Abstract
Rapid depletion of memory CD4+ T cells and delayed induction of neutralizing antibody (NAb) responses are characteristics of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections. Although it was speculated that postinfection NAb induction could have only a limited suppressive effect on primary HIV replication, a recent study has shown that a single passive NAb immunization of rhesus macaques 1 week after SIV challenge can result in reduction of viral loads at the set point, indicating a possible contribution of postinfection NAb responses to virus control. However, the mechanism accounting for this NAb-triggered SIV control has remained unclear. Here, we report rapid induction of virus-specific polyfunctional T-cell responses after the passive NAb immunization postinfection. Analysis of SIV Gag-specific responses of gamma interferon, tumor necrosis factor alpha, interleukin-2, macrophage inflammatory protein 1β, and CD107a revealed that the polyfunctionality of Gag-specific CD4+ T cells, as defined by the multiplicity of these responses, was markedly elevated in the acute phase in NAb-immunized animals. In the chronic phase, despite the absence of detectable NAbs, virus control was maintained, accompanied by polyfunctional Gag-specific T-cell responses. These results implicate virus-specific polyfunctional CD4+ T-cell responses in this NAb-triggered virus control, suggesting possible synergism between NAbs and T cells for control of HIV/SIV replication.
Virus-specific CD4+ and CD8+ T-cell responses are crucial for the control of pathogenic human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) infections (5, 6, 20, 23, 30, 39, 40). However, CD4+ T cells, especially CCR5+ memory CD4+ T cells, are themselves targets for these viruses, which may be an obstacle to potent virus-specific CD4+ T-cell induction (10, 47, 52). Indeed, HIV-1/SIV infection causes rapid, massive depletion of memory CD4+ T cells (26, 31), and host immune responses fail to contain viral replication and allow persistent chronic infection, although virus-specific CD8+ T-cell responses exert suppressive pressure on viral replication (15).
Recently, the importance of T-cell quality in virus containment has been high-lighted, and T-cell polyfunctionality, which is defined by their multiplicity of antigen-specific cytokine production, has been analyzed as an indicator of T-cell quality (4, 8, 11, 41). However, there has been no evidence indicating an association of polyfunctional T-cell responses in the acute phase with HIV-1/SIV control. Even in the chronic phase, whether polyfunctional CD4+ T-cell responses may be associated with virus control has been unclear, although an inverse correlation between polyfunctional CD8+ T-cell responses and viral loads has been shown in HIV-1-infected individuals (4).
Another characteristic of HIV-1/SIV infections is the absence of potent neutralizing antibody (NAb) induction during the acute phase (7). This is mainly due to the unusually neutralization-resistant nature of the virus, such as masking of target epitopes in viral envelope proteins (24). Whether this lack of effective NAb response contributes to the failure to control the virus, and whether NAb induction in the acute phase can contribute to virus control, remains unclear. Previous studies documenting virus escape from NAb recognition suggested that NAbs can also exert selective pressure on viral replication to a certain extent (38, 45, 49), but it was speculated that postinfection NAb induction could have only a limited suppressive effect on primary HIV-1/SIV replication (34, 37).
By passive NAb immunization of rhesus macaques after SIV challenge, we recently provided evidence indicating that the presence of NAbs during the acute phase can result in SIV control (50). In that study, passive NAb immunization 1 week after SIVmac239 challenge resulted in transient detectable NAb responses followed by reduction in set point viral loads compared to unimmunized macaques. However, the mechanism of this virus control has remained unclear. In the present study, we found rapid appearance of polyfunctional Gag-specific CD4+ T-cell responses after such passive NAb immunization postinfection. These animals maintained virus control for more than 1 year in the absence of detectable plasma NAbs, which was accompanied by potent Gag-specific T-cell responses. These results implicate virus-specific polyfunctional CD4+ T-cell responses in this NAb-triggered primary and long-term SIV control.
MATERIALS AND METHODS
Animal experiments.
We previously showed a reduction in set point viral loads by passive NAb immunization of rhesus macaques (Macaca mulatta) 1 week after SIVmac239 challenge (50). In the present study, we monitored these animals, including one additional NAb-immunized macaque (R06-023), for more than 1 year. Thus, five NAb-immunized rhesus macaques and six unimmunized controls were used in this study. Unimmunized macaque R02-021 was euthanized at week 32 because the animal showed loss of body weight, diarrhea, and general weakness. NAb-immunized macaque R02-020 was euthanized at week 42 because of a limitation on available cage numbers. Major histocompatibility complex class I (MHC-I) haplotypes were determined by reference strand-mediated conformation analysis as described previously (2, 29). A group of rhesus macaques possessing the MHC-I haplotype 90-120-Ia with the potential to efficiently elicit potent Gag-specific CD8+ T-cell responses (21, 29) were not included in this study. All animals were maintained in accordance with the guidelines for animal experiments performed at the National Institute of Infectious Diseases (33).
For passive NAb immunization, immunoglobulin G (IgG) was purified from plasma samples from SIVmac239-infected macaques with SIV-specific NAb responses in the chronic phase, as described previously (50). The SIVmac239-specific NAb titer of this IgG preparation (30 mg/ml) was 1:16 on MT-4 cells. Animals were intravenously infused with 300 mg of IgG 1 week after challenge with 1,000 50% tissue culture infective doses (TCID50) of SIVmac239 (22). Two unimmunized controls, R02-021 and R06-038, received 300 mg of control IgG prepared from noninfected rhesus macaques at week 1. Neutralizing titers were measured as described previously (50). In brief, serial twofold dilutions of heat-inactivated plasma in duplicate were incubated with 10 TCID50 of SIVmac239 for 45 min (5 μl of diluted sample was incubated with 5 μl of virus in each mixture) and added to 5 × 104 MT-4 cells/well in 96-well plates. Progeny virus production in day 12 culture supernatants was examined by enzyme-linked immunosorbent assays for detection of SIV p27 core antigen (Advanced BioScience Laboratories, Inc., Kensington, MD) to determine the 100% neutralizing endpoint. The lower limit of titration was 1:2.
Analysis of polyfunctional Gag-specific T-cell responses.
We analyzed Gag-specific induction of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-2 (IL-2), macrophage inflammatory protein 1β (MIP-1β), and CD107a in CD4+ and CD8+ T cells as described previously (1). Peripheral blood mononuclear cells (PBMCs) were cultured for 6 h in the absence or the presence of 10 μg/ml of a recombinant SIV Gag p55 (Protein Sciences, Meriden, CT) for unstimulated controls or Gag-specific stimulation (12). They were incubated with anti-human CD28 and anti-human CD49d antibodies (5 μg/ml) (BD, Tokyo, Japan) for costimulation and with anti-human CD107a antibody (BD) for immunostaining. Monensin (BD) and brefeldin A (Sigma-Aldrich, Tokyo, Japan) were added to the culture for the final 5 h of stimulation. Then, immunostaining was performed using a CytofixCytoperm kit (BD) and the following monoclonal antibodies: fluorescein isothiocyanate-conjugated anti-human IFN-γ (BD), phycoerythrin (PE)-conjugated anti-human MIP-1β (BD), peridinin chlorophyll protein-conjugated anti-human CD4 (BD), allophycocyanin (APC)-conjugated anti-human IL-2 (BD), PE-Cy7-conjugated anti-human TNF-α (BD), APC-Cy7-conjugated anti-human CD3 (BD), energy-coupled dye-conjugated anti-human CD69 (Beckman Coulter, Tokyo, Japan), biotin-conjugated anti-human CD8 (BD), and anti-human CD107a (BD) conjugated with Pacific Blue using a Zeon mouse IgG1 labeling kit (Invitrogen, Tokyo, Japan). Flow-cytometric 10-color analysis of the induction of the five marker cytokines, IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a, was performed using the FACSaria system (BD); 3 × 105 to 5 × 105 lymphocyte events were analyzed. The data were analyzed using FlowJo (version 8.2; TreeStar Inc., Ashland, OR) and FACSDiva (BD) software. Analysis of polyfunctional phenotypes of T cells was carried out using PESTLE (version 1.5.4) and SPICE (version 4.1.6) programs, which were generously provided by Mario Roederer (National Institutes of Health, Bethesda, MD). Specific T-cell levels were calculated by subtracting nonspecific T-cell frequencies from those after Gag-specific stimulation. Specific T-cell levels of less than 0.01% were considered negative.
Analysis of proliferative Gag-specific CD4+ T-cell responses.
Gag-specific CD4+ T-cell proliferation was assessed by bromodeoxyuridine (BrdU) incorporation as described previously (9). In brief, PBMCs were cultured in the absence or the presence of 10 μg/ml p55 for 6 days for unstimulated controls or Gag-specific stimulation. Then, the cells were incubated for 2 h with 100 ng/ml BrdU and immunostained using the following monoclonal antibodies: peridinin chlorophyll protein-conjugated anti-human CD4, APC-conjugated anti-human CD95 (BD), APC-Cy7-conjugated anti-human CD3, and energy-coupled dye-conjugated anti-human CD28 (Beckman Coulter, Tokyo, Japan) for surface staining and fluorescein isothiocyanate-labeled anti-human BrdU (BD) for intracellular staining. As a positive control, PBMCs were stimulated with 1 μg/ml staphylococcal enterotoxin B for 3 days. Flow-cytometric analysis was performed using the FACSaria system, and the data were analyzed using FlowJo (version 8.2).
In vitro viral suppression assay.
We examined SIVmac239 replication on CD8-depleted PBMCs in the presence of CD8+ cells positively selected from PBMCs as described previously (46). In brief, PBMCs were separated into CD8+ cells and CD8− cells by using Macs CD8 MicroBeads (Miltenyi Biotec, Tokyo, Japan). For preparing target cells, the CD8− cells negatively selected from PBMCs obtained before challenge were infected with SIVmac239 at a multiplicity of infection of 1:104 TCID50/cell and cultured in the presence of 2 μg/ml phytohemagglutinin L (Roche Diagnostics) and 20 IU/ml recombinant human IL-2 (Roche Diagnostics). Two days later, effector CD8+ cells positively selected from PBMCs obtained before challenge or at week 3 or 4 were added to the target cells at an effector/target (E/T) ratio of 1:4. The culture supernatants were harvested every other day. Reverse transcriptase activities in these supernatants were measured to confirm the peak of viral production in the control culture of target cells without CD8+ cells around day 10 after SIV infection. SIV Gag capsid protein p27 concentrations in the supernatants after 8 days of coculture (i.e., at day 10 after SIV infection) were then measured by enzyme-linked immunosorbent assay. Results from macaques R01-011, R03-005, R02-021, and R06-023 were excluded because mean p27 concentrations in the control cultures without CD8+ cells or in one of the duplicates were less than 50 ng/ml. The lower limit of p27 detection was approximately 0.2 ng/ml.
Statistical analysis.
Statistical analysis was performed with Prism software version 4.03 with significance levels set at a P value of <0.050 (GraphPad Software, Inc., San Diego, CA). Plasma viral loads and specific T-cell frequencies were log transformed and compared between unimmunized controls and NAb-immunized macaques by an unpaired two-tailed t test. Correlation was analyzed by the Pearson test.
RESULTS
Long-term SIV control after passive NAb immunization postinfection.
In order to evaluate the long-term effect on SIV replication of a single passive NAb immunization in the acute phase, we monitored animals for more than 1 year after SIVmac239 challenge (Fig. 1). Five NAb-immunized rhesus macaques and six unimmunized controls, including two animals that received control antibodies at week 1, were followed up. Of these, NAb-immunized macaque R03-011 and two unimmunized controls, R01-011 and R06-038, shared the MHC-I haplotype 90-010-Ie, and NAb-immunized R06-023 and unimmunized R01-012 shared 90-010-Id. We previously reported that a group of Burmese rhesus macaques possessing the MHC-I haplotype 90-120-Ia mounted efficient Gag-specific CD8+ T-cell responses and showed vaccine-based SIV control (21, 29), but those animals were not included in the present study.
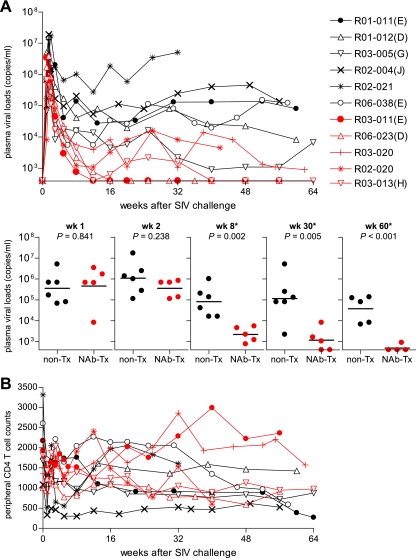
FIG. 1.
Follow up of NAb-immunized macaques. (A) Plasma viral loads (SIV gag RNA copies/ml plasma) in six unimmunized macaques (black lines) and five NAb-immunized animals (red lines) after SIVmac239 challenge. The plasma viral loads were measured as described previously (29). The lower limit of detection was approximately 4 × 102 copies/ml. The MHC-I haplotypes are shown in parentheses following the macaque numbers as follows: E, haplotype 90-010-Ie; D, 90-010-Id; G, 90-030-Ig; J, 90-088-Ij; and H, 90-030-Ih. Below are comparisons of plasma viral loads in unimmunized (non-Tx) and NAb-immunized (NAb-Tx) macaques at weeks (wk) 1, 2, 8, 30, and 60. The bars indicate the geometric mean of each group. The comparisons at weeks 8, 30, and 60 (indicated by asterisks) showed significant differences between two groups (P = 0.841 at week 1, P = 0.238 at week 2, P = 0.002 at week 8, P = 0.005 around week 30, and P < 0.001 around week 60 by t test). (B) Peripheral CD4+ T-cell counts (per μl) in unimmunized controls (black lines) and NAb-immunized macaques (red lines) after SIVmac239 challenge. The ratios of the counts around week 60 to those at week 0 in NAb-immunized macaques were significantly higher than in unimmunized controls (P = 0.028 by t test).
The plasma viral loads of both NAb-immunized and unimmunized macaques were similar at week 1, just before NAb administration (Fig. 1A). At week 2 postchallenge, i.e., 1 week after NAb administration, the geometric mean of plasma viral loads in NAb-immunized macaques was slightly lower than in unimmunized controls, but this difference did not achieve statistical significance. At week 8, however, the difference became significant, with lower plasma viral loads in NAb-immunized animals (Fig. 1A). Thereafter, the NAb-immunized macaques maintained significantly reduced viral loads for more than 1 year. In the chronic phase, plasma viral loads were less than 1 × 104 copies/ml in all five NAb-immunized macaques and were even undetectable in three of them. NAb-immunized macaque R03-011, possessing the MHC-I haplotype 90-010-Ie, contained SIV replication with undetectable viremia, whereas unimmunized macaques R01-011 and R06-038, which shared this haplotype, had high viral loads. The NAb-immunized macaque R06-023, with MHC-I haplotype 90-010-Id, contained SIV replication, whereas unimmunized macaque R01-012, which shared the same haplotype, failed to control viremia. Peripheral CD4+ T-cell counts were maintained in the NAb-immunized macaques during the observation period (Fig. 1B).
We examined SIVmac239-specific neutralizing antibody responses by determining the end point plasma titers for inhibiting 10-TCID50 virus replication on MT-4 cells (data not shown). In NAb-immunized macaques, NAb responses were detected at day 10 postchallenge but became undetectable within 1 week of passive NAb immunization, as described previously (50), implying that the infused NAbs were rapidly exhausted for virus clearance. None of the animals had detectable de novo NAb responses even around week 40 after challenge. In unimmunized controls, SIVmac239-specific NAb responses were also undetectable, except in one animal, R01-012, after week 30. Thus, passive NAb immunization 1 week after SIV challenge resulted in a transient period of NAb detection, followed by sustained virus control in the absence of detectable NAb responses.
Polyfunctional Gag-specific CD4+ T-cell responses in the acute phase in passively NAb-immunized macaques.
To investigate whether virus-specific T-cell responses were involved in this NAb-triggered SIV control, we first analyzed SIV Gag-specific CD4+ T-cell responses in the acute phase. We stimulated PBMCs obtained at week 2 with a recombinant SIV Gag p55 protein and analyzed Gag-specific induction of IFN-γ, TNF-α, IL-2, and MIP-1β and surface mobilization of CD107a (a degranulation marker) in CD4+ T cells (Fig. 2A) (14, 25, 41). The Gag-specific responses of each factor, IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a, in CD4+ T cells did not show significant differences between unimmunized and NAb-immunized animals (data not shown). We then analyzed these five factors to assess the polyfunctionality of virus-specific T cells and refer to them as marker cytokines in this study. No significant differences in the frequencies of total Gag-specific CD4+ T cells (i.e., CD4+ T cells exhibiting Gag-specific induction of one or more of the marker cytokines IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a) were observed between the two groups (Fig. 2B).
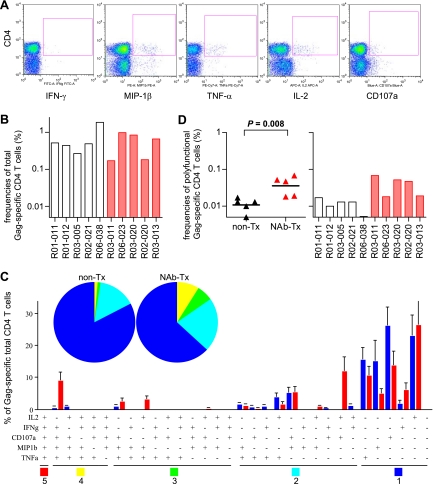
FIG. 2.
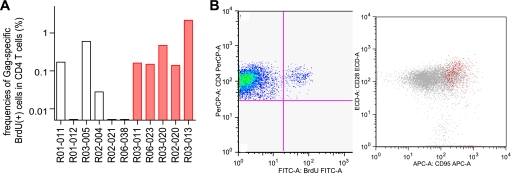
Gag-specific CD4+ T-cell responses in the acute phase. PBMCs at week 2 postchallenge were stimulated with a recombinant SIV Gag p55, and the specific induction of five marker cytokines (IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a) in CD4+ T cells was examined. PBMCs from macaque R02-004 were unavailable and therefore could not be included in this analysis. (A) Representative dot plots showing responses of IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a in CD4+ T cells in macaque R03-013 after Gag-specific stimulation. Gag-specific IFN-γ responses were undetectable at week 2 in this animal. (B) Frequencies of total Gag-specific CD4+ T cells that showed Gag-specific induction of IFN-γ, TNF-α, IL-2, MIP-1β, or CD107a in total CD4+ T cells. (C) Percentages of cells exhibiting Gag-specific induction of single or multiple marker cytokines in total Gag-specific CD4+ T cells. (Top) Mean percentages of Gag-specific CD4+ T cells producing one (dark blue), two (light blue), three (green), four (yellow), or five (red) marker cytokines in unimmunized (non-Tx) and NAb-immunized (NAb-Tx) macaques. (Bottom) Mean percentages of individual Gag-specific CD4+ T-cell subsets divided by the patterns of marker cytokine induction in unimmunized (blue bars) and NAb-immunized (red bars) macaques. (D) Frequencies of polyfunctional Gag-specific CD4+ T cells that showed Gag-specific induction of ≥3 marker cytokines in total CD4+ T cells. On the left, the bars indicate the geometric mean of each group. The frequencies in NAb-immunized macaques (n = 5) were significantly higher than in unimmunized controls (n = 5) (P = 0.008).
We examined the polyfunctionality of SIV Gag-specific CD4+ T cells, as defined by their multiplicity of marker cytokines induced by Gag-specific stimulation (11, 41) (Fig. 2C). The mean percentage of cells producing ≥3 marker cytokines (Fig. 2C, sum of red, yellow, and green) in the Gag-specific CD4+ T-cell pool was more than 15% in NAb-immunized macaques but less than 3% in unimmunized controls. The frequencies of these polyfunctional Gag-specific CD4+ T cells within the CD4+ T-cell pool were significantly higher in the immunized animals, with a solid difference (P = 0.008 by t test) (Fig. 2D). Indeed, all the NAb-immunized macaques had higher frequencies of polyfunctional Gag-specific CD4+ T cells than any of the unimmunized controls, indicating that passive NAb immunization 1 week after SIV challenge resulted in rapid induction of Gag-specific CD4+ T cells with higher polyfunctionality at week 2.
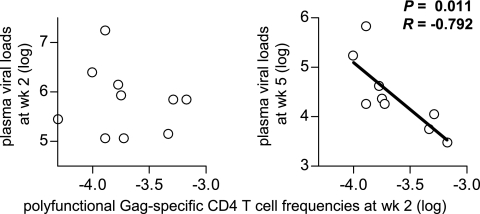
The polyfunctional Gag-specific CD4+ T-cell frequencies at week 2 were inversely correlated with plasma viral loads at week 5 (Fig. 3). The inverse correlation, however, was not indicated with plasma viral loads at week 2. These results implicate rapidly induced polyfunctional Gag-specific CD4+ T-cell responses in subsequent reduction of plasma viral loads in NAb-immunized macaques.
FIG. 3.
Analysis of correlation between polyfunctional Gag-specific CD4+ T-cell frequencies (log) at week (wk) 2 and plasma viral loads (log) at week 2 (left) or week 5 (right). Inverse correlation is shown on the right (P = 0.011; R = −0.792), but not on the left (P = 0.694; R = −0.143).
Polyfunctional Gag-specific CD4+ T-cell responses in the chronic phase in NAb-immunized macaques.
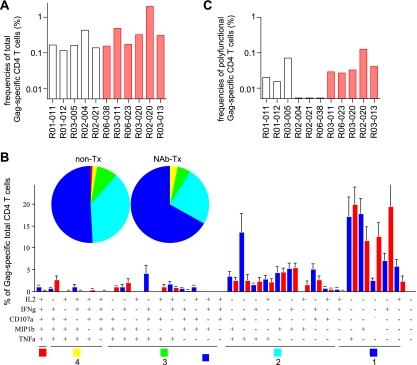
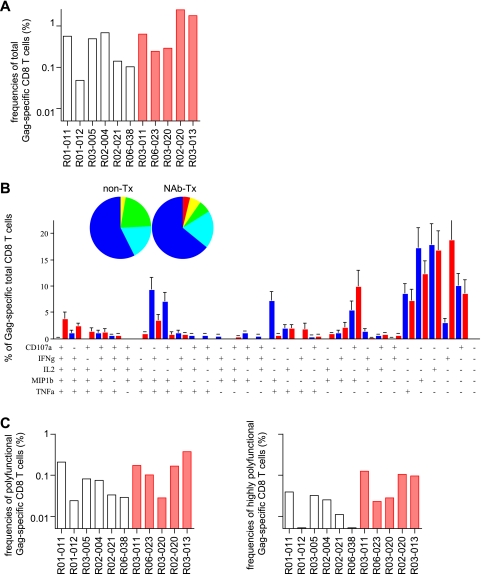
We then examined SIV Gag-specific CD4+ T-cell responses in the chronic phase. Around week 30 after challenge, total Gag-specific CD4+ T-cell frequencies in NAb-immunized animals were similar to or, if anything, higher than those in unimmunized controls (Fig. 4A). The Gag-specific responses of each marker cytokine in CD4+ T cells showed no significant difference between the two groups (data not shown). The polyfunctionalities of these Gag-specific CD4+ T cells (the percentage of cells producing ≥3 marker cytokines) within the total Gag-specific CD4+ T-cell population in both groups were similar (Fig. 4B). However, the frequencies of these polyfunctional Gag-specific CD4+ T cells as a fraction of total CD4+ T cells in NAb-immunized macaques were higher than in unimmunized controls (Fig. 4C).
FIG. 4.
Gag-specific CD4+ T-cell responses in the chronic phase. PBMCs around week 30 postchallenge were stimulated with p55, and specific induction of IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a in CD4+ T cells was examined. (A) Frequencies of total Gag-specific CD4+ T cells. (B) Percentages of cells exhibiting Gag-specific induction of single or multiple marker cytokines in total Gag-specific CD4+ T cells. See the legend to Fig. 2 for symbols. (C) Frequencies of polyfunctional Gag-specific CD4+ T cells exhibiting Gag-specific induction of ≥3 marker cytokines in total CD4+ T cells. The frequencies in NAb-immunized macaques (n = 5) were significantly higher than in unimmunized controls (n = 6) (P = 0.046).
We also examined the SIV Gag-specific proliferative responses of CD4+ T cells around week 30 by measurement of BrdU uptake after Gag-specific stimulation (Fig. 5A). This revealed higher proliferative responses of Gag-specific CD4+ T cells in NAb-immunized macaques than in unimmunized controls. Gag-specific CD4+ T-cell proliferative responses were detectable in all the NAb-immunized macaques but in only three of six unimmunized controls. Most of the BrdU+ CD4+ T cells after Gag-specific stimulation were of the central memory (CD95+ CD28+) phenotype (36) (Fig. 5B). These results suggest that NAb-immunized macaques had potent Gag-specific CD4+ T cells with efficient proliferative ability in the chronic phase.
FIG. 5.
Gag-specific CD4+ T-cell proliferative responses in the chronic phase. PBMCs around week 30 postchallenge were stimulated with p55, and specific uptake of BrdU in CD4+ T cells was examined. (A) Frequencies of Gag-specific BrdU+ CD4+ T cells in total CD4+ T cells. The frequencies in NAb-immunized macaques were significantly higher than in unimmunized controls (P = 0.042). (B) A representative density plot (gated on CD3+ lymphocytes) showing BrdU+ CD4+ T-cell induction after Gag stimulation (macaque R03-013). Most Gag-specific BrdU+ CD4+ T cells gated in the left-hand plot were CD95+ CD28+ (indicated by red) in the right-hand plot gated on CD3+ CD4+ lymphocytes. FITC, fluorescein isothiocyanate; PerCP, peridinin chlorophyll protein.
CD8+ cells with high anti-SIV efficacy in NAb-immunized macaques.
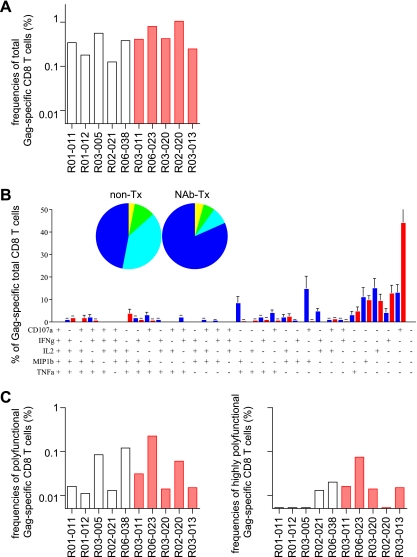
The above-mentioned results revealed higher frequencies of polyfunctional Gag-specific CD4+ T-cell responses in NAb-immunized macaques. We next analyzed Gag-specific CD8+ T-cell responses in the acute phase (Fig. 6). At week 2, total Gag-specific CD8+ T-cell frequencies were similar, and no clear difference in frequencies of Gag-specific CD8+ T cells producing ≥3 or ≥4 marker cytokines was detected between the two groups.
FIG. 6.
Gag-specific CD8+ T-cell responses in the acute phase. PBMCs at week 2 were stimulated with p55, and specific induction of IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a in CD8+ T cells was examined. (A) Frequencies of total Gag-specific CD8+ T cells. (B) Percentages of cells exhibiting Gag-specific induction of single or multiple marker cytokines in total Gag-specific CD8+ T cells. See the legend to Fig. 2 for symbols. (C) Frequencies of Gag-specific CD8+ T cells exhibiting Gag-specific induction of ≥3 marker cytokines (polyfunctional; left) or ≥4 marker cytokines (highly polyfunctional; right) in total CD8+ T cells.
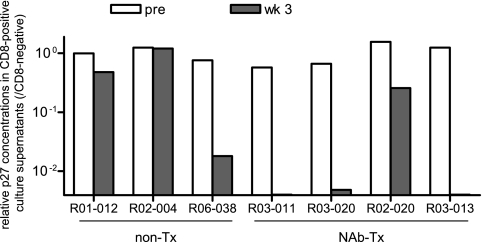
We then examined, by in vitro viral-suppression assays (13, 27, 46, 51), whether the CD8+ cells from these NAb-immunized macaques had the potential to control SIV replication more efficiently than those from the controls (Fig. 7). In this assay, CD8− target cells prepared by CD8-negative selection from PBMCs were infected with SIVmac239 and cocultured with effector CD8+ cells prepared by CD8-positive selection from PBMCs at week 3. We obtained results from four NAb-immunized macaques and three unimmunized controls.
FIG. 7.
Anti-SIV efficacy in vitro of CD8+ cells. PBMC-derived CD8− (target) cells infected with SIVmac239 were cultured alone (no CD8) or cocultured with autologous PBMC-derived CD8+ (effector) cells obtained prechallenge (pre) or at week 3 postchallenge (wk 3) at an E/T ratio of 1:4. The results were obtained from three unimmunized controls and four NAb-immunized macaques. The ratios of p27 concentrations in the culture supernatants after 8 days of coculture with pre-CD8+ or week 3 CD8+ cells to those without CD8+ cells (CD8 negative) are shown. The coculture with either R03-011 week 3 CD8+, R03-020 week 3 CD8+, and R03-013 week 3 CD8+ cells showed undetectable or marginal SIV p27 production after 8 days.
Three of four NAb-immunized macaques (R03-011, R03-020, and R03-013) showed more than 100-fold reduction in viral production at an E/T ratio of 1:4, although the remaining animal (R02-020) failed to show strong anti-SIV efficacy in vitro. Of the NAb-immunized animals, this individual R02-020 maintained the highest viral loads in the chronic phase. In contrast to CD8+ cells from the majority of immunized animals, CD8+ cells from the unimmunized controls (R01-012, R02-004, and R06-038) showed weak anti-SIV efficacy. In fact, the reduction of virus production by CD8+ cells from the unimmunized macaques R01-012 and R06-038 was less than 100-fold even in coculture at an E/T ratio of 1:1 (data not shown; not determined for R02-004). These results suggest that passive NAb immunization may facilitate the induction of potent CD8+ cells possessing higher anti-SIV efficacy.
Polyfunctional Gag-specific CD8+ T-cell responses in the chronic phase in NAb-immunized macaques.
We next examined SIV Gag-specific CD8+ T-cell responses in the chronic phase (Fig. 8). Around week 30 after challenge, the geometric means of total Gag-specific CD8+ T-cell frequencies in NAb-immunized animals were higher than in unimmunized controls, but this difference did not achieve statistical significance. In particular, NAb-immunized macaques showed significantly higher levels of Gag-specific IFN-γ responses in CD8+ T cells (data not shown). There was no clear difference in polyfunctional Gag-specific CD8+ T-cell responses between the two groups. However, highly polyfunctional Gag-specific CD8+ T cells producing ≥4 marker cytokines were detectable in all NAb-immunized macaques, and the frequencies of these highly polyfunctional Gag-specific CD8+ T cells in the total CD8+ T-cell population were higher than in unimmunized controls.
FIG. 8.
Gag-specific CD8+ T-cell responses in the chronic phase. PBMCs around week 30 postchallenge were stimulated with p55, and specific induction of IFN-γ, TNF-α, IL-2, MIP-1β, and CD107a in CD8+ T cells was examined. (A) Frequencies of total Gag-specific CD8+ T cells. (B) Percentages of cells exhibiting Gag-specific induction of single or multiple marker cytokines in total Gag-specific CD8+ T cells. See the legend to Fig. 2 for symbols. (C) Frequencies of Gag-specific CD8+ T cells exhibiting Gag-specific induction of ≥3 marker cytokines (polyfunctional; left) or ≥4 marker cytokines (highly polyfunctional; right) in total CD8+ T cells. The highly polyfunctional Gag-specific CD8+ T-cell frequencies in NAb-immunized macaques were significantly higher than in unimmunized controls (P = 0.023).
DISCUSSION
In our previous study (50), a single passive NAb immunization of rhesus macaques 1 week after SIVmac239 challenge resulted in significant reduction of set point viral loads. The present study has shown that this NAb-triggered virus control was maintained in the absence of detectable NAbs in the chronic phase. Remarkably, virus-specific CD4+ T-cell responses with higher polyfunctionality were rapidly induced in NAb-immunized macaques. These results implicate more potent induction of functional virus-specific CD4+ T-cell responses in this NAb-triggered SIV control.
All the NAb-immunized macaques had higher frequencies of polyfunctional Gag-specific CD4+ T cells than any of the unimmunized controls at week 2, although the two groups possessed similar frequencies of total Gag-specific CD4+ T cells. This implies higher polyfunctionality of Gag-specific CD4+ T cells in the acute phase in NAb-immunized macaques than in unimmunized controls. HIV-1 is known to preferentially infect HIV-1-specific CD4+ T cells (10); virus neutralization may therefore protect virus-specific CD4+ T cells from SIV infection. However, it remains unclear whether NAbs preferentially protect polyfunctional virus-specific CD4+ T cells. Our previous study suggested augmentation of the Fc-mediated uptake of NAb-virion complexes into dendritic cells following passive NAb immunization (50). This may enhance antigen presentation and induction of polyfunctional virus-specific CD4+ T-cell responses in the acute phase. Thus, both NAb-mediated effects, i.e., enhancement of antigen presentation and protection of virus-specific CD4+ T cells from viral infection, may contribute to the induction of polyfunctional virus-specific CD4+ T cells in the acute phase.
It is thought that potent virus-specific CD4+ T-cell responses are important for the control of HIV-1/SIV replication (39). Recent studies analyzing the quality of T-cell responses suggested the possible involvement of polyfunctional CD4+ T-cell responses in the control of some viral infections (8, 11, 41). However, there has been no clear evidence indicating association of polyfunctional CD4+ T-cell responses with HIV-1/SIV control. These cells are themselves targets for viral infection and killing (10), and most natural HIV-1/SIV infections fail to show efficient induction of potent virus-specific CD4+ T-cell responses (52). In the present study, passive NAb immunization of rhesus macaques 1 week after SIV infection resulted in the induction of significantly higher levels of polyfunctional Gag-specific CD4+ T-cell responses in the acute phase, followed by SIV control at the set point in the absence of NAb responses. The polyfunctional Gag-specific CD4+ T-cell frequencies at week 2 were inversely correlated with plasma viral loads, not at week 2, but at week 5. These results indicate that NAbs may facilitate the development and retention of polyfunctional virus-specific CD4+ T-cell responses in the very early phase of HIV-1/SIV infection, contributing to subsequent virus control directly or indirectly. Thus, this is the first report documenting an association between polyfunctional CD4+ T-cell responses in the acute phase and subsequent SIV control.
Previous studies of the chronic phase of HIV-1 infections have indicated an association between strong HIV-1-specific proliferative CD4+ T-cell responses and HIV-1 control, as well as their impairment in HIV-1 infection with uncontrolled viremia (3, 17, 18, 32, 39). In the present study, compared to total Gag-specific CD4+ T-cell frequencies in the acute phase, those in the chronic phase were reduced in unimmunized controls, but NAb-immunized macaques maintained similar frequencies in the chronic phase. This difference may reflect virus control in NAb-immunized macaques and high plasma viremia in unimmunized controls. Our analyses of polyfunctional and proliferative responses suggest that these animals maintained functional Gag-specific CD4+ T-cell responses in the chronic phase. This may be due to virus control and, conversely, may contribute to sustained virus control.
It has been indicated that virus-specific CD4+ T-cell responses facilitate induction of functional virus-specific CD8+ T-cell responses (19, 42, 44). Stimulation with peptides would be optimal for analysis of CD8+ T-cell responses, but in this study, our first priority was to analyze CD4+ T-cell responses, and cell samples were used for the analysis of responses after stimulation with a recombinant Gag p55 protein. Therefore, we obtained results on polyfunctional CD8+ T-cell responses after p55-specific stimulation but did not have enough cell samples for analyzing peptide-specific CD8+ T-cell responses in the acute phase. In the acute phase, no significant enhancement of polyfunctional Gag-specific CD8+ T-cell responses was detected after passive NAb immunization, but this does not exclude the possibility of functional CD8+ T-cell induction in NAb-immunized animals, which may be detected by optimal analysis. Indeed, the viral suppression assay showed that CD8+ cells able to efficiently suppress SIV replication in vitro were induced in the acute phase in those NAb-immunized macaques that contained SIV replication in vivo. These highly effective anti-SIV CD8+ cell responses, which may be affected not only by CD8+ T-cell polyfunctionality, but also by several other factors, are thus likely to be involved in NAb-triggered containment of SIV replication. Our analyses in the chronic phase indicated higher frequencies of highly polyfunctional Gag-specific CD8+ T-cell responses in NAb-immunized macaques, consistent with the previously reported observation in HIV-1-infected nonprogressors (4).
Taken together, the present study indicates that passive NAb immunization of rhesus macaques in the acute phase may be able to trigger rapid induction of polyfunctional Gag-specific CD4+ T-cell responses, followed by sustained SIV control in the absence of NAb responses in the chronic phase. These results highlight the importance of the synergy between NAb and T-cell responses in primary virus control, implying that the absence of potent NAb responses in the acute phase of HIV-1/SIV infection may be responsible for failure to control persistent viral replication.
Finally, induction of potent NAb responses is believed to be a promising strategy for AIDS vaccine development. While prechallenge passive NAb immunization studies have previously indicated the possibility of sterile protection against immunodeficiency virus infection in macaques, several studies have suggested difficulty in inducing high levels of NAb responses that are sufficient for sterile protection (16, 28, 35, 43, 48). Our results imply that prophylactic vaccination that elicits NAb responses, even if it does not achieve sterile protection, may contribute to HIV-1/SIV control by secondary NAb responses facilitating functional T-cell induction after viral exposure. Thus, this study indicates a potential for HIV-1/SIV control by synergy between NAb and T-cell responses, providing insights into the development of a prophylactic AIDS vaccine.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology; a grant from the Japan Health Sciences Foundation; and grants from the Ministry of Health, Labor, and Welfare of Japan.
The animal experiments were conducted through the Cooperative Research Program in Tsukuba Primate Research Center, National Institute of Biomedical Innovation, with the help of the Corporation for Production and Research of Laboratory Primates. We thank H. Akari, F. Ono, K. Komatsuzaki, A. Hiyaoka, H. Ogawa, K. Oto, K. Ishikawa, T. Nakasone, and H. Igarashi for assistance with the animal experiments and M. Miyazawa, M. Yasunami, T. Naruse, and A. Kimura for determining MHC-I haplotypes. We also thank M. Roederer for providing the PESTLE and SPICE software and V. Appay for his technical support and helpful suggestions on analysis of Gag-specific T-cell responses.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arguello, J. R., A. M. Little, E. Bohan, J. M. Goldman, S. G. Marsh, and J. A. Madrigal. 1998. High resolution HLA class I typing by reference strand mediated conformation analysis (RSCA). Tissue Antigens 5257-66. [DOI] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277112-116. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implication for vaccine development. Curr. Opin. Immunol. 11451-459. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 8.Casazza, J. P., M. R. Betts, D. A. Price, M. L. Precopio, L. E. Ruff, J. M. Brenchley, B. J. Hill, M. Roederer, D. C. Douek, and R. A. Koup. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2032865-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combadiere, B., A. Boissonnas, G. Carcelain, E. Lefranc, A. Samri, F. Bricaire, P. Debre, and B. Autran. 2004. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J. Exp. Med. 1991585-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 11.Duvall, M. G., M. L. Precopio, D. A. Ambrozak, A. Jaye, A. J. McMichael, H. C. Whittle, M. Roederer, S. L. Rowland-Jones, and R. A. Koup. 2008. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur. J. Immunol. 38350-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauduin, M. C., A. Kaur, S. Ahmad, T. Yilma, J. D. Lifson, and R. P. Johnson. 2004. Optimization of intracellular cytokine staining for the quantitation of antigen-specific CD4+ T cell responses in rhesus macaques. J. Immunol. Methods 28861-79. [DOI] [PubMed] [Google Scholar]
- 13.Gauduin, M. C., R. L. Glickman, R. Means, and R. P. Johnson. 1998. Inhibition of simian immunodeficiency virus (SIV) replication by CD8+ T lymphocytes from macaques immunized with live attenuated SIV. J. Virol. 726315-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauduin, M. C., Y. Yu, A. Barabasz, A. Carville, M. Piatak, J. D. Lifson, R. C. Desrosiers, and R. P. Johnson. 2006. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J. Exp. Med. 2032661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4630-640. [DOI] [PubMed] [Google Scholar]
- 16.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S. L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51107-114. [DOI] [PubMed] [Google Scholar]
- 17.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103966-972. [DOI] [PubMed] [Google Scholar]
- 18.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 7710900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421852-856. [DOI] [PubMed] [Google Scholar]
- 20.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawada, M., T. Tsukamoto, H. Yamamoto, N. Iwamoto, K. Kurihara, A. Takeda, C. Moriya, H. Takeuchi, H. Akari, and T. Matano. 2008. Gag-specific cytotoxic T lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J. Virol. 8210199-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 23.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420678-682. [DOI] [PubMed] [Google Scholar]
- 25.Lamoreaux, L., M. Roederer, and R. Koup. 2006. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 11507-1516. [DOI] [PubMed] [Google Scholar]
- 26.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 27.Loffredo, J. T., E. G. Rakasz, J. P. Giraldo, S. P. Spencer, K. K. Grafton, S. R. Martin, G. Napoe, L. J. Yant, N. A. Wilson, and D. I. Watkins. 2005. Tat28-35SL8-specific CD8+ T lymphocytes are more effective than Gag181-189CM9-specific CD8+ T lymphocytes at suppressing simian immunodeficiency virus replication in a functional in vitro assay. J. Virol. 7914986-14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 29.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 1991709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. A. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 32.McNeil, A. C., W. L. Shupert, C. A. Iyasere, C. W. Hallahan, J. A. Mican, R. T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 9813878-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute of Infectious Diseases. 2007. Guides for animal experiments performed at National Institute of Infectious Diseases. National Institute of Infectious Diseases, Tokyo, Japan. (In Japanese.)
- 34.Nishimura, Y., T. Igarashi, N. L. Haigwood, R. Sadjadpour, O. K. Donau, C. Buckler, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA 10015131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2004. Development and homeostasis of T cell memory in rhesus macaques. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 37.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10431-438. [DOI] [PubMed] [Google Scholar]
- 38.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 2781447-1450. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 41.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8247-258. [DOI] [PubMed] [Google Scholar]
- 42.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300337-339. [DOI] [PubMed] [Google Scholar]
- 43.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5204-210. [DOI] [PubMed] [Google Scholar]
- 44.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11615-622. [DOI] [PubMed] [Google Scholar]
- 46.Tsukamoto, T., M. Yuasa, H. Yamamoto, M. Kawada, A. Takeda, H. Igarashi, and T. Matano. 2007. Induction of CD8+ cells able to suppress CCR5-tropic simian immunodeficiency virus SIVmac239 replication by controlled infection of CXCR4-tropic simian-human immunodeficiency virus in vaccinated rhesus macaques. J. Virol. 8111640-11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 7457-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9343-346. [DOI] [PubMed] [Google Scholar]
- 49.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto, H., M. Kawada, A. Takeda, H. Igarashi, and T. Matano. 2007. Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE 2e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 713120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaunders, J. J., M. L. Munier, D. E. Kaufmann, S. Ip, P. Grey, D. Smith, T. Ramacciotti, D. Quan, R. Finlayson, J. Kaldor, E. S. Rosenberg, B. D. Walker, D. A. Cooper, and A. D. Kelleher. 2005. Early proliferation of CCR5+ CD38+++ antigen-specific CD4+ Th1 effector cells during primary HIV-1 infection. Blood 1061660-1667. [DOI] [PubMed] [Google Scholar]