Abstract
Influenza vaccines capable of inducing cross-reactive or heterotypic immunity could be an important first line of prevention against a novel subtype virus. Influenza virus-like particles (VLPs) displaying functional viral proteins are effective vaccines against replication-competent homologous virus, but their ability to induce heterotypic immunity has not been adequately tested. To measure VLP vaccine efficacy against a known influenza pandemic virus, recombinant VLPs were generated from structural proteins of the 1918 H1N1 virus. Mucosal and traditional parenteral administrations of H1N1 VLPs were compared for the ability to protect against the reconstructed 1918 virus and a highly pathogenic avian H5N1 virus isolated from a fatal human case. Mice that received two intranasal immunizations of H1N1 VLPs were largely protected against a lethal challenge with both the 1918 virus and the H5N1 virus. In contrast, mice that received two intramuscular immunizations of 1918 VLPs were only protected against a homologous virus challenge. Mucosal vaccination of mice with 1918 VLPs induced higher levels of cross-reactive immunoglobulin G (IgG) and IgA antibodies than did parenteral vaccination. Similarly, ferrets mucosally vaccinated with 1918 VLPs completely survived a lethal challenge with the H5N1 virus, while only a 50% survival rate was observed in parenterally vaccinated animals. These results suggest a strategy of VLP vaccination against a pandemic virus and one that stimulates heterotypic immunity against an influenza virus strain with threatening pandemic potential.
Influenza A viruses represent a substantial public health burden, with a yearly average of more than 220,000 hospitalizations and approximately 36,000 deaths in the United States alone (http://www.cdc.gov/flu/keyfacts.htm). In addition to seasonal outbreaks caused by antigenic variants of circulating influenza A and B viruses, another pandemic influenza virus strain may emerge at any time. The threat of a pandemic is greater than it has been in decades. Confirmed cases of human infection with several subtypes of avian influenza viruses have been reported since 1997 (2, 3, 38; http://www.who.int/csr/disease/avian_influenza/country/en/index.html). Of the avian subtypes that have recently been introduced into humans, the highly pathogenic avian influenza virus H5N1 subtype is the most immediate public health problem. More than 400 human H5N1 virus infections have occurred, and approximately 60% have been fatal (http://www.who.int/csr/disease/avian_influenza/country/en/index.html). Should these viruses acquire the ability to spread efficiently among humans lacking immunity to the H5 hemagglutinin (HA), a pandemic could occur. If the case fatality rate of the virus remained high, an H5 pandemic could recapitulate the devastating consequences of the “Spanish” influenza pandemic of 1918, which resulted in an estimated 50 million deaths and a 10-year reduction in the average life expectancy in the United States (13). Although recent studies suggest that most 1918 (H1N1) pandemic deaths were attributed to secondary bacterial pneumonia (5), the inherent ability of the virus to replicate efficiently and cause severe acute infection of the respiratory tract was a critical underlying cause of this historic public health disaster (24, 26, 28, 50, 72, 74, 78, 79). Thus, the 1918 influenza virus represents an ideal candidate for the study of protective immunity to a pandemic influenza virus strain.
Traditional influenza vaccines provide optimal protection against viruses that are antigenically closely matched with those contained in the vaccine but have been less effective against antigenic variants within a subtype and historically provide only minimal protection against viruses of novel HA subtypes (1). Thus, there has been interest in developing a vaccine or vaccine strategy that can induce broader cross-reactive immunity against multiple subtypes of influenza viruses containing multiple combinations of surface proteins, also known as heterosubtypic immunity. In addition to a decrease in overall morbidity following infection, heterosubtypically immune animals show decreased viral titers and duration of viral shedding within the respiratory tract (23, 27, 36, 54, 65, 70, 75).
Pandemic influenza vaccines must meet a number of criteria, which include low production cost, ease of manufacture, and rapid production and delivery. In recent years, several different approaches have been tested. A promising technology uses recombinant noninfectious virus-like particles (VLPs) that present structurally native, immunologically relevant viral antigens. VLP vaccines have proven effective in preventing diseases in humans, as exemplified by the recently approved human papillomavirus VLP vaccine for the prevention of cervical cancer (21; http://www.fda.gov/cber/vaccines.htm). Noninfectious VLPs morphologically resemble their live-virus counterparts and are recognized and processed readily by antigen-presenting cells of the immune system (4, 30, 60, 69, 76, 81). Recombinant VLPs do not involve the use of infectious influenza virus and thus require no exceptional biosafety containment to produce and can be manufactured quickly for an emergency response. Influenza VLPs have been generated in insect cells by using three influenza virus proteins, i.e., HA, neuraminidase (NA), and matrix (M1). To date, these VLPs have been produced from the H1N1, H3N2, H5N1, H5N3, and H9N2 subtypes (6, 7, 19, 41, 51-53). Unlike conventional split and inactivated vaccines based only on HA content, VLPs contain known quantities of the NA and M1 proteins. Therefore, VLPs may have a greater potential for eliciting cross-reactive antibody and T-cell immunity against newly emerging antigenic drift (61) and shift variants (71). Influenza VLPs have been shown to induce high neutralizing antibody titers against homologous and heterologous strains in mice and ferrets (6, 7, 41, 53, 54), and a phase I clinical trial with an investigational H5N1 VLP vaccine is ongoing (6).
In this study, we evaluated a 1918 VLP vaccine for the ability to protect mice against the 1918 pandemic virus in the hope that such a preclinical evaluation may pave the way for a 1918 vaccine which could be offered to laboratory workers working with the virus to mitigate biosafety concerns. In addition, the ability of the 1918 (H1N1) VLP vaccine to elicit protective heterotypic immunity against a lethal challenge with a contemporary highly pathogenic avian influenza H5N1 virus was assessed in mice and ferrets. The ferret model of influenza disease has been used to evaluate H5N1 virus virulence previously (17, 40, 82), as well as the safety and efficacy of other H5N1 vaccine candidates (6, 18, 20, 35, 41). Mice immunized intranasally (i.n.) with the 1918 VLP vaccine were protected not only from death caused by the homologous 1918 virus but also from a heterotypic H5N1 virus, whereas mice immunized parenterally were only protected against a homotypic 1918 virus challenge. A study with ferrets further demonstrates that mucosal VLP vaccination was clearly superior to parenteral vaccination for the induction of heterotypic immunity against an H5N1 virus. Although subtype-cross-reactive neutralizing antibodies were not associated with mouse and ferret survival, high circulating and respiratory mucosal immunoglobulin G (IgG) and IgA antibody levels correlated with heterotypic immunity protection in mice.
MATERIALS AND METHODS
Viruses and cells.
The replication-competent influenza viruses used in these experiments included (i) the reconstructed 1918 H1N1 (abbreviated 1918) virus (72) possessing the A/South Carolina/1/18 HA and (ii) the A/Vietnam/1203/2004 H5N1 (abbreviated VN/1203) virus previously shown to be highly virulent for both mice and ferrets (42, 74). The 1918 virus was generated with the 12-plasmid reverse genetics system in a mixture of Madin-Darby canine kidney (MDCK; ATCC, Manassas, VA) and 293T cells (ATCC) as previously described (72). The VN/1203 virus was grown in embryonating hen's eggs. All virus stock titers were determined by plaque assay on MDCK cells, and virus stocks were maintained in Dulbecco's modified Eagle's medium culture (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone, Logan, UT) and 1% penicillin/streptomycin (Gibco). All virus challenge experiments were performed under the guidance of the U.S. National Select Agent Program in negative-pressure HEPA-filtered biosafety level 3+ (BSL-3+) enhanced laboratories with the use of a battery-powered Racal HEPA filter respirator and according to Biomedical Microbiological and Biomedical Laboratory procedures (58).
VLP generation.
1918 VLPs were made with recombinant baculovirus expressing the genes for HA, NA, and M1. The genes for HA, NA, and M1 used for the generation of the VLPs were synthesized by reverse transcription-PCR with viral RNA extracted from reconstructed 1918 influenza virus (72). Following reverse transcription-PCR, the genes for the NA, M1, and HA cDNAs were combined in this order within the pFastBac1 transfer vector (Invitrogen) essentially as described previously (52). This resulted in a plasmid, pNVAX1250, that encoded the genes for HA, NA, and M1, each within its own expression cassette that included a polyhedrin promoter and transcription termination sequences. The DNA sequences of the genes and flanking regulatory sequences were confirmed. The HA gene was identical to that of influenza A virus A/South Carolina/1/18 (H1N1) (GenBank AAD17229), whereas the NA and M1 genes were identical to the influenza A/Brevig Mission/1/18 virus genes (accession no. AAF77036 and AAN06597, respectively). The DNA fragment from pNVAX1250 containing the NA, M1, and HA expression cassettes was then transferred into bacmids and transfected into Spodoptera frugiperda (Sf9) insect cells (ATCC CRL-1711) to generate recombinant baculovirus (52). Recombinant baculoviruses were used to infect Sf9 cells. VLPs were harvested at 72 h posttransfection. Recombinant VLPs were purified to approximately 90% by sucrose density gradient ultracentrifugation, followed by ion-exchange chromatography. The influenza viral proteins in the VLP preparations were confirmed by sodium dodecyl sulfate-polyacrylamide electrophoresis and Western blot analysis. Semiquantitative densitometry of the Coomassie-stained gel containing the purified VLP preparation showed that the HA, NA, and M1 proteins were present at a ratio of approximately 61:5:22. The remaining ∼12% of the Coomassie-stained material contained impurities derived from Sf9 cells and baculovirus. A single-radial-immunodiffusion assay was used to quantitate the HA content of the VLPs as described previously (80). Reference reagents for the single-radial-immunodiffusion standard curve included A/New Caledonia/20/99 (H1N1) IVR-116 (79 μg/ml of HA) antigen and anti-HA reference sheep antiserum S-6706 (obtained from the Center for Biologics Evaluation and Research, Food and Drug Administration, Rockville, MD). Control VLPs derived from human immunodeficiency virus (HIV)-type Con-S gp145 (77) were prepared and quantitated similarly.
Mouse vaccination and virus challenge.
All animal research was conducted according to the guidance of the CDC Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility. Eight- to 10-week-old female BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized by intraperitoneal injection of 0.2 ml of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich, Milwaukee, WI) (73) and vaccinated either i.n. or intramuscularly (i.m.) (right hind leg) with 5 μg (based on HA content) of 1918 VLPs in 50 μl of phosphate-buffered saline (PBS). Fourteen days post primary vaccination, mice were boosted with a second dose of 5 μg of 1918 VLPs by either the i.m. or the i.n. route. Mice were bled for collection of serum prior to and following the first and second vaccinations. Lung and nasal washes were collected as previously described in 1 ml of sterile PBS for antibody titration (75). Two weeks post boost vaccination, mice were challenged i.n. with 50 50% lethal doses (LD50) of either the 1918 or the VN/1203 virus in a volume of 50 μl. Mice were weighed daily and observed for illness. On day 5 post virus challenge (p.c.), four mice per group were exsanguinated and euthanatized and their lungs were removed for virus titration. Lungs were homogenized in 1 ml of sterile PBS, and clarified homogenate virus titers were determined by a standard plaque assay for virus infectivity. Briefly, 10-fold serial dilutions of lung homogenate were placed onto confluent monolayers of MDCK cells in the presence of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK) trypsin (1 μg/ml). Following 1 h of adsorption, cells were washed with PBS and a 2x L-15 medium (Cambrex/Lonza Inc., Walkersville, MD) agar overlay was placed into each well. The statistical significance of virus titer data was determined by using the analysis-of-variance (ANOVA) test. Mouse survival data and mean time to death (MTD) were subjected to Kaplan-Meier survival analysis with SPSS software.
Ferret vaccination and virus challenge.
Eighteen male Fitch ferrets, 10 months of age (Triple F Farms, Sayre, PA), that were serologically negative by hemagglutination inhibition (HI) assay for currently circulating influenza viruses were used in these studies. 1918 VLP vaccinations were conducted under BSL-2 conditions, and H5N1 virus challenges were performed under BSL-3+ conditions within a Duo-Flo Bioclean unit (Lab Products, Seaford, DE) with a maximum capacity of 18 ferrets. Serum samples, nasal washes, and temperature and weight measurements were taken throughout the duration of the study. Temperatures were monitored following the virus challenge by a subcutaneously implanted transponder (BioMedic Data Systems, Seaford, DE). Prior to the study, baseline serum was collected via the anterior vena cava from all 18 ferrets. Six ferrets were vaccinated with 15 μg/500 μl 1918 VLPs by the i.n. route (250 μl in each nostril), and six ferrets were vaccinated by the i.m. route. The remaining six ferrets received PBS in place of vaccine by the i.n. and i.m. routes. Fourteen days following the initial VLP or PBS inoculation, serum was collected from all of the ferrets and they were then boosted with 15 μg/500 μl by the same route used previously. Fourteen days following the VLP boost, all 18 ferrets were challenged i.n. with 50 50% ferret infectious doses in 1 ml of A/VN/1203/04 (H5N1) virus. Fifty percent ferret infectious doses were determined by inoculating groups of ferrets each at 104, 103, 102, and 101 50% egg infective doses and calculating the outcome of infection by the method of Reed and Muench (57). Ferrets were weighed daily and monitored for signs of illness. Nasal cavities of infected ferrets were washed with 1 ml of PBS every other day, beginning on day 2 p.i., and measured for virus replication by 10-fold serial titration in hen's eggs.
Serology.
Sera collected from mice and ferrets prior to a virus challenge were treated with receptor-destroying enzyme (RDE; Denka Seiken, Tokyo, Japan) and tested for reactivity to the β-propiolactone-inactivated VN/1203 and 1918 viruses by the HI assay with 1.0% horse or 0.5% turkey red blood cells, respectively, starting with a serum dilution of 1:10. Briefly, virus stocks were treated with 0.1% β-propiolactone (Sigma-Aldrich) for 24 h at 4°C. Stocks were safety tested by two serial passages in embryonating eggs before use in the HI assays. Geometric mean titers (GMTs) and their standard deviations were calculated for each treatment group. For the microneutralization assays, 100 50% tissue culture infective doses of infectious VN/1203 or 1918 virus was incubated with twofold serial dilutions of sera, starting at a 1:10 dilution, and incubated overnight on MDCK cells (59). Titers represent the reciprocal dilution of serum giving 50% neutralization activity. An indirect enzyme-linked immunosorbent assay (ELISA) was performed on RDE-treated mouse sera to determine the IgA and IgG responses following vaccination (75). Briefly, IgA and IgG titers were measured with purified, formalin-inactivated VN/1203 and recombinant 1918HA/NA:A/Texas/36/91 (49) viruses. Immulon microtiter plates (Thermo Scientific Inc., Waltham, MA) were coated with 100 hemagglutination units of each virus, and mouse antibody was detected with horseradish peroxidase-conjugated antibody (KPL Laboratories, Gaithersburg, MD). The ELISA endpoint titers were expressed as the highest dilutions that yielded an optical densities greater than the mean optical density plus 3 standard deviations of similarly diluted negative control sera. Titers are expressed in the log2 format, and the statistical significance of differences between treatment groups was measured by the ANOVA test.
RESULTS
Mucosal, but not parenteral, VLP vaccination induces heterotypic immunity in mice.
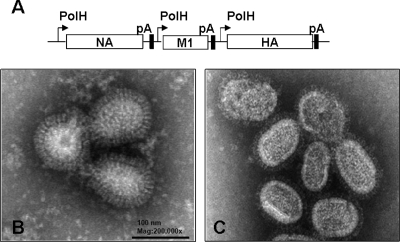
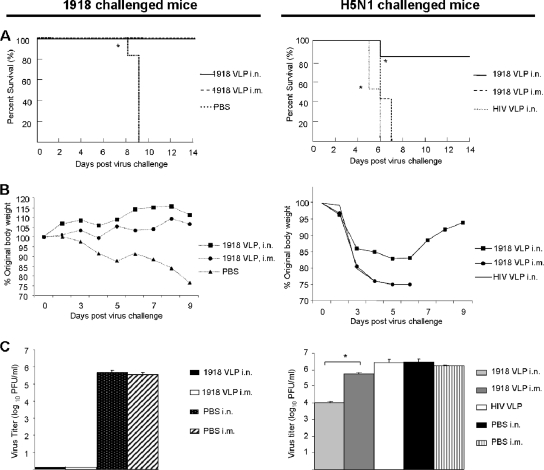
1918 (H1N1) VLPs generated in a baculovirus expression system (Fig. 1A) from Sf9 cells were purified from culture medium and negatively stained for electron microscopic examination. As shown in Fig. 1B, 1918 VLPs had a diameter of approximately 80 to 120 nm with pronounced surface protein spikes and a morphology characteristic of replication-competent 1918 virions (Fig. 1C). We compared the mucosal (i.n.) and parenteral (i.m.) vaccination routes for the ability to induce protection against a homologous 1918 virus challenge, as well as an H5N1 heterotypic virus challenge. BALB/c mice were vaccinated twice with 5 μg of 1918 VLPs or control HIV VLPs and challenged i.n. 14 days post VLP boost with 50 LD50 of either VN/1203 (H5N1) or 1918 (H1N1) virus (Fig. 2). The extent of vaccine protection was measured by (i) morbidity (weight loss), (ii) survival over a 14-day p.c. period, and (iii) virus titers in the lower respiratory tract (lung) at 5 days p.c. Mice vaccinated with 1918 VLPs by either the i.n or the i.m. vaccination route uniformly survived the lethal challenge with the homologous 1918 virus, while control animals succumbed to infection (MTD = 8.8 days; *, P < 0.05, Kaplan-Meier survival analysis) (Fig. 2A, left side). In contrast to the control animals, mice in both vaccination groups showed no signs of sickness and gained weight every day p.c. although mucosally vaccinated mice gained, on average, 5% more body mass over time than parenterally vaccinated mice (Fig. 2B, left side). The 1918 virus replicated efficiently in the lungs of control mice, with titers of approximately 5.7 log10 PFU/ml (Fig. 2C, left column). However, 1918 VLP-vaccinated/1918 virus-challenged mice did not have detectable lung virus titers on day 5 p.c.
FIG. 1.
1918 VLP generation. VLPs were constructed in a baculovirus genetic background with the sequences of the genes for HA, NA, and M1 of the 1918 pandemic virus and produced from Sf9 cells. (A) Baculovirus construct for the expression of 1918 influenza VLPs. Indicated are the polyhedrin promoter (PolH), polyadenylation signal (pA), and influenza virus genes. 1918 VLPs had an average diameter of 100 nm, as illustrated in this negatively stained transmission electron micrograph. While not replication competent, the 1918 VLPs (B) morphologically resemble the reconstructed 1918 virions that were collected from supernatants of 1918 virus-infected MDCK cell cultures (C). Mag, magnification.
FIG. 2.
1918 VLP vaccine efficacy in mice following a lethal H1N1 or H5N1 virus challenge. Mice were vaccinated with 5 μg/50 μl 1918 VLPs or control HIV VLPs and challenged with 50 LD50 of either 1918 (H1N1) (left column) or VN/1203 (H5N1) (right column) virus. Mice were monitored daily for 14 days p.c. Survival rates (A) following the challenge were calculated based percent survival within each experimental group (n = 6 mice per experimental group; *, P < 0.05, Kaplan-Meier survival analysis). Mice were weighed daily (B) to measure morbidity (loss of 25% of the original body weight necessitates euthanasia). Average weights in each treatment group were measured for the duration of the study, and percent original body weight was calculated based on the average starting weight for each group on day 0. On day 5 p.c., four mice per experimental group were euthanized and their lungs were removed for virus titration (C). Lungs were homogenized in 1 ml PBS and clarified before titer determination by standard plaque assay on MDCK cells in duplicate (limit of detection, 10 PFU). Bars represent the average titer for each experimental group; error bars indicate the standard deviation between mice in each respective group (n = 4 mice per experimental group; *, P < 0.05, ANOVA).
In contrast to the homologous subtype challenge data, all mice that received the 1918 VLPs by the i.m route succumbed to a VN/1203 (H5N1) heterotypic virus challenge (MTD = 6.4 days), as did the HIV VLP control mice (MTD = 5.5 days) (Fig. 2A, right column). Moreover, parenterally vaccinated mice exhibited dramatic weight loss from 2 days p.c. until death, similar to those animals vaccinated with the HIV VLPs (Fig. 2B, right column). In contrast, five of six mice that received the same dose of 1918 VLP vaccine administered i.n. were protected against an H5N1 heterotypic virus challenge (Fig. 2A, right column). The surviving mice exhibited morbidity that reached a mean maximum weight loss of 17% on day 5 p.c. before weight gain was observed from day 6 p.c. onward (Fig. 2B, right column). The mean lung virus titers of mice administered 1918 VLP vaccine by the i.n. route were approximately 300-fold lower than those of control mice receiving PBS or HIV VLP vaccine (Fig. 2C, right column). Mice vaccinated i.m. and challenged with the H5N1 virus exhibited titers of nearly 106 PFU/ml, only twofold lower than those of control mice. Collectively, these results demonstrate that mucosal H1N1 VLP immunization provides greater heterotypic immunity against the H5N1 virus than does parenteral VLP vaccination.
Mucosal vaccination with 1918 VLPs results in higher IgG and IgA antibody titers in mice than does parenteral vaccination.
Characterization of postvaccination antibody responses was performed in attempts to identify cross-reactive antibodies that may be responsible for conferring protection against a lethal H5N1 heterotypic virus challenge. First, the virus neutralization (v.n.) antibody titers against the 1918 (H1N1) and VN/1203 (H5N1) viruses were measured in pooled sera (n = 8 or 9 per group) collected from mice vaccinated twice with the 1918 VLPs (Table 1). Mice vaccinated by either the i.n or the i.m. route displayed v.n. antibody titers against the homologous 1918 virus (1:800 to 1:1,600) but exhibited no detectable serum v.n. antibody titer against the heterotypic H5N1 virus. As expected, mice vaccinated with the HIV VLPs had no detectable serum v.n. antibody titer (<10) to either influenza virus. The HI antibody response among individual mice was also measured 2 weeks after the vaccine boost. Similarly, vaccination of mice with 1918 VLPs elicited a detectable HI antibody response against the homologous 1918 virus and the mucosally vaccinated mice elicited a GMT (individual sera ranged from 320 to 1,280; GMT = 607) that was 3.4-fold greater than that of the parenterally vaccinated mice (range, 80 to 320; GMT = 180). However, sera from either 1918 VLP vaccination group did not exhibit any cross-reactivity to the H5N1 virus in the HI assay (<10). Mice vaccinated with HIV VLPs or given PBS did not exhibit any HI antibody titer (<10) against either the 1918 or the VN/1203 virus.
TABLE 1.
Neutralizing and HI antibody titers from mouse sera
| Vaccination group (route)c | Neutralizing antibody titera
|
HI antibody titerb
|
||
|---|---|---|---|---|
| 1918 | H5N1 | 1918 | H5N1 | |
| 1918 VLP (i.n.) | 1,600 | <10 | 607 | <10 |
| 1918 VLP (i.m.) | 800 | <10 | 180 | <10 |
| HIV VLP (i.n.) | <10 | <10 | <10 | <10 |
| PBS (i.n. or i.m.) | <10 | <10 | <10 | <10 |
Mouse serum samples from each treatment group were pooled and tested for neutralizing antibody activity against either the 1918 or the VN/1203 virus in a microneutralization assay with MDCK cells. The titers represents the reciprocal serum dilution with virus-neutralizing activity.
HI assays used either 0.5% turkey (BPL-1918 virus) or 1% horse (BPL-VN/1203 virus) red blood cells. The titers shown are GMTs.
Mice were treated twice with 1918 VLPs, HIV VLPs, or PBS.
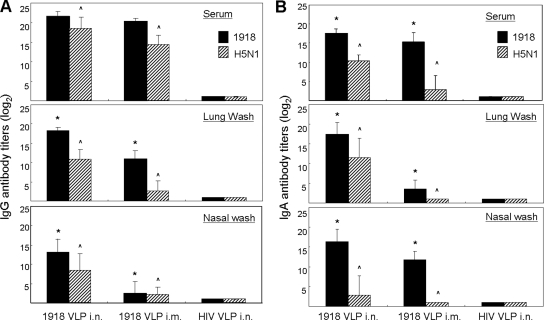
We next performed ELISAs to investigate the presence of H5N1-cross-reactive IgG and IgA antibodies in serum samples and respiratory washes from mice administered two doses of the 1918 H1N1 VLP vaccine (Fig. 3). Antiviral IgG and IgA antibodies in the sera and mucosal washes of i.n. and i.m. vaccinated mice were detected by using ELISA plates coated with purified whole H1N1 or heterologous H5N1 virus. Overall, 1918 VLP immunization by either route induced IgG antibody to the homologous 1918 virus in serum and respiratory mucosa washes and these samples also exhibited cross-reactive IgG titers against the H5N1 virus (Fig. 3A). As shown in Fig. 3A (top), i.n. vaccinated mice exhibited significantly more H5N1-cross-reactive serum IgG antibody than did mice vaccinated by the parenteral route (^, P < 0.05). Although homologous virus serum IgG titers were similar in both 1918 VLP vaccination groups (GMT [log2] = 20.3 [i.m.] versus 21.5 [i.n.]), i.n. vaccination resulted in lung mucosal IgG antibody titers against the homologous H1N1 (*, P < 0.05) and heterologous H5N1 (^, P < 0.05) viruses that were significantly higher than those measured in i.m. vaccinated mice (Fig. 3A, middle). This trend was also observed in nasal wash samples, in which the differences between the IgG titers of the two vaccination groups were even more pronounced (* and ^, P < 0.05) (Fig. 3A, bottom). IgA antibodies to whole H1N1 and H5N1 virus were also examined. As shown in Fig. 3B, mucosal administration of 1918 VLPs induced significantly higher homologous virus IgA titers in serum (top), and particularly lung (middle) and nasal mucosal (bottom) washes, than did parenteral VLP vaccination (*, P < 0.05). Likewise, circulating levels of cross-reactive IgA antibody to the H5N1 virus were significantly higher (^, P < 0.05) in mice vaccinated by the i.n. route (GMT [log2] = 10.3) than in those vaccinated by the i.m. route (GMT [log2] = 2.9) (Fig. 3B, top). The differences in IgA titer between these two vaccination groups was most pronounced in lung wash samples (Fig. 3B, middle), and these trends were also observed in nasal wash samples (Fig. 3B, bottom) (^, P < 0.05). Together, these results show that vaccination with 1918 H1N1 VLPs induced a population of HI- and v.n.-negative, cross-reactive IgG and IgA antibodies that were greater following mucosal vaccination than following parenteral vaccination.
FIG. 3.
Antibody responses in 1918 VLP-vaccinated mice. BALB/c mice were vaccinated with 1918 VLPs or HIV VLPs or given PBS. Serum, lung wash, and nasal wash sample IgG (A) and IgA (B) antibody titers were determined following two vaccinations with 1918 VLPs. Sera was treated with RDE, and all of the samples were individually tested in fourfold serial dilutions against the formalin-inactivated 1918 and VN/1203 viruses in an antibody capture ELISA. Plots show the geometric mean antibody titers (log2) of all of the mice in each treatment group, and error bars indicate the standard deviation observed among the mice in each group. Significant differences between the antibody titers elicited by the different vaccination routes are indicated (* [1918] and ^ [VN/1203], P < 0.05, ANOVA).
Ferrets mucosally vaccinated with 1918 VLPs are protected from a lethal H5N1 virus challenge.
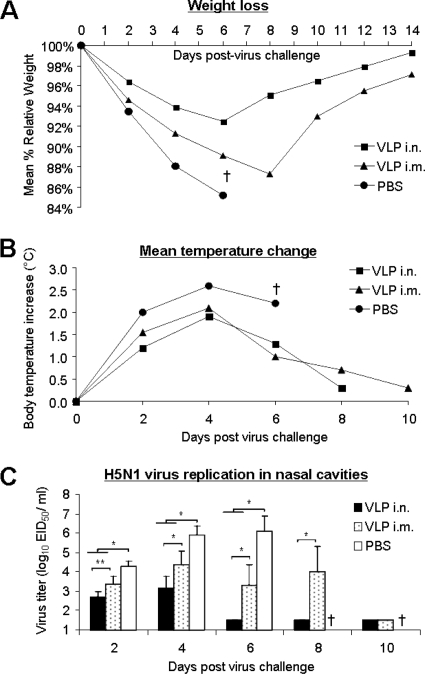
Because of its natural susceptibility to influenza A viruses, the ferret is currently accepted as an excellent mammalian host for influenza vaccine efficacy studies. We next compared mucosal versus traditional parenteral administration of the 1918 VLP vaccine for the ability to induce heterotypic immunity in this species. Ferrets were vaccinated twice with 15 μg of the 1918 VLPs either i.n. or i.m. and subsequently challenged 14 days post VLP boost with 50 LD50 of VN/1203 (H5N1) virus. Strikingly, all of the six ferrets vaccinated with the 1918 VLPs i.n. survived a lethal challenge with the H5N1 virus (Table 2), whereas 50% of the ferrets vaccinated by the i.m. route and 100% of the control animals succumbed to the lethal H5N1 virus infection. Moreover, mucosally vaccinated ferrets also exhibited less weight loss, as a group, over time than did ferrets vaccinated by the i.m route or with PBS alone (Fig. 4A). Ferrets vaccinated by the i.m route did exhibit a longer survival time (MTD = 8.3 days) than did PBS-inoculated control animals (MTD = 6.3 days). While there were no significant temperature change differences observed between ferrets in the i.n. and i.m. vaccination groups, overall the vaccinated animals exhibited less fever than did the PBS-inoculated control animals (Fig. 4B). Ferrets vaccinated i.n. also exhibited reduced virus titers in their nasal mucosa following infection than did the i.m. vaccinated ferrets, and the differences between these two groups were statistically significant on days 2, 4, 6, and 8 p.c. (Fig. 4C). All vaccinated ferrets exhibited lower virus titers than did the PBS-inoculated animals, indicating that while the i.m. vaccinated ferrets were not completely protected against death, VLP vaccination still induced an immune response capable of suppressing some virus replication in the upper respiratory tract. The HI antibody responses to the H1N1 (1918) and H5N1 (VN/1203) viruses were measured in individual serum samples collected from ferrets (n = 6) vaccinated twice with 1918 VLPs. Ferrets vaccinated by the i.n route had slightly higher circulating HI antibody titers to the homologous virus (GMT = 254) than did i.m. vaccinated ferrets (GMT = 160) (Table 2). HI antibody against the H5N1 virus was not detected in either group of VLP-vaccinated ferrets. Collectively, these results from the ferret challenge model confirm the experimental outcomes observed in the mouse studies, showing that mucosal influenza immunization with 1918 VLPs can provide greater heterotypic immunity than parenteral influenza vaccination.
TABLE 2.
Ferret mortality following 1918 VLP vaccination and lethal H5N1 challenge
| Group | No. of ferrets | No. that survived virus challenge (% mortality) | MTD (days postchallenge) | Serum HI antibody GMT prior to virus challenge
|
|
|---|---|---|---|---|---|
| 1918 | H5N1 | ||||
| VLP i.n. | 6 | 6 (0) | NAa | 254 | <20 |
| VLP i.m. | 6 | 3 (50) | 8.3 | 180 | <20 |
| PBS | 6 | 0 (100) | 6.3 | <20 | <20 |
NA, not applicable.
FIG. 4.
Ferret morbidity following 1918 VLP vaccination and an H5N1 virus challenge. Eighteen ferrets were divided into three experimental groups. Six ferrets were vaccinated by the i.n. or i.m. route with 15 μg/500 ml 1918 VLPs. Six control ferrets received the same volume of PBS i.n. plus i.m. Twenty-eight days following the administration of two doses of 1918 VLPs or PBS, ferrets were challenged i.n. with 50 LD50 of the A/Vietnam/1203/04 (H5N1) virus and monitored daily for signs of illness. The ferrets were weighed (A) and their temperatures were measured individually (B) for 14 days p.c. Control ferrets all succumbed to infection by day 6 p.c. (†). The nasal cavities of infected ferrets were washed with 1 ml PBS every other day beginning on day 2 p.c., and virus titers were determined (C). Differences in virus titers between vaccination groups were analyzed by ANOVA (*, P < 0.005).
DISCUSSION
The emergence of new influenza virus subtypes to which the human population has little immunity is of great public health concern, and vaccination remains the principal and most economically prudent public health intervention strategy against both seasonal and pandemic influenza. The ability of the 1918 (H1N1 subtype) virus to spread rapidly and cause high rates of human illness makes it an ideal candidate for studying vaccine efficacy against a pandemic strain. Furthermore, scientists working with the reconstructed 1918 virus would benefit from the development of and access to an effective vaccine against this recognized killer. The high mortality rate associated with human infections by H5N1 viruses (15, 25, 34; http://www.who.int/csr/disease/avian_influenza/country/en/index.html) highlights the need for the development of improved vaccine technologies. To date, clinical evaluations of many H5N1 vaccine candidates indicate the need for improved adjuvants or alternative approaches that could enhance vaccine immunogenicity (31-33, 46, 47). Moreover, vaccine strategies capable of inducing more cross-reactive immunity may overcome limitations in vaccine efficacy imposed by antigenic variability of influenza A viruses (11). VLPs have been generated previously from a number of subtypes and have been shown to promote a strong immune response to a challenge with a homologous virus (14, 41, 52, 53); however, their capacity to induce heterotypic immunity has not been adequately addressed.
In the present study, we tested the ability of nonadjuvanted 1918 VLPs to provide protection against the reconstructed 1918 pandemic virus, as well as elicit cross-protection against a lethal H5N1 virus challenge. Two routes of vaccination (mucosal and parenteral) were compared to assess the potential effect of the route of VLP administration on vaccine efficacy, and two mammalian models of highly pathogenic influenza disease were used (39, 42, 72, 74). All of the mice vaccinated with the 1918 VLPs and lethally challenged with the 1918 virus survived and were well protected, regardless of the vaccination route, supporting results from previous homologous virus challenge studies with baculovirus-expressed influenza VLPs (4, 41, 53, 55). Importantly, these studies showed that mucosal VLP vaccination was superior to parenteral vaccination for the induction of heterotypic immunity. The cross-protective effect of mucosal vaccination was associated with a reduction in weight loss and reduced H5N1 virus replication in the respiratory mucosa.
Antibodies are prime candidates for contributors to heterotypic immunity because they are generated to conserved antigens, and further support comes from studies in which B-cell-deficient mice failed to survive a heterotypic challenge (45, 75). In the present study, characterization of the postvaccination antibody responses identified differences between the two routes of vaccination. ELISAs revealed the presence of H5N1-cross-reactive IgG and IgA antibodies among H1N1 VLP-vaccinated animals, suggesting the presence of common cross-reactive epitopes in the HA, NA, or M1 protein. Mice vaccinated by the mucosal route generally had higher levels of antiviral IgG and IgA antibodies than did parenterally vaccinated mice. The differences in cross-reactive antibody titers between the two vaccination groups were most pronounced in the lung and upper respiratory tract. Polymeric IgA antibody may contribute to heterotypic immunity by its ability to pass through the lung epithelium and, during this transcytosis, interfere with the production of viral proteins in an infected cell (43, 44). However, heterotypic immunity can be effective in IgA−/− mice, suggesting that this class of antibody is unlikely playing a role (11). Cross-reactive IgG antibody was found in the lungs along with IgA and has been considered a correlate of heterotypic protection (75). In mice, mucosal but not parenteral vaccination induced subtype-cross-reactive lung and serum IgG anti-HA antibodies, suggesting the presence of a common cross-reactive epitope in the HA of the H3 and H5 subtypes (75).
Although mucosally vaccinated animals were largely protected against a lethal H5N1 virus challenge, neutralizing antibody against the H5N1 virus was not detected in any group of VLP-vaccinated animals. It is conceivable that the cross-reactive anti-HA antibodies produced in VLP-vaccinated hosts act by additional mechanisms in vivo to neutralize progeny virus and/or enhance the clearance of virus-infected cells (11, 16, 37, 75). While the identity between the HA1 amino acid sequences of the VN/1203 H5N1 and 1918 viruses is only 58%, antibodies to the conserved HA2 stem region are generated and may affect overall virus binding and immune recognition (13, 48, 64). More recent support for our findings presented here showed that neutralizing antibody epitopes exist in the stem region of the HA molecule and that while antibody bound to the HA2 region does not classically “neutralize” the receptor binding site, it prevents the molecule from conformationally changing upon endocytosis and exposure to a low pH, thereby preventing viral fusion with the cell membrane (9, 48, 63, 64, 67). It is reasonable to speculate that mucosal vaccination with VLPs generates a population of v.n.-negative, cross-reactive IgG and IgA antibodies to regions of the virus HA molecule that promote virus clearance. A cross-reactive epitope(s) on the NA may also be generating nonneutralizing antibodies. Anti-NA antibodies can partially reduce the viral yield and plaque size of influenza viruses from infected cells. The 1918 and VN/1203 viruses exhibited 85% identity in their NA amino acid sequences, and N1-specific antibodies have been shown to provide partial cross-protection against H5N1 virus infection (61). Studies are under way to delineate the cross-reactive epitope(s) on the HA and NA glycoproteins.
Mucosal vaccination with these VLPs may also be inducing a stronger protective immune response because of the inherent stimulation of innate immune sentinels such as Toll-like receptors on the mucosal surface (22), as these VLPs contain viral surface proteins in their native conformation and it is presumed that these particles are capable of binding to cells expressing the viral receptor α-2-3 or -2-6 sialic acid, as do infectious virus particles (66, 74). Mucosal vaccination may be better than parenteral administration at delivering viral antigens to the pertinent anatomical compartment, specifically to dendritic cells in the respiratory tissue which play a critical role in communicating with T and B cells to develop immunological memory against influenza viruses (62). Supporting this hypothesis is our observation that mice vaccinated mucosally express twofold greater amounts of interleukin-2 (IL-2) (a cytokine released by activated CD4+ T cells to allow for clonal expansion of antigen-specific T cells and also a potent activator of B cells) in the lungs 5 days following an H5N1 virus challenge than do mice vaccinated parenterally (data not shown), which may contribute to the overall survival associated with this vaccination group. Also elevated in mucosally VLP-vaccinated/H5N1-challenged surviving mice were the cytokines IL-9 and IL-17 (data not shown), both of which are recognized to facilitate B-cell proliferation. The broadly cross-neutralizing antibodies reported recently were of the IgG1 subclass (10, 67), B-cell products that help mediate the Th2 CD4+ T-cell and cytokine response, including IL-9 and L-10 production. IL-10 was recently shown to be important in regulating pulmonary inflammation in influenza virus-infected mice, and inhibition of this cytokine results in severe lung inflammation (68). We found that levels of IL-10 in lungs from VLP-vaccinated mice were three times higher in those infected with the H5N1 virus than in 1918 virus-challenged mice (data not shown), possibly contributing to their survival. While the protective role of CD8+ cytotoxic T lymphocytes and memory B cells following 1918 VLP vaccination warrants further investigation, previous studies on the induction of heterotypic immunity has revealed CD8+ T cells to be generally accessory to mouse survival following a lethal virus challenge, while B cells are critical (8, 12, 45, 56, 75).
Our studies raise important questions regarding the application of this vaccination technology, in both seasonal and epidemic outbreak situations. Specifically, could mucosal administration of influenza VLP vaccine bearing seasonal or pandemic influenza virus proteins reduce the widespread morbidity and lethality due to a newly emerging subtype prior to the production of a strain-specific vaccine? Other research has indicated that this may be possible (23, 29). A vaccine that could induce or boost heterotypic immunity through the stimulation of a cross-reactive antibody could be an important preventative measure against a novel subtype, allowing time for the development of a pandemic strain-specific vaccine.
Acknowledgments
L.A.P. was supported by a fellowship from the American Society for Microbiology and the CDC Coordinating Center for Infectious Diseases.
We thank the Vietnamese Ministry of Health for use of the A/Vietnam/1203/04 isolate and Jessica Belser for the ferret LD50 determination of that virus stock. We thank Debra Wadford, Neal Van Hoeven, Joshua DeVos, and Ebonee Butler for providing reagents and assisting with the serological assays. We also thank Ye Liu and Tom Kort for expert assistance in VLP purification and Feng Lui for his assistance in statistical analysis of mouse mortality data.
The findings and conclusions in this report are ours and do not necessarily represent the views of the funding agency.
We have no competing interests to report.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Ada, G. L., and P. D. Jones. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 1281-54. [DOI] [PubMed] [Google Scholar]
- 2.Belser, J. A., O. Blixt, L. M. Chen, C. Pappas, T. R. Maines, N. Van Hoeven, R. Donis, J. Busch, R. McBride, J. C. Paulson, J. M. Katz, and T. M. Tumpey. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl. Acad. Sci. USA 1057558-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belser, J. A., X. Lu, T. R. Maines, C. Smith, Y. Li, R. O. Donis, J. M. Katz, and T. M. Tumpey. 2007. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J. Virol. 8111139-11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessa, J., N. Schmitz, H. J. Hinton, K. Schwarz, A. Jegerlehner, and M. F. Bachmann. 2008. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur. J. Immunol. 38114-126. [DOI] [PubMed] [Google Scholar]
- 5.Beveridge, W. I. 1991. The chronicle of influenza epidemics. Hist. Philos. Life Sci. 13223-234. [PubMed] [Google Scholar]
- 6.Bright, R. A., D. M. Carter, C. J. Crevar, F. R. Toapanta, J. D. Steckbeck, K. S. Cole, N. M. Kumar, P. Pushko, G. Smith, T. M. Tumpey, and T. M. Ross. 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE 3e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright, R. A., D. M. Carter, S. Daniluk, F. R. Toapanta, A. Ahmad, V. Gavrilov, M. Massare, P. Pushko, N. Mytle, T. Rowe, G. Smith, and T. M. Ross. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 253871-3878. [DOI] [PubMed] [Google Scholar]
- 8.Droebner, K., E. Haasbach, C. Fuchs, A. O. Weinzierl, S. Stevanovic, M. Buttner, and O. Planz. 2008. Antibodies and CD4+ T-cells mediate cross-protection against H5N1 influenza virus infection in mice after vaccination with a low pathogenic H5N2 strain. Vaccine 266965-6974. [DOI] [PubMed] [Google Scholar]
- 9.Ekiert, D. C., G. Bhabha, M. A. Elsliger, R. H. Friesen, M. Jongeneelen, M. Throsby, J. Goudsmit, and I. A. Wilson. 26 February 2009. Antibody recognition of a highly conserved influenza virus epitope. Science doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed]
- 10.Epstein, S. L. 2003. Control of influenza virus infection by immunity to conserved viral features. Expert Rev. Anti. Infect. Ther. 1627-638. [DOI] [PubMed] [Google Scholar]
- 11.Epstein, S. L., A. Stack, J. A. Misplon, C. Y. Lo, H. Mostowski, J. Bennink, and K. Subbarao. 2000. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8+ cytotoxic T lymphocytes: either CD4+ or CD8+ T cells can promote survival and recovery after challenge. Int. Immunol. 1291-101. [DOI] [PubMed] [Google Scholar]
- 12.Fleury, D., B. Barrere, T. Bizebard, R. S. Daniels, J. J. Skehel, and M. Knossow. 1999. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat. Struct. Biol. 6530-534. [DOI] [PubMed] [Google Scholar]
- 13.Frost, W. H. 1920. Statistics of influenza morbidity. Public Health Rep. 35584. [Google Scholar]
- 14.Galarza, J. M., T. Latham, and A. Cupo. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18244-251. [DOI] [PubMed] [Google Scholar]
- 15.Gambotto, A., S. M. Barratt-Boyes, M. D. de Jong, G. Neumann, and Y. Kawaoka. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 3711464-1475. [DOI] [PubMed] [Google Scholar]
- 16.Gerhard, W., K. Mozdzanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 15995-103. [DOI] [PubMed] [Google Scholar]
- 17.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 792191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govorkova, E. A., R. J. Webby, J. Humberd, J. P. Seiler, and R. G. Webster. 2006. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 194159-167. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, J. R., L. Dokken, J. A. Wiley, A. G. Cawthon, J. Bigger, A. G. Harmsen, and C. Richardson. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27530-541. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, E., A. S. Lipatov, R. J. Webby, E. A. Govorkova, and R. G. Webster. 2005. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc. Natl. Acad. Sci. USA 10212915-12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung, C. F., B. Ma, A. Monie, S. W. Tsen, and T. C. Wu. 2008. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin. Biol. Ther. 8421-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinohe, T., A. Kawaguchi, S. Tamura, H. Takahashi, H. Sawa, A. Ninomiya, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, J. Chiba, T. Sata, T. Kurata, and H. Hasegawa. 2007. Intranasal immunization with H5N1 vaccine plus Poly I:Poly C12U, a Toll-like receptor agonist, protects mice against homologous and heterologous virus challenge. Microbes Infect. 91333-1340. [DOI] [PubMed] [Google Scholar]
- 23.Ichinohe, T., S. Tamura, A. Kawaguchi, A. Ninomiya, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, H. Takahashi, H. Sawa, W. M. Mitchell, D. R. Strayer, W. A. Carter, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2007. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J. Infect. Dis. 1961313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kash, J. C., T. M. Tumpey, S. C. Proll, V. Carter, O. Perwitasari, M. J. Thomas, C. F. Basler, P. Palese, J. K. Taubenberger, A. Garcia-Sastre, D. E. Swayne, and M. G. Katze. 2006. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, T. R., M. G. Hawkins, C. E. Sandrock, and W. M. Boyce. 2008. A review of highly pathogenic avian influenza in birds, with an emphasis on Asian H5N1 and recommendations for prevention and control. J. Avian Med. Surg. 221-16. [DOI] [PubMed] [Google Scholar]
- 26.Kobasa, D., S. M. Jones, K. Shinya, J. C. Kash, J. Copps, H. Ebihara, Y. Hatta, J. H. Kim, P. Halfmann, M. Hatta, F. Feldmann, J. B. Alimonti, L. Fernando, Y. Li, M. G. Katze, H. Feldmann, and Y. Kawaoka. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445319-323. [DOI] [PubMed] [Google Scholar]
- 27.Kreijtz, J. H., R. Bodewes, G. van Amerongen, T. Kuiken, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25612-620. [DOI] [PubMed] [Google Scholar]
- 28.LeCount, E. R. 1919. The pathologic anatomy of influenzal bronchopneumonia. JAMA 650-652.
- 29.Lee, L. Y., L. A. Ha do, C. Simmons, M. D. de Jong, N. V. Chau, R. Schumacher, Y. C. Peng, A. J. McMichael, J. J. Farrar, G. L. Smith, A. R. Townsend, B. A. Askonas, S. Rowland-Jones, and T. Dong. 2008. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Investig. 1183478-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 1665346-5355. [DOI] [PubMed] [Google Scholar]
- 31.Leroux-Roels, I., R. Bernhard, P. Gerard, M. Drame, E. Hanon, and G. Leroux-Roels. 2008. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS ONE 3e1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leroux-Roels, I., A. Borkowski, T. Vanwolleghem, M. Drame, F. Clement, E. Hons, J. M. Devaster, and G. Leroux-Roels. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370580-589. [DOI] [PubMed] [Google Scholar]
- 33.Levie, K., I. Leroux-Roels, K. Hoppenbrouwers, A. D. Kervyn, C. Vandermeulen, S. Forgus, G. Leroux-Roels, S. Pichon, and I. Kusters. 2008. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J. Infect. Dis. 198642-649. [DOI] [PubMed] [Google Scholar]
- 34.Li, F. C., B. C. Choi, T. Sly, and A. W. Pak. 2008. Finding the real case-fatality rate of H5N1 avian influenza. J. Epidemiol. Community Health 62555-559. [DOI] [PubMed] [Google Scholar]
- 35.Li, S., C. Liu, A. Klimov, K. Subbarao, M. L. Perdue, D. Mo, Y. Ji, L. Woods, S. Hietala, and M. Bryant. 1999. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 1791132-1138. [DOI] [PubMed] [Google Scholar]
- 36.Liang, S., K. Mozdzanowska, G. Palladino, and W. Gerhard. 1994. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 1521653-1661. [PubMed] [Google Scholar]
- 37.Lu, X., L. E. Edwards, J. A. Desheva, D. C. Nguyen, A. Rekstin, I. Stephenson, K. Szretter, N. J. Cox, L. G. Rudenko, A. Klimov, and J. M. Katz. 2006. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 246588-6593. [DOI] [PubMed] [Google Scholar]
- 38.Lu, X., M. Renshaw, T. M. Tumpey, G. D. Kelly, J. Hu-Primmer, and J. M. Katz. 2001. Immunity to influenza A H9N2 viruses induced by infection and vaccination. J. Virol. 754896-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 735903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maher, J. A., and J. DeStefano. 2004. The ferret: an animal model to study influenza virus. Lab. Anim. 3350-53. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood, K., R. A. Bright, N. Mytle, D. M. Carter, C. J. Crevar, J. E. Achenbach, P. M. Heaton, T. M. Tumpey, and T. M. Ross. 2008. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 265393-5399. [DOI] [PubMed] [Google Scholar]
- 42.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L. M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. Nguyen, J. H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 7911788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 691339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 896901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen, H. H., F. W. van Ginkel, H. L. Vu, J. R. McGhee, and J. Mestecky. 2001. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J. Infect. Dis. 183368-376. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson, K. G., A. E. Colegate, A. Podda, I. Stephenson, J. Wood, E. Ypma, and M. C. Zambon. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 3571937-1943. [DOI] [PubMed] [Google Scholar]
- 47.Nolan, T. M., P. C. Richmond, M. V. Skeljo, G. Pearce, G. Hartel, N. T. Formica, K. Hoschler, J. Bennet, D. Ryan, K. Papanaoum, R. L. Basser, and M. C. Zambon. 2008. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine 264160-4167. [DOI] [PubMed] [Google Scholar]
- 48.Okuno, Y., Y. Isegawa, F. Sasao, and S. Ueda. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 672552-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pappas, C., P. V. Aguilar, C. F. Basler, A. Solorzano, H. Zeng, L. A. Perrone, P. Palese, A. Garcia-Sastre, J. M. Katz, and T. M. Tumpey. 2008. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc. Natl. Acad. Sci. USA 1053064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrone, L. A., J. K. Plowden, A. Garcia-Sastre, J. M. Katz, and T. M. Tumpey. 2008. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 4e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prel, A., G. Le Gall-Recule, M. Cherbonnel, B. Grasland, M. Amelot, and V. Jestin. 2007. Assessment of the protection afforded by triple baculovirus recombinant coexpressing H5, N3, M1 proteins against a homologous H5N3 low-pathogenicity avian influenza virus challenge in Muscovy ducks. Avian Dis. 51484-489. [DOI] [PubMed] [Google Scholar]
- 52.Pushko, P., T. M. Tumpey, F. Bu, J. Knell, R. Robinson, and G. Smith. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 235751-5759. [DOI] [PubMed] [Google Scholar]
- 53.Pushko, P., T. M. Tumpey, N. Van Hoeven, J. A. Belser, R. Robinson, M. Nathan, G. Smith, D. C. Wright, and R. A. Bright. 2007. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine 254283-4290. [DOI] [PubMed] [Google Scholar]
- 54.Quan, F. S., R. W. Compans, H. H. Nguyen, and S. M. Kang. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 821350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quan, F. S., D. Steinhauer, C. Huang, T. M. Ross, R. W. Compans, and S. M. Kang. 2008. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 263352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raulet, D. H. 1994. MHC class I-deficient mice. Adv. Immunol. 55381-421. [DOI] [PubMed] [Google Scholar]
- 57.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 58.Richmond, J. Y., and R. W. McKinney (ed.). 2008. Biosafety in microbiological and biomedical laboratories, 5th ed., p. 28-58. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA.
- 59.Rowe, T., R. A. Abernathy, J. Hu-Primmer, W. W. Thompson, X. Lu, W. Lim, K. Fukuda, N. J. Cox, and J. M. Katz. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudolf, M. P., S. C. Fausch, D. M. Da Silva, and W. M. Kast. 2001. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J. Immunol. 1665917-5924. [DOI] [PubMed] [Google Scholar]
- 61.Sandbulte, M. R., G. S. Jimenez, A. C. Boon, L. R. Smith, J. J. Treanor, and R. J. Webby. 2007. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.See, H., and P. Wark. 2008. Innate immune response to viral infection of the lungs. Paediatr. Respir. Rev. 9243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smirnov, Y. A., A. S. Lipatov, A. K. Gitelman, E. C. Claas, and A. D. Osterhaus. 2000. Prevention and treatment of bronchopneumonia in mice caused by mouse-adapted variant of avian H5N2 influenza A virus using monoclonal antibody against conserved epitope in the HA stem region. Arch. Virol. 1451733-1741. [DOI] [PubMed] [Google Scholar]
- 64.Smirnov, Y. A., A. S. Lipatov, A. K. Gitelman, Y. Okuno, R. Van Beek, A. D. Osterhaus, and E. C. Claas. 1999. An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol. 43237-244. [PubMed] [Google Scholar]
- 65.Song, J. H., H. H. Nguyen, N. Cuburu, T. Horimoto, S. Y. Ko, S. H. Park, C. Czerkinsky, and M. N. Kweon. 2008. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 1051644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens, J., O. Blixt, T. M. Tumpey, J. K. Taubenberger, J. C. Paulson, and I. A. Wilson. 2006. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 312404-410. [DOI] [PubMed] [Google Scholar]
- 67.Sui, J., W. C. Hwang, S. Perez, G. Wei, D. Aird, L. M. Chen, E. Santelli, B. Stec, G. Cadwell, M. Ali, H. Wan, A. Murakami, A. Yammanuru, T. Han, N. J. Cox, L. A. Bankston, R. O. Donis, R. C. Liddington, and W. A. Marasco. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun, J., R. Madan, C. L. Karp, and T. J. Braciale. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swenson, D. L., K. L. Warfield, D. L. Negley, A. Schmaljohn, M. J. Aman, and S. Bavari. 2005. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 233033-3042. [DOI] [PubMed] [Google Scholar]
- 70.Takada, A., S. Matsushita, A. Ninomiya, Y. Kawaoka, and H. Kida. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 213212-3218. [DOI] [PubMed] [Google Scholar]
- 71.Tompkins, S. M., Z. S. Zhao, C. Y. Lo, J. A. Misplon, T. Liu, Z. Ye, R. J. Hogan, Z. Wu, K. A. Benton, T. M. Tumpey, and S. L. Epstein. 2007. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 13426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 31077-80. [DOI] [PubMed] [Google Scholar]
- 73.Tumpey, T. M., A. Garcia-Sastre, J. K. Taubenberger, P. Palese, D. E. Swayne, M. J. Pantin-Jackwood, S. Schultz-Cherry, A. Solorzano, N. Van Rooijen, J. M. Katz, and C. F. Basler. 2005. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 7914933-14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tumpey, T. M., T. R. Maines, N. Van Hoeven, L. Glaser, A. Solorzano, C. Pappas, N. J. Cox, D. E. Swayne, P. Palese, J. M. Katz, and A. Garcia-Sastre. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315655-659. [DOI] [PubMed] [Google Scholar]
- 75.Tumpey, T. M., M. Renshaw, J. D. Clements, and J. M. Katz. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 755141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ulrich, R., D. Koletzki, S. Lachmann, A. Lundkvist, A. Zankl, A. Kazaks, A. Kurth, H. R. Gelderblom, G. Borisova, H. Meisel, and D. H. Kruger. 1999. New chimaeric hepatitis B virus core particles carrying hantavirus (serotype Puumala) epitopes: immunogenicity and protection against virus challenge. J. Biotechnol. 73141-153. [DOI] [PubMed] [Google Scholar]
- 77.Wang, B. Z., W. Liu, S. M. Kang, M. Alam, C. Huang, L. Ye, Y. Sun, Y. Li, D. L. Kothe, P. Pushko, T. Dokland, B. F. Haynes, G. Smith, B. H. Hahn, and R. W. Compans. 2007. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 8110869-10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winternitz, M. C., I. M. Wason, and F. P. McNamara. 1920. The pathology of influenza. Yale University Press, New Haven, CT.
- 79.Wolbach, S. B. 1919. Comments on the pathology and bacteriology of fatal influenza cases, as observed at Camp Devins, Mass. Bull. Johns Hopkins Hosp. 338104-109. [Google Scholar]
- 80.Wood, J. M., J. Mumford, G. C. Schild, R. G. Webster, and K. G. Nicholson. 1986. Single-radial-immunodiffusion potency tests of inactivated influenza vaccines for use in man and animals. Dev. Biol. Stand. 64169-177. [PubMed] [Google Scholar]
- 81.Yao, Q., Z. Bu, A. Vzorov, C. Yang, and R. W. Compans. 2003. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine 21638-643. [DOI] [PubMed] [Google Scholar]
- 82.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 764420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]