Fig. 1) and because the cellular PKR kinase binds dsRNA, as does the viral E3L protein (5, 24, 33), we presumed that viral dsRNA is likely a trigger of the enhanced phosphorylation of the p38 and JNK MAPKs seen in vivo in ΔE3L and Δ26C virus-infected cells with sufficient PKR. To further test the notion that vaccinia virus dsRNA is a key factor in PKR-dependent MAPK activation, we examined the effect of Ara C treatment on the phosphorylation of p38 and JNK induced by E3L mutant virus infection. The pharmacologic agent Ara C inhibits DNA replication and reduces viral dsRNA production by ∼85% (7, 53). As shown in Fig. 2, treatment with Ara C reduced the phosphorylation of both p38 and JNK MAPKs in ΔE3L mutant-infected PKR+ and PKRkd-con cells, which have sufficient PKR, to a level near that in uninfected cells (Fig. 2, lanes 3 and 4 and lanes 11 and 12) but did not alter the p38 or JNK phosphorylation status in PKRkd cells (Fig. 2, lanes 7 and 8). As a control, the treatment of uninfected HeLa cells, either those with sufficient PKR or PKR-deficient cells, with Ara C had no measurable effect on the phosphorylation pattern of either p38 or JNK (Fig. 2, lanes 2, 6, and 10).
FIG. 2.
Ara C blocks PKR-dependent MAPK activation in ΔE3L mutant-infected cells. Whole-cell extracts were prepared from uninfected (−) or ΔE3L virus-infected (+) HeLa cells at 6 h after infection, either left untreated (−) or treated (+) with Ara C, and analyzed by a Western immunoblot assay with antibodies against phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, PKR, and β-actin as a loading control.
PKR dependency of MAPK activation in human amnion U cells.
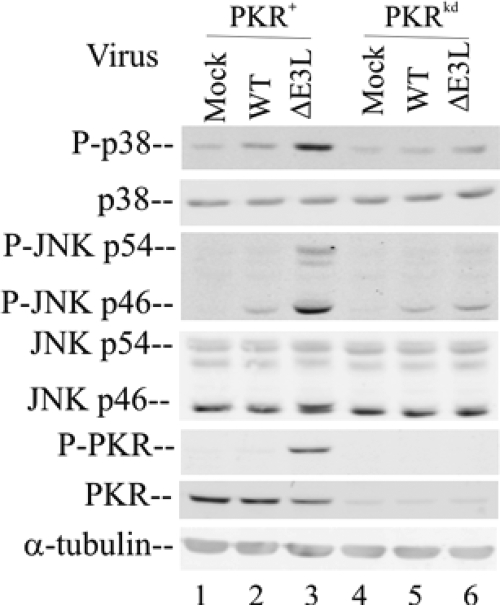
Our results obtained with a HeLa cell line with the stable knockdown of PKR provide strong evidence that the hPKR protein is an important mediator of MAPK activation in these cells following infection with E3L mutant vaccinia virus (Fig. 1 and 2). To test the generality of the PKR dependency of p38 and JNK activation following infection, we established a PKR knockdown line from a second human cell line, human amnion U cells, from which we had previously isolated a PKR cDNA that we then characterized (24, 34, 40). The same shRNA interference silencing strategy and targeting sequence that were used to generate the HeLa PKRkd cells (52) were successfully employed to generate a U cell line with the stable knockdown of the PKR protein. As measured by Western analysis, the PKRkd U cell clone showed <10% of the PKR protein seen in the parental PKR+ U cells in the absence of IFN treatment (Fig. 3). While the amount of phospho(Thr446)-PKR was greatly increased following the infection of the PKR+ parental U cells with the ΔE3L virus (Fig. 3, lane 3), little increase in the phosphorylation of PKR in WT-virus infected U cells with sufficient PKR was seen (Fig. 3, lane 2). The amounts of both the PKR protein and, following vaccinia virus infection, the phospho(Thr446)-PKR protein in the PKRkd U cells were extremely small and marginally detectable (Fig. 3, lanes 4 to 6).
FIG. 3.
The activation of p38 and JNK MAPKs in human amnion U cells is enhanced by PKR. Whole-cell extracts were prepared from either uninfected (mock) or ΔE3L vaccinia virus-infected U cells at 6 h after infection and were analyzed by Western immunoblotting (with 30 μg of protein per lane) with antibodies against phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, phospho-PKR (P-PKR), PKR, and α-tubulin as a loading control.
The PKR knockdown U cells displayed the same MAPK activation phenotype seen in HeLa cells: the phosphorylation of both p38 and JNK was greatly impaired following ΔE3L mutant virus infection of PKRkd U cells (Fig. 3, lane 6) compared to the increased phosphorylation of p38 and JNK seen in ΔE3L virus-infected PKR+ U cells (Fig. 3, lane 3). WT vaccinia virus infection did not significantly increase the phosphorylation of either p38 or JNK in the PKR+ U cells (Fig. 3), a result similar to our findings for HeLa cells (Fig. 1). The levels of p38 and JNK proteins in both PKR+ and PKRkd U cells with and without infection were comparable (Fig. 3), again similar to those in HeLa cells (Fig. 1).
PKR plays a stimulus-dependent role in the activation of MAPK phosphorylation.
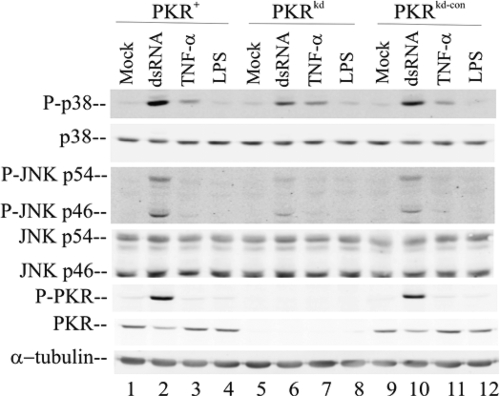
Because the activation of p38 and JNK was increased in a PKR-dependent manner following the infection of HeLa and U cells with vaccinia virus in which the dsRNA binding protein E3L was deleted, we examined the role of PKR in the activation of these same MAPKs in response to stress stimuli other than viral infection. As shown in Fig. 4, transfection with the synthetic dsRNA poly(I:C) enhanced the phosphorylation of both p38 and JNK in HeLa cells. The most pronounced increases in phosphorylation were those in cells with sufficient PKR, approximately 17- and 8-fold, respectively, for p38 and JNK (Fig. 4, lanes 2 and 10). In the dsRNA-stimulated PKR-deficient cells, the increases in phosphorylation were somewhat smaller, about 8- and 3-fold, respectively (Fig. 4, lane 6). Treatment with TNF-α increased p38 phosphorylation only modestly in a PKR-independent manner and did not alter JNK phosphorylation in any of the cells (Fig. 4, lanes 3, 7, and 11). Likewise, LPS treatment of the HeLa cells, either those with sufficient PKR or PKR-deficient cells, did not significantly activate the p38 or JNK kinases (Fig. 4, lanes 4, 5, and 12). Finally, as an independent beacon of the dsRNA, TNF-α, and LPS stimuli, the phosphorylation status of PKR was measured using the phospho(Thr446)-PKR antibody. dsRNA transfection effectively activated PKR Thr446 phosphorylation in the HeLa cells with sufficient PKR (Fig. 4, lanes 2 and 10), while neither TNF-α nor LPS treatment did (Fig. 4, lanes 3 and 4, 7 and 8, and 11 and 12).
FIG. 4.
dsRNA-mediated activation of MAPK phosphorylation is enhanced by PKR. Whole-cell extracts were prepared from cells with sufficient PKR (PKR+ and PKRkd-con cells) and PKRkd HeLa cells that were untreated (mock), transfected with 3 μg/ml poly(I:C) (dsRNA) by using Lipofectamine 2000, or treated with 10 ng/ml TNF-α or 100 ng/ml LPS for 2 h. Immunoblot analyses were carried out (with 30 μg of protein) with antibodies against phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, phospho-PKR (P-PKR), PKR, and α-tubulin as a loading control.
PKR-dependent activation of p38 and JNK requires the kinase activity of PKR.
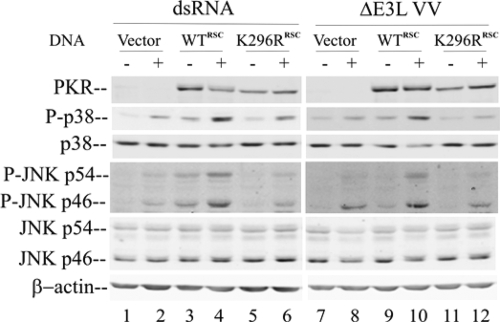
As an approach to begin to assess whether PKR catalytic activity is required to modulate the activation of the p38 and JNK MAPKs in human cells or whether the PKR protein without catalytic activity is sufficient, we expressed either WT hPKR or a catalytic activity-defective PKR mutant (the K296R mutant) in the PKRkd HeLa cells and then either treated them with dsRNA or infected them with the ΔE3L mutant. The PKR expression plasmids, both pSG5-WTRSC and pcDNA6-K296RRSC, were mutated (without altering the PKR coding sequence) in positions targeted by the silencing RNA in order to circumvent the knockdown of the plasmids in transfected cells by the stably expressed short hairpin silencing RNA.
As shown in Fig. 5, the expression of both the WT (lanes 3 and 4 and lanes 9 and 10) and K296R (lanes 5 and 6 and lanes 11 and 12) proteins, to comparable levels, was achieved by using different-strength promoters, pSG5 for the WT and pcDNA6 for the K296R protein. As a control, the cells transfected with the empty vector (Fig. 5, lanes 1 and 2 and lanes 7 and 8) did not show detectable PKR protein. The complementation of the PKRkd cells with the WT PKR protein (Fig. 5, lanes 4 and 10), but not with the K296R mutant (Fig. 5, lanes 6 and 12), restored the phosphorylation of p38 and JNK MAPKs either by dsRNA treatment or by ΔE3L mutant infection. These results suggest that the kinase activity of hPKR is required for MAPK activation. Interestingly, in the absence of the stress stimulus of transfection with dsRNA or virus infection, the expression of the WT hPKR in transfected PKRkd HeLa cells led to slightly higher levels of phosphorylated MAPKs (Fig. 5, lanes 3 and 9) than those in cells transfected with either the empty vector or the K296R PKR mutant plasmid (Fig. 5, lanes 1 and 5 and lanes 7 and 11), further indicating that the catalytically active PKR protein expressed ectopically is sufficient to mediate the activation of the p38 and JNK MAPK pathway.
FIG. 5.
The expression of hPKR in PKRkd cells rescues MAPK activation by ΔE3L mutant infection. PKRkd HeLa cells were transfected with equimolar amounts of empty vector or the PKR WT (WTRSC) or PKR catalytic mutant (K296RRSC) expression construct. At 24 h after transfection, cells were either transfected with dsRNA or infected with the vaccinia virus ΔE3L mutant. (Left panel) Cells were mock transfected (−) or transfected with dsRNA (+) for 2 h; (right panel) cells were mock infected (−) or infected with ΔE3L virus (+) for 6 h. Whole-cell extracts were prepared and analyzed by Western immunoblotting (with 30 μg per lane). Membranes were probed with antibodies against PKR, phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, and β-actin as a loading control.
PKR mediates MAPK activation through the RLR signaling pathway and the IPS-1 adapter.
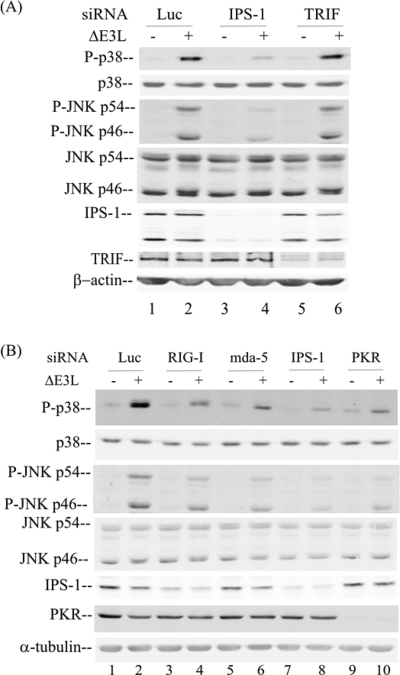
Both the cytoplasmic RLRs (26, 50) and membrane-bound Toll-like receptor 3 (TLR3) (43) sense viral dsRNA to initiate the host innate immune response. To test which of these dsRNA sensors may be involved in the PKR-dependent MAPK activation, we transiently knocked down the corresponding adapter proteins, IPS-1 for RLR (15) and TRIF for TLR3 (43), using chemically synthesized siRNAs. As shown in Fig. 6A, the knockdown of IPS-1 (lane 4), but not that of TRIF (lane 6), impaired the activation of p38 and JNK MAPKs in response to ΔE3L virus infection. At 6 h after ΔE3L virus infection, the phosphorylation of both p38 and JNK was increased in the HeLa cells with sufficient PKR that had been transfected with the control siRNA against luciferase (Fig. 6A, lane 2) or siRNA against TRIF (Fig. 6A, lane 6).
FIG. 6.
The activation of MAPKs following infection with ΔE3L vaccinia virus occurs through RLR signaling. Whole-cell extracts were prepared from parental PKR+ HeLa cells, either uninfected (−) or infected with ΔE3L vaccinia virus (+) for 6 h, following siRNA transient knockdown by chemically synthesized siRNAs as described in Materials and Methods. (A) Analysis of the effects of siRNAs against luciferase (Luc) as a control, IPS-1, and TRIF. (B) Analysis of the effects of siRNAs against luciferase as a control, RIG-I, mda-5, IPS-1, and PKR. Western immunoblot analyses were carried out with antibodies against phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), JNK, PKR, IPS-1, TRIF, and either β-actin (A) or α-tubulin (B) as a loading control.
Next, we examined whether the RIG-I or the mda-5 sensor is the major component involved in the recognition of dsRNA and signaling through the IPS-1 adapter following ΔE3L virus infection. The transfection of cells with siRNA against either RIG-1 (Fig. 6B, lane 4) or mda-5 (Fig. 6B, lane 6) partially reduced p38 and JNK phosphorylation induced by ΔE3L infection compared to that in cells transfected with siRNA against luciferase. In addition, the transient knockdown of PKR in the PKR+ HeLa cells impaired the phosphorylation of the p38 and JNK MAPKs induced by infection with the ΔE3L mutant (Fig. 6B, lane 10), consistent with our results obtained with the stable PKR knockdown cell line (Fig. 1 and 2). The transient knockdowns were verified by Western immunoblot analyses (Fig. 6), which revealed a reduction of more than 80% in the steady-state levels of the targeted proteins, including IPS-1 (Fig. 6A, lanes 3 and 4, and B, lanes 7 and 8), TRIF (Fig. 6A, lanes 5 and 6), and PKR (Fig. 6B, lanes 9 and 10). Although inadequate antibody reagents to assess the knockdown of endogenous RIG-I and mda-5 in human cells were available, functional evidence consistent with their loss in addition to the knockdown of their adapter IPS-1, which was verified by Western immunoblot analysis, was obtained in the observed effect on MAPK activation (Fig. 6).
DISCUSSION
The objective of our study described herein was to determine the role of the PKR kinase in MAPK activation and signaling in human cells either transfected with dsRNA or infected with vaccinia virus. We found, utilizing PKR-deficient human cell lines, that the PKR protein is an important mediator of p38 and JNK activation in cells infected with vaccinia virus or transfected with dsRNA and that the vaccinia virus E3L gene function effectively antagonized MAPK activation. Furthermore, PKR catalytic activity was required for the enhanced activation of p38 and JNK, and importantly, RLR signaling pathway components, including the mitochondrial adapter protein IPS-1, mediated the PKR-dependent activation of MAPKs by transfection with dsRNA and virus infection.
In two types of human cells (HeLa and U) in which the knockdown of PKR expression was achieved either stably or transiently, the phosphorylation of p38 and JNK was impaired following infection with ΔE3L mutant vaccinia virus. The knockdown of PKR protein expression in these human cell lines was achieved using two different strategies: the generation of cells stably deficient in PKR by an shRNA interference approach for HeLa cells (Fig. 1) and amnion U cells (Fig. 3) and the use of chemically synthesized siRNA to transiently knock down PKR expression (Fig. 6). The results for independent cell lines and approaches, all of which demonstrated PKR dependency for MAPK activation, argue against either off-target effects of the silencing or clonal variance of the knockdown and indicate that the PKR protein is indeed required to achieve maximal activation of p38 and JNK MAPKs.
Several lines of evidence point toward PKR as a sensor of viral dsRNA and a subsequent mediator of the p38 and JNK activation seen following vaccinia virus infection of HeLa or U cells with sufficient PKR but not PKR-deficient HeLa or U cells. Robust activation of the phosphorylation of both p38 and JNK was seen in cells with sufficient PKR that were infected with vaccinia virus deletion mutants either not expressing the E3L protein (the ΔE3L mutant) or expressing a C-terminally truncated E3L protein that lacks the dsRNA binding domain (the Δ26C mutant). While MAPK phosphorylation in cells with sufficient PKR that were infected with these E3L mutants was enhanced, infection with either WT virus or an N-terminally truncated E3L (Δ83N) mutant, both of which express E3L proteins that possess dsRNA binding activity (6, 19), led to little, if any, enhancement of MAPK phosphorylation. The observation that Ara C treatment impaired the activation of p38 and JNK in ΔE3L mutant virus-infected cells implies that transcription following DNA replication enhances the production of viral RNA at a concentration and with the structural properties suitable to mediate MAPK activation and PKR activation (53). Our findings for the activation of MAPK phosphorylation by ΔE3L and Δ26C mutant viruses but not WT or Δ83N mutant virus at 6 h after infection are fully consistent with prior observations regarding the role of E3L proteins in the suppression of proinflammatory signal transduction (19).
The depletion of PKR by stable or transient knockdown largely abolished the MAPK activation by ΔE3L mutant infection or dsRNA transfection, establishing PKR as a key mediator of the p38 and JNK MAPK signaling pathway in human cells. These findings suggest an additional molecular mechanism by which the vaccinia virus E3L protein interferes with signal transduction relevant to IFN induction—by inhibiting the activation of p38 and JNK MAPKs, in addition to antagonizing the activation of the transcription factor IRF-3 (48, 53). The substantial loss of p38 and JNK phosphorylation in ΔE3L mutant virus-infected PKRkd cells is consistent with the notion that PKR, not other dsRNA binding proteins, is the principal cellular determinant of the phenotype characteristic of ΔE3L mutant virus-infected cells (51). The modest MAPK phosphorylation still seen in the PKRkd cells transfected with dsRNA or infected with ΔE3L virus may be attributed either to the residual 2 to 5% PKR that remains in the PKRkd cells or alternatively to the presence of a redundant PKR-independent MAPK signaling pathway, such as that mediated by RNase L (13, 20), activated by dsRNA or ΔE3L mutant virus.
Our data indicate that not only the PKR protein per se, but also catalytically active PKR is required to mediate the activation of MAPKs in human cells. PKR kinase activity was found to be indispensable in PKR-mediated MAPK signaling in a complementation assay. When expressed ectopically in PKRkd cells, the catalytically inactive K296R mutant protein failed to autoactivate, as indicated by the lack of Thr446 phosphorylation, and importantly, the mutant K296R protein also failed to restore p38 and JNK phosphorylation following either dsRNA transfection or ΔE3L mutant virus infection. In contrast, the expression of WT PKR in the PKRkd cells was sufficient to mediate increased phosphorylation of p38 and JNK MAPKs, in addition to yielding clearly detectable Thr446 autophosphorylation of PKR in the absence of any other inducers such as dsRNA transfection or ΔE3L virus infection. Finally, the phosphorylation of PKR in TNF-α- or LPS-treated cells with sufficient PKR was either not or only minimally enhanced, and this result correlated with the very weak activation of MAPK phosphorylation, even though normal levels of PKR protein were present, consistent with the notion that PKR protein alone is unable to mediate the activation.
Vaccinia virus is a DNA virus that multiplies in the cytoplasm of infected cells (25), and dsRNA has been detected in situ in vaccinia virus-infected cells (4, 45). We therefore tested whether PKR functioned in the RLR pathway that senses cytosolic dsRNA (50) or the TLR3 pathway that senses endosomal dsRNA (43) for the activation of p38 and JNK. We found that the transient knockdown of IPS-1, the mitochondrial adapter for RLR signaling, nearly completely abolished the PKR-enhanced phosphorylation of p38 and JNK induced by infection with ΔE3L virus. In contrast, the knockdown of TRIF, the adapter for TLR3, had no effect. These results indicate that the sensing of vaccinia virus infection, leading to the enhanced phosphorylation of the MAPKs, occurred predominantly if not exclusively through the cytoplasmic helicases RIG-I and/or mda-5 and not through the membrane-bound TLR3 sensor. These findings for p38 and JNK mirror our observations regarding the PKR-dependent activation of IRF-3 in ΔE3L virus-infected cells, which likewise occurs through adapter IPS-1 signaling (53), and the observations of Deng et al. (9), who found that the response of mouse keratinocytes to ΔE3L mutant vaccinia virus is unaffected by TRIF or MyD88 loss but that the IPS-1/MAVS adapter is required to generate an innate immune response. Our findings, following the transient knockdown of RIG-I and mda-5 individually and then in combination, are consistent with the possibility that mda-5 and perhaps also RIG-I sense vaccinia virus dsRNA.
How does PKR function to enhance the activation of p38 and JNK in response to dsRNA transfection or vaccinia virus infection in an IPS-1-dependent manner? One possibility is through protein-protein interaction with an RLR signal transduction pathway component, for example, TNF receptor-associated factor 6 (TRAF6). The PKR protein possesses two putative TRAF-interacting motifs and physically interacts in vivo with TRAF proteins, a family of adapter molecules linking different pathways with IκB kinase activation (10). The TRAF-PKR binding is dependent on PKR dimerization (9). PKR may mediate the p38 and JNK activation through interaction with the upstream protein TRAF6. A second potential mechanism involves direct PKR interaction with MAPK kinases (MAPKKs). PKR is reported to interact with MAPKK6 in MEFs and to regulate the phosphorylation of MAPKK6 and its downstream p38 MAPK in a dsRNA-dependent manner (35). A third possibility is that PKR interacts directly with p38 or JNK MAPKs. Alisi et al. and Spaziani et al. reported that PKR physically interacts with the p38 protein and that this interaction requires both the N- and C-terminal regions of PKR (2, 36). Among these three PKR-protein interaction models, we favor that of the interaction between PKR and TRAF as the most likely mechanism by which PKR regulates the p38 and JNK MAPK activation by dsRNA, based on our observations and those of others that PKR is required for the activation of multiple downstream transcription factors, including IRF-3, NF-κB, and ATF-2/c-Jun, in the RLR signaling pathway induced by dsRNA (1, 4, 16, 23, 53).
Our results obtained with human cells may be related to those of previously reported studies with PKR null mouse cells that yielded seemingly inconsistent and apparently contradictory observations on the role of PKR in MAPK activation (2, 11, 12, 35, 37). Our findings for human cells with the stable knockdown of PKR are consistent with some results obtained with PKR null MEF cells (11, 12, 37); for example, in both human and mouse cells, PKR enhances p38 MAPK activation in response to dsRNA. However, the activation of p38 by TNF-α in HeLa cells was not PKR dependent, unlike that in MEF cells, in which PKR genetic disruption has been reported to both impair (11, 12) and potentiate (37) p38 activation in response to TNF-α. Whether these observed differences reflect differences between MEF and HeLa cells or mouse PKR and hPKR proteins or simply the conditions of culture is unclear. Studies with poxvirus K3L suggest that mouse PKR and hPKR proteins are differentially antagonized by the K3L viral pseudosubstrate (30), and it has long been known that the mouse PKR protein, at 517 aa, is smaller than the hPKR protein, at 551 aa (29, 39). We earlier did not find a requirement for PKR in TNF-α-triggered eIF-2α phosphorylation in HeLa cells, in contrast to that reported to have been found in MEFs (52). And neither HeLa cells with sufficient PKR nor PKR-deficient HeLa cells were responsive to bacterial LPS as a p38 activator, presumably because these cells are deficient in the LPS receptor TLR4 (8).
Taken together, our findings have established that in cultured human cell lines, the PKR protein and its catalytic activity are required for the activation of the p38 and JNK MAPKs by stresses including dsRNA transfection and vaccinia virus infection. Furthermore, the RLR signaling components, including IPS-1, are required for PKR-mediated MAPK phosphorylation. The role of PKR in regulating stress-activated MAPKs, in addition to the well-established role of the phosphorylation of eIF-2α, provides an explanation for how PKR functions in a multiple different cellular stress responses, including apoptosis, innate antiviral immune responses, and cell proliferation.
Acknowledgments
This work was supported in part by research grants AI-12520 and AI-20611 (C.E.S.) and AI-52347 and AI-66326 (B.L.J.) from the National Institute of Allergy and Infectious Diseases, NIH, Public Health Service.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 2.Alisi, A., A. Spaziani, S. Anticoli, M. Ghidinelli, and C. Balsano. 2008. PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cell. Signal. 20534-542. [DOI] [PubMed] [Google Scholar]
- 3.Ashwell, J. D. 2006. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6532-540. [DOI] [PubMed] [Google Scholar]
- 4.Bayliss, C. D., and R. C. Condit. 1993. Temperature-sensitive mutants in the vaccinia virus A18R gene increase double-stranded RNA synthesis as a result of aberrant viral transcription. Virology 194254-262. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology 194537-547. [DOI] [PubMed] [Google Scholar]
- 6.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 696605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colby, C., C. Jurale, and J. R. Kates. 1971. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J. Virol. 771-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Correia, J., and R. J. Ulevitch. 2002. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 2771845-1854. [DOI] [PubMed] [Google Scholar]
- 9.Deng, L., P. Dai, T. Parikh, H. Cao, V. Bhoj, Q. Sun, Z. Chen, T. Merghoub, A. Houghton, and S. Shuman. 2008. Vaccinia virus subverts a mitochondrial antiviral signaling protein-dependent innate immune response in keratinocytes through its double-stranded RNA binding protein, E3. J. Virol. 8210735-10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil, J., M. A. Garcia, P. Gomez-Puertas, S. Guerra, J. Rullas, H. Nakano, J. Alcami, and M. Esteban. 2004. TRAF family proteins link PKR with NF-κB activation. Mol. Cell. Biol. 244502-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh, K. C., M. J. deVeer, and B. R. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 194292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 20617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsoulidis, E., Y. Li, H. Mears, and L. C. Platanias. 2005. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J. Interferon Cytokine Res. 25749-756. [DOI] [PubMed] [Google Scholar]
- 15.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6981-988. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 16406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299133-141. [DOI] [PubMed] [Google Scholar]
- 18.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324419-429. [DOI] [PubMed] [Google Scholar]
- 19.Langland, J. O., J. C. Kash, V. Carter, M. J. Thomas, M. G. Katze, and B. L. Jacobs. 2006. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J. Virol. 8010083-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, G., Y. Xiang, K. Sabapathy, and R. H. Silverman. 2004. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. J. Biol. Chem. 2791123-1131. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Y., E. G. Shepherd, and L. D. Nelin. 2007. MAPK phosphatases—regulating the immune response. Nat. Rev. Immunol. 7202-212. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig, H., J. Mages, C. Staib, M. H. Lehmann, R. Lang, and G. Sutter. 2005. Role of viral factor E3L in modified vaccinia virus Ankara infection of human HeLa cells: regulation of the virus life cycle and identification of differentially expressed host genes. J. Virol. 792584-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAllister, C. S., and C. E. Samuel. 2009. The RNA-activated protein kinase enhances the induction of interferon-β and apoptosis mediated by cytoplasmic RNA sensors. J. Biol. Chem. 2841644-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack, S. J., D. C. Thomis, and C. E. Samuel. 1992. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology 18847-56. [DOI] [PubMed] [Google Scholar]
- 25.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 26.Onomoto, K., M. Yoneyama, and T. Fujita. 2007. Regulation of antiviral innate immune responses by RIG-I family of RNA helicases. Curr. Top. Microbiol. Immunol. 316193-205. [DOI] [PubMed] [Google Scholar]
- 27.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 28.Rochester, S. C., and P. Traktman. 1998. Characterization of the single-stranded DNA binding protein encoded by the vaccinia virus I3 gene. J. Virol. 722917-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothenburg, S., N. Deigendesch, M. Dey, T. E. Dever, and L. Tazi. 2008. Double-stranded RNA-activated protein kinase PKR of fishes and amphibians: varying the number of double-stranded RNA binding domains and lineage-specific duplications. BMC Biol. 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenburg, S., E. J. Seo, J. S. Gibbs, T. E. Dever, and K. Dittmar. 2009. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat. Struct. Mol. Biol. 1663-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadler, A. J., and B. R. Williams. 2007. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316253-292. [DOI] [PubMed] [Google Scholar]
- 32.Samuel, C. E. 1993. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 2687603-7606. [PubMed] [Google Scholar]
- 33.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel, C. E., and G. S. Knutson. 1983. Mechanism of interferon action: human leukocyte and immune interferons regulate the expression of different genes and induce different antiviral states in human amnion U cells. Virology 130474-484. [DOI] [PubMed] [Google Scholar]
- 35.Silva, A. M., M. Whitmore, Z. Xu, Z. Jiang, X. Li, and B. R. Williams. 2004. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 27937670-37676. [DOI] [PubMed] [Google Scholar]
- 36.Spaziani, A., A. Alisi, D. Sanna, and C. Balsano. 2006. Role of p38 MAPK and RNA-dependent protein kinase (PKR) in hepatitis C virus core-dependent nuclear delocalization of cyclin B1. J. Biol. Chem. 28110983-10989. [DOI] [PubMed] [Google Scholar]
- 37.Takada, Y., H. Ichikawa, A. Pataer, S. Swisher, and B. B. Aggarwal. 2007. Genetic deletion of PKR abrogates TNF-induced activation of IκBα kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene 261201-1212. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka, H., and C. E. Samuel. 1994. Mechanism of interferon action: structure of the mouse PKR gene encoding the interferon-inducible RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 917995-7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomis, D. C., and C. E. Samuel. 1992. Mechanism of interferon action: alpha and gamma interferons differentially affect mRNA levels of the catalytic subunit of protein kinase A and protein Mx in human cells. J. Virol. 662519-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomis, D. C., J. P. Doohan, and C. E. Samuel. 1992. Mechanism of interferon action: cDNA structure, expression, and regulation of the interferon-induced, RNA-dependent P1/eIF-2 alpha protein kinase from human cells. Virology 18833-46. [DOI] [PubMed] [Google Scholar]
- 41.Thomis, D. C., and C. E. Samuel. 1995. Mechanism of interferon action: characterization of the intermolecular autophosphorylation of PKR, the interferon-inducible, RNA-dependent protein kinase. J. Virol. 695195-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toth, A. M., P. Zhang, S. Das, C. X. George, and C. E. Samuel. 2006. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog. Nucleic Acid Res. Mol. Biol. 81369-434. [DOI] [PubMed] [Google Scholar]
- 43.Uematsu, S., and S. Akira. 2007. Toll-like receptors and type I interferons. J. Biol. Chem. 28215319-15323. [DOI] [PubMed] [Google Scholar]
- 44.Watson, J. C., H. W. Chang, and B. L. Jacobs. 1991. Characterization of a vaccinia virus-encoded double-stranded RNA-binding protein that may be involved in inhibition of the double-stranded RNA-dependent protein kinase. Virology 185206-216. [DOI] [PubMed] [Google Scholar]
- 45.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 805059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, B. R. 1999. PKR: a sentinel kinase for cellular stress. Oncogene 186112-6120. [DOI] [PubMed] [Google Scholar]
- 47.Winter-Vann, A. M., and G. L. Johnson. 2007. Integrated activation of MAP3Ks balances cell fate in response to stress. J. Cell. Biochem. 102848-858. [DOI] [PubMed] [Google Scholar]
- 48.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 765251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, Z., and B. R. Williams. 2000. The B56α regulatory subunit of protein phosphatase 2A is a target for regulation by double-stranded RNA-dependent protein kinase PKR. Mol. Cell. Biol. 205285-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoneyama, M., and T. Fujita. 2007. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 28215315-15318. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, P., B. L. Jacobs, and C. E. Samuel. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, P., and C. E. Samuel. 2007. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J. Virol. 818192-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, P., and C. E. Samuel. 2008. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J. Biol. Chem. 28334580-34587. [DOI] [PMC free article] [PubMed] [Google Scholar]