Abstract
We developed a high-throughput, cell-based screen to identify chemicals that inhibit infection by the primate polyomaviruses. The screen is based on the detection of compounds that inhibit the ability of a replication-defective simian virus 40 (SV40)-based viral vector to cause growth arrest in HeLa cells by repressing the expression of the endogenous human papillomavirus E7 oncogene in these cells. We identified two compounds, ellagic acid and spiperone, that suppressed the ability of the SV40 recombinant virus to inhibit cellular DNA synthesis. These compounds caused a marked reduction of the ability of wild-type SV40 to productively infect permissive monkey cells, even when the compounds were added several hours after infection. The fraction of cells expressing SV40 large T antigen and the levels of T antigen mRNA were reduced in infected human and monkey cells treated with ellagic acid and spiperone, suggesting that these compounds block a step in the virus life cycle prior to SV40 early gene expression. Ellagic acid and spiperone also inhibited large T antigen expression by BK virus and JC virus, two important, pathogenic human polyomaviruses.
The Polyomaviridae are nonenveloped, small DNA tumor viruses of great medical and scientific interest. Simian virus 40 (SV40), the first primate polyomavirus isolated, is one of the best-studied animal viruses. Study of SV40 has provided important insights into many aspects of cellular and molecular biology, molecular oncology, and virology. In addition, SV40 has been implicated in several human cancers, although its role in human diseases remains controversial (10). BK virus (BKV) and JC virus (JCV) are human polyomaviruses closely related to SV40. BKV and JCV infect the great majority of humans worldwide and cause serious disease in immunosuppressed individuals. BKV causes BK nephropathy, a frequent cause of graft failure in renal transplant recipients, and hemorrhagic cystitis, a common complication of allogeneic bone marrow and hematopoietic stem cell transplants (4, 6, 17, 24). JCV causes progressive multifocal leukoencephelopathy (PML), most commonly in AIDS patients, but JCV-induced PML has also occurred in patients receiving cancer chemotherapy or monoclonal antibody therapy for autoimmune diseases (4, 16, 20). BKV and JCV have potent tumorigenic activity in experimental animals, and both have been linked to human cancers (3, 23). The ubiquitous nature of these infections may mask as-yet-unrecognized roles of these viruses in other diseases. In the last 2 years, three new polyomaviruses have been isolated from humans and implicated in respiratory disease and skin cancer, suggesting that additional human polyomaviruses remain to be discovered (1, 9, 11). Despite the medical importance of the polyomaviruses, there are no effective antiviral therapies (30).
A major limitation to the discovery and development of antiviral drugs is the dearth of sensitive and specific cell-based assays that are suitable for high-throughput chemical screening, especially for viruses such as polyomaviruses that express relatively few gene products. It is possible to insert an easily scorable gene, such as one encoding a fluorescent protein, into the viral genome for use as a reporter of virus infection. However, assays based on the reduction in protein expression tend to identify toxic compounds that kill the host cell, as well as compounds that inhibit virus infection, thus reducing the efficiency of the screen. To eliminate this problem, we exploited our understanding of the growth regulatory circuits in a human cancer cell line to design a rapid, efficient, cell-based screen for polyomavirus infection. In this screen, inhibition of infection by an active compound restores cell proliferation, whereas this screen does not score compounds that are toxic to cells. By using this assay, we discovered two compounds that inhibit the early phase of infection by SV40, JCV, and BKV.
MATERIALS AND METHODS
Cells and reagents.
CV1 and Vero cells were purchased from the American Type Culture Collection [ATCC]. CMT4 cells were provided by Y. Gluzman (12). HeLa-E6 cells are a clonal line of HeLa cells in which HPV16 E6 is expressed from an integrated retrovirus from its long terminal repeat, which does not respond to the BPV E2 protein (5). Low-passage, primary human foreskin fibroblasts were obtained from the Yale Skin Diseases Research Center. The SV40-transformed fetal glial cell line SVG-A has been described elsewhere (33). CV1 and Vero cells were grown in the minimal essential medium E, and all other cells in Dulbecco minimal essential medium, both containing 10% fetal bovine serum (FBS), standard antibiotics, and 10 mM HEPES (pH 7.2). The anti-SV40 T antigen mouse monoclonal antibodies MS-1832 and MS-1833 were obtained from NeoMarkers (Fremont, CA), and the anti-JCV T antigen JC/BK antibody was obtained from Abcam, Inc. (Cambridge, MA). Alexa Fluor 488-labeled donkey anti-mouse immunoglobulin G (IgG; H+L) was obtained from Invitrogen (Carlsbad, CA) and used as a secondary antibody for fluorescent detection. Ellagic acid, spiperone, N-methylspiperone, and related chemicals were obtained from the Sigma-Aldrich Corp. (St. Louis, MO). Compounds were freshly dissolved to 10 mM in dimethyl sulfoxide (DMSO) for each experiment. Ellagic acid was left at 37°C for 1 h to allow complete solubilization. CulturPlate-384 tissue culture and assay plates were purchased from Perkin-Elmer (Waltham, MA). Opti-MEM and Lipofectamine were obtained from Invitrogen (Carlsbad, CA). DNA synthesis was measured by using a cell proliferation enzyme-linked immunosorbent assay (ELISA) bromodeoxyuridine (BrdU) chemiluminescence kit from Roche Applied Science (Indianapolis, IN) after adaptation for 384-well plates.
Viruses.
Production and titering of the Pava1 recombinant SV40 in CMT4 cells was performed as described previously (14, 25). SV40 (strain B2E Baylor) was generated from pUCSV40-B2E DNA (obtained from the ATCC) in CV1 cells. A final, high-titer stock was prepared by using the Pava1 protocol and yielded a titer of 3.8 × 109 infectious units per ml as determined by plaque formation or titering via flow cytometry in CV1 cells (see below). BKV was obtained as a viral stock from the ATCC (catalogue VR-837) and amplified in Vero cells as described previously (21). JCV strain MAD-4 was obtained as a viral stock from the ATCC and used directly. Adenovirus type 5-green fluorescent protein (Ad5-GFP) was obtained as a viral stock from Vector Biolabs (Philadelphia, PA).
High-throughput drug screen.
The chemical compound screen was conducted at the Yale University Center for Genomics and Proteomics in the Chemical Genomics Screening Facility. The Gen-Plus library of 960 active chemical compounds from MicroSource Discovery Systems (Gaylordsville, CT) was used in the primary screen. HeLa-E6 cells were diluted to 7.5 × 104 cells/ml in screening medium (Opti-MEM supplemented with 2% FBS and antibiotics), and 20 μl of cells was added to each well in six CulturPlate-384 plates. After 24 h, Pava1 was diluted to 6 × 106 infectious units/ml in screening medium, and 10 μl of virus or screening medium alone was added to each well for a final multiplicity of infection (MOI) of 20. Four hours later ∼10 mM chemical compound stocks in DMSO or DMSO alone were added one to a well in duplicate by using an Aquarius liquid handling system (Tecan Group, Ltd., Durham, NC) equipped with a 384 pin tool for a final compound concentration of ∼10 μM in 0.1% DMSO. Two days later, 10 μl of a 40 μM solution of BrdU in screening medium was added to a final concentration of 10 μM, and the cells were incubated for 4 h. We then followed the directions of the Roche cell proliferation kit, scaling the volumes for a 384-well plate versus the 96-well plate. Briefly, we fixed and denatured the cells, washed the wells, incubated the cells with the peroxidase-labeled BrdU antibody provided with the kit, washed the wells six times, and then added the chemiluminescence detection reagent. After a 4-min incubation, we used an Envision plate reader (Perkin-Elmer, Waltham, MA) to measure the emitted light. The data for the mock-infected samples were averaged, and all data were normalized to that value. Initial chemicals were scored as positive that allowed DNA synthesis at least four standard deviations above the values for averaged infected, mock-treated samples in both replicates.
Microscopy.
HeLa-E6 cells were plated at 104 cells per well in an eight-chamber slide (BD Falcon, Bedford, MA) and infected with SV40 the next day at an MOI of ∼1 in 100 μl of medium. At 2 h postinfection, 100 μl of fresh medium containing drugs at twice the final concentration was added. After 2 days, the cells were gently washed with phosphate-buffered saline (PBS), the slide was placed on ice, and ice-cold methanol was slowly added to each well. After 20 min of fixation, the cells were washed twice with PBS and once with 0.5% bovine serum albumin in PBS with 0.01% sodium azide (flow cytometry staining buffer [FSB]). The T-antigen antibodies were diluted to 1 μg/ml in 5% normal donkey serum in PBS (NDS-PBS), and 100 μl was added per well, followed by incubation for 1 h at 37°C. After four washes with FSB, 100 μl of a 1:500 dilution of Alexa Fluor 488-conjugated donkey anti-mouse IgG supplemented with 300 nM DAPI (4′,6′-diamidino-2-phenylindole) in NDS-PBS was added to each well, and the samples were then incubated at 37°C for 30 min. The cells were washed three times in FSB and once in PBS before removal of the chamber assembly. The excess PBS was aspirated, and the slides were mounted using Gel-Mount (Accurate Chemical & Scientific Corp. Westbury, NY) and a 22-by-50-mm coverslip. SVG-A cells were stained for JCV T antigen in a similar fashion except that 5 × 103 cells were plated per chamber, the cells were incubated 4 days after infection, and the primary antibody was a tissue culture cell supernatant of JC/BK diluted 1:10 in NDS-PBS. Staining was visualized by using a Zeiss Axioskop microscope equipped with a ×20 objective lens and a ×10 eyepiece and fluorescence filters appropriate for DAPI and fluorescein isothiocyanate (Zeiss, Thornwood, NY). Cell images were captured using a QImaging camera and QCapture Pro 6.0 software (Surrey, British Columbia, Canada) using the same exposure settings for all samples.
Flow cytometry.
Cells were plated at 1.5 × 105 cells per well of a six-well dish. The next day, the cells were mock infected or infected with virus and treated with test compound or DMSO. The cells were harvested at the indicated times by using 0.25% trypsin-0.56 mM EDTA in PBS (Gibco-BRL), resuspended in complete medium, and pelleted by 5 min of low-speed centrifugation in a clinical centrifuge. The cell pellet was resuspended in 5 ml of ice-cold PBS and again isolated by centrifugation. Ice-cold 100% methanol was added dropwise to the cell pellet with gentle vortexing, and the fixation continued for >20 min on ice. The cells were then washed once with PBS and once with FSB. After the cell pellet was resuspended in 100 μl of NDS-PBS containing 1 μg of primary antibody/ml, the cells were incubated at 37°C for 1 h, washed twice with FSB, and resuspended in 100 μl of NDS-PBS containing a 1:500 dilution of Alexa Fluor 488-conjugated donkey anti-mouse IgG and incubated at 37°C for 30 min. After two washes in FSB, the cell pellet was resuspended in 300 μl of FSB and kept on ice. Flow cytometric analysis was performed with a FACSVantage flow cytometer (Becton Dickinson) using CellQuest software. The cells were excited at 488 nm, and the forward scatter, side scatter, and fluorescence were collected through a band-pass filter (530 ± 15 nm). To detect Ad5-GFP positive cells, the cells were collected as described above and washed in PBS, and the live cells were kept on ice until flow cytometry.
qRT-PCR.
HeLa-E6 or SVG-A cells were plated at 105 in a six-well plate and infected the next day. Cells were infected with SV40 at an MOI ∼0.5 (HeLa-E6 cells) or with 10 μl of JCV stock at an MOI of ≪1 (SVG-A cells). After 2 h, the compounds were added to the indicated concentrations. The HeLa-E6 cells were harvested 2 days after infection, and the SVG-A cells were harvested 4 days after infection. Total RNA was prepared by using an RNeasy minikit with on-column DNase digestion (Qiagen, Valencia, CA) to remove any viral DNA, which would otherwise confound the analysis. Then, 1 μg of total RNA was converted to cDNA by using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The cDNA was subjected to quantitative reverse transcriptase real-time PCR (qRT-PCR) in triplicate using the IQ SYBR green Supermix and a MyIQ single color real-time PCR detection system (both from Bio-Rad) and specific DNA primers. The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers (GAG TCA ACG GAT TTG GTC GT [forward] and TTG ATT TTG GAG GGA TCT CG [reverse]), the SV40 T-antigen primers (GGT GGG TTA AAG GAG CAT GA [forward] and TAG TGG CTG GGC TGT TCT TT [reverse]), and the JCV T antigen primers (AAA GGG TTG ACA GCA GCC AC [forward] and CTG TCT ATT GGC CCC TTG AA [reverse]) were all obtained from Integrated DNA Technologies (Coralville, IA).
SV40 production assay.
A total of 105 CV1 cells were seeded into 35-mm wells of six-well tissue culture plates in Eagle modified minimal essential medium supplemented with 10% FBS. After 24 h, the medium was replaced with 2 ml of fresh medium containing infectious SV40 (MOI of 0.5), and the cells were incubated for 1.5 h at 37°C. Ellagic acid or spiperone were dissolved in DMSO to 10 mM and added with swirling to the medium in each well to yield the indicated final concentration of drug. The well without drug was treated with the same amount of DMSO as in the most concentrated drug sample. All conditions were performed in triplicate. At 55 h after infection, the plates containing cells and spent medium were subjected to three freeze-thaw cycles to release virus from the cells. Virus production was assayed by infecting 3 × 105 CV1 cells with 25 μl of the lysate, followed by 23 h of growth at 37°C, immunostaining, and flow cytometry to detect T antigen-positive cells as described above. The highest concentration of ellagic acid or spiperone carried over in the 25-μl virus lysates did not inhibit SV40 infection (data not shown). The percentages of positive cells, in triplicate, for each condition were corrected for the possibility of multiple infections per cell by using the Poisson distribution, averaged, and expressed as a fraction of the amount of virus produced by infected cells in the absence of test compound.
Transfection assay.
SV40 DNA was prepared by digesting plasmid pUCSV40-B2E with EcoRI to release the full-length viral genome. After heat inactivation of EcoRI, the SV40 DNA was recircularized by dilution to 1 ml in T4 DNA ligase buffer and overnight incubation at 15°C with 800 U of T4 DNA ligase (EcoRI and T4 DNA ligase were obtained from New England Biolabs, Ipswich, MA). The DNA was concentrated by ethanol precipitation and diluted to 0.1 μg/ml. For each transfection, 2 × 105 CV1 cells were plated in a 35-mm well. After 16 h, a transfection mix was prepared by diluting 1 μg of SV40 DNA to 100 μl with Opti-MEM, followed by mixing with 8 μl of Lipofectamine in 92 μl of Opti-MEM. After 30 min, 800 μl of Opti-MEM was added to the transfection mix, which was then added to cells, followed by incubation for 4 h at 37°C. The transfection mix was replaced with Opti-MEM containing 10% FBS, but no antibiotics, and the indicated concentration of ellagic acid or a DMSO control. The cells were harvested 26 h after the start of transfection and processed for large T antigen staining and flow cytometry as described above.
RESULTS
Design of a high-throughput assay for SV40 infection.
We developed a sensitive cell-based assay for SV40 infection of a human cancer cell line and used it to screen a chemical library for small molecules that inhibit infection (Fig. 1). Human cervical carcinoma cells continuously express the human papillomavirus (HPV) E6 and E7 proteins, which inactivate the cellular p53 and retinoblastoma (RB) tumor suppressor pathways, respectively. Papillomaviruses also encode a transcription factor, the E2 protein, which can bind directly to the HPV E6/E7 promoter and repress it. In HeLa cervical carcinoma cells, the HPV18 E2 gene is disrupted by integration of the viral DNA into the cellular genome, so that the E2 protein can no longer repress the E6/E7 promoter. We developed an SV40-based recombinant virus (designated Pava1) to express the bovine papillomavirus (BPV) E2 gene in HeLa cells (34). In this vector, the SV40 large T antigen, which is required for SV40 DNA replication, is replaced with the BPV E2 gene, but the SV40 capsid protein genes are retained. Replication-defective Pava1 virus particles containing the BPV E2 gene packaged in an SV40 capsid were generated in permissive monkey CMT4 cells. These cells express SV40 large T antigen in trans, allowing high-level viral DNA replication, capsid protein expression, and virus production (12). Infection of HeLa cells with Pava1 delivers the E2 gene into essentially every cell in the population and represses the HPV18 E6 and E7 genes (18), thereby activating the endogenous cellular p53 and RB pathways in these cells and rapidly imposing a profound growth arrest (13, 14, 18). Agents that block infection by Pava1 and subsequent expression of the E2 protein can be readily identified because they allow continued DNA synthesis in HeLa cells despite exposure to Pava1. To simplify this screen, we used HeLa-E6 cells, which are engineered to constitutively express the HPV16 E6 gene from a promoter that is not repressed by the E2 protein (5). In these cells, Pava1 infection and E2 expression cause HPV18 E7 repression and RB activation, resulting in growth arrest (5, 28), whereas the p53 pathway remains repressed by the HPV16 E6 protein, thus eliminating the recovery of agents that inactivate the p53 growth-inhibitory pathway.
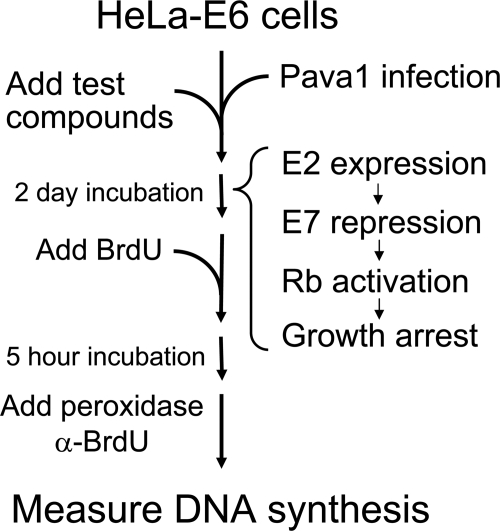
FIG. 1.
Flow chart of the strategy to identify inhibitors of SV40 infection. HeLa-E6 cells in wells were infected with a recombinant SV40 virus (Pava1) coding for the BPV E2 protein, and various test compounds were added 4 h later. If Pava1 successfully infects HeLa cells, the E2 protein is expressed and represses the endogenous HPV18 E7 oncogene, leading to activation of the RB tumor suppressor pathway and growth arrest. Wells containing compounds that interfere with infection prevent growth arrest and can be identified by BrdU incorporation.
Identification of compounds that inhibit infection by a recombinant SV40 virus.
To screen for chemicals that block SV40 infection, we used a robotic, high-throughput assay for cellular DNA synthesis. HeLa-E6 cells in 384-well plates were either mock-infected or infected once with Pava1 at an MOI of ∼20 to repress HPV18 E7 expression and induce growth arrest. After 4 h, the cells were treated in duplicate with DMSO or DMSO containing test compounds at a final concentration of ∼10 μM. We screened 960 compounds in duplicate from the GenPlus Chemical Library from MicroSource Discovery Systems, which consists of known bioactive compounds. After 45 h of incubation at 37°C, BrdU was added to the plates. After incubation for an additional 5 h, BrdU incorporation was measured by using a horseradish peroxidase-conjugated anti-BrdU antibody and a cell proliferation ELISA kit, followed by chemiluminescence detection with a plate reader. As shown in Fig. 2A, in the absence of E2 expression, the cells displayed robust DNA synthesis, whereas DNA synthesis was markedly inhibited in infected cells. We calculated a measure of high-throughput screen quality, termed the Z-factor, which incorporates both the dynamic range of the signal and its variability (36). The Z-factor in this assay was 0.54, which is deemed excellent. We readily identified compounds that resulted in substantial preservation of DNA synthesis despite exposure to Pava1. The positive hit in Fig. 2A is one of these active compounds, spiperone.
FIG. 2.
Identification of compounds that inhibit Pava1 infection. (A) A three-dimensional plot of the DNA synthesis activity in a portion of a 384-well plate. HeLa-E6 cells were plated into 384-well plates and either mock-infected (columns 1 and 2) or infected with Pava1 to express the E2 protein (columns 3 to 24). After 4 h, the cells in columns 3 to 22 were treated with ∼10 μM test compounds dissolved in DMSO, while the mock-infected wells and mock-treated wells (columns 23 and 24) received an equivalent amount of DMSO without test compound. After 2 days, the cells were pulse-labeled with BrdU, and DNA synthesis was measured by a chemiluminescent ELISA and normalized to the average of the mock-infected wells. The mock-treated wells averaged 16.8% of uninfected cells. The arrowhead points to a well containing spiperone. (B) Chemical structures of the two compounds that inhibit Pava1 growth-inhibitory activity (the diagrams were obtained from http://pubchem.ncbi.nlm.nih.gov/).
The four compounds that inhibited the ability of Pava1 to block DNA synthesis in HeLa-E6 cells in the primary screen were subjected to further analysis. Two of these compounds did not display activity upon repeat testing. The two other compounds, ellagic acid and spiperone, displayed a reproducible, robust, dose-dependent ability to abrogate the inhibition of DNA synthesis caused by Pava1 infection (data not shown). Figure 2B shows the chemical structures of these two compounds.
qRT-PCR for BPV E2 and HPV18 E7 mRNA 48 h after Pava1 infection revealed that treatment of HeLa-E6 cells with ellagic acid or spiperone markedly inhibited the expression of the BPV E2 gene and the repression of the HPV18 E7 gene (data not shown). These results suggested that ellagic acid and spiperone did not inhibit the cellular RB signaling pathway responsible for growth arrest, but rather that they inhibited infection by the SV40 viral vector expressing the E2 gene. Because Pava1 does not express large T antigen, viral DNA does not replicate in this system, implying that the block is at an early step in infection.
Specific inhibition of authentic SV40 in human cells.
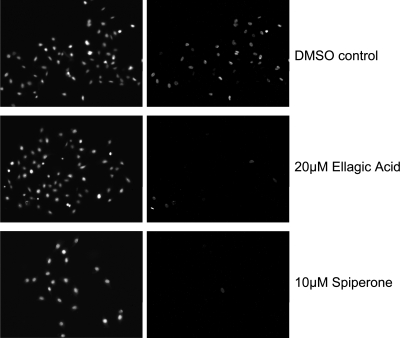
As described above, the active compounds were identified based on their ability to allow robust DNA synthesis in HeLa-E6 cells exposed to the Pava1 recombinant virus. We next tested whether ellagic acid and spiperone inhibited infection by wild-type SV40. HeLa-E6 cells were infected with wild-type SV40 at an MOI of ∼1 and subsequently treated with ellagic acid or spiperone. After 48 h, cells were assayed for large T antigen expression by indirect immunofluorescence and fluorescence microscopy. Uninfected cells displayed a low level of background staining (data not shown), whereas cells infected with SV40 showed strong nuclear staining in the great majority of cells (Fig. 3). Treatment with 20 μM ellagic acid or 10 μM spiperone resulted in a > 5-fold reduction in the fraction of cells staining positive for large T antigen.
FIG. 3.
Ellagic acid and spiperone inhibit the ability of SV40 to infect HeLa-E6 cells. HeLa-E6 cells on glass slides were infected with wild-type SV40. Two hours after infection, the medium was replaced with fresh medium containing the indicated compound or DMSO carrier control. Two days later, the cells were immunostained for large T antigen and examined by fluorescence microscopy. The left panels show nuclei stained with DAPI. The right panels show the nuclear large T antigen staining of infected cells within the same field.
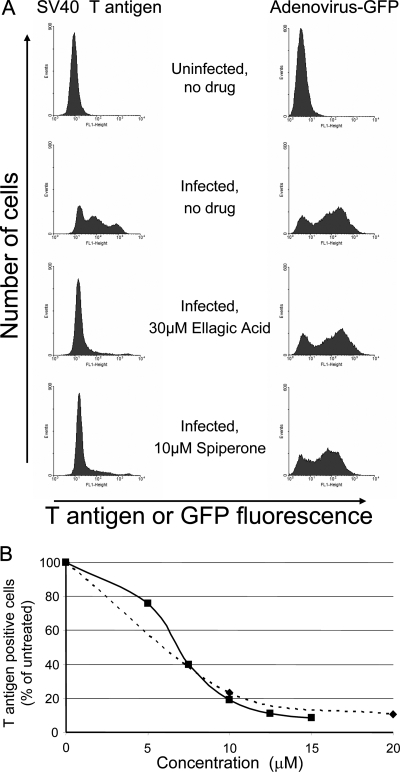
To obtain a quantitative measure of the extent of inhibition of SV40 infection, large T antigen expression was also assessed by immunostaining and flow cytometry. As shown in Fig. 4A, uninfected HeLa-E6 cells displayed a low level of background staining. At 48 h after infection with SV40 at an MOI of 1, approximately one-third of the cells showed low-level staining, and two-thirds expressed a high amount of large T antigen, as expected for infection at this MOI. The fraction of large T antigen positive cells was reduced by treatment with ellagic acid or spiperone by eight- or fivefold, respectively (Fig. 4A). To examine the specificity of inhibition, we also assessed the ability of ellagic acid and spiperone to inhibit infection by a replication-defective recombinant Ad5-GFP. As shown in Fig. 4A, neither ellagic acid nor spiperone inhibited expression of GFP from adenovirus in HeLa-E6 cells, indicating that the inhibitory effect on SV40 infection was specific for the polyomaviruses and not due to nonspecific cell toxicity. Figure 4B shows that ellagic acid and spiperone caused a dose-dependent reduction in the fraction of HeLa-E6 cells expressing SV40 large T antigen, as assessed by flow cytometry. Similar results were obtained in primary human foreskin fibroblasts (data not shown).
FIG. 4.
Ellagic acid and spiperone cause concentration-dependent inhibition of SV40 but not adenovirus. (A) In the left panels, mock-infected or SV40-infected HeLa-E6 cells were treated with the indicated compounds for 2 days. The cells were then harvested, stained for large T antigen expression, and subjected to flow cytometry. In the right panels, HeLa-E6 cells were mock infected or infected with a recombinant adenovirus expressing GFP and then treated with the indicated compounds for 2 days. The cells were then harvested and subjected to flow cytometry to quantify GFP expression as a marker for adenovirus infection. (B) HeLa-E6 cells were infected with SV40 and treated with various concentrations of ellagic acid (dashed line) and spiperone (solid line). Large T antigen positive cells were enumerated by flow cytometry, corrected for multiple viral infections per cell by using the Poisson distribution, and expressed as the percentage of T antigen positive cells in the absence of compound. Solid line, spiperone; dashed line, ellagic acid. In the absence of test compound, ca. 60% of infected cells were large T antigen positive in this experiment. The experiment shown is representative of five independent experiments with similar results.
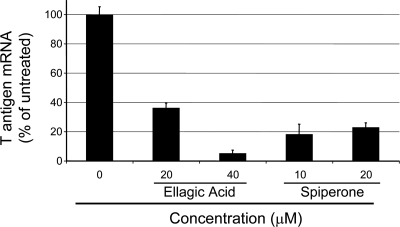
We also determined whether the reduced expression of SV40 large T antigen was due to reduced levels of viral early region transcripts. RNA was purified from HeLa-E6 cells 48 h after infection with SV40 in the presence or absence of ellagic acid or spiperone, and qRT-PCR was used to measure T antigen mRNA. Consistent with the reduction in large T antigen expression as assessed by immunological methods, there was a dose-dependent reduction in the levels of T antigen mRNA in cells treated with ellagic acid, attaining ∼20-fold inhibition compared to untreated control cells (Fig. 5). Spiperone caused an ∼5-fold reduction in large T antigen mRNA. These results indicated that these compounds inhibited the accumulation of large T antigen mRNA.
FIG. 5.
Ellagic acid and spiperone inhibit the expression of SV40 T antigen RNA. SV40-infected HeLa-E6 cells were treated with the indicated concentrations of ellagic acid and spiperone, and total RNA was prepared 2 days later. T antigen and GAPDH mRNA were quantified by using qRT-PCR. The GAPDH mRNA levels were used to normalize the signal between different samples. The relative levels of T antigen mRNA in treated cells is expressed as the percentage of the amount of mRNA in untreated cells.
Inhibition of SV40 in permissive cells.
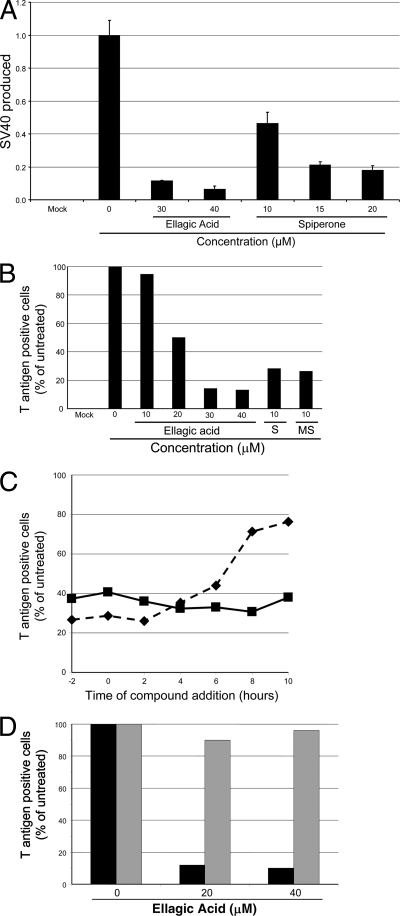
We also tested whether ellagic acid and spiperone inhibited production of SV40 in permissive monkey cells capable of supporting the entire SV40 life cycle. CV1 cells were infected in triplicate with wild-type SV40 at an MOI of ∼0.5, and 2 h later the cells were treated with various concentrations of spiperone or ellagic acid. At 55 h after initial exposure to SV40, the cells were lysed, and the titer of infectious SV40 was determined by infecting untreated CV1 cells and conducting flow cytometry for large T antigen expression 22 h later. As shown in Fig. 6A, production of infectious SV40 in CV1 cells was inhibited in a dose-dependent fashion by both compounds. The virus yield was inhibited ∼12-fold by 40 μM ellagic acid and ∼5-fold by 20 μM spiperone.
FIG. 6.
Ellagic acid and spiperone reduce SV40 infection and virus production in permissive CV1 cells. (A) Ellagic acid and spiperone reduce the amount of SV40 produced from a single round of virus growth. CV1 cells were plated in triplicate for each condition and mock-infected or infected with SV40 1 day later. Two hours after infection, the medium was replaced with fresh medium containing the indicated concentration of ellagic acid or spiperone or a DMSO control. At 55 h postinfection, the newly produced SV40 was released from the cells by multiple freeze-thaw cycles. The virus titer was determined by infecting fresh, untreated CV1 cells and quantifying T antigen expression 22 h later via immunostaining and flow cytometry. The percentages of positive cells, in triplicate, for each condition were corrected for multiple infections by using a Poisson distribution, averaged, and then expressed as a fraction of SV40 produced by the infected cells in the absence of compound. The error bars represent one standard deviation. The experiment shown is representative of two independent experiments with similar results. (B) Ellagic acid and spiperone (column S) inhibit SV40 infection in CV1 cells. CV1 cells were infected with SV40 for 2 h and then treated with test compound or DMSO for an additional 21 h. Cells were then harvested and large T antigen expression was assayed by immunostaining and flow cytometry. We also tested the activity of N-methylspiperone (column MS). The experiment shown is representative of 10 independent experiments with similar results. (C) Timing of compound efficacy. Ellagic acid (40 μM) and spiperone (10 μM) were added to CV1 cells at times ranging from 2 h before SV40 infection (−2) to 10 h postinfection. At 22 h postinfection, the cells were harvested and large T antigen was quantified by immunostaining and flow cytometry and expressed as the percentage of T antigen-positive infected cells in the absence of compound. Solid line, spiperone; dashed line, ellagic acid. At this multiplicity, ca. 78% of infected, untreated cells displayed large T antigen fluorescence in this experiment. (D) Transfection overcomes the block to SV40 infection. In parallel, CV1 cells were infected with SV40 (▪) or transfected with SV40 DNA (░⃞). Four hours later, cells were treated with DMSO or 20 or 40 μM ellagic acid. After an additional 22 h, the fraction of T antigen-positive cells were quantified by immunostaining and flow cytometry. In this experiment, ca. 60% of infected and 21% of transfected cells displayed T antigen fluorescence in the absence of ellagic acid.
To determine whether the block to SV40 production in monkey cells occurred prior to viral early gene expression, CV1 cells were infected with SV40 at an MOI of 1, treated with spiperone or ellagic acid, and subjected to flow cytometry after 23 h to acutely assess large T antigen expression. At this time point, there is no spread of virus to secondarily infected cells (data not shown). Both compounds caused a dose-dependent reduction in the fraction of cells expressing large T antigen, although the extent of inhibition was not as dramatic as in HeLa cells, with ellagic acid causing a 7-fold reduction and spiperone a 3.5-fold reduction in T antigen-positive cells (Fig. 6B and data not shown). Combined treatment with 40 μM ellagic acid and 10 μM spiperone caused an ∼20-fold inhibition of SV40 infection (data not shown).
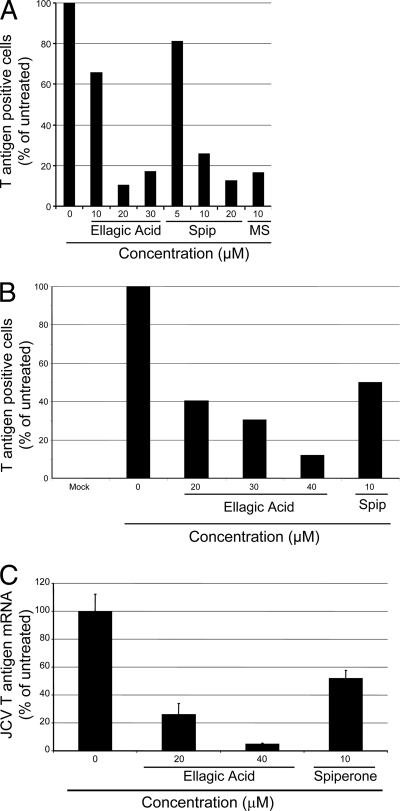
We also tested compounds related to ellagic acid or spiperone for their ability to block SV40 infection. These included flavellagic acid (compound identification number in Pubchem [CID] 5384468), gallein (CID 73685), and tetrafluoro-gallein (CID 24202920) for ellagic acid, and N-methylspiperone (CAS 87539-19-3), afluorspiperone (CID 98814), fluspiperone (CID 166535), phenylspiperone (CID 70419), Declenperone (CID 71744), aminospiperone (CID 298412), and haloperidol (CID 3559) for spiperone. Flavellagic acid differs from ellagic acid only by the addition of a single hydroxyl group. None of the ellagic acid relatives possessed anti-SV40 activity as measured by T antigen staining and flow cytometry (data not shown). N-Methylspiperone consistently showed anti-SV40 activity greater than or equal to that of spiperone (Fig. 6B). The other spiperone relatives had little if any anti-SV40 activity (data not shown).
We tested the time course of inhibition of SV40 large T antigen expression by spiperone and ellagic acid in CV1 cells. At various times before and after infection, 40 μM ellagic acid or 10 μM spiperone was added to the culture medium. The fraction of cells expressing large T antigen was assessed by immunostaining and flow cytometry 22 h after infection (Fig. 6C). Spiperone exerted strong inhibitory activity on large T antigen expression even if it was added as late as 10 h after infection. Large T antigen expression was strongly inhibited if ellagic acid was added within the first 2 h after infection. If ellagic acid was added after this time, the inhibitory effect declined but was still evident if the compound was added 10 h after infection. The ability of these compounds to inhibit large T antigen expression even if they are added several hours after infection indicates that they do not affect virus binding to cells or the initial steps of virus entry into the cell.
The experiments described above showed that the block to infection occurred prior to early viral gene expression, suggesting that spiperone and ellagic acid inhibited cell binding or entry, intracellular trafficking or disassembly of virus particles. If this is the case, then the block should be circumvented by transfection of viral DNA, which delivers the DNA directly to the nucleus without engaging the virus trafficking and disassembly machinery. To test this, CV1 cells were transfected with SV40 DNA and treated with 20 or 40 μM ellagic acid 4 h later. In parallel, cells were infected with SV40 and treated with the same concentrations of ellagic acid. At 26 h after transfection or infection, the fraction of cells expressing large T antigen was determined by flow cytometry. As shown in Fig. 6D, although ellagic acid caused an ∼8-fold inhibition of T antigen expression after infection with virus particles, it did not inhibit T antigen expression in the transfected cells. These results confirmed that the ellagic acid-induced block to infection occurred prior to the delivery of viral DNA to the nucleus. We were not able to test whether transfection circumvented the inhibition caused by spiperone because combined spiperone treatment and transfection was toxic to the cells.
To address the possibility that the observed inhibition of infection in CV1 cells was due to toxicity of the compounds, we subjected CV1 cells to a range of concentrations of the compounds and then used metabolic monitoring with MTT [3-(4.5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to identify any toxic effects. The results of this experiment demonstrated that CV1 cells tolerated the concentrations and time of exposure of ellagic acid, spiperone, and DMSO used here without any apparent change in their viability (data not shown), suggesting that these compounds inhibited a function required for viral infection rather than causing a widespread disruption of cellular metabolism.
Inhibition of human polyomaviruses.
We tested the ability of ellagic acid and spiperone to inhibit infection by the pathogenic human polyomaviruses, BKV and JCV. Primary human foreskin fibroblasts were infected with BKV and, after 4 h, cells were washed and incubated for 4 days with medium containing various concentrations of spiperone and ellagic acid. Infection was scored by immunostaining and flow cytometry for BKV large T antigen by using a mouse monoclonal antibody against SV40 large T antigen that cross-reacts with BKV large T antigen. Ellagic acid or spiperone treatment caused up to a 10-fold inhibition of the fraction of cells expressing BKV large T antigen (Fig. 7A). Similar results were obtained in Vero cells, in cells infected with a second (Dunlop) strain of BKV, or when V antigen expression was assessed (data not shown).
FIG. 7.
Ellagic acid and spiperone block infection by BKV and JCV. A. Human foreskin fibroblasts were infected with BKV, and the indicated compounds or DMSO (column 0) were added after 2 h. After 4 days, the cells were harvested, fixed, immunostained for large T antigen, and quantified by flow cytometry. Spip, spiperone; MS, N-methylspiperone. Approximately 16% of cells were T antigen positive in the absence of test compound in this experiment. The experiment shown is representative of five independent experiments with similar results. (B) SVG-A cells on glass slides were infected with wild-type JCV. Two hours after infection, the medium was replaced with fresh medium containing the indicated compounds or DMSO (column 0). After 4 days, the cells were fixed and immunostained for JCV large T antigen with the antibody JC/BK, which does not recognize SV40 large T antigen, and counterstained with DAPI. T antigen-positive cells in multiple random fields were examined microscopically, and the ratio of T antigen-positive to DAPI-positive nuclei was determined and normalized to that of cells infected in the absence of compound. Approximately 600 to 1,000 cells were counted for each determination, and ca. 4% of infected cells were T antigen positive in the absence of test compound in this experiment. The experiment shown is representative of two independent experiments with similar results. (C) SVG-A cells were infected with wild-type JCV and compounds were added as described above. Total RNA was prepared 4 days after infection and converted into cDNA. JCV T antigen- or GAPDH-specific primers were used to determine mRNA levels by qRT-PCR. The GAPDH levels were used to normalize the signal between different samples, and the relative levels of JCV T antigen mRNA were determined.
SVG-A cells, human glial cells transformed with SV40 large T antigen, support JCV replication. A total of 5,000 SVG-A cells were infected with JCV for 2 h, and then ellagic acid, spiperone, or DMSO control was added. At 4 days postinfection the cells were stained for indirect immunofluorescence microscopy with a monoclonal antibody specific for JCV large T antigen, which does not recognize the SV40 T antigen in these cells. As shown in Fig. 7B, ellagic acid and spiperone caused a 10- or 2-fold, respectively, reduction in the fraction of cells expressing JCV large T antigen expression. Infection by JCV strain Mad1/SVEΔ (35) was also inhibited by ellagic acid and spiperone (data not shown). Because JCV large T antigen was detected in only a small fraction of untreated cells, we also measured the effect of ellagic acid and spiperone on the expression of JCV T antigen mRNA. We prepared mRNA from JCV-infected SVG-A cells and subjected it to qRT-PCR using primers specific for JCV T antigen mRNA. Ellagic acid treatment caused a 20-fold reduction of JCV T antigen mRNA, while spiperone caused a 2-fold decrease. (Fig. 7C).
DISCUSSION
BKV and JCV are important human pathogens. Although infection with these viruses is very common and they can cause serious diseases, there are no effective treatments or preventive measures for BKV and JCV infection. Here, we describe a novel, cell-based, high-throughput screen to identify chemicals that block infection by the human polyomaviruses in cultured cells. The screen is based on a detailed molecular understanding of the growth regulatory circuitry in human cervical carcinoma cells and on extensive work showing that repression of the HPV oncogenes in these cells causes rapid and profound growth arrest. Several features of this screen should be noted. This nonpermissive screening system uses a replication-defective SV40 vector, so replicated virus is not produced that could amplify, spread, and overwhelm an incomplete block to infection. Another major advantage of this positive growth assay is that active compounds cause an increase in cellular DNA synthesis, so chemicals that inhibit cell growth are eliminated. Indeed, subsequent testing confirmed that two of the four compounds identified in the initial screen displayed specific antiviral activity. Finally, although the screen used a recombinant SV40-based vector, both of the active compounds we identified inhibited infection by wild-type SV40, as well as by the closely related human viruses, BKV and JCV. Therefore, this screen can identify compounds with potential clinical utility. We also tested a variety of related compounds, but only N-methylspiperone showed inhibitory activity.
The active compounds we identified or related compounds could themselves have therapeutic potential, and the screen we developed can be modified and expanded to identify or test additional compounds with antiviral activity. Although neither ellagic acid nor spiperone completely blocked infection, they showed additive antiviral activity. It is possible that chemical modifications could increase the potency of these lead compounds. It is also possible that SV40 initiates infection in a small fraction of cells by using an alternative mechanism that is not sensitive to these chemicals.
The mechanism of action of ellagic acid and spiperone is not known, but the block is prior to expression of large T antigen mRNA, the major viral early gene product. Experiments involving the addition of neutralizing antibodies to infected cells indicate that all three of these viruses are internalized within the first few hours after infection (2, 7, 27). Therefore, the ability of ellagic acid and spiperone to inhibit infection even though they were added up to 10 h after exposure to SV40 suggests that the block is not at the level of virus binding to cells or the first steps in virus entry. The finding that SV40 remains sensitive to spiperone considerably longer than to ellagic acid implies that spiperone acts at a later step of infection. This time of action of ellagic acid and spiperone is not unexpected, because test compounds were added 4 h after infection during the screen. It will presumably be possible to identify compounds that act earlier by conducting a screen in which chemicals are added at earlier times.
SV40, BKV, and JCV utilize different cell surface receptors, and the former two viruses enter cells primarily through caveoli, whereas JCV utilizes clathrin-coated pits (2, 7, 26, 27). The entry pathways for all three viruses later converge at the caveosome in transit to the endoplasmic reticulum (26, 29). The inhibition of all three viruses by ellagic acid and spiperone implies that inhibition occurs at distal steps shared by these three viruses. In contrast, Ad5, which is not inhibited by these compounds, is known to use a different entry pathway (15). The inability of ellagic acid to inhibit the activity of transfected SV40 DNA indicates that this compound blocks virus infection at a step prior to delivery of viral DNA to the nucleus. Taken together, these results suggest that the compounds inhibited intracellular trafficking or disassembly of virus particles. Similarly, at the nonpermissive temperature, SV40 tsD mutants show a defect after infection with virus particles but not after transfection of viral DNA, a phenotype that was attributed to an uncoating defect (31). The steps in SV40 entry and capsid disassembly are known in some detail, and similar information is becoming available for the human polyomaviruses (see, for example, references 19 and 32). By following the progress of infection in the presence of ellagic acid and spiperone, it may be possible to identify the step at which these chemicals inhibit infection. Furthermore, the biochemical targets of these compounds may serve as antiviral targets for other agents or approaches.
We observed some cell type specificity in the response to drug treatment. In general, ellagic acid and particularly spiperone were more active HeLa-E6 cells, in which the screen was performed, than in CV1 cells. Although ellagic acid strongly inhibited SV40 infection in Vero cells, spiperone had no effect in these cells. These results suggest that these compounds may be less effective in certain cell types or in nonhuman cells.
Spiperone and ellagic acid are not chemically related (Fig. 2B). Ellagic acid, a component of pomegranates, raspberries, and other fruits, is a known antioxidant. It has been previously claimed to have activity against a variety of enveloped viruses, but this activity has not been characterized (reviewed in reference 22). Flavellagic acid, which has a very similar structure, does not inhibit SV40 infection (unpublished results), suggesting that the antiviral effect of ellagic acid is likely to be independent of its antioxidant properties. Spiperone, also known as spiroperidol, is a butyrophenone derivative structurally related to haloperidol and is an agonist of seratonin and dopamine receptors. Although JCV can utilize the serotonin (5-HT) receptor for cell entry (8), this seems unrelated to the inhibitory activity of spiperone, which displays a relatively late time of action and inhibits SV40 and BKV, which do not utilize this receptor. Spiperone has been used in humans as an antipsychotic drug, and its ability to cross the blood-brain barrier suggests that spiperone or related compounds might be useful in the treatment of PML.
As well as identifying lead compounds with antiviral activity in cultured cells, these results validate the use of this high-throughput chemical screen to identify compounds that block infection by primate polyomaviruses. Our results provide proof-of-principle for a strategy to identify novel antiviral compounds that may provide significant medical benefit. In addition, it may also be possible to insert the BPV E2 gene into other viral vectors and use the same platform to search for agents that inhibit these viruses as well.
Acknowledgments
We thank Jamie Merkle and Paul Fletcher for assistance with assay development and execution, Thomas Magaldi and Sara Marlatt for critical reading of the manuscript, and Jan Zulkeski for assistance in preparing the manuscript.
This study was supported by a grant from the NIH to D.D. (CA16038) and by a pilot grant from the Yale Center for Genomics and Proteomics.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Allander, T., K. Andreasson, S. Gupta, A. Bjerkner, G. Bogdanovic, M. A. Persson, T. Dalianis, T. Ramqvist, and B. Andersson. 2007. Identification of a third human polyomavirus. J. Virol. 814130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 71825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbanti-Brodano, G., S. Sabbioni, F. Martini, M. Negrini, A. Corralini, and M. Tognon. 2006. BK virus, JC virus, and simian virus 40 infection in humans, and association with human tumors. Adv. Exp. Med. Biol. 577319-341. [DOI] [PubMed] [Google Scholar]
- 4.Bohl, D. L., and D. C. Brennan. 2007. BK virus nephropathy and kidney transplantation. Clin. J. Am. Soc. Nephrol. 2(Suppl. 1)S36-S46. [DOI] [PubMed] [Google Scholar]
- 5.DeFilippis, R. A., E. C. Goodwin, L. Wu, and D. DiMaio. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 771551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dropulic, L. K., and R. J. Jones. 2008. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transpl. 4111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eash, S., W. Querbes, and W. J. Atwood. 2004. Infection of Vero cells by BK virus is dependent on caveolae. J. Virol. 7811583-11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elphick, G. F., W. Querbes, J. A. Jordan, G. V. Gee, S. Eash, K. Manley, A. Dugan, M. Stanifer, A. Bhatnagar, W. K. Kroeze, B. L. Roth, and W. J. Atwood. 2004. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 3061380-1383. [DOI] [PubMed] [Google Scholar]
- 9.Feng, H., S. M., Y. Chang, and P. S. Moore. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 3191049-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcea, R. L., and M. J. Imperiale. 2003. Minireview: simian virus 40 infection of humans. J. Virol. 775039-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynor, A. M., M. D. Nissen, D. M. Whiley, I. M. Mackay, S. B. Lambert, G. Wu, D. C. Brennan, G. A. Storch, T. P. Sloots, and D. Wang. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 3e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerard, R. D., and Y. Gluzman. 1985. New host cell system for regulated simian virus 40 DNA replication. Mol. Cell. Biol. 53231-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin, E. C., and D. DiMaio. 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 9712513-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin, E. C., L. K. Naeger, D. E. Breiding, E. J. Androphy, and D. DiMaio. 1998. Transactivation-competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J. Virol. 723925-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greber, U. F. 2002. Signalling in viral entry. Cell. Mol. Life Sci. 59608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, H. E. 2008. Progressive multifocal leucoencephalopathy in a patient with systemic lupus erythematosus treated with rituximab. Rheumatology 47224-225. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 791277-1286. [DOI] [PubMed] [Google Scholar]
- 18.Hwang, E.-S., D. J. Riese III, J. Settleman, L. A. Nilson, J. Honig, S. Flynn, and D. DiMaio. 1993. Inhibition of cervical carcinoma cell line proliferation by introduction of a bovine papillomavirus regulatory gene. J. Virol. 673720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, M., J. R. Abend, B. Tsai, and M. J. Imperiale. 2009. Early events during BK virus entry and disassembly. J. Virol. 831350-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalili, K., M. K. White, F. Lublin, P. Ferrante, and J. R. Berger. 2007. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology 68985-990. [DOI] [PubMed] [Google Scholar]
- 21.Knowles, W. A. 2000. Propagation and assay of BK virus. Methods Mol. Biol. 16519-31. [DOI] [PubMed] [Google Scholar]
- 22.Kotwal, G. J. 2008. Genetic diversity-independent neutralization of pandemic viruses (e.g., HIV), potentially pandemic (e.g., H5N1 strain of influenza) and carcinogenic (e.g., HBV and HCV) viruses and possible agents of bioterrorism (variola) by enveloped virus neutralizing compounds (EVNCs). Vaccine 263055-3058. [DOI] [PubMed] [Google Scholar]
- 23.Lee, W.-H., and E. Langhoff. 2006. Polyomavirus in human cancer development. Adv. Exp. Med. Biol. 577310-318. [DOI] [PubMed] [Google Scholar]
- 24.Mischitelli, M., A. Bellizzi, E. Anzivino, D. Fioriti, R. Boldorini, U. Miglio, F. Chiarini, F. Di Monaco, and V. Pietropaolo. 2008. Complications post renal transplantation: literature focus on BK virus nephropathy and diagnostic tools actually available. Virol. J. 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naeger, L. K., E. C. Goodwin, E.-S. Hwang, R. A. DeFilippis, H. Zhang, and D. DiMaio. 1999. Bovine papillomavirus E2 protein activates a complex growth-inhibitory program in p53-negative HT-3 cervical carcinoma cells that includes repression of cyclin A and cdc25A phosphatase genes and accumulation of hypophosphorylated retinoblastoma protein. Cell Growth Differ. 10413-422. [PubMed] [Google Scholar]
- 26.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3473-483. [DOI] [PubMed] [Google Scholar]
- 27.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 742288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psyrri, A., R. A. DeFilippis, A. P. B. Edwards, K. E. Yates, L. Manuelidis, and D. DiMaio. 2004. Role of the retinoblastoma pathway in senescence triggered by repression of the human papillomavirus E7 protein in cervical carcinoma cells. Cancer Res. 643079-3086. [DOI] [PubMed] [Google Scholar]
- 29.Querbes, W., B. A. O'Hara, G. Williams, and W. J. Atwood. 2006. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J. Virol. 809402-9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldo, C. H., and H. H. Hirsch. 2007. Antivirals for the treatment of polyomavirus BK replication. Expert Rev. Anti-Infect. Ther. 5110-115. [DOI] [PubMed] [Google Scholar]
- 31.Robb, J. A., and R. G. Martin. 1972. Genetic analysis of simian virus 40. III. Characterization of a temperature sensitive mutant blocked at an early stage of productive infection in monkey cells. J. Virol. 9956-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schelhaas, M., J. Malmstrom, L. Pelkmans, J. Haugstetter, L. Ellgaard, K. Grunewald, and A. Helenius. 2007. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131516-529. [DOI] [PubMed] [Google Scholar]
- 33.Schweighardt, B., and W. J. Atwood. 2001. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res. Hum. Retrovir. 171133-1142. [DOI] [PubMed] [Google Scholar]
- 34.Settleman, J., and D. DiMaio. 1988. Efficient transactivation and morphologic transformation by bovine papillomavirus genes expressed from a bovine papillomavirus/simian virus 40 recombinant virus. Proc. Natl. Acad. Sci. USA 859007-9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170353-361. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, J.-H., T. D. Y. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 467-73. [DOI] [PubMed] [Google Scholar]