Abstract
Previous studies demonstrated that immunization of macaques with simian immunodeficiency virus (SIV) Gag-Pol and Env recombinants of the attenuated poxvirus modified vaccinia virus Ankara (MVA) provided protection from high viremia and AIDS following challenge with a pathogenic strain of SIV. Although all animals became infected, plasma viremia was significantly reduced in animals that received the MVA-SIV recombinant vaccines compared with animals that received nonrecombinant MVA. Most importantly, the reduction in viremia resulted in a significant increase in median and cumulative survival. Continued analysis of these animals over the subsequent 9 years has shown that they maintain a survival advantage, although all but two of the macaques have progressed to AIDS. Importantly, improved survival correlated with preservation of memory CD4+ T cells in the peripheral blood. The greatest survival advantage was observed in macaques immunized with regimens containing SIV Env, and the titer of neutralizing antibodies to the challenge virus prior to or shortly following challenge correlated with preservation of CD4+ T cells. These data are consistent with a role for neutralizing antibodies in nonsterilizing protection from high viremia and associated memory CD4+ T-cell loss.
Despite intensive efforts, access to successful antiretroviral therapy remains elusive for a large number of human immunodeficiency virus (HIV)-infected people in Africa and other developing areas (8, 91), and an HIV vaccine remains the most promising strategy for controlling the AIDS pandemic (16, 44, 65). Ideally such a vaccine would elicit broadly reactive neutralizing antibodies (NAb) as well as cellular immune responses (12, 39). However, the difficulties associated with inducing broadly NAb responses combined with the urgent need for a vaccine to limit HIV transmission resulted in a shift in research priorities toward strategies that elicit strong cellular immunity (59, 75, 87, 97, 102). The rationale for this approach was based on the observation that the reduction of acute HIV viremia occurs contemporaneously with the appearance of virus-specific CD8 cytotoxic T lymphocytes (CTL) and before the detection of NAb responses (10, 41). The crucial role of CTL in the control of HIV infection has also been supported by CD8 depletion studies with the simian immunodeficiency virus (SIV) macaque model (23, 37, 54, 71, 90).
Several vaccine candidates eliciting strong virus-specific CTL responses and capable of protecting macaques from CD4+ T-cell loss and AIDS following CXCR4-tropic simian-human immunodeficiency virus (SHIV) challenge were developed (3, 6, 53, 88, 95). However, similar or identical approaches resulted in only modest reductions in virus load following rigorous challenge of macaques with CCR5-tropic pathogenic SIVs (7, 31-33, 36, 74). The recent failure of one such approach, a replication-defective adenovirus-based vaccine, in a phase III clinical trial of almost 3,000 healthy uninfected volunteers suggests that the SIV model may be more predictive of efficacy than the X4-SHIV model (66). Although this vaccine induced cellular immunity against the HIV Gag, Pol, and Nef proteins, the rates of infection were similar in volunteers administered placebo and those administered vaccine. In fact, vaccination appeared to increase the rate of acquisition of HIV infection in a subset of volunteers with prior immunity against the adenoviral vector. These disappointing results suggest that more comprehensive assessment of vaccine-induced immune responses and modulation of these responses by HIV infection is urgently needed. In retrospect, the failure of this vaccine was predictable from SIV-macaque challenge models rather than the more promising results using the SHIV-macaque model. Therefore, more emphasis should be placed on the more rigorous SIV-macaque model before further efficacy trials are undertaken in humans (31, 92, 102, 103).
SIV shows a great many parallels to HIV infection in modeling AIDS pathogenesis (35). SIV infection usually induces a slow progressive disease in macaques that is characterized by the gradual loss of circulating CD4+ T lymphocytes (67). Infected monkeys generally develop strong cell-mediated and humoral immune responses against SIV that partially control, but do not eliminate, the virus. Infected animals eventually develop immunodeficiency and succumb to opportunistic infections with a median survival time of 1 to 2 years Like HIV, SIV preferentially utilizes the CCR5 coreceptor and therefore targets the memory CD4+ T-cell subset during infection (68). Recent studies have shown the highly destructive nature of this assault on preexisting memory CD4+ T cells throughout the body during acute HIV/SIV infection. Since loss of this critical subset precedes the development of an antiviral response, it may well set the stage for the subsequent development of immunodeficiency (27, 28, 56, 81, 99). Therefore, an important goal of an HIV vaccine is to prevent, or at least contain, this early destruction of CD4+ T cells with a goal of preserving the ability of the immune system to generate secondary immune responses to infection.
Earlier we reported that immunization of rhesus macaques with modified vaccinia virus Ankara (MVA) recombinants that efficiently expressed high levels of either SIVsmH4 Gag-Pol (MVA-gag-pol) or Env (MVA-env), alone or in combination (MVA-gag-pol-env), significantly reduced acute-phase plasma viremia after challenge with uncloned, pathogenic SIVsmE660, compared with control animals vaccinated with nonrecombinant MVA (74). Most importantly, this reduction in levels of viremia resulted in a significant increase in cumulative survival in all three groups of macaques immunized with MVA-gag-pol, MVA-env, or MVA-gag-pol-env recombinant vaccines. This present study describes the continued observation of these macaques over the subsequent 9 years after challenge. All but two of the immunized animals developed immunodeficiency during the period of observation. In this present study, we monitored the dynamics of CD4+ T-lymphocyte subsets in peripheral blood mononuclear cell (PBMC) samples acquired during the early stages of infection and evaluated correlations between viral load, memory CD4+ T-cell preservation, and survival. In addition we used more sensitive neutralizing assays to evaluate vaccine-induced NAb as an immune correlate of protection from memory CD4+ T-cell loss.
MATERIALS AND METHODS
Vaccinia virus recombinants.
The generation of MVA recombinants expressing SIVsmH4 Env, Gag-Pol, or Gag-Pol and Env has been described previously (63). MVA was originally obtained from A. Mayr, Veterinary Faculty, University of Munich, Munich, Germany, and virus stocks were routinely propagated in chicken embryo fibroblasts (CEF). The SIVsmH4 sequences encoding Env or Gag-Pol protein precursors were inserted into the PmeI site of the MVA transfer plasmids under the control of the efficient synthetic early/late vaccinia virus promoter (13, 15) and are subsequently referred to as pLW22env and pMC03gag-pol, respectively. The expression cassette of pLW22env was flanked by the sequences for homologous recombination into the site of deletion II (60) in the MVA genome and contained the Escherichia coli β-galactosidase gene driven by the vaccinia virus early/late promoter (P7.5). The expression cassette of pMC03gag-pol was flanked by sequences homologous to deletion III in the MVA genome and contained the P7.5-GUS (E. coli β-glucuronidase) operon. To generate recombinant MVA virus, monolayers of nearly confluent CEF were infected with 0.05 PFU of MVA per cell in six-well plates and were transfected with 10 μg of plasmid DNA using the PerFect transfection kit (Invitrogen, San Diego, CA) at 90 min after infection, as recommended by the manufacturer. At 48 h after infection, the cells were harvested and processed as described previously (96). MVA recombinants expressing the SIVsmH4 env (MVA-E) and gag-pol (MVA-GP) genes were selected by β-galactosidase or β-glucuronidase screening in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) or X-Glu (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) (Gold BioTechnology, St. Louis, MO), respectively. Expression of SIV Env and Gag-Pol proteins was demonstrated by immunostaining of recombinant plaques with sera from SIV-infected macaques and Western blot analysis of CEF and BS-C-1 cells infected with selected recombinant clones of MVA. After five consecutive rounds of plaque purification, recombinant viruses were amplified in CEF and purified by centrifugation through a 36% sucrose cushion. The titers of these stocks were determined by immunostaining of infected CEF with SIV-specific monkey sera. The double recombinant MVA-GPE was selected after transfection of transfer plasmid pMC03gag-pol into CEF infected with the MVA-env recombinant.
Cells and viruses.
Monkey BS-C-1 and CEF were grown in minimal essential medium with nonessential amino acids supplemented with 10% fetal calf serum. CEMx174 cells used for SIV rescue were grown in RPMI 1640 supplemented with glutamine and 10% fetal calf serum. TZM-bl cells, a genetically engineered HeLa cell clone that expresses CD4, CXCR4, and CCR5 and contains Tat-responsive reporter genes for firefly luciferase and Escherichia coli β-galactosidase under the regulatory control of an HIV type 1 (HIV-1) long terminal repeat, were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu (83, 104). TZM-bl cells were maintained in Dulbecco's modified Eagle's medium (Gibco BRL Life Technologies) containing 10% heat-inactivated fetal bovine serum and 50 μg gentamicin/ml. All cultures were incubated at 37°C in a humidified 5% CO2-95% air environment. PBMC were separated by centrifugation through lymphocyte separation medium (ICN Biomedicals Inc., Aurora, OH) and maintained for 4 days in RPMI 1640 supplemented with 10% interleukin-2 and 5 μg/ml of phytohemagglutinin and subsequently in a similar medium lacking phytohemagglutinin.
The challenge virus was a cell-free virus stock of uncloned SIVsmE660 (24) which had been cultured in pig-tailed macaque PBMC and titrated for infectivity by intravenous inoculation of 10-fold serial dilutions into rhesus macaques to determine a 50% monkey infectious dose. This virus was highly pathogenic and highly related but not identical to the molecular clone SIVsmH4, used to construct the recombinant vaccinia viruses.
Animals and immunization schedule.
Twenty-four juvenile, simian retrovirus- and simian T-cell leukemia virus type 1-seronegative colony-bred rhesus macaques of Indian origin (Macaca mulatta) were immunized intramuscularly with 108 PFU of recombinant MVA-gag-pol (group A, n = 6), MVA-env (group B, n = 6), MVA-gag-pol-env (group C, n = 6), or nonrecombinant MVA (group D, n = 6) at 0, 4, 16, and 28 weeks. The animals were bled periodically throughout the immunization protocol, and plasma samples were assayed for SIV-specific antibody by enzyme-linked immunosorbent assay and NAb assays. The animals were then challenged intravenously 4 weeks later with 50 50% monkey infectious doses of the SIVsmE660 virus stock described earlier (24, 34). Blood and plasma samples were collected biweekly before challenge, on the day of challenge, and subsequently at 3, 7, 10, and 14 days after challenge; 3, 4, 6, and 8 weeks after challenge; and monthly thereafter.
Quantitative real-time PCR of plasma SIV RNA.
Viral RNA was isolated from cryopreserved plasma samples using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany). Reverse transcription was carried out using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) and random RETROscript decamers (Ambion) for priming. Viral RNA levels in plasma were then determined by quantitative real-time PCR using a Prism 7700 sequence detector (Applied Biosystems, Foster City, CA) as detailed previously (94). A serial fivefold dilution series of the standard RNA template was assayed in duplicate to generate a standard curve for each assay. Real-time PCR for each plasma sample was performed in triplicate, including one control reaction processed without addition of reverse transcriptase for potential DNA contamination. Results of the assay were normalized to the volume of plasma extracted and expressed as SIV RNA copy equivalents per ml of plasma, as described for HIV-1 (77, 78). Interassay variation was less than 25% (coefficient of variation).
Flow cytometric analysis of CD4+ T cells.
Blood samples were drawn directly into BD Vacutainer blood collection tubes containing EDTA (Becton Dickinson), and a complete blood count was performed (24) to determine the absolute white blood cell count. Plasma samples were collected after low-speed blood cell sedimentation, and PBMC were isolated by density gradient centrifugation over lymphocyte separation medium, as recommended by the manufacturer (MP Biomedicals). Density gradient-isolated PBMC were washed with Hanks balanced salt solution and counted. For cryopreservation, isolated PBMC were resuspended in ice-cold fetal bovine serum with 10% dimethyl sulfoxide at 5 × 106 to 10 × 106 cells/ml, and the cell suspension was placed in cryovials, transferred into a precooled (4°C) Nalgene Cryofreezing container (Nalge Nunc International), and placed in a −70°C freezer overnight (101). Frozen samples were transferred to a liquid nitrogen freezer and were stored in liquid nitrogen before being thawed and assayed. Recovery and immunophenotyping of cryopreserved PBMC were performed according to AIDS Clinical Trials Group guidelines. Frozen PBMC samples were thawed in a 37°C water bath with continuous agitation until completely thawed and then placed on ice for 2 min. Each 1 ml of thawed cell suspension was slowly diluted with 10 ml of RPMI 1640 medium supplemented with 20% fetal bovine serum and 25 mM HEPES buffer at room temperature, incubated for 10 min, pelleted by centrifugation, and washed a second time with 10 ml of medium (85). Recovered cells were resuspended in staining buffer and stained for flow cytometric analysis using combinations of the following fluorochrome-conjugated monoclonal antibodies: CD3 (allophycocyanin), CD4 (fluorescein isothiocyanate [FITC] or phycoerythrin [PE]), CD8 (FITC or PE), CD28 (FITC), CD95 (allophycocyanin), CCR5 (PE), and isotype-matched controls (mouse immunoglobulin G1 [IgG1] and IgG2). All antibodies were obtained from BD Biosciences. To avoid interference of nonviable (dead) cells with accurate flow cytometric data analysis from nonspecific binding of antibodies and increased autofluorescence, live CD4+ T cells were distinguished by the absence of staining with 7-amino-actinomycin D (89). Four-color flow cytometry analysis was performed on a FACSCalibur cytometer (BD Biosciences Immunocytometry Systems). Data were acquired using CellQuest Pro (BD Biosciences) and analyzed with FlowJo (TreeStar) software. CD4+ T-lymphocyte memory subset phenotypic analysis was performed as described elsewhere (68, 82). For Ki-67 staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm fixation/permeabilization kit buffers (BD Biosciences) and stained with Ki-67 or a control isotype IgG1 monoclonal antibody.
Analysis of NAb titers.
NAb were measured as reductions in Luc reporter gene expression in TZM-bl cells after infection with SIVsmE660 challenge virus preincubated with macaque plasma samples as described previously (62). This assay is a modified version of the assay used by Wei et al. (105). Briefly, 50 50% tissue culture infective doses of virus was incubated with various dilutions of test samples (starting with 1 to 20 with eight threefold stepwise dilutions) in triplicate for 1 h at 37°C in a total volume of 100 μl growth medium in 96-well flat-bottomed culture plates (Corning Costar). Freshly trypsinized cells (10,000 cells in 100 μl of growth medium) were added to each well. One set of six control wells received cells plus virus (virus control), and another set of six wells received cells only (background control). Approximately 36 h after incubation, a culture medium was removed from each well and 50 μl of cell lysing buffer (Promega) was added to the cells. After a 15-min incubation at room temperature to allow cell lysis, 30 μl of cell lysate was transferred to 96-well black solid OptiPlates-96F plates (Perkin-Elmer) for measurements of luminescence using a Mithras LB940 luminometer (Bethold Technologies)) that inject luciferase assay substrate (Promega) into each well. The 50% inhibitory dose (ID50) was defined as the serum dilution that caused a 50% reduction in luciferase activity compared to virus control wells after subtraction of background. To calculate the dilution of serum that neutralized 50% of infectious virus (ID50), the inhibitory dose-response curve was fit with a nonlinear function (a four-parameter dose-response curve equation) using GraphPad Prism 5 software (GraphPad Software, Inc.).
Rhesus macaque genotyping for MHC class I alleles.
Genomic DNA was extracted from frozen PBMC using the QIAamp DNA blood kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The concentration and quality of the recovered genomic DNA were determined using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington DE), and a major histocompatibility complex A2 (MHC-A2) locus site-specific primer PCR assay (SSP-PCR) was performed to assess for the absence of amplification inhibitors (43). Genotyping for Mamu-A*01 was performed using an SSP-PCR assay as described previously (47). Macaques carrying the Mamu-B*08 allele in their genome were identified by using the SSP-PCR assay described by Loffredo et al. (48). Genotyping for Mamu-B*290101 and Mamu-B*7301 was performed by SSP-PCR using MamuB29S1 sense (CGGAGTATTGGGAAGAGGAAA) and MamuB29R1 reverse (CGCTCCGCATAACGGT) primers. The PCR mixture contained 50 mM KCl; 1.5 mM MgCl2; 10 mM Tris, pH 8.3; 0.2 mM (each) dATP, dTTP, dCTP, and dGTP; 50 pmol of each Mamu-B*290101-specific primer; and 5 U of AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) in a final volume of 50 μl. The reaction mixtures were heated at 95°C for 3 min, and amplification was conducted for 25 cycles as follows: mixtures were denatured for 30 s at 95°C, annealed for 30 s at 60°C, and extended for 30 s at 72°C. A final extension was conducted for 7 min at 72°C. Subsequently, 15 μl of the PCR mixture was loaded on a 1% agarose Tris-acetate-EDTA gel and separated by electrophoresis. This SSP-PCR generates a 539-bp-long amplicon in Mamu-B*290101- and Mamu-B*7301-positive macaques. To distinguish between these two MHC alleles, a second PCR was performed using the Superstar Plus DNA polymerase (Ambion, Austin, TX) for all macaques having a positive result. PCR amplicons were gel purified using the Qiaquick gel extraction kit and directly sequenced using an Applied Biosystems 3130XL genetic analyzer. Allele identification was achieved after sequence alignment with Mamu-B*290101 (GenBank accession number EU305650) and Mamu-B*7301 (GenBank accession number AM902578) reference sequences.
Statistical analyses.
Scientific graphing, curve fitting linear and nonlinear regression, and other statistical procedures were performed using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA) as indicated in the figure legends. The statistical significance of differences between control and vaccinated groups was analyzed by using an unpaired t test. The statistical significance of correlation between different parameters was determined by Spearman ranking. The comparison of cumulative survival for different groups of vaccinated monkeys was performed using a Gehan-Wilcoxon test.
RESULTS
Vaccination did not prevent infection but significantly reduced viremia following SIV challenge.
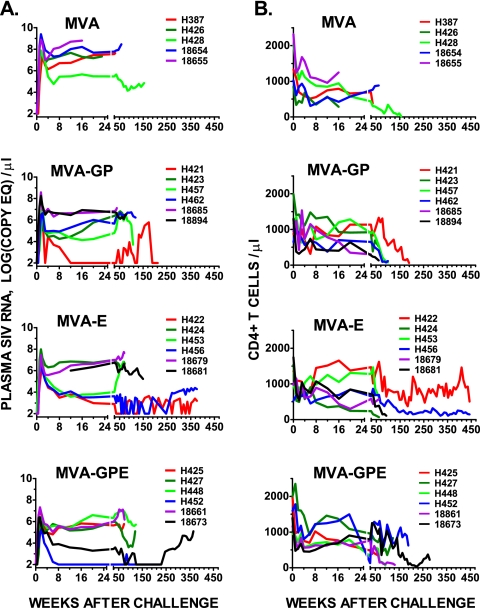
Twenty-four rhesus macaques, six animals per group, were vaccinated with nonrecombinant MVA or recombinant MVA viruses efficiently expressing either Gag-Pol (MVA-GP) or Env (MVA-E) or both Gag-Pol and Env precursors of the SIVsmH4 structural proteins. Previous reports followed the outcome of these macaques for the first 85 weeks of observation after challenge with pathogenic uncloned SIVsmE660 (73, 74). The animals vaccinated with recombinant MVA vaccines showed a transient reduction in plasma viremia that translated into a clinical survival benefit. We have now followed these animals for a further 450 weeks. Only two survivors remain; both of these were immunized with the MVA-E recombinant, and only one of these animals (H422) has maintained healthy levels of CD4+ T cells in the peripheral blood (Fig. 1). As shown in Table 1, macaques were euthanized at various time points during the study as a result of a variety of opportunistic infections (Pneumocystis and Cryptosporidium species), overwhelming bacterial infections, neoplasia (multicentric lymphoma), or severe enteritis with or without amyloidosis. All of the macaques that received nonrecombinant MVA progressed to AIDS within 150 weeks. In contrast, the majority of SIV vaccinees survived for at least 105 weeks and some survived much longer. The sequential plasma viral load and CD4+ T-cell levels are shown graphically in Fig. 1. A number of animals in the SIV-vaccinated groups (H421, H422, H456, H452, and 18673) showed sustained control of viremia. One of these animals (H452) was euthanized 4 years postchallenge due to osteoarthritis that was unrelated to SIV infection, since this animal had maintained normal levels of CD4+ T cells and an undetectable plasma viral load. Increasing plasma viremia was observed in some of the controllers and was frequently associated with a decline in peripheral CD4+ T-cell counts (Fig. 1B). This pattern is suggestive of escape from immune control in these animals. As we published previously (74), SIV was isolated from PBMC of all 24 macaques after challenge. However, virus isolation was transient from PBMC samples of four macaques (H421, H457, H422, and H452). Isolation of virus was not successful for samples collected from macaques H422 and H452 after 12 weeks postinoculation, consistent with extremely low viral loads in these animals.
FIG. 1.
Plasma viral load (A) and peripheral blood CD4+ T-lymphocyte levels (B) in immunized and control macaques after challenge with uncloned SIVsmE660. (A) Sequential levels of plasma viral RNA are shown for animals immunized prior to challenge with nonrecombinant MVA as control or recombinant MVA expressing SIVsm gag-pol (MVA-GP), env (MVA-E), or both gag-pol and env precursors (MVA-GPE). Results are expressed as the number of SIV RNA copy equivalents per ml of plasma. Plasma samples having values under assay threshold sensitivity were given a value of 100 copy equivalents per ml. (B) Sequential CD4+ T-lymphocyte levels evaluated by fluorescence-activated cell sorting on whole-blood samples from the same animals as those in panel A.
TABLE 1.
Clinical and pathological outcome in MVA-SIV-vaccinated macaques following SIVsmE660 challenge
| Group and macaque | MHC-Ia | Survival (wks) | Pathology or clinical findingsc |
|---|---|---|---|
| Gag-Pol | |||
| H421 | B*29 | 212 | Pneumocystis pneumonia, LD |
| H423 | 70 | Bacterial bronchopneumonia, LD | |
| H457 | 107 | Amyloidosis, lymphoid and intestinal | |
| H462 | 118 | Enterocolitis | |
| 18685 | B*29 | 39 | Bacterial endocarditis with microabscesses |
| 18894 | 84 | Enteritis, mild SIVE, interstitial nephritis, LD | |
| Env | |||
| H422 | 450+ | Healthy | |
| H424 | 72 | Protozoal enteritis, interstitial pneumonia, SIV | |
| H453 | B*29 | 66 | Pneumocystis pneumonia, LD |
| H456 | B*29 | 450+ | Low CD4 cell count |
| 18679 | 70 | Multicentric lymphoma (liver, kidneys, heart) | |
| 18681 | 149 | Endocarditis, enteritis with intestinal amyloidosis | |
| Gag-Pol-Env | |||
| H425 | B*29 | 73 | Enteritis (Strongyloides), lymphoma |
| H427 | 120 | Enteritis, LD | |
| H448 | 123 | Enteritis | |
| H452b | 225 | Suppurative arthritis, all other tissues WNL | |
| 18661 | B*08 | 75 | Pneumocystis enteritis with amyloid (LP) |
| 18673 | A*01, B*29 | 365 | Cryptosporidial cholecystitis, pancreatitis, intestinal amyloidosis, colitis |
| Control | |||
| H386b | A*01 | 16 | Anesthetic death, not SIV related |
| H387 | 32 | Severe bilateral bacterial pyelonephritis, LD | |
| H426 | 23 | SIVE and SIV pneumonia | |
| H428 | 150 | Cholangiohepatitis and enteritis (Cryptosporidium) | |
| 18654 | B*08 | 58 | Severe enteritis with edema, crypt abscesses |
| 18655 | 16 | SIVE and SIV pneumonia |
All animals were genotyped for three of the MHC-I alleles associated with control of viremia and slow disease progression in SIV-infected macaques, Mamu-A*01, -B*08, and -B*29, as described in Materials and Methods.
Premature death, not directly related to SIV infection.
SIVE, SIV encephalitis; LD, lymphoid depletion; NA, not applicable; WNL, within normal limit; LP, lamina propria.
Reduction of viremia after challenge significantly correlates with survival.
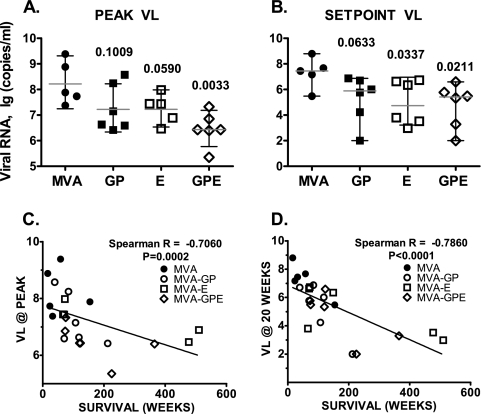
Plasma viral RNA levels at peak viremia and set point (defined as the mean of 16- to 20-week values) in each of the vaccinated groups are shown in Fig. 2A and B. The peak viral load was significantly lower in animals immunized with MVA-GPE than in the control group (P = 0.0033) and approached significance in the MVA-E group (P = 0.059). Both MVA-GPE- and MVA-E-immunized groups showed a significant reduction in plasma viral load at set point compared with the control group (P = 0.0337 and 0.0211, respectively). Although there was a trend toward virus load reduction in the MVA-GP group, this did not achieve statistical significance. Some macaques in all three groups of vaccinated animals showed substantial control of viremia at set point (<10,000 copies per ml of plasma) (Fig. 2B). All but one of these macaques showed prolonged survival. Moreover, the levels of viremia for all control and vaccinated animals both at peak (Fig. 2C) and at set point (Fig. 2D) after challenge were significantly correlated with survival (P < 0.0001). Lower levels of viremia were associated with prolonged survival of the animals. To examine the potential contribution of genetic factors, we performed genotyping of all 24 rhesus macaques for three MHC class I (MHC-I) alleles previously associated with control of viremia and slow disease progression in SIV-infected macaques: Mamu-A*01, -B*08, and -B*29 (38, 47-49). The results of this analysis, presented in Table 1, did not reveal preferential distribution of these MHC-I alleles among the groups or direct association of “elite controller” genotypes with control of viremia and survival of infected monkeys after challenge with SIVsmE660. For example, one of the Mamu-B*08-positive macaques, 18654, showed the highest plasma viral load in the control group. Macaques expressing the Mamu-B*29 MHC-I allele were evenly distributed among all three vaccine groups. Notably, one of the long-term nonprogressors, rhesus macaque 18673, expressed both Mamu-A*01 and Mamu-B*29 MHC-I alleles. However, the longest-surviving animal (H422) in the cohort did not express any of these three MHC-I alleles.
FIG. 2.
Immunization with MVA-SIVsmH4 recombinants reduces viremia levels during acute and set point phases of pathogenic SIVsmE660 infection. (A and B) Viral RNA levels for individual macaques in the control group and each vaccinated group are presented, with bars indicating the geometric means of plasma viral loads with 95% confidence intervals for each group at peak (A) and set point (B) of viremia. The significance of differences between the control group and each individual vaccinated group was analyzed using an unpaired t test, and P values for these comparisons of vaccinated groups are indicated. (C) The survival of macaques challenged with SIVsmE660 significantly correlated with peak and set point (20 weeks). (D) Plasma viral RNA levels as shown by Spearman rank correlation test.
Preservation of memory CD4+ T cells in vaccinees.
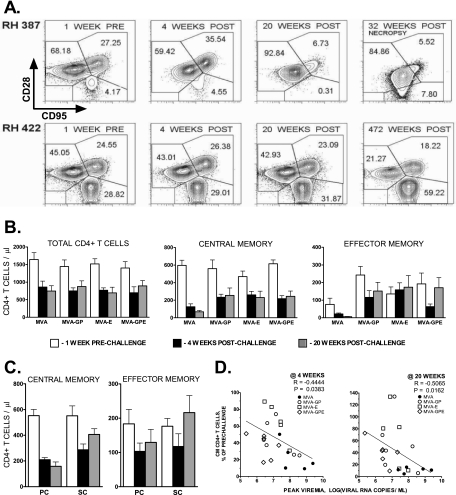
Cryopreserved PBMC collected from vaccinated monkeys were analyzed by flow cytometry (85). Memory and naïve CD4+ T-cell subsets were identified on the basis of expression of CD28 and CD95 (82), as shown for representative samples in Fig. 3A. To exclude nonspecific staining and autofluorescence, dead lymphocytes were differentiated from live cells by staining with 7-amino-actinomycin D (89). As can be observed in the samples from H387, a macaque from the control group immunized with nonrecombinant MVA, significant depletion of effector and central memory CD4+ T cells was observed by 4 weeks postchallenge and became more pronounced by viremia set point at 20 weeks. This pattern of CD4 memory cell depletion was observed in all of the control animals to various degrees. In contrast, samples from the same time points of H422, a long-term survivor vaccinated with the MVA-E recombinant, showed significant preservation of memory CD4+ T-cell subsets. Figure 3B shows the mean values of various subsets of CD4+ T cells in the control group and each of the three groups of macaques immunized with MVA-GP, MVA-E, or MVA-GPE prechallenge, after primary peak, and at set point postchallenge. All four groups showed similar degrees of depletion of total CD4+ T cells at both time points postchallenge. However, more-pronounced depletion of central memory CD4+ T cells during primary viremia peak was observed in the control group compared with that in any of the groups immunized with MVA-SIV recombinants. This difference in degree of central memory CD4+ T-lymphocyte depletion in peripheral blood samples became even more pronounced at set point of viremia (20 weeks); continued depletion of this cell subset occurred in macaques from the control group, whereas this subset was preserved or even partially restored in all three groups of monkeys immunized with MVA-SIV recombinants. Reflecting the loss of central memory cells, more-pronounced depletion of effector memory CD4+ T cells was observed in the control animals compared with that in the animals immunized with MVA-SIV immunogens. Animals immunized with MVA-SIV recombinants also had a higher number of effector memory CD4+ T cells prior to challenge and showed expansion up to prechallenge levels after primary viremia, suggestive of increased levels of SIV-specific cells. Despite similar prechallenge numbers of circulating memory CD4+ T lymphocytes in vaccinated monkeys showing partial control and in those showing substantial control of virus replication (Fig. 3C), macaques with substantial control of viremia showed significantly more memory CD4+ T cells at set point (P = 0.0006 by unpaired t test and P = 0.0044 by nonparametric Mann-Whitney U test). As seen in Fig. 3D, a significant inverse correlation was observed between the peak of viremia and the percentage of central memory CD4+ T cells remaining at either 4 or 20 weeks; the higher the peak of viremia, the greater the loss of CD4+ T cells.
FIG. 3.
Immunization with MVA-SIVsmH4 recombinants is associated with preservation of central memory CD4+ T lymphocytes following SIVsmE660 challenge. (A) Flow cytometric gating strategy for identification of naïve (CD28high CD95low), central memory (CD28high CD95high), and effector memory (CD28low CD95high) CD4+ T cells is shown for a representative macaque from the control group (H387) and a long-term-surviving macaque with sustained control of viremia vaccinated with MVA-Env (H422). Profiles are shown for samples collected prior to challenge, 4 and 20 weeks post-SIV challenge, and time of death (H387) or 472 weeks postchallenge (H422). As indicated by the percentages of central and effector memory CD4+ T cells, progressive loss of these populations was observed in the control macaque. In contrast, these populations were preserved in the vaccinated macaque with sustained control of viremia. (B) Bar graphs depicting mean cell numbers of total (left), central memory (center), or effector memory (right) CD4+ T cells in PBMC samples of the various groups for 1 week (white) prechallenge and 4 weeks (black) and 20 weeks (gray) postchallenge show loss of central and effector memory cells in the control macaques and significantly better preservation of these cells in each of the three vaccinated groups. (C) Better preservation of mean central memory and effector memory CD4+ T cells in macaques showing sustained control (SC) of viremia compared to those with partial control (PC). (D) A significant inverse correlation of peak viremia was observed with the percentage of CD4+ T cells remaining at 4 weeks (P < 0.0383) and 20 weeks (P < 0.0162) postchallenge.
Improved survival in macaques immunized with Env recombinants.
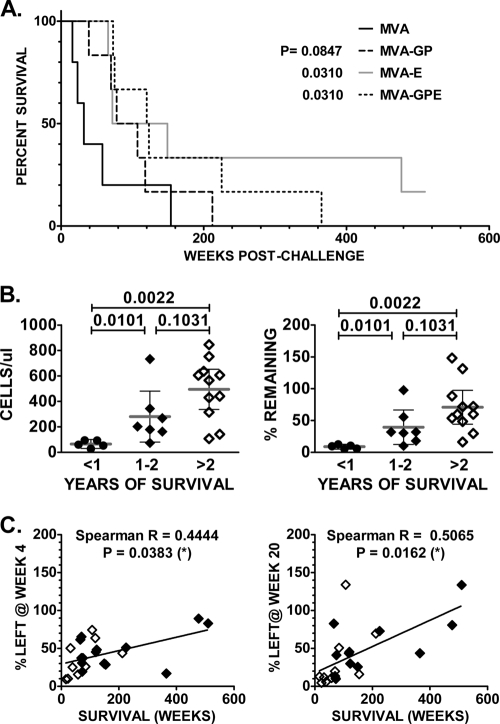
In our previous report, a significant difference in survival was observed between macaques immunized with MVA-SIV recombinant and the controls. However, we were unable to reveal substantial differences in survival between the various groups of vaccinated macaques. With longer follow-up, the survival of animals immunized with either MVA-GPE or MVA-E was significantly greater than survival of the control group, as shown in the Kaplan-Meier cumulative survival plot in Fig. 4A. Although there was a trend toward longer survival of animals immunized with MVA-GP, the difference in cumulative survival between this group of animals and the control group did not reach statistical significance. Since our analysis of memory CD4+ T cells showed that vaccination was associated with preservation of these subsets, we evaluated whether the absolute numbers of memory CD4+ T cells or the percentage of this subset remaining at 20 weeks postchallenge correlated with survival of these animals. Animals were divided into three groups based on survival: those surviving less than 1 year, those surviving 1 to 2 years, and those surviving longer than 2 years. As shown in Fig. 4B, survival for 1 to 2 years or >2 years was associated with significant preservation of memory CD4+ T cells compared to survival of <1 year. This difference was observed when comparing the absolute numbers of memory CD4+ T cells at set point (left panel) or the percentage of remaining cells normalized to prechallenge numbers (right panel). There was no significant difference in terms of preservation of CD4+ T cells between groups of monkeys surviving 1 to 2 years and those surviving more than 2 years. However, a significant correlation was observed between survival and the percentage of memory CD4+ T cells remaining at 4 or 20 weeks postchallenge, with most of the long-term-surviving macaques coming from groups vaccinated with MVA-E and MVA-GPE recombinants expressing SIVsmH4 Env (Fig. 4C).
FIG. 4.
Improved survival rates for macaques vaccinated with MVA-SIV recombinants are associated with preservation of CD4+ central memory T cells. (A) Kaplan-Meier plot of cumulative survival indicates significant differences between the control group and groups immunized with MVA-SIV recombinants expressing SIV env. The comparison of survival in these groups of monkeys was done using a Gehan-Wilcoxon test. P values for groups of vaccinated macaques compared to the control group are indicated. (B) The absolute number of memory CD4+ T cells (left) or percentage of prechallenge memory CD4+ cells remaining (right) at 20 weeks postchallenge is shown for animals that survived less than 1 year, between 1 and 2 years, or more than 2 years. Significant preservation of memory CD4+ T cells was observed in animals that survived 1 to 2 years or longer. (C) Scatter plots of the correlation between the percentage of central memory CD4+ T cells left in peripheral blood at week 4 (left) or week 20 (right) post-SIVsm infection and survival (weeks) of control and vaccinated macaques. Monkeys immunized with MVA-SIV recombinants expressing SIV env (MVA-env or MVA-gag-pol-env) are shown by solid diamonds, and open diamonds indicate animals immunized with MVA-GP or control MVA. P values for the Spearman rank correlation test are indicated.
NAb responses before and shortly after challenge correlated with preservation of memory CD4+ T cells.
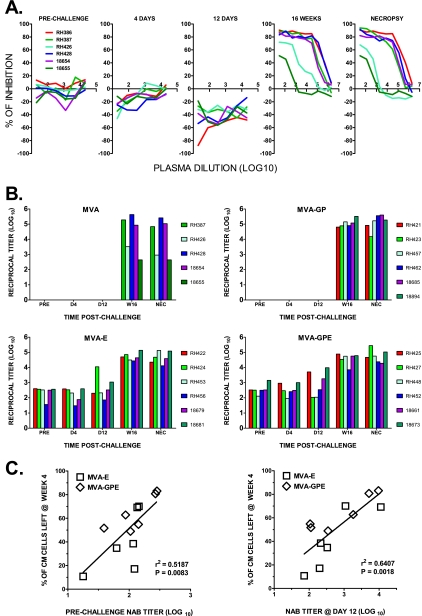
Animals immunized with MVA recombinants that included SIV Env showed significantly improved survival, consistent with a role for immune responses to this immunogen in protection. We previously evaluated NAb responses prior to and following challenge using a cell-killing assay (73). Based on this assay, vaccination induced a type-specific NAb response effective only against the vaccine virus, SIVsmH4; antibody that neutralized the challenge virus was observed only by 12 weeks postchallenge (73). However, higher-throughput and more-sensitive assays have been developed since our previous report (86). We therefore evaluated the titers of NAb to the challenge virus SIVsmE660 by using the TZM-bl cell-based luciferase reporter assay and the actual challenge virus generated in pig-tailed macaque PBMC as a target for neutralization. Figure 5A shows representative titration curves of plasma samples collected sequentially from macaques in the control group. As expected, no neutralization was detected in samples collected prechallenge or at 4 or 12 days postchallenge but a range of neutralization activity was detected by 16 weeks and at necropsy. Different individual plasmas showed a variable degree of test virus infectivity augmentation during primary infection (i.e., 4 and 12 days) as shown by a shift of the titration curves to less than 0%. This may be explained by the presence of significant amounts of inactivated challenge virus in these samples. To minimize these nonspecific effects, the ID50s were determined after nonlinear regression of the dose-response curve by fitting the model equation normalized to the top and bottom curve plateaus obtained for each plasma sample. A bar graph in Fig. 5B shows the NAb titers for plasma samples collected from all four groups of macaques prechallenge and at day 4 (early postchallenge), day 12 (peak viremia), week 16 postchallenge (set point), and the day of necropsy. NAb were not detected in prechallenge, day 4, and day 12 plasma samples from the macaques immunized with MVA or MVA-GP. However, high titers of antibodies were observed in the majority of animals by the set point of infection at week 16, regardless of the immunogen used for vaccination. The titers of NAb achieved by set point positively correlated with levels of viremia at the peak of infection, consistent with a role of the extent of antigenic exposure in driving antibody responses. Presumably the antigenic evolution of virus infecting these animals outstrips the development of new NAb specificities, as observed in HIV infection (86). Titers were significantly reduced in two control animals, H426 and 18655, which progressed rapidly to disease. In contrast, NAb were detected in all prechallenge plasma samples from animals immunized with MVA recombinant vaccines expressing SIVsmH4 Env with titers increasing exponentially by week 16 postchallenge. To evaluate the role of NAb induced by vaccination with MVA-E and MVA-GPE in protection from SIVsmE660 challenge, we examined whether ID50 titers correlated with the degree of central memory CD4+ T-cell preservation. As shown in Fig. 5C, a statistically significant inverse correlation was observed between the percentage of memory CD4+ T lymphocytes remaining at week 4 and NAb titers prechallenge in animals immunized with Env recombinants (left; P = 0.0083), titers at day 4 (not shown), or titers at day 12 (right; P = 0.0018). Thus, preexisting NAb titers were predictive of the degree of subsequent preservation of central memory CD4+ T cells.
FIG. 5.
Prechallenge and early postchallenge levels of SIVsmE660-NAb in plasma of vaccinated macaques correlate with the degree of central memory CD4+ T-cell preservation in peripheral blood. (A) Titration curves of percent inhibition of virus infectivity by plasma samples of each macaque of the control group are shown for plasma samples collected prechallenge; at 4 days, 12 days, and 16 weeks postchallenge; and at necropsy. No neutralizing activity was detected until 16 weeks, with two animals (18655 and H426) showing very low titers of neutralizing activity consistent with rapid disease progression. (B) Bar graphs of reciprocal titers of NAb (log10) to the challenge virus SIVsmE660 prechallenge; at day 4, day 12, and week 16 postchallenge; and at the time of necropsy in each vaccinated group. Titers of NAb are presented for each of the six monkeys per immunization group. Data are presented as the ID50 neutralization titers determined by nonlinear regression of obtained virus infectivity inhibition curve as described in Materials and Methods. The assay limit of detection, which was the lowest reciprocal plasma dilution tested, was an ID50 titer of >20. (C) Scatter plots of the correlations between percentage of central memory CD4+ T cells remaining in peripheral blood at week 4 post-SIVsmE660 infection and log of SIVsmE660-NAb titers in plasma from macaques immunized with MVA-SIV recombinants expressing SIV env (MVA-env or MVA-gag-pol-env). Data for prechallenge and day 12 postchallenge plasma samples are shown. P values for the Spearman rank correlation test are indicated.
DISCUSSION
In this study, long-term follow-up of macaques immunized with recombinant MVA vaccines that expressed either SIV Gag-Pol (MVA-GP) or Env (MVA-E), alone or in combination (MVA-GPE), for over 9 years revealed the following: (i) significant correlations between virus load, survival, and preservation of blood memory CD4+ T cells; (ii) significantly improved survival in animals immunized with Env recombinants compared with either control macaques or macaques immunized with Gag-Pol alone; and (iii) correlation of prechallenge NAb responses with better outcome. These results suggest that despite the failure to achieve sterilizing protection, suboptimal NAb appeared to play a significant role in disease amelioration.
In the past decade, immunopathological studies have shown that the high viral replication in the course of primary infection in SIV-infected macaques, and HIV-infected humans, efficiently targets and destroys most of the body's CD4+ memory T cells, especially CCR5+ CD4+ effector memory T cells in mucosal and peripheral tissues (11, 26, 28, 46, 56, 58, 79, 80, 99). This acute destruction, together with a state of persistent immune activation, has been implicated in the subsequent immune failure and development of immunodeficiency (11, 26, 27, 56, 72, 79, 82, 98). These studies suggest that the initial virus-mediated depletion and the immune activation-mediated apoptotic death of CD4+ effector memory T cells at extralymphoid sites make this population highly dependent on continuous replenishment. Since replenishing cells are derived from the proliferation and differentiation of the CCR5− CD4+ central memory T-cell subset, preservation of CD4+ central memory cell homeostasis may play a key role in maintaining immune competence and delaying the onset of AIDS in SIV-infected macaques (72, 79, 80). These observations are supported by a positive correlation between the numbers of circulating CD4+ central memory T cells and long-term survival in vaccinated macaques challenged with SIVmac that partially control viremia (40, 45, 50, 55, 57). The degree of protection of blood memory CD4+ T cells observed in our MVA-SIV vaccinees was similar to that previously reported for studies of regimens incorporating DNA priming with recombinant adenovirus boosting and SIVmac251 challenge (45). In our study, preservation clearly correlated with reductions in plasma viral load at the primary peak of infection. Most of the animals sustaining lower peak plasma viral loads also exhibited lower levels of CD4+ central memory T-cell loss measured at 4 and 20 weeks after challenge. These results can be explained by greater direct destruction of the CD4+ memory compartment during the acute stage of infection in animals with higher peak viremia. Thus, during primary infection, macaques from the control group lost more than 85% of their prechallenge central memory CD4+ T cells, compared with a 40 to 60% loss in vaccinated animals; this preservation was associated with efficient expansion and restoration of effector memory CD4+ T cells to prechallenge levels. The association of levels of plasma viremia with survival benefits and the preservation of CD4+ memory T-cell compartments in vaccinated animals became even more obvious when we compared macaques that managed only partial control of viremia with those showing substantial control of virus replication (i.e., set point plasma viral loads of <104 copies per ml). Early preservation of central memory CD4+ T cells and expansion of virus-specific secondary immune responses may have a significant impact on the outcome of the disease course. Indeed, at the set point of infection, vaccinated macaques that survived more than 2 years after challenge with highly pathogenic SIVsmE660 showed preservation of more than 80% of the prechallenge level of central memory CD4+ T cells. In contrast, monkeys from the control group that survived less than 1 year had less than 10% of central memory CD4+ T cells remaining. Moreover, survival was significantly correlated with degree of central memory CD4+ T-cell preservation after primary peak and at set point of infection for all animals. Better central memory CD4+ T-cell preservation was associated with better long-term survival in the groups vaccinated with MVA recombinants expressing SIV Env.
Consistent with growing evidence of the important role of CTL in protection from HIV and SIV infections (41, 42, 90), the mechanism of protection of viral vectors such as MVA and recombinant adenovirus type 5 has been presumed to be due to cell-mediated immunity. Indeed, evaluation of cellular immune responses in DNA/recombinant adenovirus type 5-immunized macaques showed significant correlations with cellular immune responses as measured by ELISPOT and intracellular cytokine assays (45). The challenge virus used in this latter study, SIVmac251, is difficult to neutralize in vitro, and as expected, NAb responses were not predictive of the degree of protection of memory CD4+ T cells. In our initial report on MVA-SIV, we suggested that the control of viremia observed in vaccinated macaques during the acute phase after challenge with SIVsmE660 was mediated by cellular immune responses (74). This mechanism was suggested based upon our inability to detect NAb to the challenge virus prior to challenge and a parallel study of CTL responses in MVA-immunized rhesus macaques expressing the MHC-I allele Mamu-A*01 showing that Gag-specific CTL responses correlated with viremia reduction (94). Cellular immune responses were not reevaluated in this present follow-up study. However, it is clear based on the parallel study that MVA immunization induced robust Gag-specific CTL in rhesus macaques following immunization with the same MVA-GP recombinant virus as that used in the present study (93). Following SIV challenge, we observed a reduction in plasma viremia in MVA-GP vaccinees compared with macaques immunized with nonrecombinant MVA that was correlated with the magnitude of the vaccine-elicited Gag-specific CTL response prior to SIV challenge (94). In the present study, protection was also observed in macaques immunized with MVA-GP recombinant expressing only Gag-Pol, where NAb could play no role in protection. However, we observed significantly better survival in macaques that were immunized with MVA recombinants that contained envelope (MVA-E and MVA-GPE). Envelope glycoprotein immunization could also generate protection through generation of cellular immune responses as well as antibody responses. At least eight Env epitopes are highly conserved among SIVsm and SIVmac viruses (49, 102). One potential explanation that we did not explore, due to limited cryopreserved PBMC samples, was the induction of Env-specific CTL responses.
In the current study we evaluated the role of antibodies in observed protection from SIV-induced memory CD4+ T-cell loss. Protective antibody responses documented in other vaccine studies include classical NAb, antibody-dependent cellular cytotoxicity, or antibody-mediated and antibody-dependent cell-mediated virus inhibition (17, 25). Non-NAb that bind defective Env glycoprotein spikes (64) could contribute to the control of viral replication through complement opsonization or Fc receptor-mediated functions (63), particularly in the presence of natural killer cells (18-21). In the present study, we focused on NAb responses. Reestimation of antibody-mediated neutralization using the TZM-bl assay revealed detectable neutralizing activity in prechallenge plasma samples from all macaques vaccinated with recombinants expressing SIV Env. Moderate titers of NAb were also readily detectable in these monkeys shortly after challenge (day 4), with some increase by the peak of primary viremia (day 12) and an exponential increase by week 16 (set point of infection). In contrast, groups of animals vaccinated with MVA or MVA-GP showed no detectable NAb responses until the set point of infection. Significantly reduced titers detected in two macaques from the control group were associated with rapid onset of immunodeficiency.
Most importantly, prechallenge titers of NAb revealed in this assay correlated with the extent of subsequent preservation of memory CD4+ T cells, consistent with a functional role in modifying the disease course. Similar effects of Env-specific antibody responses in the long-term control of viral replication have been documented previously. Passive transfer of high-titer SIV-specific immunoglobulin or neutralizing anti-HIV antibodies can alter the courses of SIV and SHIV infections (4, 29, 30, 51, 52, 69, 76). For example, early postinfection treatment with polyclonal immune globulin with high neutralizing titers against SIV may significantly accelerate de novo production of NAb in SIV-specific immunoglobulin-treated macaques (29). In addition, de novo antiviral antibody responses were also critical for maintaining the stable set point viral replication levels after intravaginal SIVmac239 inoculation of chronically CD20+ cell-depleted macaques (61). Even the relatively modest anti-SIV IgG antibody responses in B-cell-depleted monkeys were sufficient to prevent uncontrolled viral replication such as that seen in the short-term survivor animals that failed to mount antibody responses. Finally, passive immunization at day 7 after SIVmac239 challenge of rhesus macaques (preceding the peak of viral replication) resulted in significant sustained suppression of SIV replication (107, 108). In addition to passive immunization studies, the role of NAb in protection against homologous SIV or SHIV challenge viruses has also been demonstrated in macaques immunized either with Env protein-based vaccines or using various prime-boost vaccination strategies with recombinant DNA and viral vectors (1-3, 14, 22, 55, 84, 100, 106). A recent report also showed a strong correlation between NAb titers and protection against mucosal challenge with pathogenic CCR5-tropic SHIVSF162p4 in macaques immunized with replicating host range mutants of adenovirus expressing the HIV-189.6p env gene following by boosting with HIV-1SF162p4 Env recombinant protein (9). Similarly, immunization with an HIV-1SF162 envelope protein vaccine conferred protection from intravaginal challenge with the closely related SHIVSF162p4. Prechallenge serum NAb titers against the challenge virus appeared to correlate with protection (5).
Results of the present study suggest that NAb, present at the initial stage of infection, may play a critical role in protection of SIV-specific memory CD4+ cells, perhaps by transiently containing viremia. We demonstrated a significant correlation of prechallenge and early postchallenge titers of NAb in MVA-E- and MVA-GPE-immunized monkeys with the degree of central memory CD4+ lymphocyte protection during the acute phase of infection. In conjunction with other studies of the role of NAb in control of SIV infection (29, 30, 61, 70, 107), our results underline that an early antiviral antibody response is required to limit virus replication and preserve CD4+ memory cells during the acute phase of infection.
In the absence of a vaccine providing sterilizing immunity, the long-term control of established chronic SIV infection and disease amelioration may be maintained in the presence and interplay of both cellular and humoral immune antiviral effector mechanisms. The ability of HIV vaccine to provide an optimal balanced combination of cellular and humoral immune responses capable of protecting the CD4+ central memory cell during the early phase of infection and of slowing down the destruction of this key compartment of immune system in chronic infection may significantly increase AIDS-free survival. Nonetheless, despite sustained suppression of viremia in some animals and the accompanying preservation of CD4+ T cells, the majority of the immunized animals eventually progressed to AIDS. Thus, while these data are encouraging, it is clear that regimens such as these that primarily generate cellular immune responses are inadequate and the field should strive to develop strategies capable of generating robust cellular as well as humoral immune responses.
Acknowledgments
We thank Russ Byrum for excellent care of the study animals and David Montefiori and John Mascola for helpful discussions on the development of NAb assays.
This work was supported by the intramural research program of NIAID, NIH.
Footnotes
Published ahead of print on 25 March 2009.
REFERENCES
- 1.Amara, R. R., C. Ibegbu, F. Villinger, D. C. Montefiori, S. Sharma, P. Nigam, Y. Xu, H. M. McClure, and H. L. Robinson. 2005. Studies using a viral challenge and CD8 T cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 343246-255. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 766138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6200-206. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22339-348. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290486-492. [DOI] [PubMed] [Google Scholar]
- 7.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 724170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertozzi, S., J. P. Gutierrez, M. Opuni, N. Walker, and B. Schwartlander. 2004. Estimating resource needs for HIV/AIDS health care services in low-income and middle-income countries. Health Policy 69189-200. [DOI] [PubMed] [Google Scholar]
- 9.Bogers, W. M., D. Davis, I. Baak, E. Kan, S. Hofman, Y. Sun, D. Mortier, Y. Lian, H. Oostermeijer, Z. Fagrouch, R. Dubbes, M. van der Maas, P. Mooij, G. Koopman, E. Verschoor, J. P. Langedijk, J. Zhao, E. Brocca-Cofano, M. Robert-Guroff, I. Srivastava, S. Barnett, and J. L. Heeney. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenchley, J. M., D. A. Price, and D. C. Douek. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7235-239. [DOI] [PubMed] [Google Scholar]
- 12.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 231094-1097. [DOI] [PubMed] [Google Scholar]
- 14.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162DeltaV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 751547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., N. Cooper, L. S. Wyatt, M. W. Carroll, O. Elroy-Stein, and B. Moss. 1998. Expression of proteins in mammalian cells using vaccinia viral vectors, p. 16.15.1-16.19.11. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 16.Emini, E. A., and W. C. Koff. 2004. AIDS/HIV. Developing an AIDS vaccine: need, uncertainty, hope. Science 3041913-1914. [DOI] [PubMed] [Google Scholar]
- 17.Florese, R. H., K. K. Van Rompay, K. Aldrich, D. N. Forthal, G. Landucci, M. Mahalanabis, N. Haigwood, D. Venzon, V. S. Kalyanaraman, M. L. Marthas, and M. Robert-Guroff. 2006. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J. Immunol. 1774028-4036. [DOI] [PubMed] [Google Scholar]
- 18.Forthal, D. N., P. B. Gilbert, G. Landucci, and T. Phan. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J. Immunol. 1786596-6603. [DOI] [PubMed] [Google Scholar]
- 19.Forthal, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 756953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forthal, D. N., G. Landucci, R. Haubrich, B. Keenan, B. D. Kuppermann, J. G. Tilles, and J. Kaplan. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 1801338-1341. [DOI] [PubMed] [Google Scholar]
- 21.Forthal, D. N., G. Landucci, T. B. Phan, and J. Becerra. 2005. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J. Virol. 792042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchini, G., J. Nacsa, Z. Hel, and E. Tryniszewska. 2002. Immune intervention strategies for HIV-1 infection of humans in the SIV macaque model. Vaccine 20(Suppl. 4)A52-A60. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich, T. C., L. E. Valentine, L. J. Yant, E. G. Rakasz, S. M. Piaskowski, J. R. Furlott, K. L. Weisgrau, B. Burwitz, G. E. May, E. J. Leon, T. Soma, G. Napoe, S. V. Capuano III, N. A. Wilson, and D. I. Watkins. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 813465-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein, S., W. R. Elkins, W. T. London, A. Hahn, R. Goeken, J. E. Martin, and V. M. Hirsch. 1994. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J. Med. Primatol. 2375-82. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 1742185-2189. [DOI] [PubMed] [Google Scholar]
- 26.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12289-295. [DOI] [PubMed] [Google Scholar]
- 27.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8319-323. [DOI] [PubMed] [Google Scholar]
- 28.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 29.Haigwood, N. L., D. C. Montefiori, W. F. Sutton, J. McClure, A. J. Watson, G. Voss, V. M. Hirsch, B. A. Richardson, N. L. Letvin, S. L. Hu, and P. R. Johnson. 2004. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 785983-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S. L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51107-114. [DOI] [PubMed] [Google Scholar]
- 31.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 1694778-4787. [DOI] [PubMed] [Google Scholar]
- 32.Hel, Z., J. Nacsa, W. P. Tsai, A. Thornton, L. Giuliani, J. Tartaglia, and G. Franchini. 2002. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology 304125-134. [DOI] [PubMed] [Google Scholar]
- 33.Hel, Z., W. P. Tsai, E. Tryniszewska, J. Nacsa, P. D. Markham, M. G. Lewis, G. N. Pavlakis, B. K. Felber, J. Tartaglia, and G. Franchini. 2006. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J. Immunol. 17685-96. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch, V. M., and P. R. Johnson. 1994. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 32183-203. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch, V. M., and J. D. Lifson. 2000. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv. Pharmacol. 49437-477. [DOI] [PubMed] [Google Scholar]
- 36.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 767187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaizu, M., G. J. Borchardt, C. E. Glidden, D. L. Fisk, J. T. Loffredo, D. I. Watkins, and W. M. Rehrauer. 2007. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics 59693-703. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson Hedestam, G. B., R. A. Fouchier, S. Phogat, D. R. Burton, J. Sodroski, and R. T. Wyatt. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6143-155. [DOI] [PubMed] [Google Scholar]
- 40.Kawada, M., T. Tsukamoto, H. Yamamoto, A. Takeda, H. Igarashi, D. I. Watkins, and T. Matano. 2007. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J. Virol. 815202-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1625127-5133. [PubMed] [Google Scholar]
- 43.Lafont, B. A., C. M. McGraw, S. A. Stukes, A. Buckler-White, R. J. Plishka, R. A. Byrum, V. M. Hirsch, and M. A. Martin. 2007. The locus encoding an oligomorphic family of MHC-A alleles (Mane-A*06/Mamu-A*05) is present at high frequency in several macaque species. Immunogenetics 59211-223. [DOI] [PubMed] [Google Scholar]
- 44.Letvin, N. L. 2007. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity 27366-369. [DOI] [PubMed] [Google Scholar]
- 45.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 3121530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 47.Lobashevsky, A. L., and J. M. Thomas. 2000. Six mamu-A locus alleles defined by a polymerase chain reaction sequence specific primer method. Hum. Immunol. 611013-1020. [DOI] [PubMed] [Google Scholar]
- 48.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 818827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loffredo, J. T., L. E. Valentine, and D. I. Watkins. 2007. Beyond Mamu-A*01+ Indian rhesus macaques: continued discovery of new MHC class I molecules that bind epitopes from the simian AIDS viruses, p. 29-51. In B. T. M. Korber, C. Brander, B. F. Haynes, R. Koup, J. P. Moore, B. D. Walker, and D. I. Watkins (ed.), HIV molecular immunology 2006/2007. Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, NM.
- 50.Mao, H., B. A. Lafont, T. Igarashi, Y. Nishimura, C. Brown, V. Hirsch, A. Buckler-White, R. Sadjadpour, and M. A. Martin. 2005. CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype. J. Virol. 7914887-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 734009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 53.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 7511891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2031533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 57.Mattapallil, J. J., B. Hill, D. C. Douek, and M. Roederer. 2006. Systemic vaccination prevents the total destruction of mucosal CD4 T cells during acute SIV challenge. J. Med. Primatol. 35217-224. [DOI] [PubMed] [Google Scholar]
- 58.Mattapallil, J. J., N. L. Letvin, and M. Roederer. 2004. T-cell dynamics during acute SIV infection. AIDS 1813-23. [DOI] [PubMed] [Google Scholar]
- 59.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24227-255. [DOI] [PubMed] [Google Scholar]
- 60.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 721031-1038. [DOI] [PubMed] [Google Scholar]
- 61.Miller, C. J., M. Genesca, K. Abel, D. Montefiori, D. Forthal, K. Bost, J. Li, D. Favre, and J. M. McCune. 2007. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J. Virol. 815024-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays p. 12.11.1-12.11.15. In A. M. Kruisbeek, J. E. Coligan, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 63.Montefiori, D. C. 1997. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin. Immunopathol. 18371-390. [DOI] [PubMed] [Google Scholar]
- 64.Moore, P. L., E. T. Crooks, L. Porter, P. Zhu, C. S. Cayanan, H. Grise, P. Corcoran, M. B. Zwick, M. Franti, L. Morris, K. H. Roux, D. R. Burton, and J. M. Binley. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 802515-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nathanson, N., and B. J. Mathieson. 2005. Development of an AIDS vaccine: a daunting epidemiological challenge. Eur. J. Epidemiol. 20123-126. [DOI] [PubMed] [Google Scholar]
- 66.Nature. 2007. HIV vaccine failure prompts Merck to halt trial. Nature 449390. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura, Y., T. Igarashi, A. Buckler-White, C. Buckler, H. Imamichi, R. M. Goeken, W. R. Lee, B. A. Lafont, R. Byrum, H. C. Lane, V. M. Hirsch, and M. A. Martin. 2007. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in simian immunodeficiency virus-infected macaques. J. Virol. 81893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 10112324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 762123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura, Y., T. Igarashi, N. L. Haigwood, R. Sadjadpour, O. K. Donau, C. Buckler, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA 10015131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 2792103-2106. [DOI] [PubMed] [Google Scholar]
- 72.Okoye, A., M. Meier-Schellersheim, J. M. Brenchley, S. I. Hagen, J. M. Walker, M. Rohankhedkar, R. Lum, J. B. Edgar, S. L. Planer, A. Legasse, A. W. Sylwester, M. Piatak, Jr., J. D. Lifson, V. C. Maino, D. L. Sodora, D. C. Douek, M. K. Axthelm, Z. Grossman, and L. J. Picker. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 2042171-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ourmanov, I., M. Bilska, V. M. Hirsch, and D. C. Montefiori. 2000. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 742960-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 742740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 76.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piatak, M., Jr., K. C. Luk, B. Williams, and J. D. Lifson. 1993. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques 1470-81. [PubMed] [Google Scholar]
- 78.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 2591749-1754. [DOI] [PubMed] [Google Scholar]
- 79.Picker, L. J. 2006. Immunopathogenesis of acute AIDS virus infection. Curr. Opin. Immunol. 18399-405. [DOI] [PubMed] [Google Scholar]
- 80.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2001299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Picker, L. J., and D. I. Watkins. 2005. HIV pathogenesis: the first cut is the deepest. Nat. Immunol. 6430-432. [DOI] [PubMed] [Google Scholar]
- 82.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 83.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 722855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polacino, P. S., V. Stallard, J. E. Klaniecki, S. Pennathur, D. C. Montefiori, A. J. Langlois, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S. L. Hu. 1999. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in macaques. J. Virol. 738201-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reimann, K. A., M. Chernoff, C. L. Wilkening, C. E. Nickerson, A. L. Landay, and The ACTG Immunology Advanced Technology Laboratories. 2000. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. Clin. Diagn. Lab. Immunol. 7352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson, H. L., and R. R. Amara. 2005. T cell vaccines for microbial infections. Nat. Med. 11S25-S32. [DOI] [PubMed] [Google Scholar]
- 88.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106539-549. [DOI] [PubMed] [Google Scholar]
- 89.Schmid, I., J. Ferbas, C. H. Uittenbogaart, and J. V. Giorgi. 1999. Flow cytometric analysis of live cell proliferation and phenotype in populations with low viability. Cytometry 3564-74. [DOI] [PubMed] [Google Scholar]
- 90.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 91.Schwartlander, B., J. Stover, N. Walker, L. Bollinger, J. P. Gutierrez, W. McGreevey, M. Opuni, S. Forsythe, L. Kumaranayake, C. Watts, and S. Bertozzi. 2001. AIDS.Resource needs for HIV/AIDS. Science 2922434-2436. [DOI] [PubMed] [Google Scholar]
- 92.Sekaly, R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seth, A., I. Ourmanov, M. J. Kuroda, J. E. Schmitz, M. W. Carroll, L. S. Wyatt, B. Moss, M. A. Forman, V. M. Hirsch, and N. L. Letvin. 1998. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl. Acad. Sci. USA 9510112-10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 742502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 96.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 8910847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thorner, A. R., and D. H. Barouch. 2007. HIV-1 vaccine development: progress and prospects. Curr. Infect. Dis. Rep. 971-75. [DOI] [PubMed] [Google Scholar]
- 98.Vaccari, M., A. Boasso, Z. M. Ma, V. Cecchinato, D. Venzon, M. N. Doster, W. P. Tsai, G. M. Shearer, D. Fuchs, B. K. Felber, G. N. Pavlakis, C. J. Miller, and G. Franchini. 2008. CD+ T-cell loss and delayed expression of modulators of immune responses at mucosal sites of vaccinated macaques following SIVmac251 infection. Mucosal Immunol. 1497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 100.Verschoor, E. J., P. Mooij, H. Oostermeijer, M. van der Kolk, P. ten Haaft, B. Verstrepen, Y. Sun, B. Morein, L. Akerblom, D. H. Fuller, S. W. Barnett, and J. L. Heeney. 1999. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in rhesus macaques: evidence for viral clearance. J. Virol. 733292-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vingerhoets, J., G. Vanham, L. Kestens, and P. Gigase. 1995. A convenient and economical freezing procedure for mononuclear cells. Cryobiology 32105-108. [DOI] [PubMed] [Google Scholar]
- 102.Watkins, D. I. 2008. The hope for an HIV vaccine based on induction of CD8+ T lymphocytes—a review. Mem. Inst. Oswaldo Cruz 103119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watkins, D. I., D. R. Burton, E. G. Kallas, J. P. Moore, and W. C. Koff. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 106.Xu, R., I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos. 2006. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res. Hum. Retrovir. 221022-1030. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto, H., M. Kawada, A. Takeda, H. Igarashi, and T. Matano. 2007. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE 2e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto, H., and T. Matano. 2008. Anti-HIV adaptive immunity: determinants for viral persistence. Rev. Med. Virol. 18293-303. [DOI] [PubMed] [Google Scholar]