Abstract
Human immunodeficiency virus type 1 (HIV-1) subtype C is the dominant subtype globally, due largely to the incidence of subtype C infections in sub-Saharan Africa and east Asia. We compared the relative replicative fitness (ex vivo) of the major (M) group of HIV-1 subtypes A, B, C, D, and CRF01_AE and group O isolates. To estimate pathogenic fitness, pairwise competitions were performed between CCR5-tropic (R5) or CXCR4-tropic (X4) virus isolates in peripheral blood mononuclear cells (PBMC). A general fitness order was observed among 33 HIV-1 isolates; subtype B and D HIV-1 isolates were slightly more fit than the subtype A and dramatically more fit than the 12 subtype C isolates. All group M isolates were more fit (ex vivo) than the group O isolates. To estimate ex vivo transmission fitness, a subset of primary HIV-1 isolates were examined in primary human explants from penile, cervical, and rectal tissues. Only R5 isolates and no X4 HIV-1 isolates could replicate in these tissues, whereas the spread to PM1 cells was dependent on active replication and passive virus transfer. In tissue competition experiments, subtype C isolates could compete with and, in some cases, even win over subtype A and D isolates. However, when the migratory cells from infected tissues were mixed with a susceptible cell line, the subtype C isolates were outcompeted by other subtypes, as observed in experiments with PBMC. These findings suggest that subtype C HIV-1 isolates might have equal transmission fitness but reduced pathogenic fitness relative to other group M HIV-1 isolates.
Human immunodeficiency virus type 1 (HIV-1) subtypes (A through J) have a common ancestor, one that is most closely related to SIVcpz from the chimpanzee subspecies Pan troglodytes troglodytes, suggesting a jump across a species barrier into humans (25, 51). Since their introduction, HIV-1 group M subtypes have expanded rapidly into humans in west and central Africa and established multiple epidemics around the world. Regional expansion of HIV-1 has come in waves with the rapid emergence of specific subtypes due in part to specific modes and routes of transmission. For example, intravenous drug use in Southeast Asia in the mid-1980s and in Eastern Europe and Russia during the early 1990s led to the rapid spread of CRF01_AE and of subtype A, respectively (3, 27). A similar expansion of subtype B HIV-1 transmission occurred among men who have sex with men in North America and Europe in the early 1980s. However, HIV-1 subtype C appears to have slowly emerged throughout the world over the past 10 to 15 years following multiple introductions (3).
To date, more than 20 million humans are infected (over 50%) with “pure” HIV-1 subtype C or HIV-1 recombinant forms containing at least the envelope gene of subtype C (3, 13). This subtype C dominance has involved its gradual increase among preexisting HIV-1 subtypes following an initial founder event to introduce subtype C. Subtype C or a recombinant form has now appeared to displace subtype B and CRF01_AE in south China (40); the plethora of diverse subtypes in Kinshasa, Democratic Republic of the Congo (60); and subtype B in southern Brazil (52). Nonetheless, subtype C does appear to increase over subtypes A and D in Kenya (45). In all likelihood, expansion of subtype C HIV-1 relates to transmission of subtype C out pacing transmission of other subtypes in these populations, resulting in this perceived displacement. However, this increased expansion should not be interpreted as direct competition between two viruses of different subtypes in a population. Differential spread of subtypes has been attributed to specific transmission groups, but there is little supporting evidence to suggest that subtypes A, B, C, D, and CRF01_AE (also referred to as subtype E) are any more or less transmissible by a specific route, in a specific ethnic group, or in specific cell types (5, 10, 12, 42, 48, 63). The continued circulation of two distinct subtypes may eventually converge through the selection of intersubtype HIV-1 recombinants. For example, HIV-1 subtype B and CRF01_AE were segregated to the intravenous drug user and heterosexual groups in Thailand (respectively) in the late 1980s (24, 62) but are now more uniformly mixed and have now recombined in the Thai population (46, 55, 56). Another example is the dominant emergence of CRF07_BC and CRF08_BC across most of China in the late 1990s and over the past decade (53, 64).
Virulence is defined as the rate of host mortality due to infection but can be further refined to the reproduction rate and pathogenic potential of the parasite (6). We have previously compared replicative fitness in ex vivo systems to virulence (44, 57). However, this comparison is only based on the direct correlation between the ex vivo replicative fitness with markers of disease progression. Virulence and replicative fitness are not synonymous, but the ability of a virus to reproduce in susceptible primary human cells may be considered a surrogate for their pathogenicity or virulence in humans (3). We have utilized dual virus competitions in peripheral blood mononuclear cells (PBMC) as a model to estimate “pathogenic” fitness (1, 5, 44, 57), whereas competitions performed in vaginal, rectal, or penile explants may provide a complementary model for “sexual transmission” fitness (3). The terms “pathogenic” and “transmission” fitness are used to contrast the different systems.
There is evidence from clinical cohorts of slower disease progression, as estimated by CD4 cell decline in individuals infected with subtype C compared to other group M subtypes (4a). A critical caveat in generalizing from clinical markers in natural history cohorts and ex vivo experiments with virus isolates is the potential for confounding factors such as host genetics and secondary infections. However, we tested here the hypothesis that subtype C HIV-1 isolates are less fit (ex vivo) than any other HIV-1 group M subtype by competing 12 subtype C HIV-1 isolates against 17 group M HIV-1 isolates in over 500 dual infections of PBMC and cell lines, as well as a subset of competitions in human vaginal, cervical, penile, and rectal explants.
The virus isolates were obtained from patients with either early or late disease and, with respect to coreceptor tropism, were CCR5-tropic (R5), CXCR4-tropic (X4), or dual/mixed (DM)-tropic. The vast majority of subtype C HIV-1 isolates were outcompeted by HIV-1 of the dominant group M subtypes (A, B, D, and CRF01_AE) in PBMC. By utilizing R5 and X4 viruses, we examined the possibility that X4 viruses were more pathogenic in the ex vivo model and may have “regained” fitness relative to X4 viruses of other HIV-1 subtypes but instead observed the opposite. Although pathogenic fitness of subtype C is reduced, a small subset of these viruses could compete with other group M isolates in human vaginal/cervical, penile, and rectal explants. Since subtype C isolates as a group are the outlier in “pathogenic” fitness (ex vivo) among the dominant group M subtypes, we hypothesize that this dramatic difference in ex vivo replicative fitness coupled with evidence of similar transmissibility might be related to the evolving, global epidemiologic picture.
MATERIALS AND METHODS
Cells.
PBMC were isolated from heparin-treated venous blood of HIV-seronegative donors by Ficoll-Paque density-grade centrifugation. Prior to infection, cells were stimulated with 2 μg of phytohemagglutinin (PHA; Gibco-BRL)/ml for 3 days in RPMI (Cellgro) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES buffer, 1 ng of recombinant interleukin-2 (IL-2; Gibco-BRL)/ml, 100 U of penicillin/ml, and 100 μg of streptomycin (Cellgro)/ml. U87 cell lines (human glioma cell line) expressing CD4 and either CCR5 or CXCR4 were obtained from D. Littman and the AIDS Research and Reagent Program. These cells were maintained in Dulbecco modified Eagle medium supplemented with 15% FBS, 100 U of penicillin/ml, 100 mg of streptomycin, 1 mg of Geneticin (G418; Life Technologies, Inc.)/ml, and 5 μg of puromycin/ml to maintain receptor and coreceptor expression.
Viruses.
In the present study, eight syncytium-inducing subtype C isolates from Zimbabwean patients were propagated by PBMC cocultivation. Six of these were X4-tropic, and two were dualtropic (R5X4). These isolates and four HIV-1 group M primary subtype C NSI/R5 obtained from the National Institutes of Health AIDS Research and Reference Reagent Program were used in pairwise competition experiments with a panel of SI/X4 and NSI/R5 isolates of subtypes A, B, C, D, and CRF01 (E) and group O (33 in all; see Fig. 1). The tissue culture dose for 50% infectivity (TCID50) of propagated viruses was calculated by utilizing the accumulative Reed and Muench method (30). In brief, 10-fold serial dilutions of each stock of HIV isolate were added to 105 PHA-stimulated PBMC in a 96-well plates in triplicates. All TCID50 calculations and subsequent fitness assays were determined by using PBMC from one blood draw from one donor (unless indicated otherwise). The virus infectivity in each well was tested by a reverse transcriptase (RT) assay which measures the activity of the RT enzyme present in HIV supernatant. Titers were expressed as infectious units (IU) per milliliter and are shown in Fig. 1A. The infectious titers ranged from 2.0 to 4.5 log10 IU/ml and were significantly different between subtypes. Virus titers were also confirmed by measuring the RT activity in triplicate 1:4 serial dilutions and comparing these “virtual TCID50 titers” to the actual infectious titers (30). With all viruses, the virtual and actual TCID50 values were not significantly different and were within a 10% variance.
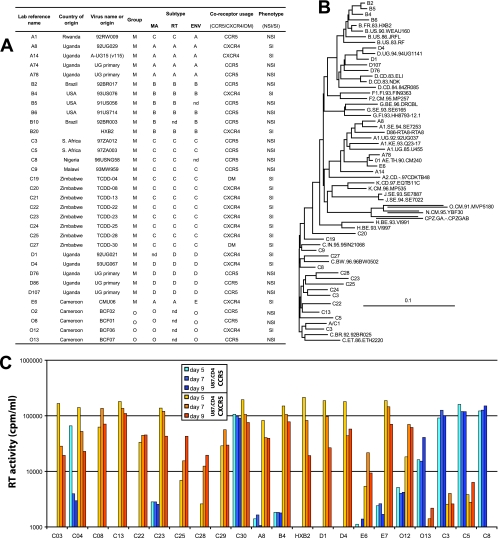
FIG. 1.
Genotypic and phenotypic characteristics of the HIV-1 isolates. All of the HIV-1 isolates were designated with a laboratory reference name, but the country of origin and other virus names are listed in panel A. Subtype was determined by sequencing the C2-C4 region of the env, the p27 coding regions of gag, and the RT coding region of pol. (B) Sequences of the 33 HIV-1 isolates were aligned with reference strains (http://www.hiv.lanl.gov/content/index) using neighbor joining and then mapped onto a tree using TreeView (bootstrapping values are not shown). Most of the isolates were pure subtypes with the exception of A/C1 (termed A1) and A/D86 (termed D86). Coreceptor usage was determined by exposing U87/CD4/CCR5 and U87/CD4/CXCR4 cells to each HIV-1 isolate. (C) Virus production in the supernatant was monitored on days 5, 7, and 9 using radioactive RT assays. The assay has a background cutoff of 2,000 counts/min/ml. As shown, most of the isolates were CXCR4-tropic, four were CCR5-tropic, and three were dualtropic.
Sequence analyses.
A region of the HIV-1 env gene (C2-V3 [480 nucleotides]), the HIV-1 gag (MAp17 [350 nucleotides]), and the pol gene (polymerase region of RT [460 nucleotides]) were PCR amplified (nested) from extracted proviral DNA of monoinfected PMBC. PCR products were purified by using QIAquick PCR purification kit (Qiagen) and sequenced with the forward and reverse primers from the nested reactions as described(5, 49, 57). All of the sequencing reactions were performed using an ABI 377 sequencer at the University of California-Davis. The chromatograms were manually edited for alignment by using BioEdit. Sequence alignments and neighbor-joining phylogenetic trees were constructed by using CLUSTALX and viewed by using TreeView (37). All sequences have been submitted to GenBank (accession numbers FJ541288 to FJ541309).
Competitions in genital tissue.
Cervical mucosal tissue was collected from premenopausal women undergoing therapeutic hysterectomies at the St. George's and Kingston Hospitals (London, United Kingdom), and male genital mucosal tissue (penile glans) was collected from patients undergoing gender reassignment surgery at Charing Cross Hospital, London, United Kingdom (all with informed consent). Tissue was cut into explants of approximately 3 by 3 by 2 mm prior to culture as previously described for cervical tissue and more recently established for male genital tissue (18, 29; L. Fischetti, S. M. Barry, T. J. Hope, and R. J. Shattock, unpublished data). Colorectal tissue was obtained from patients undergoing rectocele repair and colectomy from colorectal cancer at St. George's Hospital. Only healthy tissue obtained at 10 to 15 cm away from the tumor was used. On arrival, muscle was stripped from the resected tissue, which was then cut into 2- to 3-mm3 explants comprising both epithelial and muscularis mucosae as previously described (15). Ex vivo culture of human colorectal tissue was performed for the evaluation of candidate microbicides. Colorectal explants were exposed to 2,500 IU of each HIV-1 pair or a single virus (per each of five explants) for 2 h. Viral inoculum was removed by four washes with phosphate-buffered saline, after which explants were transferred onto gelfoam rafts (Welbeck Pharmaceuticals, United Kingdom). Colorectal explants were harvested at 72 h or at day 10 postinfection and kept at −80°C before being digested for DNA extraction. Genital explants used for infection included the epithelial layer and underlying stromal tissue and were from the penile glans or the ectocervical or endocervical areas of the cervix. In brief, five explants were exposed to 2,500 IU of each HIV-1 pair or a single virus (per each of five explants) for 2 h. After removal of the viral inoculum by extensive washing with phosphate-buffered saline, explants were incubated overnight at 37°C. Migratory cells (MCs) present in the overnight cultures were collected and cocultured with 2 × 104 PM-1 cells for 10 days postinfection prior to digestion for DNA extraction. Genital tissue explants were transferred to different wells in 96-well plates, followed by incubation at 37°C for 10 days postinfection prior to digestion for DNA extraction. Cells and tissue explants were digested with 500 mg of proteinase K/ml and 0.1% (vol/vol) IGEPAL CA-630 in PCR buffer II 10X (PE) overnight at 56°C (200 ml per explant of cell pellet). Proteinase K was inactivated at 95°C for 5 min. DNA was extracted from the harvested cell and tissue digests using a DNA extraction kit (Qiagen, Valencia, CA) and then subjected to HIV-1 DNA PCR amplification and heteroduplex tracking assay (HTA) analyses as described below.
Coreceptor determination.
The virus phenotype for each virus was determined by infecting U87 cell lines expressing either CD4/CCR5 or CD4/CXCR4. Each HIV-1 isolate in the present study was used to infect 25,000 U87/CD4/CCR5 and U87/CD4/CXCR4 cells in duplicate in a 48-well plate as previously described (54). Virus production was determined by assaying the RT activity in the cell-free supernatants at different time points after infection, as well as monitoring for syncytium formation in each of the cell lines.
Ex vivo growth competition assays.
Ex vivo pathogenic fitness in the present study was determined as previously described (1, 5, 44, 57). To determine the pathogenic fitness of group M NSI/R5 HIV-1 isolates, full pairwise dual-infection/competition was performed with four subtype C, three subtype A, four subtype B, and two subtype D isolates. For the pathogenic ex vivo fitness of SI/X4 HIV-1 group M, eight HIV-1 group M subtype C isolates (two of which were dualtropic); eight HIV-1 group M isolates of subtypes A, B, D, and CRF01 (E) (two of each); and two HIV-1 group O (SI and NSI) were used in a full pairwise dual-infection/competition experiment. Competitions were performed in 2 × 105 PHA-stimulated PBMC (except where noted) by adding both viruses at equal multiplicities of infection (MOIs) of 0.0004. A monoinfection representing each of the viruses in competition was included at the same MOI. For all of the competitions performed, virus production was monitored by RT assay on cell-free supernatants. At peak RT activity (ranging from 9 to 11 days depending on the cell type used), cells and supernatants were harvested and stored at −80°C.
HTA analysis for estimation of viral fitness.
HTA was used to quantify production of HIV-1 isolates in dual infections/competitions as previously described (1, 5, 44, 57). Briefly, DNA was extracted from the harvested cell using a Qiagen DNA extraction kit. Nested PCR products in env (inter- and intra-HIV-1 group M competitions) was performed with Env B-ED14 as external primers and E80-E125 as nested primers for a 480-bp C2-V3 Env PCR product (1, 5, 44, 57). For competitions involving group O HIV-1 isolates, a 460-bp HIV-1 RT PCR product (amplified with RTS1-RTA4 and then RTS2-RTA3) was utilized for the HTA as described previously (1). As a control, we amplify for HIV-1 Env DNA in the inoculating virus to ensure the lack of significant HIV-1 DNA in the virus stocks. The quantity of HIV-1 DNA in virus preparations is 1,000-fold less than that of the viral RNA, and the HIV-1 DNA found in cells is the result of de novo reverse transcription (4). Radiolabeled DNA probes were also amplified using the same set of primers in the env and pol RT regions. The antisense primer for these amplifications was end labeled with 5′-[γ-32P]ATP as described elsewhere (44). Radiolabeled PCR-amplified DNA probes were separated on 1% ethidium bromide-stained agarose gels and purified with a QIAquick gel extraction kit. Purified probe (300 cpm) was mixed with 5 μl of PCR products in the presence of annealing buffer and loading dye. This mixture was denatured at 95°C for 3 min, annealed at 37°C for 5 min, and then kept on ice during loading on a 6% nondenaturing polyacrylamide gel. Heteroduplexes corresponding to each HIV-1 isolate in a dual infection and monoinfection were identified by using a Molecular Imager FX (Bio-Rad) phosphorimager and then quantified by using QuantityOne software. The relative viral fitness and fitness difference was estimated by HTA analysis as described previously (1, 5, 44, 57). The final ratio of the two viruses produced from each dual infection was determined by comparing the virus production in the competition to the virus production in the monoinfection. The production of each HIV-1 isolate in a dual infection (f0) was divided by the initial proportion in the inoculum (i0) to determine the relative fitness (W = f0/i0). The fitness difference (Wd) is the ratio of the relative fitness values of each HIV-1 isolate in the competition (Wd = wM/wL) (1, 5, 44, 57).
RESULTS
Phenotypic and genotypic analyses on a cohort of primary HIV-1 isolates.
HIV-1 sequence data are available from thousands of infected individuals throughout the global epidemic. In contrast, there are relatively few primary HIV-1 isolates propagated from patient samples that are readily available for phenotypic analyses. Many of these have been poorly characterized, lack sequence data for the majority of the genome, have not been typed for coreceptor usage, and are predominantly subtype B. In attempts to measure the relative replicative fitness (ex vivo) among group M isolates, we obtained over a hundred primary HIV-1 from various sources including the NIH AIDS Reagent Program and directly from laboratory sites in Uganda, Zimbabwe, Argentina, and Brazil. A subset of 29 group M and four group O HIV-1 isolates were selected for detailed fitness analyses (Fig. 1A). The RT coding region of pol, the MA p17 coding region of gag, and the C2-C4 region of HIV-1 env were sequenced, aligned, and then utilized to construct neighbor-joining phylogenetic trees (Fig. 1B). All primary HIV-1 isolates were propagated on PHA/IL-2-treated PBMC to obtain sufficient titers for subsequent coreceptor usage and fitness analyses. The rationale for utilizing the 33 viruses as opposed to the others is simply based on (i) the ability to propagate the virus on PBMC and obtaining sufficient stock for the pairwise competitions; (ii) obtaining sequences from MA, RT, and env and thus eliminating most recombinants, especially those within a gene; and (iii) the ability to characterize coreceptor usage and MT2 phenotype.
Of the 29 group M viruses, we had an overrepresentation of subtype C (twelve isolates and one A env/C RT recombinant), followed by six subtype B isolates, five subtype A isolates (including one A env/C RT recombinant), five subtype D isolates, and one CRF01_AG isolate (Fig. 1A). One of the primary objectives of the present study was to establish the ex vivo relative fitness of subtype C in the context of other group M isolates. Preliminary data suggested that NSI/R5 subtype C isolates were considerably less fit than subtype B (5) and possibly other group M isolates, which were more fit (ex vivo) than the rare HIV-1 group O isolates (1).
Coreceptor usage was determined by exposing U87/CD4/CCR5 or U87/CD4/CXCR4 cells to 0.01 IU (as measured by TCID50 assays in PBMC) of each virus. Figure 1C represents a repeat analyses of coreceptor usage on a subset of primary HIV-1 isolates used in the present study. All coreceptor usage is reported in Fig. 1A. Virus production as measured by RT activity is presented in Fig. 1C. In this panel, 15 of the 22 HIV-1 isolates were CXCR4-tropic, four were exclusively CCR5-tropic, and three were dual or dual mix (DM). We had nearly equal representation of R5 and X4 phenotype for subtypes A, B, and D and group O. Unlike other HIV-1 group M infections, a switch from a CCR5-tropic virus to a CXCR4-tropic or DM phenotype is rare in subtype C infections. However, we obtained a panel of X4 and DM subtype C isolates from Zimbabwe for these fitness determinations (20). Coreceptor usage on U87 cells was confirmed by the predicted phenotype by applying the 11/25 rule to the V3 sequences of each virus. The GENO2PHENO, PSSM, or VSM algorithms provide weaker predictions of coreceptor usage for non-subtype B HIV-1 isolates (data not shown). Finally, the SI versus the NSI phenotype was determined through the detection of syncytium formation, following a 7-day incubation of each virus with MT2 cells. All X4 or DM viruses were SI and all R5 were NSI as determined by the MT2 assay.
Relative replicative fitness of primary HIV-1 group M isolates.
Previous studies indicate that, regardless of subtype, X4 HIV-1 isolates completely outcompete R5 HIV-1 isolates in PBMC cultures (1, 2, 44). Thus, the 14 primary NSI/R5 and 15 primary SI/X4 HIV-1 isolates were utilized in separate pairwise competitions experiments to establish two different fitness matrices. A total of 364 separate pairwise competitions were performed for the two matrices. Briefly, each NSI/R5 HIV-1 isolate was added to PBMC (the same donor and blood draw), along with each of the other 13 NSI/R5 isolates at an MOI of 0.0004. The same pairwise competition strategy was used with the SI/X4 HIV-1 isolates. Titers of all viruses were determined on the same PBMC (same donor and blood draw) as those used for the competitions. Previous studies have indicated that the infectious titers of the stock virus were unrelated to the ex vivo replicative fitness, which is not surprising considering equal quantities of infectious virus were used in all dual-virus competitions (30). A subset of competitions was repeated in PBMC from different donors of different ethnicities as described below. Cells were harvested at peak virus production based on RT activity in the supernatant, and dual virus production was assessed by using HTAs (1, 2, 5, 44, 57). In our previous competitions involving time courses and measuring dual-virus production at various steps in the life cycle (i.e., entry, reverse transcription, integration, transcription, and virus release), we determined that the replicative fitness was best estimated by viral DNA in infected cells at the stage of peak virus production (1, 2, 5, 44, 57). Relative fitness values and fitness differences were calculated based on the relative intensities of heteroduplex bands on a nondenaturing polyacrylamide gel (see Materials and Methods).
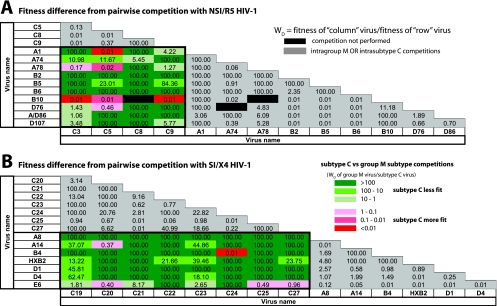
The fitness differences for the NSI/R5 and SI/X4 fitness matrices are shown as the relative fitness of the “column” virus over the “row” virus (Fig. 2). These matrices revealed that fitness differences between the subtype A, B, D, and CRF01_AE viruses fluctuated between the limits of detections (0.01 and 100) (gray boxes, Fig. 2) but had a overall mean relative fitness of ∼1 (or equal fitness). In contrast, nearly all of the NSI/R5 subtype C isolates were outcompeted by the other group M isolates (e.g., subtypes A, B, D, and CRF01_AE). Lighter to darker green in Fig. 2A represents the relative outgrowth of a group M isolate over subtype C. In 20 of the 39 subtype C versus group M competitions, the NSI/R5 subtype C HIV-1 isolate was completely outcompeted by a group M virus (dark green, Fig. 2A). The subtype C NSI/R5 HIV-1 isolates only won 8 of 39 competitions (pink to red colors, Fig. 2A). These findings were even more dramatic when the SI/X4 subtype C viruses competed against the other SI/X4 group M strains. SI/X4 subtype C isolates lost 51 of 56 competitions (green boxes, Fig. 2B), and in 39 of these 51 loses there was a complete outgrowth of the M over the subtype C isolate (dark green, Fig. 2B).
FIG. 2.
Ex vivo replicative fitness matrices derived from pairwise competitions within NSI/R5 and SI/X4 HIV-1 isolates. (A) Each of the R5/NSI isolates was competed against all of the other R5 isolates. (B) Each SI/X4 isolate was competed against all of the other SI/X4 isolates. For both sets of experiments, the fitness difference for the pairwise competitions were determined as outlined in Materials and Methods. The magnitudes of increased fitness difference of the non-subtype C over the subtype C HIV-1 isolates in competitions are indicated by the shade of green: dark green, a >100 fitness difference (Wd) or wnon-subtype C/wsubtype C; medium green, a 100-to-10 fitness difference; and light green, a 10-to-1 fitness difference. The abilities of subtype C isolates to outcompete non-subtype C, group M isolates are indicated by the shade of red: dark red, a <0.01 fitness difference (Wd) or wnon-subtype C/wsubtype C; medium red, a 0.1-to-0.01 fitness difference; and light red, a 1-to-0.1 fitness difference.
Comparison of the ex vivo fitness of HIV-1 isolates of different phenotypes.
Although SI/X4 HIV-1 isolates typically outcompete NSI/R5 HIV-1 isolates regardless of the subtype or group, a small subset of SI/X4 subtype C isolates (C20, C21, and C22) were still competed against three NSI/R5 HIV-1 isolates (C3, B10, and D76) in PHA/IL-2-treated PBMC (see Fig. S1 in the supplemental material). It is important to note that the SI/X4 and NSI/R5 HIV-1 isolates do not “compete” for the same susceptible cells because the CCR5 and CXCR4 receptors are commonly expressed on different CD4+ T-cell subsets (CCR5 predominantly on memory T cells and CXCR4 on naive T cells) or at different levels on macrophages (higher CCR5 over CXCR4 levels) (28, 65). Despite being less fit than all tested SI/X4 group M isolates, the subtype C SI/X4 isolates could still outcompete the group M NSI/R5 HIV-1 isolates. However, the D76 HIV-1 isolate could compete and replicate as efficiently as C22, i.e., the least fit of all of the SI/X4 isolates (see Fig. S1 in the supplemental material). Thus, a subtype C HIV-1 isolate (when SI/X4) can successfully outcompete and even win over a group M isolate (when NSI/R5) in PBMC but, again, these dual-infection experiments do not represent “true” competition except in cells expressing both CCR5 and CXCR4.
Effect of donor host cells on ex vivo replicative fitness.
A subset of 23 competitions was repeated in PBMC from three different donors. Eight of these competitions involved NSI/R5 HIV-1 pairs, and fifteen were with SI/X4 virus pairs. The total virus replication varied considerably in PBMC cultures derived from the three HIV-negative donors of different ethnicities (African American, Chinese, and Bantu African). PHA/IL-2-treated PBMC of donor 3 supported higher HIV-1 replication than did the PBMC of donors 1 and 2 (data not shown). Regardless of this difference in overall virus replication, the relative replication of the two viruses in each competition (or the relative fitness values) remained constant. The same “winner” was observed in all three of the 23 competition subsets performed on the PBMC of each of the three different donors (see Fig. S2 in the supplemental material). In 11 of the 23 competitions there was, however, some variation in the relative fitness values between the different donors. Donor 3 supported the fastest replication kinetics and, as a result, outgrowth of the winner over the losing virus was beyond the limit of assay detection (>100-fold) in the majority of the competitions. Donors 1 and 2 supported slightly slower replication kinetics and, in these few competitions, the more-fit virus had not yet completely outcompeted the less-fit virus in the dual infection.
Determining whether group O HIV-1 isolates remain low fit outliers.
We have previously shown that HIV-1 group O isolates are less fit (ex vivo) than HIV-1 group M and HIV-2 isolates through pairwise competitions (1). In the present study, we established that all 13 HIV-1 subtype C isolates were less fit than any other group M isolate. All of the group M isolates (even the subtype C viruses) were more fit (ex vivo) than the two group O HIV-1 isolates, O12 and O13 (see Fig. S3 in the supplemental material). The only exception to this dominance was the ability of the NSI/R5 O8 isolate to outcompete the least fit NSI/R5 subtype C isolate, C9 (see Fig. S3 in the supplemental material).
Ranking the pathogenic fitness of different HIV-1 group M subtypes.
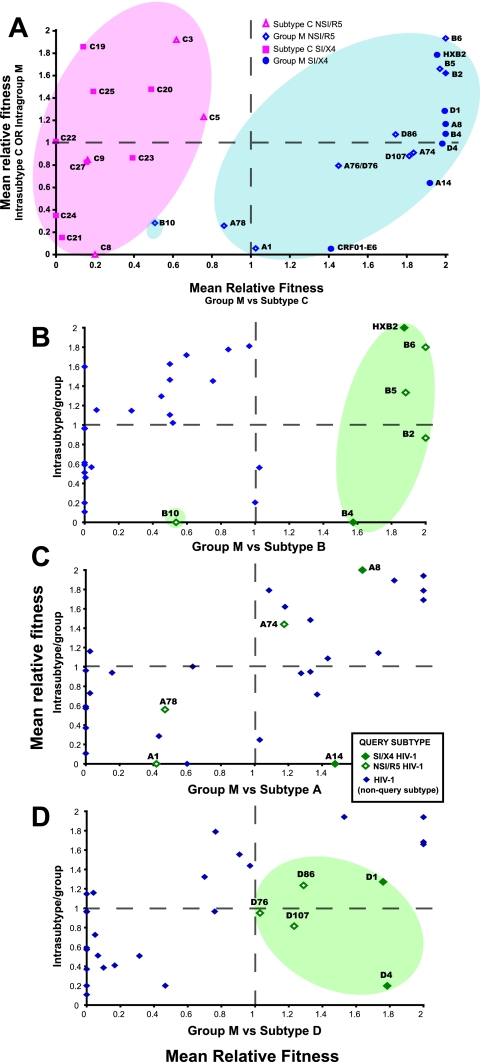
The most striking observation from the pairwise competitions was the poor replicative fitness of subtype C isolates when competed against HIV-1 isolates of other group M subtypes. However, differences in fitness among other subtypes may be still significant but not as striking as those observed with subtype C. Thus, we compared the relationship between intra- and intersubtype fitness derived from over 400 competitions (Fig. 3). The mean relative fitness of each HIV isolate derived from competitions against all others in the same subtype or group (intrasubtype or intragroup M fitness) are plotted on the y axis, whereas the x axis represents the mean relative fitness of each isolate competed against all isolates from the different subtypes(1, 2).
FIG. 3.
Comparison of intra- and intersubtype fitness of different HIV-1 group M isolates. (A, B, C, and D) Mean relative fitness values were determined for each HIV-1 isolate from pairwise competitions between isolates of the same subtype (mean intrasubtype relative fitness) and of different subtypes (mean intersubtype relative fitness). The mean intrasubtype and intersubtype fitness values for each HIV-1 isolate was them plotted as the x and y coordinates. Panel A plots the intrasubtype and intersubtype fitness values for the subtype C versus non-subtype C, group M isolates. Panels B, C, and D are plots of the intrasubtype versus intersubtype fitness values for the subtypes B, A, or D versus the other group M isolates, respectively. All intra- and intersubtype mean relative fitness values were only derived from competitions between isolates of the same phenotype (NSI/R5 or SI/X4).
In Fig. 3A, it is clear that intrasubtype fitness of each subtype C isolate (derived from all competitions with the other subtype C viruses) dispersed below and above a relative fitness value of 1 or equal fitness (x axis). In other words, some subtype C isolates such as C19 and C3 were more fit (ex vivo) than other C isolates (e.g., C21 and C8 had the lowest intrasubtype C fitness). However, all of the subtype C isolates were substantially less fit (range, 0 to 0.7) than the other group M isolates (range, 0.8 to 2) in the subtype C versus group M competitions (y axis). When we stratified the data to compare other subtypes against group M, HIV-1 subtype B and D isolates were more fit (ex vivo) than the other group M isolates with HIV-1 B10 as an outlier (Fig. 3B and D, respectively). As discussed below, subtypes B and D are also more closely related than any other pair of “pure” subtype lineages. When we compared the mean relative fitness of HIV-1 subtype A against the other group M isolates, these subtype A-derived values scattered across the y axis, unlike what we observed with the more-fit subtype B and D and less-fit subtype C isolates. These intra- and intersubtype fitness comparisons were derived from two separate fitness matrices, and yet the relative fitness values remained interspersed for both NSI/R5 and SI/X4 HIV-1 isolates within a subtype.
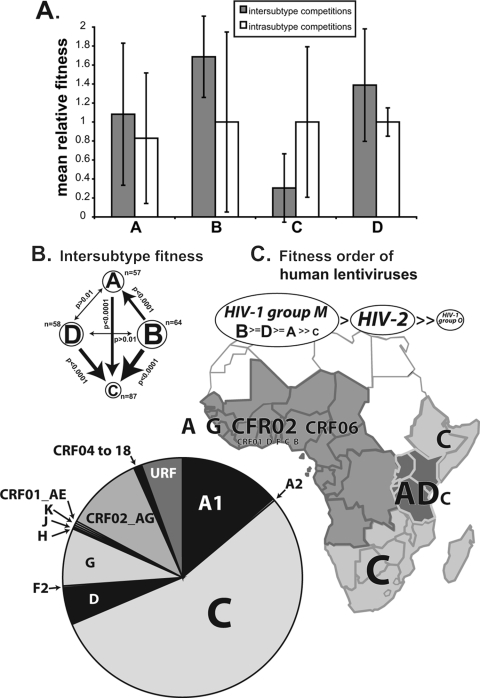
We have previously described a possible fitness order for the groups (M and O) and types (1 and 2) for human lentiviruses from pairwise competition but were unable to resolve possible fitness difference between the group M subtypes(1). To determine whether pathogenic fitness (ex vivo) differed from group M isolates, we determined for each subtype the mean relative fitness values and variance of all intrasubtype competitions (intrasubtype) and of competitions with other group M isolates (intersubtype) (Fig. 4A). One- and two-tailed t tests were then used to compare the intersubtype fitness values derived from each virus within a subtype competed against each virus of a different subtype. For example, five subtype B isolates were competed against the non-B group M isolates for a total of 64 competitions. Compared to the 57 competitions involving subtype A isolates, subtype B was significantly more fit than subtype A (P < 0.000001). As expected, subtype C isolates (n = 87) were less fit (ex vivo) than each of the subtypes A, B, and D. However, differences between subtype B and D fitness or between subtype A and D fitness were not significant. More competitions analyses with other HIV-1 isolates are necessary to confirm a replicative fitness order (Fig. 4B and C) of B ≥ D ≥ A > C. This pathogenic fitness order follows a transitive relationship such that both subtypes B and D are significantly more fit than subtype A. In Fig. 4B and C, we depict the most-fit subtype with the largest font, the relative significance of a fitness difference by the width of the line, and more- over less-fit subtype by an arrow.
FIG. 4.
Fitness order of group M HIV-1 subtypes. (A) The mean relative fitness values derived from competitions for all of the HIV-1 isolates in a subtype were plotted as mean intra- and intersubtype relative fitness values. (B) The intersubtype values between subtypes were then compared for significance by using two-tailed t tests. (C) Estimated rank order of fitness for HIV-1 types, groups, and subtypes based on >2,000 competitions performed in the present study, by Arien et al. (1), or by Ball et al. (5). The relative proportions of each HIV-1 subtype in the African epidemic, as well as the general geographical distribution, are schematically illustrated.
Competing HIV-1 isolates in vaginal/cervical, penile, and rectal explants as a model for “transmission” fitness.
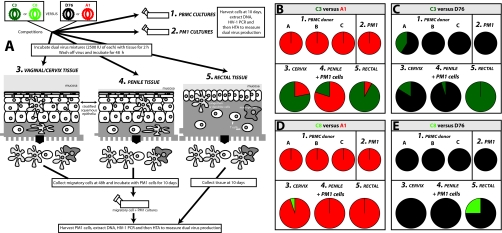
To estimate “transmission” fitness, we performed dual virus competitions in primary human cervical (group 3 in Fig. 5A), penile (group 4), and rectal explants (group 5). “Transmission” fitness (ex vivo) is a term used solely to define competition results in these genital and rectal explants systems and to contrast and compare them to “pathogenic” fitness (ex vivo), a term defined by our group (3, 43) as ex vivo competitive fitness in PBMC, macrophages, or T cells (5). Ex vivo pathogenic fitness appears to be related to disease progression (44, 57). Each competition involved five explants (3 by 2 by 2 mm), each of which was exposed to 2,500 IU of the two viruses for 2 h. MCs were harvested from cervical and penile explants at 24 h and then incubated with the PM1 cell line (e.g., cervix MC+PM1, Fig. 5B to E). HIV-1 DNA was PCR amplified from lysed PM1 and MCs at day 10 and then used in HTAs to determine dual-virus production. Rectal explants showing increased susceptibility to infection were cultured for 10 days (in the absence of PM1 cells) prior to digestion and analysis by HTA (rectal, Fig. 5B to E). These competitions for “transmission” fitness were compared to that of “pathogenic” fitness performed in PBMC of different donors (group 1 in Fig. 5A) and in PM1 cells (group 2). The outcome of competitions between C3+A1, C3+D76, C8+A1, and C8+D76 are shown as percentages in Fig. 5B to E, respectively. As described earlier, the C3 and C8 HIV-1 isolates were completely outcompeted by A1 and D76 in PBMC. Only C3 could compete with D76 in one of the three PBMC from different donors. The same dominance over C3 and C8 was observed in the PM1 cells. However, in the cervical, penile, and rectal tissue experiments, the C3 and C8 viruses could now compete with the A1 and D76 primary HIV-1 isolates. In the C3+A1 competition, C3 replicated in all three conditions, and in the cervix (where migratory tissue cells were incubated with PM1 cells) and rectal tissue (without the addition of PM1 cells) C3 actually outcompeted A1. The ability of C3 to compete with D76 in genital tissue (penile, cervix) was less evident, although it completely outcompeted D76 in rectal tissue. Pairwise competition of C8 with A1 or D76 in these tissue explant experiments was not as evident as that observed with C3 versus A1. It is important to note that this increase in C3 and C8 fitness, using “transmission” versus “pathogenic” models, was observed in a limited subset of the competitions performed in Fig. 2.
FIG. 5.
Estimation of an HIV-1 “transmission” fitness using a cervical, penile, and rectal explants tissue model. (A) Schematic of the experimental protocol used to determine an ex vivo “transmission” fitness. The C3 and C8 HIV-1 isolates were competed against the D76 and A1 isolates (all NSI/R5) in PBMC cultures of three different donors (part 1), PM1 cells (part 2), vaginal/cervix tissue (part 3), penile (part 4), and rectal (part 5). MCs from the genital tissue explants (cervix, penile) were collected and incubated with PM1 cells for 10 days prior to determination of the relative fitness values as described in Materials and Methods. Rectal tissue explants were cultured for 10 days postinfection prior to digestion of the explants and extraction of DNA for analysis. (B to E) Relative fitness values are presented as the percent production of one virus versus the other in competition. Panels B, C, D, and E provide the fitness values in all five culture conditions for competitions between C3 versus A1, C3 versus D76, C8 versus A1, and C8 versus D76, respectively.
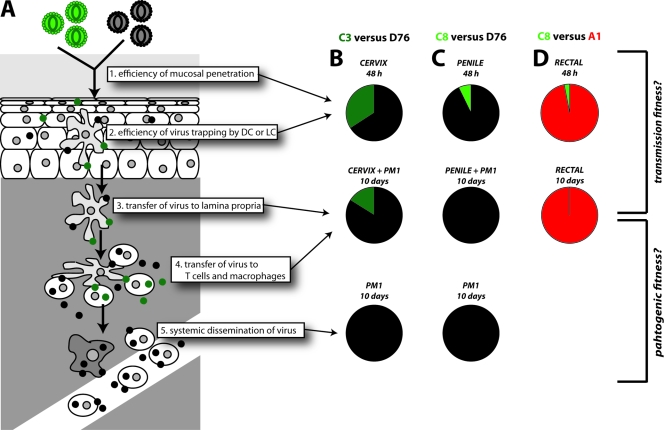
As described below, the ability of the virus to establish an infection in genital tissue, i.e., through Langerhans cells, dendritic cells and, to a lesser extent, macrophages or T cells, may be the consequence of virus trapping prior to direct infection and/or transfer of the virus to PM1 cells (41, 65). This “trapping” and transfer of the virus may be less dependent on “pathogenic” fitness (3, 5, 43). Nonetheless, C3 was significantly more fit (ex vivo) than C8 in the intrasubtype and intersubtype competition analyses (P < 0.001), i.e., the mean relative fitness from competitions within subtype C or with the non-subtype C, group M viruses (Fig. 3A). Due to low number of susceptible cells in the five genital explants used for each competition (∼5,000 to 10,000), the initial infection of the tissue was difficult to access, and the addition of PM1 cells was necessary to amplify the virus. However, as indicated in the PM1 competitions, the A1 and D76 viruses completely outcompeted the C2 and C8 isolates. Thus, PM1 cells will skew the results in the genital explant experiments to the virus with higher “pathogenic” fitness. Furthermore, for rectal tissue explants replication could be detected within the tissue 10 days postinfection. Here, C3 completely outcompeted A1 or D76. In 3 of these 12 competitions, we were also able to PCR amplify HIV-1 DNA from extracted genital tissue explants, which was incubated for 10 days in the absence of PM1 cells. In the cervical tissue, C3 could compete with D76 (34 to 66%, Fig. 6B), but after transfer of the virus from MCs to the PM1 cells the dominance of D76 fitness was more apparent. In the penile tissue, C8 was able to replicate to small extent with D76 but was not observed in the penile MC+PM1 cell competitions. Again, when only PM1 cells were used, D76 completely outcompeted C3 or C8, which is similar to what was observed in the PBMC competitions. For rectal explants, we were also able to amplify DNA from tissue harvested at 72 h; here, low but detectable replication of C8 was apparent but had been outcompeted by day 10 of culture (Fig. 6B).
FIG. 6.
Determining the relative HIV-1 replication and/or transfer during passage through the genital tissue. (A) Schematic of the proposed model for HIV-1 infection of the genital explant tissue and then transfer to susceptible immune cells in the lamina propria and subsequent systemic dissemination. (B, C, and D) Dual virus competitions were arrested at different stages of this process by accessing dual-virus production in the actual explants. Charts in the top row denote tissue DNA (for cervix and penile tissue harvested at 10 days and for rectal tissue at 72 h). Charts in the second row depict replication in PM1 exposed to the MCs from cervical and penile explants and direct replication (in the absence of PM-1 cells) for rectal tissue, all harvested after 10 days in culture. The bottom row shows PM1 competitions alone (or PBMC competitions in Fig. 5) and may provide an estimate of the “pathogenic” fitness of the circulating virus, long after transmission. Panels B, C, and D provide the fitness values as the percent virus production for competitions between C3+D76, C8+D76, and C8+A1 in the explants described above. In the remaining nine competitions performed in the various genital explant conditions, HIV-1 DNA could not be amplified from the tissue.
During primary acute infection, a donor is often exposed to virus populations harboring NSI/R5 strains mixed with X4 strains. Previous studies suggest that X4 isolates may not replicate as efficiently as R5 HIV-1 isolates in penile gland explant tissues. To test this hypothesis, we competed NSI/R5 C3 and C8 HIV-1 isolates against the SI/X4 C21 HIV-1 isolate. Even though equal infectious titers were added, only C3 or C8 and not C21 were detected after a 10-day incubation in the tissue or in the cocultivation of PM1 cells with MCs (from the infected tissue). The ability of the NSI/R5 C3 isolate to outcompete the SI/X4 C21 in the penile tissue is in sharp contrast to the 100-fold increase of C21 over C3 replication in PBMC.
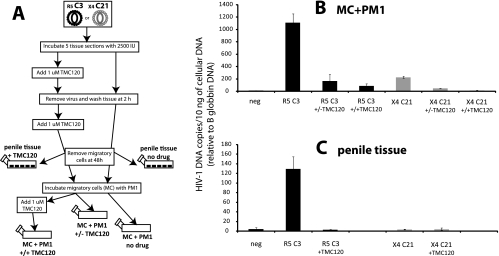
Although only HIV-1 DNA is PCR amplified from the tissue or MC+PM1 cells, there is still the possibility that virus is trapped by Langerhans or dendritic cells in the tissue and then transferred to the PM1 cells in the absence of MC infection. To test this hypothesis (schematically illustrated in Fig. 7A), we exposed the penile tissue to 1 μM TMC120 (or the samples were mock treated) at the time of virus exposure. This nonnucleoside RT inhibitor was added again after virus was removed from the tissue samples. At 24 h, PM1 cells were cocultivated with MC (either mock treated or treated with 1 μM TMC120). The MC+PM1 cultures were then left untreated or treated with 1 μM TMC120 over a 10-day incubation. Virus infection (as opposed to free virus particles) was monitored by real-time PCR for the long-terminal-repeat (LTR) DNA (10). As expected, the SI/X4 C21 virus, unlike the NSI/R5 C3 virus, could not establish a robust infection in the tissue or in MC+PM1 cells (Fig. 7B and C). TMC120, added only in the tissue, blocked 80% of the virus transmitted from MC to PM1 cells (Fig. 7B). The addition of 1 μM TMC120 to the MC+PM1 cultures only decreased infection a further 7%. It is important to note that TMC120 has not been demonstrated to have a virucidal effect, and incubation of the drug with virus prior to infection does not result in appreciable inhibition (data not shown). Over the same 10-day incubation, TMC120 completely blocked the low-level replication in the penile tissue. As described below, these findings suggest that infection of the tissue and of the PM1 cells was dependent on initial infection of the dendritic cells, Langerhans cells, or other cell resident in the tissue. Trapping of free virus, in these competitions and in this tissue, was not a major route for subsequent PM1 infections.
FIG. 7.
Comparison of virus trapping versus infection during passage from MCs to the PM1 indicator cell line. (A) Schematic showing the outline for treating an NSI/R5 C3 or SI/X4 C21 infection of tissue in the presence or absence of TMC120 (1 μM), followed by the subsequent transfer of MCs to PM1 cocultivation in the presence or absence of TMC120 (1 μM). (B and C) Levels of HIV-1 LTR DNA, measured by TaqMan real-time PCR (11) and detected in approximately 10 ng of cellular DNA, for MC+PM1 (B) and penile tissue (C). The levels of HIV-1 LTR were standardized against 10-fold serial dilutions of pNL4-3 HIV-1 plasmid and normalized for the level of β-globin DNA, also measured by TaqMan real-time PCR (11).
DISCUSSION
The disproportional expansion of HIV-1 subtype C in the global epidemic could be the result of random introduction into high-risk transmission groups, specific host genetics leading to greater susceptibility, and/or some intrinsic property of the virus. The impact of host genetics is dampened considering the diversity of the human population in southern Africa, India, China, and Brazil, i.e., regions of dominant or emerging subtype C (3, 40, 52, 60). It is also possible that subtype C HIV-1 expanded in these distal geographic regions through a preferential introduction into a high-risk heterosexual transmission group (61). However, the notion that subtype C is more apt for heterosexual transmission than other group M subtypes implies differential phenotypic characteristics specific to subtype C. Innate or acquired immunity may be more or less active with respect to infection with subtype C HIV-1 than other group M subtypes (32, 36, 50), which again implies a genotypic/phenotypic difference between subtypes having a consequence on disease. Finally, but not exclusively, HIV-1 subtype C may differ from other subtypes in terms of lower virulence, which could lead to slower disease and greater opportunity for transmission(3, 4a). In the present study, we are not excluding any of these possible factors contributing to disproportional expansion of subtype C. Instead, we have tested whether HIV-1 subtype C and the other group M subtypes differ in relation to phenotype as opposed to just nucleotide sequence similarity. To do so, we competed over 33 HIV-1 isolates in more than 400 pairwise competitions to determine the relative ex vivo replicative fitness in PBMC from different human donors of different ethnicities. As outlined above, HIV-1 replicative fitness in PBMC has been termed “pathogenic” fitness due to correlation between increasing ex vivo HIV-1 fitness, decreasing CD4 cell counts, and increasing viral load during disease.
To date, the present study is the first to perform a comprehensive fitness analysis of primary HIV-1 isolates utilizing CCR5 or CXCR4 coreceptors for entry. Previous studies have generally compared different HIV-1 subtypes by extracting and/or analyzing short genetic elements such as the LTR in a heterologous HIV-1 backbone (19, 33, 34, 58, 59). These analyses help to tease apart specific phenotypes such as the increased transcription activation from subtype C versus subtype B LTRs but do provide the relative impact of these effects on the entire virus in the native genetic context. Our findings now clearly show that regardless of coreceptor usage, the 12 subtype C HIV-1 isolates are clearly less fit than the 17 group M isolates of other dominant HIV-1 subtype and CRFs (A, C, D, CRF01_AE, and CRF02_AG). This 100-fold difference in fitness sets subtype C HIV-1 isolates apart from other group M subtypes or recombinants, for which the fitness differences are much more subtle. The relative fitness order within group M (as described by these 33 HIV-1 isolates) can be teased apart, with subtype B and D being the most fit (ex vivo), followed by subtype A and then subtype C. However, there is considerable overlap in subtype fitness aside from that of subtype C. We could not discern a significant fitness difference when comparing subtype B versus subtype D and subtype D versus subtype A, whereas subtypes A, B, and D were all significantly more fit (ex vivo) than subtype C. Thus, subtype C is clearly the outlier in the pathogenic fitness of group M HIV-1.
It is important to note that our sampling of 12 subtype C and 17 group M isolates is not a thorough representation of all of the circulating HIV-1 isolates in the epidemic. However, these types of studies are limited by sample and virus availability, as well as the complexity of the experimentation. If the fitness differences had been subtle, as with the non-subtype C group M isolates, more primary HIV-1 isolates would have been necessary to suggest that subtype C is less fit in PBMC cultures. At present, we are using a larger number of non-subtype C group M HIV-1 isolates to segregate the possible fitness differences between subtypes A, B, D, and F, as well as CRF01_AE, CRF02_AG, CRF12_BF, and CRF33_BC. Preliminary data again suggest minor differences in replicative fitness between these subtypes, but they all are significantly more fit (ex vivo) than subtype C. Another confounding factor for these fitness studies may be the PBMC utilized for these competitions. To standardize the fitness of the primary HIV-1 isolates, all pairwise competitions were purposely performed on PBMC from a single donor. However, a subset of 69 competitions was performed on PBMC derived from three HIV-negative donors of different ethnicities (African American, Chinese, and Bantu African). The relative fitness values for each HIV-1 isolate did not significantly vary in these different PBMC. The lack of PBMC donor effect on replicative fitness in competition assays has been described in previous studies (1, 5). Although the relative production of the two competing viruses does not vary (e.g., fitness value), the overall production of the two HIV-1 isolates can vary 100-fold in PBMC from different donors.
Why are subtype C HIV-1 isolates less fit (ex vivo) than other group M strains? Early genetic analyses of subtype C suggest that various components of this virus are actually more efficient than those of subtype B (19, 33, 34, 58, 59). Again, it is difficult to access individual genetic coding regions or components of a virus in phenotypic assays and not account for the effects of the entire genome. We initially described six NSI/R5 subtype C HIV-1 isolates being significantly less fit than nine subtype B isolates (5). By monitoring the steps of the retroviral lifecycle over the course of 12 subtype B versus C dual infections, it was clear that the outcome of these competitions was dependent on the efficiency of virus entry into the host cell (5). In subsequent studies, the dominance of entry on replicative fitness over all other retroviral steps was further confirmed by cloning the env genes into a neutral, NL4-3 laboratory strain (31, 47). It is important to note that replicative fitness is controlled by all regions of HIV-1 genome and steps in the replication cycle but, barring a highly deleterious mutation (e.g., nef deletion in long-term nonprogressors), the env gene and the efficiency of host entry appear to override minor differences in efficiency of intracellular replication events (31, 47). We are now testing for “a single trait” hypothesis (e.g., env) controlling replicative fitness. Lingo optimization modeling software (Lindo Systems) has been used to test for a transitive relationship throughout the pairwise fitness matrices (T. Immomen and E. J. Arts, unpublished data). Preliminary data on the pairwise competitions indicate that the vast majority of HIV-1 isolates fall into a linear ranking system, suggesting a dominant gene or trait. The strains that fall out of this transitive relationship are being tested to identify other genes and/or coding sequences that impact replicative fitness.
The relevance of this reduced subtype C fitness to HIV-1 virulence or even global spread is debatable. However, infection with an HIV-1 isolate with poor replicative fitness in PBMC typically often leads to slower disease progression. This was best described in the long-term nonprogressing individuals infected with the replication-defective, nef-mutated virus (8, 9, 26). Preliminary studies now suggest that elite controllers, infected individuals maintaining undetectable viral loads without treatment, are infected with HIV-1 isolates of poor ex vivo fitness. The replicative fitness of the infecting virus is not static but rather continually increases during disease progression as significant correlate of increasing viral load and decreasing CD4 cell count (26a). This mounting evidence suggests that ex vivo replicative fitness of HIV-1 in PBMC may be associated with pathogenesis or virulence. By analogy, poor replicative fitness of subtype C HIV-1 isolates could be related to reduced virulence and slow disease progression. Most clinical studies on cross-sectional cohorts have, however, suggested higher viral loads in subtype C-infected individuals compared to subtype B infections of historical cohorts (17, 35). More recently, conference reports have suggested slower disease progression in individuals infected with subtype C than in individuals infected with other group M subtypes, most notably, subtypes A and D (4a). It has already been established that subtype A HIV-1 infections are associated with slightly slower progression than subtype D infections (21, 23). However, the subtype A versus D difference in CD4 cell count declines was relatively minor compared to very slow declines in CD4 cell counts of subtype C-infected individuals. Even though these clinical studies involve natural history cohorts, decisive conclusions describing differences in subtype virulence must be preceded by rigorous analyses to discount for any confounding factors such as host genetics, secondary infections, and social and environmental factors.
Another difference segregating subtype C from other group M subtypes is the low frequency of SI/X4 isolates in patients at late stages of disease (7, 38, 39). The infrequent switch to subtype C virus from R5 to X4 or a dual tropism may also reflect slower disease progression. SI/X4 phenotype has been described as a marker or contributing factor to faster disease progression. Interestingly, faster progression with subtype D infections appears related to higher proportions of the SI/X4 phenotype (22). For the present study, eight rare SI/X4 or dualtropic subtype C HIV-1 isolates were obtained at late disease from HIV-infected Zimbabweans (20) and competed against other SI/X4 group M isolates. We had hypothesized that the switch from R5 to X4 may increase the replicative fitness of HIV-1 subtype C. However, all eight of the X4 subtype C isolates were at least 100-fold less fit (ex vivo) than the seven X4 isolates of subtypes A, B, D, and CRF01_AE. These findings suggest that R5, X4, and dual-subtype C HIV-1 isolates are intrinsically less fit than the other dominant group M subtypes of the respective coreceptor phenotype.
The dominance of subtype C in the HIV-1 epidemic and rapid expansion into new geographic regions such as southern Brazil (3, 52) appears to contradict our findings on the poor replicative or “pathogenic” fitness of subtype C. However, a slower disease progression may also result in more opportunity for transmission from donors in the chronic stages of disease as opposed to just at acute or early infection (3, 16). In contrast to its low replicative fitness, several reports suggest that subtype C HIV-1 is transmitted at least as efficiently as other group M subtypes (5, 48, 61). To further test the “transmission” fitness of subtype C HIV-1 isolates, we competed two subtype C isolates against a subtype A and D isolate in human cervical, penile, and rectal tissue. We also performed direct competitions between R5 and X4 subtype C isolates in penile tissue compared to PBMC cultures. The subtype A and D isolates were at least 100-fold more fit (ex vivo) than the subtype C isolates in PBMC of three different donors and in PM1 cells. Subtype C HIV-1 replication was evident in the cervix, penile, and rectal tissue, whereas these subtype C isolates could not be detected in 11 of 12 competitions performed in PBMC. In this set of experiments, MCs from the tissue were incubated with PM1 cells to expand the virus for detection and fitness analyses. The addition of a nonnucleoside RT inhibitor to the tissue infections blocked the transfer of virus from the MCs to PM1 cells (in the absence of drug), suggesting that the majority of infection in this cocultivations was dependent of the original MC infection in the tissue and not due to “trapped” virus. The X4 subtype C HIV-1 isolate could not establish infection in the tissue and could not be sufficiently amplified in the MC-PM1 cocultivations such that R5 subtype C HIV-1 isolates completely outcompeted the X4 HIV-1 isolate (in contrast to the outgrowth of X4 over R5 HIV-1 isolates in PBMC [see Fig. S1 in the supplemental material]). Although highly speculative, these findings suggest that R5 HIV-1 strains may be selected over the X4 strains in a virus population during transmission. These findings also support our recent study describing the lack of tissue infections with X4 primary HIV-1 isolates (14).
Interestingly, direct infection was most efficient in rectal tissue explants, detectable in all competitions by day 10, most likely reflecting the higher density of activated CD4 T cells within this mucosal site. Here, subtype C HIV isolate C3 outcompeted A1 and D76 and isolate C8 was able to compete with D76. Furthermore, while A1 was seen to outcompete C8 at day 10, detectable competition was seen at day 3. Although preliminary, differential fitness for replication in gastrointestinal tissue may have important implications for pathogenesis given its acknowledged role in disease progression. In summary, these findings suggest that subtype C HIV-1 is transmissible in genital and rectal tissue models of infection and that poor replicative fitness is only observed when the virus was transferred to the PM1, which appears analogous to PBMC in terms of HIV-1 fitness outcomes. However, it must be stressed that these competitions in explants are preliminary and were only performed with 5 of the 29 primary isolates used for fitness determination in PBMC. As described above, HIV-1 “pathogenic” fitness appears to be governed by the rate of host cell entry. In the case of genital explant infections, the virus may be trapped on the surface of dendritic and/or Langerhans cells prior to infection or transfer to T cells or macrophages in the lamina propria. This trapping could reduce or dampen the rate-limiting step of HIV-1 entry. This mechanism may be absent in rectal tissue, where virus may have direct access to a higher abundance of activated CD4 T cells, leading to rapid amplification of an infectious event. However, once the virus enters the systemic system, the virulence of the infecting strain may be more related to “pathogenic” fitness, as well as various host factors.
Supplementary Material
Acknowledgments
This study was supported by the Case/UHC CFAR Biosafety Core (AI36219) and by research grants awarded to E.J.A. (NIH/NIAID AI49170). A.S., L.F., and R.J.S. are supported by the Center for HIV/AIDS Vaccine Immunology. C.H. is supported by NIH IP/CP grant U19 AI060614, and D.K. is supported by NIH/NIAID grant AI060399.
Footnotes
Published ahead of print on 18 March 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Arien, K. K., A. Abraha, M. E. Quinones-Mateu, L. Kestens, G. Vanham, and E. J. Arts. 2005. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J. Virol. 798979-8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arien, K. K., R. M. Troyer, Y. Gali, R. L. Colebunders, E. J. Arts, and G. Vanham. 2005. Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS 191555-1564. [DOI] [PubMed] [Google Scholar]
- 3.Arien, K. K., G. Vanham, and E. J. Arts. 2007. Is HIV-1 evolving to a less virulent form in humans? Nat. Rev. Microbiol. 5141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts, E. J., J. Mak, L. Kleiman, and M. A. Wainberg. 1994. DNA found in human immunodeficiency virus type 1 particles may not be required for infectivity. J. Gen. Virol. 75(Pt. 7)1605-1613. [DOI] [PubMed] [Google Scholar]
- 4a.Arts, E. J., et al. 2006. AIDS 2006-XVI International AIDS Conference, abstr. no. TUAA0204.
- 5.Ball, S. C., A. Abraha, K. R. Collins, A. J. Marozsan, H. Baird, M. E. Quinones-Mateu, A. Penn-Nicholson, M. Murray, N. Richard, M. Lobritz, P. A. Zimmerman, T. Kawamura, A. Blauvelt, and E. J. Arts. 2003. Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J. Virol. 771021-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremermann, H. J., and J. Pickering. 1983. A game-theoretical model of parasite virulence. J. Theor. Biol. 100411-426. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia, D., S. S. Kulkarni, S. P. Tripathy, R. R. Gangakhedkar, R. S. Paranjape, and D. A. Gadkari. 2000. Absence of coreceptor switch with disease progression in human immunodeficiency virus infections in India. Virology 271253-258. [DOI] [PubMed] [Google Scholar]
- 8.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 2581938-1941. [DOI] [PubMed] [Google Scholar]
- 9.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 10.Dittmar, M. T., G. Simmons, S. Hibbitts, M. O'Hare, S. Louisirirotchanakul, S. Beddows, J. Weber, P. R. Clapham, and R. A. Weiss. 1997. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J. Virol. 718008-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley, D. M., J. McGhee, M. S. Lalonde, R. S. Veazey, and E. J. Arts. A single dose of an HIV-specific, vaginally applied microbicide, PSC-RANTES (PSC) can select for a drug-resistant SHIV in a macaque model. J. Virol., in press.
- 12.Eshleman, S. H., L. A. Guay, A. Mwatha, E. Brown, P. Musoke, F. Mmiro, and J. B. Jackson. 2005. Comparison of mother-to-child transmission rates in Ugandan women with subtype A versus D HIV-1 who received single-dose nevirapine prophylaxis: HIV Network For Prevention Trials 012. J. Acquir. Immune. Defic. Syndr. 39593-597. [PubMed] [Google Scholar]
- 13.Essex, M. 1999. Human immunodeficiency viruses in the developing world. Adv. Virus Res. 5371-88. [DOI] [PubMed] [Google Scholar]
- 14.Fischetti, L., S. M. Barry, T. J. Hope, and R. J. Shattock. 2009. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 23319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher, P. S., J. Elliott, J. C. Grivel, L. Margolis, P. Anton, I. McGowan, and R. J. Shattock. 2006. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 201237-1245. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, C., T. D. Hollingsworth, R. Chapman, F. de Wolfe, and W. P. Hanage. 2007. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc. Natl. Acad. Sci. USA 10417441-17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray, C. M., C. Williamson, H. Bredell, A. Puren, X. Xia, R. Filter, L. Zijenah, H. Cao, L. Morris, E. Vardas, M. Colvin, G. Gray, J. McIntyre, R. Musonda, S. Allen, D. Katzenstein, M. Mbizo, N. Kumwenda, T. Taha, S. A. Karim, J. Flores, and H. W. Sheppard. 2005. Viral dynamics and CD4+ T-cell counts in subtype C human immunodeficiency virus type 1-infected individuals from southern Africa. AIDS Res. Hum. Retrovir. 21285-291. [DOI] [PubMed] [Google Scholar]
- 18.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 745577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeeninga, R. E., M. Hoogenkamp, M. Armand-Ugon, M. de Baar, K. Verhoef, and B. Berkhout. 2000. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J. Virol. 743740-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston, E. R., L. S. Zijenah, S. Mutetwa, R. Kantor, C. Kittinunvorakoon, and D. A. Katzenstein. 2003. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J. Virol. 777682-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaleebu, P., N. French, C. Mahe, D. Yirrell, C. Watera, F. Lyagoba, J. Nakiyingi, A. Rutebemberwa, D. Morgan, J. Weber, C. Gilks, and J. Whitworth. 2002. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J. Infect. Dis. 1851244-1250. [DOI] [PubMed] [Google Scholar]
- 22.Kaleebu, P., I. L. Nankya, D. L. Yirrell, L. A. Shafer, J. Kyosiimire-Lugemwa, D. B. Lule, D. Morgan, S. Beddows, J. Weber, and J. A. Whitworth. 2007. Relation between chemokine receptor use, disease stage, and HIV-1 subtypes A and D: results from a rural Ugandan cohort. J. Acquir. Immune. Defic. Syndr. 4528-33. [DOI] [PubMed] [Google Scholar]
- 23.Kaleebu, P., A. Ross, D. Morgan, D. Yirrell, J. Oram, A. Rutebemberwa, F. Lyagoba, L. Hamilton, B. Biryahwaho, and J. Whitworth. 2001. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS 15293-299. [DOI] [PubMed] [Google Scholar]
- 24.Kalish, M. L., A. Baldwin, S. Raktham, C. Wasi, C.-C. Luo, G. Schochetman, T. D. Mastro, N. Young, S. Vanichseni, H. Rübsamen-Waigmann, H. von Briesen, J. I. Mullins, E. Delwart, B. Herring, J. Esparza, W. L. Heyward, and S. Osmanov. 1995. The evolving molecular epidemiology of HIV-1 envelope subtypes in injecting drug users in Bangkok, Thailand: implications for HIV vaccine trials. AIDS 9851-857. [DOI] [PubMed] [Google Scholar]
- 25.Keele, B. F., H. F. Van, Y. Li, E. Bailes, J. Takehisa, M. L. Santiago, F. Bibollet-Ruche, Y. Chen, L. V. Wain, F. Liegeois, S. Loul, N. E. Mpoudi, Y. Bienvenue, E. Delaporte, J. F. Brookfield, P. M. Sharp, G. M. Shaw, M. Peeters, and B. H. Hahn. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 26a.Lassen, K. G., M. A. Lobritz, J. R. Bailey, S. Johnston, S. Nguyen, B. Lee, T. Chou, R. F. Siliciano, M. Markowitz, and E. J. Arts. Elite suppressor-derived HIV-1 envelope glycoproteins exhibit reduced entry efficiency and kinetics. PLoS Pathog. [epub 10 April 2009.] [DOI] [PMC free article] [PubMed]
- 27.Lau, K. A., B. Wang, and N. K. Saksena. 2007. Emerging trends of HIV epidemiology in Asia. AIDS Rev. 9218-229. [PubMed] [Google Scholar]
- 28.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 965215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madan, R. P., P. M. Mesquita, N. Cheshenko, B. Jing, V. Shende, E. Guzman, T. Heald, M. J. Keller, S. L. Regen, R. J. Shattock, and B. C. Herold. 2007. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J. Virol. 817636-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marozsan, A. J., E. Fraundorf, A. Abraha, H. Baird, D. Moore, R. Troyer, I. Nankja, and E. J. Arts. 2004. Relationships between infectious titer, capsid protein levels, and reverse transcriptase activities of diverse human immunodeficiency virus type 1 isolates. J. Virol. 7811130-11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marozsan, A. J., D. M. Moore, M. A. Lobritz, E. Fraundorf, A. Abraha, J. D. Reeves, and E. J. Arts. 2005. Differences in the fitness of two diverse wild-type human immunodeficiency virus type 1 isolates are related to the efficiency of cell binding and entry. J. Virol. 797121-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masemola, A. M., T. N. Mashishi, G. Khoury, H. Bredell, M. Paximadis, T. Mathebula, D. Barkhan, A. Puren, E. Vardas, M. Colvin, L. Zijenah, D. Katzenstein, R. Musonda, S. Allen, N. Kumwenda, T. Taha, G. Gray, J. McIntyre, S. A. Karim, H. W. Sheppard, and C. M. Gray. 2004. Novel and promiscuous CTL epitopes in conserved regions of Gag targeted by individuals with early subtype C HIV type 1 infection from southern Africa. J. Immunol. 1734607-4617. [DOI] [PubMed] [Google Scholar]
- 33.Montano, M. A., C. P. Nixon, T. Ndung'u, H. Bussmann, V. A. Novitsky, D. Dickman, and M. Essex. 2000. Elevated tumor necrosis factor-alpha activation of human immunodeficiency virus type 1 subtype C in Southern Africa is associated with an NF-κB enhancer gain-of-function. J. Infect. Dis. 18176-81. [DOI] [PubMed] [Google Scholar]
- 34.Montano, M. A., V. A. Novitsky, J. T. Blackard, N. Cho, D. A. Katzenstein, and M. Essex. 1997. Divergent transcriptional regulation among expanding human immunodeficiency virus type 1 subtypes. J. Virol. 718657-8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neilson, J. R., G. C. John, J. K. Carr, P. Lewis, J. K. Kreiss, S. Jackson, R. W. Nduati, D. Mbori-Ngacha, D. D. Panteleeff, S. Bodrug, C. Giachetti, M. A. Bott, B. A. Richardson, J. Bwayo, J. Ndinya-Achola, and J. Overbaugh. 1999. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 734393-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novitsky, V., H. Cao, N. Rybak, P. Gilbert, M. F. McLane, S. Gaolekwe, T. Peter, I. Thior, T. Ndung'u, R. Marlink, T. H. Lee, and M. Essex. 2002. Magnitude and frequency of cytotoxic T-lymphocyte responses: identification of immunodominant regions of human immunodeficiency virus type 1 subtype C. J. Virol. 7610155-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12357-358. [DOI] [PubMed] [Google Scholar]
- 38.Peeters, M., R. Vincent, J. L. Perret, M. Lasky, D. Patrel, F. Liegeois, V. Courgnaud, R. Seng, T. Matton, S. Molinier, and E. Delaporte. 1999. Evidence for differences in MT2 cell tropism according to genetic subtypes of HIV-1: syncytium-inducing variants seem rare among subtype C HIV-1 viruses. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 20115-121. [DOI] [PubMed] [Google Scholar]
- 39.Ping, L. H., J. A. Nelson, I. F. Hoffman, J. Schock, S. L. Lamers, M. Goodman, P. Vernazza, P. Kazembe, M. Maida, D. Zimba, M. M. Goodenow, J. J. Eron, Jr., S. A. Fiscus, M. S. Cohen, and R. Swanstrom. 1999. Characterization of V3 sequence heterogeneity in subtype C human immunodeficiency virus type 1 isolates from Malawi: underrepresentation of X4 variants. J. Virol. 736271-6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piyasirisilp, S., F. E. McCutchan, J. K. Carr, E. Sanders-Buell, W. Liu, J. Chen, R. Wagner, H. Wolf, Y. Shao, S. Lai, C. Beyrer, and X. F. Yu. 2000. A recent outbreak of human immunodeficiency virus type 1 infection in southern China was initiated by two highly homogeneous, geographically separated strains, circulating recombinant form AE and a novel BC recombinant. J. Virol. 7411286-11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78389-398. [DOI] [PubMed] [Google Scholar]
- 42.Pope, M., S. S. Frankel, J. R. Mascola, A. Trkola, F. Isdell, D. L. Birx, D. S. Burke, D. D. Ho, and J. P. Moore. 1997. Human immunodeficiency virus type 1 strains of subtypes B and E replicate in cutaneous dendritic cell-T-cell mixtures without displaying subtype-specific tropism. J. Virol. 718001-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinones-Mateu, M. E., and E. J. Arts. 2006. Virus fitness: concept, quantification, and application to HIV population dynamics. Curr. Top. Microbiol. Immunol. 29983-140. [DOI] [PubMed] [Google Scholar]
- 44.Quinones-Mateu, M. E., S. C. Ball, A. J. Marozsan, V. S. Torre, J. L. Albright, G. Vanham, G. van Der Groen, R. L. Colebunders, and E. J. Arts. 2000. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 749222-9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rainwater, S., S. Devange, M. Sagar, J. Ndinya-Achola, K. Mandaliya, J. K. Kreiss, and J. Overbaugh. 2005. No evidence for rapid subtype C spread within an epidemic in which multiple subtypes and intersubtype recombinants circulate. AIDS Res. Hum. Retrovir. 211060-1065. [DOI] [PubMed] [Google Scholar]
- 46.Ramos, A., L. Nguyen, D. J. Hu, S. Vanichseni, K. Choopanya, N. L. Young, J. W. Tappero, T. D. Mastro, T. M. Folks, and S. Subbarao. 2003. New HIV type 1 CRF01_AE/B recombinants displaying unique distribution of breakpoints from incident infections among injecting drug users in Thailand. AIDS Res. Hum. Retrovir. 19667-674. [DOI] [PubMed] [Google Scholar]
- 47.Rangel, H. R., J. Weber, B. Chakraborty, A. Gutierrez, M. L. Marotta, M. Mirza, P. Kiser, M. A. Martinez, J. A. Este, and M. E. Quinones-Mateu. 2003. Role of the human immunodeficiency virus type 1 envelope gene in viral fitness. J. Virol. 779069-9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renjifo, B., P. Gilbert, B. Chaplin, G. Msamanga, D. Mwakagile, W. Fawzi, and M. Essex. 2004. Preferential in utero transmission of HIV-1 subtype C as compared to HIV-1 subtype A or D. AIDS 181629-1636. [DOI] [PubMed] [Google Scholar]
- 49.Richard, N., M. Juntilla, A. Abraha, K. Demers, E. Paxinos, J. Galovich, C. Petropoulos, C. C. Whalen, F. Kyeyune, D. Atwine, C. Kityo, P. Mugyenyi, and E. J. Arts. 2004. High prevalence of antiretroviral resistance in treated Ugandans infected with non-subtype B human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 20355-364. [DOI] [PubMed] [Google Scholar]
- 50.Rousseau, C. M., M. G. Daniels, J. M. Carlson, C. Kadie, H. Crawford, A. Prendergast, P. Matthews, R. Payne, M. Rolland, D. N. Raugi, B. S. Maust, G. H. Learn, D. C. Nickle, H. Coovadia, T. Ndung'u, N. Frahm, C. Brander, B. D. Walker, P. J. Goulder, T. Bhattacharya, D. E. Heckerman, B. T. Korber, and J. I. Mullins. 2008. HLA class I-driven evolution of human immunodeficiency virus type 1 subtype c proteome: immune escape and viral load. J. Virol. 826434-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santiago, M. L., C. M. Rodenburg, S. Kamenya, F. Bibollet-Ruche, F. Gao, E. Bailes, S. Meleth, S. J. Soong, J. M. Kilby, Z. Moldoveanu, B. Fahey, M. N. Muller, A. Ayouba, E. Nerrienet, H. M. McClure, J. L. Heeney, A. E. Pusey, D. A. Collins, C. Boesch, R. W. Wrangham, J. Goodall, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2002. SIVcpz in wild chimpanzees. Science 295465. [DOI] [PubMed] [Google Scholar]
- 52.Soares, E. A., A. M. Martinez, T. M. Souza, A. F. Santos, H. Da, V. J. Silveira, F. I. Bastos, A. Tanuri, and M. A. Soares. 2005. HIV-1 subtype C dissemination in southern Brazil. AIDS 19(Suppl. 4)S81-S86. [DOI] [PubMed] [Google Scholar]
- 53.Tee, K. K., O. G. Pybus, X. J. Li, X. Han, H. Shang, A. Kamarulzaman, and Y. Takebe. 2008. Temporal and spatial dynamics of human immunodeficiency virus type 1 circulating recombinant forms 08_BC and 07_BC in Asia. J. Virol. 829206-9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torre, V. S., A. J. Marozsan, J. L. Albright, K. R. Collins, O. Hartley, R. E. Offord, M. E. Quinones-Mateu, and E. J. Arts. 2000. Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs. J. Virol. 744868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tovanabutra, S., C. Beyrer, S. Sakkhachornphop, M. H. Razak, G. L. Ramos, T. Vongchak, K. Rungruengthanakit, P. Saokhieo, K. Tejafong, B. Kim, S. M. De, M. L. Robb, D. L. Birx, J. Jittiwutikarn, V. Suriyanon, D. D. Celentano, and F. E. McCutchan. 2004. The changing molecular epidemiology of HIV type 1 among northern Thai drug users, 1999 to 2002. AIDS Res. Hum. Retrovir. 20465-475. [DOI] [PubMed] [Google Scholar]
- 56.Tovanabutra, S., V. Polonis, M. De Souza, R. Trichavaroj, P. Chanbancherd, B. Kim, E. Sanders-Buell, S. Nitayaphan, A. Brown, M. R. Robb, D. L. Birx, F. E. McCutchan, and J. K. Carr. 2001. First CRF01_AE/B recombinant of HIV-1 is found in Thailand. AIDS 151063-1065. [DOI] [PubMed] [Google Scholar]
- 57.Troyer, R. M., K. R. Collins, A. Abraha, E. Fraundorf, D. M. Moore, R. W. Krizan, Z. Toossi, R. L. Colebunders, M. A. Jensen, J. I. Mullins, G. Vanham, and E. J. Arts. 2005. Changes in human immunodeficiency virus type 1 fitness and genetic diversity during disease progression. J. Virol. 799006-9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Opijnen, T., R. E. Jeeninga, M. C. Boerlijst, G. P. Pollakis, V. Zetterberg, M. Salminen, and B. Berkhout. 2004. Human immunodeficiency virus type 1 subtypes have a distinct long terminal repeat that determines the replication rate in a host-cell-specific manner. J. Virol. 783675-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velazquez-Campoy, A., M. J. Todd, S. Vega, and E. Freire. 2001. Catalytic efficiency and vitality of HIV-1 proteases from African viral subtypes. Proc. Natl. Acad. Sci. USA 986062-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vidal, N., C. Mulanga, S. E. Bazepeo, J. K. Mwamba, J. W. Tshimpaka, M. Kashi, N. Mama, C. Laurent, F. Lepira, E. Delaporte, and M. Peeters. 2005. Distribution of HIV-1 variants in the Democratic Republic of Congo suggests increase of subtype C in Kinshasa between 1997 and 2002. J. Acquir. Immune. Defic. Syndr. 40456-462. [DOI] [PubMed] [Google Scholar]
- 61.Walker, P. R., O. G. Pybus, A. Rambaut, and E. C. Holmes. 2005. Comparative population dynamics of HIV-1 subtypes B and C: subtype-specific differences in patterns of epidemic growth. Infect. Genet. Evol. 5199-208. [DOI] [PubMed] [Google Scholar]
- 62.Wasi, C., B. Herring, S. Raktham, S. Vanichseni, T. D. Mastro, N. L. Young, H. Rubsamen-Waigmann, H. von Briesen, M. L. Kalish, and C. C. Luo. 1995. Determination of HIV-1 subtypes in injecting drug users in Bangkok, Thailand, using peptide-binding enzyme immunoassay and heteroduplex mobility assay: evidence of increasing infection with HIV-1 subtype E. AIDS 9843-849. [DOI] [PubMed] [Google Scholar]
- 63.Yang, C., M. Li, R. D. Newman, Y. P. Shi, J. Ayisi, A. M. van Eijk, J. Otieno, A. O. Misore, R. W. Steketee, B. L. Nahlen, and R. B. Lal. 2003. Genetic diversity of HIV-1 in western Kenya: subtype-specific differences in mother-to-child transmission. AIDS 171667-1674. [DOI] [PubMed] [Google Scholar]
- 64.Yang, R., X. Xia, S. Kusagawa, C. Zhang, K. Ben, and Y. Takebe. 2002. On-going generation of multiple forms of HIV-1 intersubtype recombinants in the Yunnan Province of China. AIDS 161401-1407. [DOI] [PubMed] [Google Scholar]
- 65.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 31369-1375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.