Abstract
KAP1 is an essential cofactor of KRAB zinc finger proteins, a family of vertebrate-specific epigenetic repressors of largely unknown functions encoded in the hundreds by the mouse and human genomes. So far, KRAB/KAP1-mediated gene regulation has been studied within the environment of chromosomal DNA. Here we demonstrate that KRAB/KAP1 regulation is fully functional within the context of episomal DNA, such as adeno-associated viral and nonintegrated lentiviral vectors, and is correlated with histone modifications typically associated with this epigenetic regulator.
The mouse and human genomes each encode about four hundred KRAB (Krüppel-associated box)-containing zing finger proteins (KRAB-ZFPs). These tetrapod-specific transcriptional repressors thus constitute the single largest family of gene regulators found in mammals, yet their functions remain collectively ill defined, and very few of their targets have been identified unequivocally (13, 14, 17). In contrast, a good understanding of their mechanisms of action has been achieved, notably through the identification and characterization of KAP1, believed to represent their universal corepressor. KAP1 (KRAB-associated protein 1), also known as TIF1β (transcription intermediary factor 1β), KRIP1 (KRAB-interacting protein 1), and TRIM28 (tripartite motif protein 28) (5), harbors the following, from its N to its C terminus: a RING finger, B boxes, a coiled-coil region, a PHD finger, and a bromodomain (10, 20). The first three of these motifs define the so-called RBCC or TRIM domain, which is both necessary and sufficient for homo-oligomerization and direct binding to KRAB. Upon recruitment to DNA loci, KAP1 functions as a scaffold for the formation of a multimolecular complex, comprising heterochromatin protein 1 (HP1), histone deacetylases, and histone methyltransferases, which induces transcriptional repression through the formation of heterochromatin (1, 27).
We recently described a lentiviral vector (LV) system for conditional transgenesis and knockdown based on the tetracycline-controllable trans-repressor tTRKRAB, a chimeric protein built by fusing KRAB with the DNA binding domain of the Escherichia coli tetracycline repressor (tetR). Upon binding tetracycline operator (teto) sequences, tTRKRAB induces transcriptional repression, which can silence RNA polymerase I, II, and III promoters situated nearby, thus allowing for doxycycline (Dox)-regulated gene expression and knockdown (4, 7, 28, 32). Using this system, we demonstrated that KRAB-induced silencing is fully reversible in vitro and in vivo, even after prolonged periods of repression, whether in a variety of established human and murine cell lines, in primary cells, or in the brains of rats injected with an LV expressing the tTRKRAB repressor and a teto-controlled target. One exception to this rule was the early embryonic period, where tTRKRAB induced irreversible silencing through promoter DNA methylation (31). The latter observation suggested that the KRAB/KAP1 pathway plays an essential role in DNA methylation events taking place during the early embryonic period, a model supported by the recent finding that mice with a knockout of ZFP57, a member of the KRAB-ZFP family, exhibit a failure to establish maternal methylation imprints at specific loci and defects in the postfertilization maintenance of maternal and paternal methylation imprints at multiple imprinted domains (14).
KRAB/KAP1-mediated gene regulation has been studied so far either by using integrating gene delivery systems or through the analysis of cellular genes, that is, within the environment of chromosomal DNA. Here we asked whether this system could similarly regulate episomal gene expression. For this purpose, we examined the tTRKRAB-mediated regulation of transgenes carried by adeno-associated viral (AAV) vectors and integration-defective lentiviral vectors (IDLV) and investigated the types of chromatin modifications induced by this transcriptional modulator in such environments. We chose AAV vectors and IDLV because of their place in gene therapy, where nonintegrating systems are the focus of intense study to avoid insertional mutagenesis and cis-acting influences from the local chromosomal environment. Additionally, these vectors provide experimentally convenient paradigms for episomal DNA generated by a large number of viruses, for instance, members of the herpesvirus group.
MATERIALS AND METHODS
Cell culture.
293T cells, kindly provided by L. Naldini (Tiget, Milan, Italy), and HCT116 cells, obtained from ATCC (Manassas, VA), were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (HyClone), 50 IU/ml penicillin, and 50 μg/ml streptomycin (Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2.
Dox (Sigma Aldrich) was used at a standard concentration of 1 μg/ml.
For primary embryonic fibroblast isolation, pregnant females were sacrificed at day 13 of gestation by cervical dislocation. The uterine horns were dissected and place into a 10-cm petri dish. Each embryo was separated from its placenta and surrounding membranes, and the head and viscera were removed. The embryos were rinsed several times with 1× phosphate-buffered saline (PBS) and finely minced with scissors. The tissue suspension was then treated with trypsin (about 1 to 2 ml per embryo) for 5 to 6 min at 37°C. The resulting cell suspension, which should have been essentially free of any larger pieces of tissue, was transferred to 50-ml Falcon tubes, and trypsin was inactivated by adding fresh MEF medium. This medium is composed of Dulbecco's modified Eagle's medium supplemented with 10% non-heat-inactivated fetal calf serum (HyClone characterized fetal bovine serum), 50 IU/ml penicillin, and 50 μg/ml streptomycin. The cells were then plated out at 1 embryo equivalent per 10-cm dish, and the medium was changed on the following day. The fibroblasts would be the only cells that attached to the dishes. Plates that were normally confluent within 1 day were either frozen or split 1/5.
Vectors.
A recombinant AAV vector genome encoding enhanced green fluorescent protein (EGFP) under the control of the murine phosphoglycerate kinase (mPGK) promoter flanked by AAV serotype 2 inverted repeats was generated from pAAV-MCS (Stratagene) in the P. Aebischer lab (29). TetO sequences were introduced downstream of the polyadenylation site from the bovine growth hormone gene. Vector DNA was packaged into rAAV serotype 6 by cotransfection of a 293AAV cell line that stably expresses the E1 gene, which is needed for activation of the rep and cap promoters, with the pDF6 helper plasmid (9). Cell lysates were purified 48 h later on a heparin affinity column, using high-pressure liquid chromatography. Viral titers (infectious particles) were determined by 2-h transductions of 105 293T cells with serial dilutions of the vector preparation in a 12-well plate. Forty-eight hours later, transduced cells were analyzed for EGFP expression by fluorescence-activated cell sorting (FACS).
pFUT, pLV-tTR-KRAB-Red, and a self-deleting LV expressing CRE were described previously (3, 31, 32). The integrase-defective third-generation packaging plasmid pMD.Lg/pRRE.D64VInt was provided by L. Naldini (16). IDLV and control stocks were produced as previously described (35), with the pFUT vector plasmid, the pMD.Lg/pRRE(D64VInt) packaging plasmid, the pMD2.G envelope-encoding plasmid, and pRSV-Rev in the following amounts: 22.5, 14.6, 7.9, and 6 μg DNA per 15-cm dish, respectively. Culture medium was collected at 24 and 36 h, pooled, filtered through a 0.2-μm filter, concentrated approximately 1,000-fold by ultracentrifugation, aliquoted, and stored at −80°C until used. The amount of p24 capsid protein was determined using HIV-1 p24 core profile ELISA (NEN/Perkin-Elmer) as described by the manufacturer. Viral titers (infectious particles) were determined by 2-h transductions of 105 HCT116 cells with serial dilutions of the vector preparation in a 12-well plate. Seventy-two hours later, transduced cells were analyzed for EGFP expression by FACS.
In vivo analysis.
The generation and genotyping of floxed KAP1 mice have been described previously (30).
Andreas Trumpp provided the hPGK::tTRKRAB transgenic mice. For the generation of this transgenic line, a linearized construct (2,034 bp) containing the human PGK (hPGK) promoter, tTRKRAB, and rBG poly(A) was injected into fertilized eggs at the single-cell stage. Pronuclear injections were performed at the ISREC mouse mutant core facility under the supervision of Friedrich Beerman. Putative founders from the F0 generation were screened by PCR with the following primers: GAGTGGAAGCTGCTGGACAC as the forward primer and CAGGATGGGTCTCTTGGTGA as the reverse primer.
Dox was administered in drinking water at a concentration of 2 g/liter supplemented with 4% sucrose.
AAV vectors were administered through intravenous injection of 150 μl of PBS containing 107 transducing units of vector into the tail vein, using a 29-gauge U-100 insulin syringe (BD Micro-Fine +).
For whole-body in vivo analysis, anesthetized mice were imaged using a charge-coupled device camera in a Xenogen IVIS imaging system. The fluorescence was quantified using Living Image software (Xenogen).
For histology analysis, mice were perfused with cold PBS (Gibco) followed by 50 ml of 4% paraformaldehyde (Sigma). Organs were dissected, postfixed for 2 h at 4°C, washed three times with 1 × PBS, and embedded in OCT compound. Organs were sectioned on a cryostat at 10 μm and collected on Superfrost+ slides. For immunofluorescence, sections were pretreated in 1× PBS containing 0.25% Triton X-100 and blocked with 1% bovine serum albumin and with a streptavidin-biotin blocking kit (Vector Labs). A polyclonal rabbit anti-GFP antibody (Molecular Probes, Invitrogen) (1:100) was incubated overnight at 4°C. The next day, sections were incubated for 40 min at room temperature (RT) with a biotin anti-rabbit antibody (Jackson Immunoresearch) (1:300), followed by 30 min at RT with streptavidin-Alexa 568 (Molecular Probes, Invitrogen) (1:1,000). DAPI (4′,6-diamidino-2-phenylindole) (1:10,000) was used as a nuclear marker. Sections were coverslipped with 1× PBS-glycerol containing DABCO (Sigma) and then analyzed with a Leica DMI 4000 microscope (×20 objective).
ChIP.
Chromatin immunoprecipitation (ChIP) was performed according to the Upstate protocol, with minor modifications. In brief, 107 cells per precipitating antibody were cross-linked in 10 ml at a final concentration of 1% formaldehyde for 15 min at room temperature. Fixation was quenched by adding glycine at a final concentration of 0.125 M. The cells were then rinsed three times with cold 1× PBS and lysed on ice with 1 ml buffer containing 50 mM Tris-Cl, pH 8, 10 mM EDTA, 1% sodium dodecyl sulfate (SDS), and protease inhibitors. The cell lysate was sheared into about 300- to 700-bp pieces, using a Branson digital sonicator (model 102C). Each sample was sonicated on ice four times for 20 s each at 30% of maximum power.
One hundred microliters of sonicated lysate was directly de-cross-linked by adding 1% SDS, 100 mM NaOH, and 50 μg/ml RNase A for 2.5 h at 55°C and then adding 200 μg/ml proteinase K overnight at 65°C. DNA was extracted with phenol-chloroform, ethanol precipitated, and resuspended in 50 μl H2O. A 1/350 dilution of this sample was used for normalization (total input [TI]).
One hundred microliters was used for each ChIP reaction and was diluted in 900 μl ChIP dilution buffer containing 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris, pH 8.1, 167 mM NaCl, and protease inhibitors. The samples were precleared with 80 μl salmon sperm DNA-protein A agarose before the precipitating antibodies were added for overnight incubation at 4°C in a rotator. The lysate was precleared with 80 μl salmon sperm DNA-protein A agarose and immunoprecipitated overnight at 4°C with 5 μg of antibody directed against trimethylated H3K9 (Abcam) or KAP1 (rabbit polyclonal; a gift of Dave Schultz, Wistar Institute, Philadelphia, PA). Chromatin-antibody complexes were isolated with 60 μl of salmon sperm DNA-protein A agarose beads for 1 h. Beads were sequentially washed with low-salt, high-salt, LiCl, and Tris-EDTA (twice) buffers. Chromatin was eluted twice with 150 μl 100 mM NaHCO3, 1% SDS buffer. The cross-link between DNA and histones was reversed under high-salt conditions at 65°C overnight. Samples were then treated with RNase A for 30 min at 37°C and with proteinase K for 3 h at 55°C, extracted with phenol-chloroform, ethanol precipitated, and resuspended in 50 μl H2O. A 1/5 dilution of the samples was then subjected to quantitative real-time PCR (ABI Prism 7900). Primers specific for the promoters present in the rAAV IDLV and LV were used. Primers for rAAV were targeted to the mPGK promoter (GGC ACT TGG CGC TAC ACA A as the forward primer and CCT ACC GGT GGA TGT GGA AT as the reverse primer), and those for LV were targeted to the ubiquitin (Ub) promoter (GGC CCG CTG CTC ATA AGA as the forward primer and AAG TCC CGT CCT AAA ATG TCC TT as the reverse primer). The silenced MyoD gene and ZNF77 served as normalizers for H3K9 trimethylation and KAP1 IP, respectively. The primers used were as follows: MyoD forward and reverse, CCG CCT GAG CAA AGT AAA TGA and GGC AAC CGC TGG TTT GG, respectively; and ZNF77 forward and reverse, GGCTGCAGTTGAGCCTTCA and CACTGTCTGCCTGGTTTCTATGG, respectively. SYBR green quantitative real-time PCRs were run in triplicate with Power SYBR green PCR master mix (Applied Biosystems), using standard procedures. Negative control reactions without antibody were run for each sample and in all cases gave negligible results. To ensure the specificity of the PCR products, these were subsequently evaluated by dissociation curve analysis. To quantify the relative enrichment of H3K9 trimethylation or KAP1 at each promoter sequence, a ratio of the relative quantities of IP and TI was calculated with the help of a standard curve made with serial dilutions. To compare the relative enrichments (IP/TI) at promoters of interest (mPGK and Ub), we then normalized our IP/TI values by dividing them by either the IP/TI for MyoD (for H3K9 trimethylation) or that for ZNF77 (for KAP1).
All data are expressed as means ± standard errors of the means, based on the results of two independent experiments.
RESULTS
tTRKRAB can regulate transgenes on episomal DNA both in vitro and in vivo.
We previously exploited KRAB/KAP1-mediated regulation to achieve drug-controllable transgene expression within the setting of an integrating LV expressing EGFP from the Ub C promoter and harboring teto sequences in the U3 region of the long terminal repeat (LTR) (28, 31) (Fig. 1). In this study, we produced virions containing this vector, using either a prototypic, integrase-competent packaging construct or its mutated counterpart. Previous studies demonstrated that IDLV are competent for delivering transgenes to the nuclei of target cells, where they are expressed in the absence of integration (22, 24, 33). In parallel, we inserted teto sequences into a rAAV2 encoding EGFP under the control of the mPGK promoter (Fig. 1) and produced viral particles coated with the AAV serotype 6 capsid.
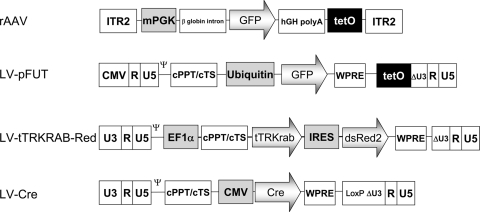
FIG. 1.
Schematic diagram of the vectors used in the study. The rAAV vector encodes EGFP under the control of the mPGK promoter, with teto sequences in one copy downstream of the EGFP gene. ITR, AAV inverted terminal repeats for AAV2; β-globin intron, beta-globin mRNA slice donor/splice acceptor; hGH polyA, the polyadenylation site from the bovine growth hormone gene. The LV are self-inactivated; the 5′ U3 region is wild type except for that in LV-pFUT, which comes from cytomegalovirus (CMV). The teto sequences in pFUT are located in the deleted part of the 3′ U3 region. All vectors bear the encapsidation signal, the Rev-responsive element (RRE), the central polypurine tract with its termination sequence (cPPT/cTS), and the woodchuck hepatitis virus responsive element (WPRE). The internal promoter is the one from Ub C (Ubiquitin) for LV-pFUT, the one from human elongation factor 1 alpha (EF1α) for LV-tTRKrabRed, and the one from cytomegalovirus for LV-Cre. IRES, internal ribosome entry site derived from the encephalomyocarditis virus; tTRKRAB, cDNA encoding the tetracycline transrepressor (tTR) from Escherichia coli Tn10 fused to the KRAB domain of human Kox1; Cre, Cre recombinase; dsRed2, variant red fluorescent protein modified with six point mutations that improve solubility by reducing the tendency to form aggregates.
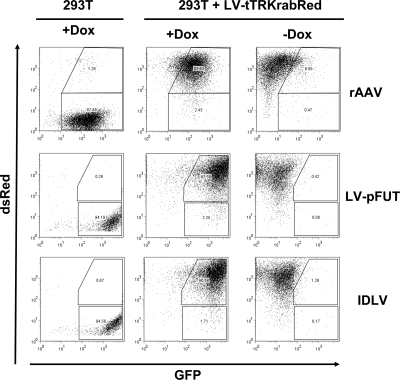
We used the LV, IDLV, and AAV particles to transduce 293T cells that stably express the tTRKRAB trans-repressor owing to prior transduction with the LV-tTRKRAB-Red vector (Fig. 1). These cells were then cultured for 2 days in the presence of absence of Dox and analyzed by FACS for GFP expression (Fig. 2). All three vectors induced tTRKRAB-controllable GFP expression, with high levels in the presence of Dox and full silencing in the absence of drug. Regulation was as efficient with the episomal rAAV and IDLV as with the integrated LV, indicating that the KRAB/KAP1 pathway can repress nonintegrated DNA in vitro.
FIG. 2.
In vitro regulation of EGFP expression from AAV and IDLV by tTRKRAB. 293T cells carrying 20 copies of LV-tTR-KRAB-Red were transduced in the presence or absence of Dox with either rAAV encoding mPGK-eGFP-tetO at a multiplicity of infection (MOI) of 1.5 or LV-pFUT or IDLV at an MOI of 30 or 40, respectively. 293T cells containing no LV-tTR-KRAB-Red vector were transduced under the same conditions in the presence of Dox. The cells were FACS analyzed at 48 h posttransduction. For the LV-tTR-KRAB-Red-transduced cells, the percentage of EGFP-positive cells is gated on the dsRed-positive population.
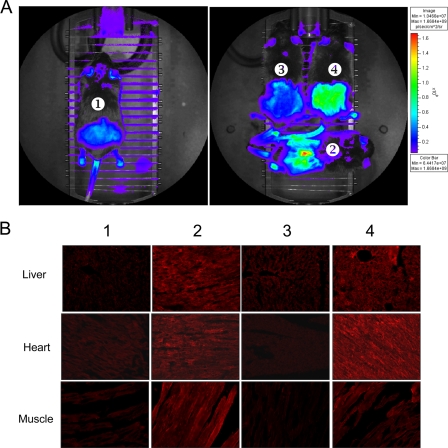
The functionality of tTRKRAB-mediated regulation of the episomal transgenes was then examined in vivo. Given that intravenous administration of rAAV serotype 6 has been demonstrated to result in systemic delivery (8), 3-month-old transgenic mice (n = 6) expressing tTRKRAB under the control of the ubiquitous hPGK promoter were administered 2 × 108 transducing units of rAAV through the tail vein. Animals were then given Dox, or not (n = 3 in each group), in the drinking water for 3 weeks. Two control groups were represented by mice that were either noninjected or injected with a homologous, albeit nonregulatable rAAV vector devoid of teto sequences. At 3 weeks post-rAAV injection, mice were analyzed for EGFP expression by Xenogen IVIS three-dimensional imaging, which allows for noninvasive yet quantitative measurement of fluorescence in live animals (Fig. 3A). GFP expression in muscle was scored by shaving the overlying hair, which otherwise blocks emission. Fluorescence was as intense in animals injected with the regulatable rAAV and kept on Dox as in the controls injected with the nonregulatable rAAV, while in the absence of Dox it dropped to the background levels measured in the noninjected mice. In order to confirm these data, the rAAV-induced GFP expression profile was assessed by GFP-specific immunofluorescence analysis of muscles, hearts, and livers harvested from animals sacrificed at 4 months postinjection. These tissues were previously identified as the most efficiently transduced tissues after intravenous injection of AAV6 capsid-coated vectors (29). The results confirmed the Dox-dependent transgene expression from the tTRKRAB-controllable rAAV (Fig. 3B).
FIG. 3.
In vivo regulation of EGFP expression from AAV vector by tTRKRAB. Transgenic mice expressing the tTRKRAB protein under the control of the hPGK promoter were injected intravenously with rAAV. (A) At 3 weeks postinjection, EGFP fluorescence was measured with a Xenogen IVIS imaging system on live animals treated (4) or not treated (3) with Dox. A noninjected animal was used as a negative control (1), and a tTRKRAB transgenic mouse injected with a nonregulatable AAV encoding EGFP under the control of the hPGK promoter without the teto sequence was used as a positive control (2). The color scale next to the images indicates the signal intensity. (B) At 4 months postinjection, animals were sacrificed and GFP expression analyzed by immunofluorescence with an antibody directed against EGFP on different tissues (liver, heart, and muscle). The animal numbers are the same as those in the whole-animal images: panels 1 and 2 are the negative and positive controls, respectively, and panels 3 and 4 are hPGK::tTRKRAB mice injected with the regulatable AAV, treated (4) or not (3) with Dox.
Episomal tTRKRAB silencing is KAP1 dependent.
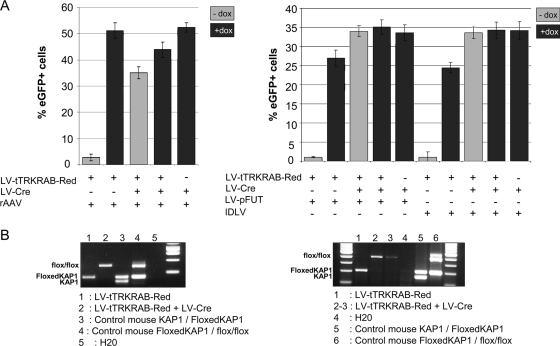
KAP1 is essential for KRAB-mediated repression within the context of integrating LV (31). To confirm that this corepressor is also involved in KRAB-induced silencing of episomal transgenes, we used primary embryonic fibroblasts obtained from mice carrying Cre recombinase-excisable KAP1 alleles (30), as previously described (31). These cells were first transduced with the LV-tTRKRAB-Red construct to induce stable tTRKRAB expression before transduction with an LV expressing the Cre recombinase to inactivate KAP1 (3) or with an empty vector as a control. Finally, the resulting KAP1-competent and KAP1-defective primary embryonic fibroblasts were exposed to the teto- and GFP-containing rAAV, IDLV, and LV vectors, cultured in the presence or absence of Dox for 2 days, and examined by FACS for EGFP expression. PCR analysis of Cre-treated cells confirmed the efficient excision of the loxP-containing KAP1 alleles (Fig. 4B). In these targets, tTRKRAB-mediated control of transgene expression was lost from all three types of vectors (Fig. 4A), indicating that KRAB-induced silencing is as KAP1 dependent for episomal as for chromosomal DNA.
FIG. 4.
tTRKRAB-mediated regulation of AAV and IDLV is KAP1 dependent. (A) GFP expression in primary embryonic fibroblasts derived from mice homozygous for a floxed KAP1 allele first transduced with LV-tTRKRAB-Red and LV-Cre, followed by transduction with AAV, LV-pFUT, or IDLV, as indicated under the graphics. Cells were cultured with or without Dox and FACS analyzed 48 h after the last transduction. (B) PCR analysis of primary embryonic fibroblasts after transduction with LV-tTRKrabRed and/or LV-Cre to check for KAP1 excision. The flox/flox band represents a null allele.
tTRKRAB/KAP1 silencing of episomal DNA is mediated by histone modification.
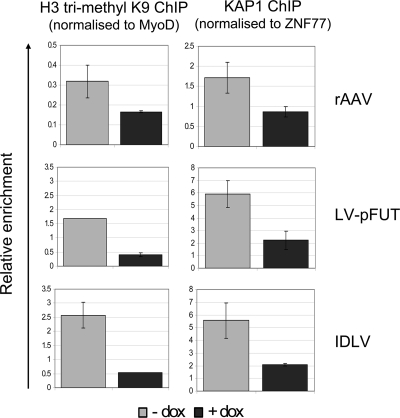
In order to investigate the mechanism of tTRKRAB/KAP1 transcriptional repression of episomal vectors, we performed ChIP on DNA isolated from tTRKRAB-expressing 293T cells transduced with the teto- and GFP-containing rAAV, IDLV, or LV, using antibodies directed against either KAP1 or histone 3 trimethylated on lysine 9 (H3K9me3). Methylation of H3K9 is a chromatin modification implicated in gene silencing and notably mediated by the KAP1-associated SETDB1 histone methyltransferase (12, 27). Chromatin immunoprecipitates were amplified with primers specific for the promoters present in rAAV, IDLV, and LV, as well as those of MyoD, a muscle-specific master regulator used as a reference for a gene repressed in 293T cells, and ZNF77, a KAP1-binding KRAB-ZNF (23). Promoter sequences present in all three vectors exhibited the same degrees of Dox-inhibited enrichment in H3K9 trimethylation and KAP1 binding (Fig. 5), a pattern fully consistent with drug-controllable, tTRKRAB-mediated, KAP1-induced repression through the recruitment of H3K9 histone methyltransferase (27).
FIG. 5.
tTRKRAB/KAP1 silencing on episomal DNA is mediated by histone modifications. 293T cells carrying 20 copies of the LV-tTRKrabRed vector were transduced with AAV at an MOI of 0.1 or with LV-pFUT or IDLV at an MOI of 1 and cultured in the presence or absence of Dox. The histone trimethylation profile (H3 tri-methyl K9) and KAP1 binding on AAV and LV promoters were investigated by ChIP and quantitative SYBR green PCR. The cellular promoter of the muscle-specific master regulator MyoD gene served as a control for methylation, and that for ZNF77 served as a control for KAP1 binding.
DISCUSSION
This study demonstrates that the tTRKRAB/KAP1 system can be applied successfully to episomal DNA produced by AAV and IDLV to achieve drug-controllable, epigenetically regulated transgene expression in tissue culture and in vivo. Nonintegrating vectors are of particular interest to avoid insertional mutagenesis (11, 21, 26) and have become first-choice gene delivery systems in some applications, for instance, in the brain (18, 24). The present work expands their versatility by conferring external controllability, an asset of importance, for instance, to express therapeutic genes that might be detrimental if constantly expressed, such as those encoding growth factors (6).
A KRAB fusion protein was previously used to tighten the regulation of tetracycline-controlled artificial promoters within the setting of episomal DNA, whether from Epstein-Barr virus-based plasmids (2), helper-dependent adenoviral vectors (25), or rAAV vectors (19). In this study, we used this system to confer external regulation to natural promoters. While only polymerase II promoters were tested here, our previous results indicating that tTRKRAB can regulate the polymerase III-mediated expression of small hairpin RNAs (32) suggests that the vectors described here could also be used for drug-controllable gene knockdown.
Importantly, our experiments also demonstrate that KRAB/KAP1 epigenetic regulation is fully functional within the context of episomal DNA. Indeed, the drug controllability of our AAV and IDLV (i) was completely dependent upon KAP1 and (ii) correlated with histone modifications typically associated with this epigenetic regulator. The involvement of the KRAB/KAP1 pathway in the regulation of episomal DNA was previously observed within the context of herpesviruses. First, the KRAB-ZNF ZBRK1, along with KAP1, was found to interact with the EBV replication protein BBLF2/3, thus partaking in a complex that provides an origin-tethering function for EBV replication (15). Also, the replication and transcription activator (RTA) protein of Kaposi's sarcoma-associated herpesvirus (KSHV), a transactivator of the lytic cascade of this pathogen, is inhibited by K-RBP (KSHV RTA-binding protein), a KAP1-recruiting KRAB-containing transcriptional repressor that can suppress KSHV passage from latency to lytic replication (34). Our data indicate that KRAB/KAP1-induced epigenetic alterations may regulate the biology of nonintegrating DNA viruses at large.
Acknowledgments
We thank Luigi Naldini for providing the IDLV packaging vector, Florence Cammas and Régine Losson for providing the floxed KAP1 transgenic mice, Jessica Dessimoz and all of the EPFL Histology Core Facility for the development of immunostaining, and Johan Jackobson for helpful discussions.
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Ayyanathan, K., M. S. Lechner, P. Bell, G. G. Maul, D. C. Schultz, Y. Yamada, K. Tanaka, K. Torigoe, and F. J. Rauscher III. 2003. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 171855-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornkamm, G. W., C. Berens, C. Kuklik-Roos, J. M. Bechet, G. Laux, J. Bachl, M. Korndoerfer, M. Schlee, M. Holzel, A. Malamoussi, R. D. Chapman, F. Nimmerjahn, J. Mautner, W. Hillen, H. Bujard, and J. Feuillard. 2005. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 33e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cudre-Mauroux, C., T. Occhiodoro, S. Konig, P. Salmon, L. Bernheim, and D. Trono. 2003. Lentivector-mediated transfer of Bmi-1 and telomerase in muscle satellite cells yields a Duchenne myoblast cell line with long-term genotypic and phenotypic stability. Hum. Gene Ther. 141525-1533. [DOI] [PubMed] [Google Scholar]
- 4.Deuschle, U., W. K. Meyer, and H. J. Thiesen. 1995. Tetracycline-reversible silencing of eukaryotic promoters. Mol. Cell. Biol. 151907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman, J. R., W. J. Fredericks, D. E. Jensen, D. W. Speicher, X. P. Huang, E. G. Neilson, and F. J. Rauscher III. 1996. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 102067-2078. [DOI] [PubMed] [Google Scholar]
- 6.Georgievska, B., D. Kirik, and A. Bjorklund. 2002. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp. Neurol. 177461-474. [DOI] [PubMed] [Google Scholar]
- 7.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 895547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregorevic, P., M. J. Blankinship, J. M. Allen, R. W. Crawford, L. Meuse, D. G. Miller, D. W. Russell, and J. S. Chamberlain. 2004. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm, D., M. A. Kay, and J. A. Kleinschmidt. 2003. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 7839-850. [DOI] [PubMed] [Google Scholar]
- 10.Kim, S. S., Y. M. Chen, E. O'Leary, R. Witzgall, M. Vidal, and J. V. Bonventre. 1996. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc. Natl. Acad. Sci. USA 9315299-15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohn, D. B., M. Sadelain, and J. C. Glorioso. 2003. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer 3477-488. [DOI] [PubMed] [Google Scholar]
- 12.Kouzarides, T. 2007. Chromatin modifications and their function. Cell 128693-705. [DOI] [PubMed] [Google Scholar]
- 13.Krebs, C. J., L. K. Larkins, R. Price, K. M. Tullis, R. D. Miller, and D. M. Robins. 2003. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 172664-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, X., M. Ito, F. Zhou, N. Youngson, X. Zuo, P. Leder, and A. C. Ferguson-Smith. 2008. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell 15547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao, G., J. Huang, E. D. Fixman, and S. D. Hayward. 2005. The Epstein-Barr virus replication protein BBLF2/3 provides an origin-tethering function through interaction with the zinc finger DNA binding protein ZBRK1 and the KAP-1 corepressor. J. Virol. 79245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardo, A., P. Genovese, C. M. Beausejour, S. Colleoni, Y. L. Lee, K. A. Kim, D. Ando, F. D. Urnov, C. Galli, P. D. Gregory, M. C. Holmes, and L. Naldini. 2007. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 251298-1306. [DOI] [PubMed] [Google Scholar]
- 17.Looman, C., M. Abrink, C. Mark, and L. Hellman. 2002. KRAB zinc finger proteins: an analysis of the molecular mechanisms governing their increase in numbers and complexity during evolution. Mol. Biol. Evol. 192118-2130. [DOI] [PubMed] [Google Scholar]
- 18.Mandel, R. J., F. P. Manfredsson, K. D. Foust, A. Rising, S. Reimsnider, K. Nash, and C. Burger. 2006. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol. Ther. 13463-483. [DOI] [PubMed] [Google Scholar]
- 19.McGee Sanftner, L. H., K. G. Rendahl, D. Quiroz, M. Coyne, M. Ladner, W. C. Manning, and J. G. Flannery. 2001. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol. Ther. 3688-696. [DOI] [PubMed] [Google Scholar]
- 20.Moosmann, P., O. Georgiev, B. Le Douarin, J. P. Bourquin, and W. Schaffner. 1996. Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 244859-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai, H., S. R. Yant, T. A. Storm, S. Fuess, L. Meuse, and M. A. Kay. 2001. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 756969-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nightingale, S. J., R. P. Hollis, K. A. Pepper, D. Petersen, X. J. Yu, C. Yang, I. Bahner, and D. B. Kohn. 2006. Transient gene expression by nonintegrating lentiviral vectors. Mol. Ther. 131121-1132. [DOI] [PubMed] [Google Scholar]
- 23.O'Geen, H., S. L. Squazzo, S. Iyengar, K. Blahnik, J. L. Rinn, H. Y. Chang, R. Green, and P. J. Farnham. 2007. Genome-wide analysis of KAP1 binding suggests autoregulation of KRAB-ZNFs. PLoS Genet. 3e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippe, S., C. Sarkis, M. Barkats, H. Mammeri, C. Ladroue, C. Petit, J. Mallet, and C. Serguera. 2006. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. USA 10317684-17689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salucci, V., A. Scarito, L. Aurisicchio, S. Lamartina, G. Nicolaus, S. Giampaoli, O. Gonzalez-Paz, C. Toniatti, H. Bujard, W. Hillen, G. Ciliberto, and F. Palombo. 2002. Tight control of gene expression by a helper-dependent adenovirus vector carrying the rtTA2(s)-M2 tetracycline transactivator and repressor system. Gene Ther. 91415-1421. [DOI] [PubMed] [Google Scholar]
- 26.Schnepp, B. C., K. R. Clark, D. L. Klemanski, C. A. Pacak, and P. R. Johnson. 2003. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J. Virol. 773495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sripathy, S. P., J. Stevens, and D. C. Schultz. 2006. The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Mol. Cell. Biol. 268623-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szulc, J., M. Wiznerowicz, M. O. Sauvain, D. Trono, and P. Aebischer. 2006. A versatile tool for conditional gene expression and knockdown. Nat. Methods 3109-116. [DOI] [PubMed] [Google Scholar]
- 29.Towne, C., C. Raoul, B. L. Schneider, and P. Aebischer. 2008. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol. Ther. 161018-1025. [DOI] [PubMed] [Google Scholar]
- 30.Weber, P., F. Cammas, C. Gerard, D. Metzger, P. Chambon, R. Losson, and M. Mark. 2002. Germ cell expression of the transcriptional co-repressor TIF1beta is required for the maintenance of spermatogenesis in the mouse. Development 1292329-2337. [DOI] [PubMed] [Google Scholar]
- 31.Wiznerowicz, M., J. Jakobsson, J. Szulc, S. Liao, A. Quazzola, F. Beermann, P. Aebischer, and D. Trono. 2007. The Kruppel-associated box repressor domain can trigger de novo promoter methylation during mouse early embryogenesis. J. Biol. Chem. 28234535-34541. [DOI] [PubMed] [Google Scholar]
- 32.Wiznerowicz, M., and D. Trono. 2003. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 778957-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanez-Munoz, R. J., K. S. Balaggan, A. MacNeil, S. J. Howe, M. Schmidt, A. J. Smith, P. Buch, R. E. MacLaren, P. N. Anderson, S. E. Barker, Y. Duran, C. Bartholomae, C. von Kalle, J. R. Heckenlively, C. Kinnon, R. R. Ali, and A. J. Thrasher. 2006. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12348-353. [DOI] [PubMed] [Google Scholar]
- 34.Yang, Z., and C. Wood. 2007. The transcriptional repressor K-RBP modulates RTA-mediated transactivation and lytic replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 816294-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15871-875. [DOI] [PubMed] [Google Scholar]