Abstract
BK virus (BKV) causes persistent and asymptomatic infections in most humans and is the etiologic agent of polyomavirus-associated nephropathy (PVAN) and other pathologies. Unfortunately, there are no animal models with which to study activation of BKV replication in the human kidney and the accompanying PVAN. Here we report studies of the restriction of BKV replication in murine cells and extracts and the cause(s) of this restriction. Upon infection of murine cells, BKV expressed large T antigen (TAg), but viral DNA replication and progeny were not detected. Transfection of murine cells with BKV TAg expression vectors also caused TAg expression without accompanying DNA replication. Analysis of the replication of DNAs containing chimeric BKV and murine polyomavirus origins revealed the importance of BKV core origin sequences and TAg for DNA replication. A sensitive assay was developed with purified BKV TAg that supported TAg-dependent BKV DNA replication with human but not with murine cell extracts. Addition of human replication proteins, DNA polymerase α-primase, replication protein A, or topoisomerase I to the murine extracts with BKV TAg did not rescue viral DNA replication. Notably, addition of murine extracts to human extracts inhibited BKV TAg-dependent DNA replication at a step prior to or during unwinding of the viral origin. These findings and differences in replication specificity between BKV TAg and the TAgs of simian virus 40 (SV40) and JC virus (JCV) and their respective origins implicate features of the BKV TAg and origin distinct from SV40 and JCV in restriction of BKV replication in murine cells.
Persistent and asymptomatic infections by the human polyomavirus BK virus (BKV) occur in most humans (44) and have been implicated in pulmonary, ophthalmologic, hepatic, autoimmune, neurological, and renal disease (76). Free and integrated BKV genomes also have been detected in human tumor cells and tissues (10, 18); however, their significance in human cancer has not been established (46). The most significant and frequently noted consequence of BKV infection is polyomavirus-associated nephropathy (PVAN) with resulting risk of allograft loss (35). Activation of latent BKV replication in kidney allografts leading to PVAN has been suggested to be caused by inhibition of gamma interferon (1, 6), inflammation or stress, and ischemia/reperfusion (27, 34).
Animal models of BKV-associated diseases would be a great help in dissecting the cause(s) of PVAN and other BKV-related pathologies. Some rodent cells have been reported to be semipermissive for BKV infection (17, 82, 101). BKV infection or expression of the viral early region can cause malignant transformation in cultured cells (53, 72, 101) and hepatocellular carcinomas and renal and other tumors in rodents (21, 38, 88). As in human cells, BKV DNAs are maintained in some rodent cells at low levels, perhaps as episomes (10, 62); however, the processes limiting viral replication have not been defined. Murine polyomavirus (mPyV) DNA replication in mouse kidneys following injury (2, 3) or renal transplant (31) has been suggested to provide a useful model for the study of PVAN. However, replication of BKV in human and murine kidney cells must be better understood to judge the relevance of such models.
Previous studies of simian virus 40 (SV40), mPyV, and JC virus (JCV) have provided numerous insights into the processes of viral DNA replication. The viral large T antigen (TAg) helps to initiate replication by binding to multiple G(A/G)GGC motifs within the core origin, forming a dodecameric structure that distorts duplex DNA, opening DNA on one side of the origin and untwisting the other side (8, 22, 64). In an ATP- and phosphorylation-dependent process, the TAg helicase activity unwinds DNA in a bidirectional manner (81, 95, 107). Single-stranded DNA is coated with replication protein A (RPA), and topoisomerase I relieves torsional stress ahead of the replication fork (7, 37, 86). DNA polymerase α-primase (Pol α-primase) synthesizes short RNA primers that are elongated by DNA polymerase α (28, 65), and leading-strand synthesis is completed by DNA polymerase δ, RPA, proliferating cell nuclear antigen (PCNA), and replication factor C (45, 57, 110). Replication of the lagging strand is mediated by Pol α-primase, DNA polymerase δ, and accessory proteins (69, 102).
In addition to these shared properties, differences in replication between viruses have been reported to occur because of structural variation between viral core origins and TAg proteins (4, 5, 47, 51, 52); differences in TAg acetylation that either stimulate replication (109), or regulate TAg stability (73, 84); selective interactions with the host p180 DNA Pol α-primase and p48 primase subunits (9, 94) and with RPA (103); steps in replication beyond initiation (89); and modulation of replication by 5′ and 3′ cis-acting origin proximal sequences that alter activities of replication proteins and determine chromatin structure and intranuclear DNA localization (14, 29, 63, 91, 100, 104, 106, 109). Such virus- and host-specific features may affect the outcomes of viral infection.
Despite its importance in human disease, BKV DNA replication in human kidney epithelial cells and its role in PVAN are not well understood. Early studies documented that archetype BKV isolated from human tissues does not replicate in cultured human cells, and consequently, most analyses have utilized naturally occurring BKV variants with genomic alterations that promote growth in cell culture (58, 96). The consequences of such alterations for BKV infection of humans and possible viral pathogenesis are unknown. Robust BKV DNA replication in cell culture requires a 76-base-pair “core origin” very similar to that of SV40 but which does not by itself suffice for DNA replication (when mediated by SV40 TAg) (23, 24). Binding sites for cellular factors that might activate or modulate replication during PVAN are located in the core origin flanking sequences, termed the enhancer (25, 54, 59, 60). Also, although BKV TAg resembles SV40 TAg in its J domain, DNA binding domain (42, 85), helicase domain, and interactions with p53 and pRb (33), physical or functional BKV TAg interactions with cellular replication factors such as Pol α-primase subunits p180 and p48, RPA, and topoisomerase I have not been characterized.
Here, we report sensitive assays of BKV replication and comparative studies of BKV replication in human and murine cells and extracts that point to an early, TAg-dependent step as being a likely cause of the replication block in murine cells and extracts, and we speculate about possible mechanisms.
MATERIALS AND METHODS
Viral infections.
Primary human renal proximal tubule epithelial (RPTE) cells (Lonza) were maintained in renal epithelial cell growth medium as previously described (1). Murine embryonic fibroblasts (40) were immortalized using the 3T3 protocol (99) as previously described (16) and were maintained in Dulbecco modified Eagle medium (DMEM) (Gibco/BRL) containing 10% fetal bovine serum (FBS) (HyClone) and supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Lonza). Both RPTE and 3T3 cells were grown at 37°C with 5% CO2 in a humidified incubator. BKV strains TU and Proto-2 were propagated as previously described (1). Purified stocks of BKV strain TU were produced by infecting Vero cells (ATCC CCL-81) for 4 weeks and harvesting progeny virus by centrifugation through a 20% sucrose cushion followed by centrifugation in a 1.2- to 1.4-g/cm3 cesium chloride gradient (49).
BKV Proto-2 and BKV TU were used to infect 70% confluent RPTE or 3T3 cells at a multiplicity of infection of 5 infectious units per cell for 1 h at 37°C. 3T3 cells were infected and maintained in DMEM containing 2% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Total cell lysates were collected at 4 and 7 days postinfection (dpi) using E1A lysis buffer (32) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, as previously described (1). Low-molecular-weight DNA was isolated at 0, 4, and 7 dpi using the Hirt protocol (36). Each sample was spiked with unrelated plasmid (pRL-Null; Promega) as a control for DNA isolation.
Real-time PCR.
The following primers were designed using Primer3 software (77) to amplify 125- and 84-base-pair fragments of the TU and Proto-2 noncoding control regions, respectively: TUNCCRFor (5′CGCCCCTAAAATTCTCTCTT3′) and TUNCCRRev (5′ATGTCTGTCTGGCTGCTTTC3′), and ProtoNCCRFor (5′CCAGCCAGTGGCAGTTAATA3′) and ProtoNCCRRev (5′CATGGCCTTTGTCCAGTTTA3′). In addition, primers RTAmpFor (5′TCGCCGCATACACTATTCTC3′) and RTAmpRev (5′GCCGCAGTGTTATCACTCAT3′) were used to amplify a 129-base-pair fragment of the β-lactamase-coding region of the pRL-Null plasmid for normalization of the samples. All primers were synthesized by Invitrogen. Reactions were performed in a total volume of 25 μl using 2× Power Sybr green PCR master mix (Applied Biosystems), 2.5 μl template diluted 1:10,000, and 300 nM of each primer. Amplification was performed in 96-well PCR plates (Bio-Rad) using the iCycler iQ5 real-time detection system (Bio-Rad) with the following conditions: 2 min at 50°C; 10 min at 95°C; and 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 56°C (Proto-2) or 58°C (TU) for 1 min. Samples were analyzed in triplicate and normalized by amplification of the β-lactamase coding region fragment using the 2−ΔΔCT method (48).
Plasmids.
pOriBKV (termed B-B-B in Fig. 2 to 4) was generated by inserting the HindIII-SphI fragment (positions 5031 to 282) of archetype BKV Dik strain (kindly provided by J. Lednicky) into the polylinker region of pUC18. Other similar pUC18-based plasmid DNAs with complete viral origins included pOriJCV (Mad-1 strain [68]), pOriSV40 (SV-S strain [49]), and pOrimPyV (P-P-P; A3 strain [79]). The pUC18 plasmid without an insert served as negative control (pOri−) for cell-free DNA replication as well as a vector for cloning all viral origins. DNAs for replication assays were verified by sequencing and purified with Qiagen Midiprep kits.
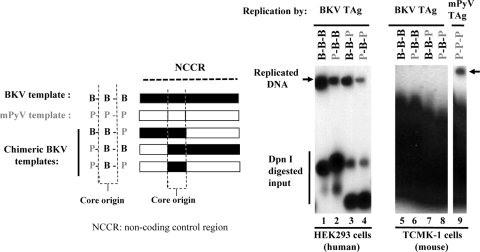
FIG. 2.
In vivo DNA replication of BKV and mPyV in human and mouse cells. Vectors expressing BKV TAg (lanes 1 to 8) or mPyV TAg (lane 9) were cotransfected into human HEK293 (lanes 1 to 4) or mouse TCMK-1 (lanes 5 to 9) cells together with plasmids containing the complete BKV origin (lanes 1 and 5, B-B-B), the complete mPyV origin (lane 9, P-P-P), and BKV-mPyV chimeric origins (lanes 2 to 4 and 6 to 8). At 48 h after transfection, DNA was isolated and analyzed by Southern blotting. DNA replication products are marked by arrows.
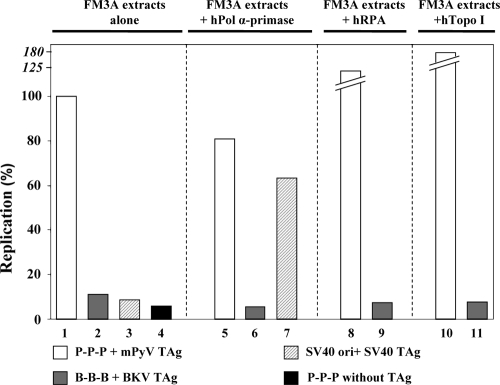
FIG. 4.
Modulation of polyomavirus DNA replication in murine cell extracts by human replication factors. In vitro DNA replication in the presence of equal amounts of purified recombinant BKV, mPyV, and SV40 TAg (shaded bars) using mouse FM3A cell extracts is shown. Bars 1 to 4 show DNA synthesis in the presence of mouse cell extracts and either mPyV TAg/P-P-P, BKV TAg/B-B-B, or SV40 TAg/pOriSV40. Incorporation of dNMPs into P-P-P in the absence of TAg served as a negative control. DNA synthesis with mouse cell extract and additional human DNA Pol α-primase (2 units of hPol α-primase) (66) is depicted in bars 5 to 7 (mPyV TAg/P-P-P, BKV TAg/B-B-B, and SV40 TAg/pOriSV40, respectively). The DNA synthesis of mouse cell extracts with an additional 0.5 μg of hRPA using mPyV TAg/P-P-P and BKV TAg/B-B-B is presented in bars 8 and 9, respectively, whereas the influence of human topoisomerase I (hTopo I, 120 ng) on the DNA synthesis in the presence of mouse cell extracts with mPyV TAg/P-P-P and BKV TAg/B-B-B is shown in bars 10 and 11, respectively. All assays were carried out in triplicate, and the results presented are the averages from two independent experiments.
In vivo DNA replication assays.
Murine TCMK-1 cells were grown in DMEM with 10% FBS, seeded in eight-well plates (1 × 105 cells/well), and incubated overnight at 37°C. Cells were transfected with Lipofectamine and Plus reagent (Invitrogen, Carlsbad, CA) with expression vectors for TAg (0.6 μg of DNA) and template plasmid (0.4 μg). After incubation of cells with DNA-Lipofectamine and PLUS reagent mixture for 4 h in 500 μl serum-free DMEM, the transfection solution was replaced with 2 ml of DMEM containing 20% FBS. Similarly, human HEK 293 cells were grown in DMEM with 10% FBS, seeded in 12-well plates (4 × 105 cells/well), and incubated overnight at 37°C. Cells were transfected with expression vector for TAg (5 ng), template plasmid (50 ng), and pUC18 empty vector (0.65 μg) as carrier DNA with Lipofectamine and Plus reagent. DNA-Lipofectamine and Plus reagent mixtures were incubated with HEK 293 cells as described above. Cells were harvested at 48 h after transfection, and low-molecular-weight DNAs were isolated by the Hirt protocol with Promega Miniprep columns, digested with EcoRI to linearize the plasmid, and digested with DpnI to distinguish input from replicated DNA (36). The DpnI-resistant DNA was resolved from digested DNA by agarose gel electrophoresis (1%). After transfer of the DNA to a nylon membrane, DpnI-resistant DNA was detected by Southern blotting with a biotinylated probe of the lacZ gene (∼400 nucleotides) of the pUC18 vector and visualized by chemiluminescent nucleic acid detection (Pierce).
Expression of TAg in mammalian cells.
Murine TCMK-1 cells (1.5 × 106 cells/60-mm plate) were transfected with 10.5 μg of TAg expression vectors as indicated using Lipofectamine and Plus transfection reagents as described above. At 48 h after transfection, cells were harvested and lysed in lysis buffer (150 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Roche]) by rolling at 4°C for 30 min. The cell lysates were centrifuged for 30 min at 10,000 × g. Supernatants were collected and incubated with anti-Flag beads (Sigma) for 2 h. The beads were washed three times in ice-cold lysis buffer, and proteins bound to the beads were eluted with 50 μl of SDS loading buffer and then analyzed by SDS-PAGE and Western blotting using an anti-Flag antibody to detect TAg expression.
Expression of TAg in insect cells and purification.
The BKV TAg cDNA was subcloned from pGEM 3Zf(−) vectors into pFastBac vector (Invitrogen, United Kingdom) with EcoRI and NotI sites. Recombinant baculoviruses containing BKV TAg were generated using the Bac-to-Bac baculovirus expression system (Invitrogen) and amplified in insect SF-9 cells in TC-100 medium plus 10% FBS (both from Lonza) (105). For high yields of protein expression, High Five insect cells (Invitrogen) were infected with the amplified virus and expression of TAg was confirmed by Western blot analysis with polyclonal antibodies against SV40 TAg that cross-react with BKV TAg (kindly provided by W. Deppert [Hamburg]). It is noteworthy that baculovirus vectors expressed BKV TAg at significantly lower levels than similar vectors coding for SV40 TAg and JCV TAg (68), in part due to the presence of inhibitory 5′ leader sequences upstream of BKV TAg cDNA and alternative splicing of BKV TAg mRNAs (data not shown). Infected cells were harvested at 48 h, homogenized (Dounce pestle A, 20 strokes) in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM KCl, 0.5% MgCl2, 0.5% Igepal CA630 [Sigma], 10% glycerol, 1× phosphatase and protease inhibitors [Sigma]), and clarified by centrifugation at 18,000 × g for 30 min. The resulting lysate was subjected to immobilized metal affinity chromatography using Talon resins (Clontech). After binding, the resin was washed with 20 column volumes of buffer A (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM KCl, 0.5% MgCl2, 0.01% Igepal CA630) and then with 20 column volumes of buffer A containing 5 mM imidazole (pH 7.5). BKV TAg was eluted in buffer A containing 500 mM imidazole (pH 7.5), and fractions containing TAg were pooled and dialyzed against 500 ml of buffer containing 20 mM HEPES [pH 7.5], 5 mM NaCl, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, and 20% glycerol and stored at −80°C. The purity and amount of BKV TAg were determined by SDS-PAGE using Roti-mark standard (150 to 10 kDa; Carl Roth GmbH & Co.) or prestained molecular weight marker proteins (New England Bioscience). Proteins were detected either by staining with Roti-Blue colloidal Coomassie blue staining reagent (Carl Roth) or by Western blot analysis (68). The purity of BKV TAg was estimated as ∼90% by Coomassie blue staining. SV40 TAg, JCV TAg, and mPyV TAg proteins were purified from extracts of insect High Five cells infected with recombinant baculoviruses by immunoaffinity chromatography using monoclonal antibodies PAb101 and 5F (for SV40 and mPyV TAg, respectively) coupled to protein A-Sepharose or by immobilized metal chelate chromatography (for JCV TAg) using Talon resin as described previously (9, 68).
Cellular replication proteins and extracts.
Pol α-primase (80), topoisomerase I (92), and RPA (67, 71) were expressed and purified and their concentrations and activities determined as previously described (66). Logarithmically growing adherent HeLa S3 cells in DMEM and suspension FM3A cells in RPMI, supplemented with 10% and 5% FBS, respectively, were collected by centrifugation, washed once with phosphate-buffered saline, and then washed extensively with hypotonic buffer (20 mM HEPES-KOH [pH 7.8], 5 mM potassium acetate, 0.5 mM DTT, and 1× phosphatase and protease inhibitors). Cells were homogenized (Dounce homogenizer B pestle, 20 strokes), adjusted to 50 mM NaCl, and incubated on ice for 30 min. Extracts were clarified by centrifugation twice at 20,000 × g for 30 min and stored at −80°C. Extracts of High Five insect cells containing BKV TAg were prepared as described above except that after centrifugation, extracts were dialyzed into 20 mM HEPES (pH 7.8), 5 mM KCl, and 1 mM DTT with 1× protease inhibitors and stored at −80°C.
In vitro DNA replication assays.
Replication of DNAs in vitro was assayed as described by Stadlbauer and coworkers with slight modifications (94). Briefly, the reaction mixtures (30 μl) contained 20 mM HEPES (pH 7.8); 7 mM magnesium acetate; 1 mM DTT; 4 mM ATP; 200 μM each CTP, UTP, and GTP; 50 μM dCTP; 100 μM each dATP, dTTP, and dGTP; 40 mM creatine phosphate di-Tris (pH 7.8); 40 μg/ml creatine kinase plus 5 μCi of [α32P]dCTP (3,000 Ci/mmol); 0.25 μg test plasmid DNA; FM3A or HeLa cell extract (25 to 75 μg of protein); and purified TAgs at the indicated concentrations. After incubation for 60 min at 37°C, reaction products were precipitated with cold 10% (wt/vol) trichloroacetic acid containing 2.5% (wt/vol) sodium pyrophosphate, spotted on glass fiber filters (GF/C; Whatman), washed with 1 M HCl, and analyzed by scintillation counting.
The monopolymerase replication assay (89) was assembled on ice with 0.5 μg of pOriBKV DNA or 0.5 μg of pUC-HS DNA (containing the SV40 replication origin) (79), 50 ng topoisomerase I, 100 ng Pol α-primase, and 1 μg RPA in 30 mM HEPES-KOH (pH 7.8); 7 mM magnesium acetate; 0.1 mM EGTA; 0.5 mM DTT; 200 μM each UTP, GTP, and CTP; 4 mM ATP; 100 μM each dATP, dGTP, and dTTP; 10 μM dCTP; 40 mM creatine phosphate; 1 μg creatine kinase; 0.1 mg/ml heat-treated bovine serum albumin; and 5 μCi [α-32P]dCTP (3000 Ci/mmol; Perkin-Elmer) in 40 μl. Purified BKV or SV40 TAg (0.2 μg) was added to start the reaction, and after incubation for 60 min at 37°C, the reaction products were precipitated with cold 10% (wt/vol) trichloroacetic acid containing 2.5% (wt/vol) sodium pyrophosphate, spotted on glass fiber filters (GF/C; Whatman), washed with 1 M HCl, and analyzed by scintillation counting.
RESULTS
Comparison of BKV infection of human and murine cells.
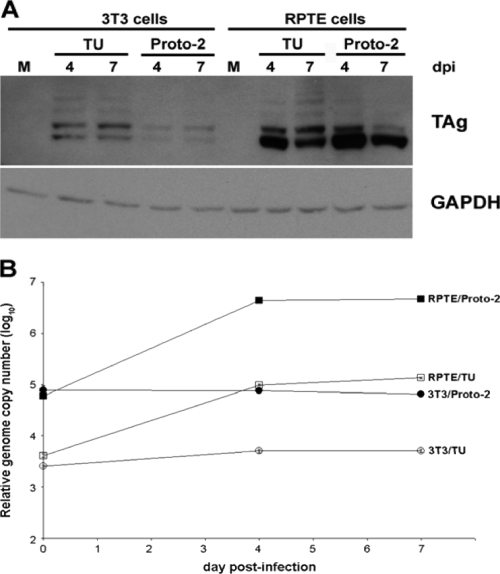
Human RPTE and mouse 3T3 cells were infected with the TU and Proto-2 BKV strains and at 4 and 7 dpi, proteins and low-molecular-weight DNA were extracted and analyzed by Western blotting and quantitative real-time PCR, respectively. Although BKV TAg was expressed in both the human and murine cells, viral DNA replication was observed only in the human RPTE cells, with levels increasing between 4 and 7 dpi (Fig. 1). Incubating the infected murine cells for an additional week did not increase viral DNA, and a fluorescent-focus assay revealed that progeny were produced by infected RPTE cells but not by murine cells (data not shown).
FIG. 1.
Lack of BKV DNA replication in murine cells during viral infection. Human RPTE cells or murine 3T3 cells were infected with BKV. (A) Total cell lysates were harvested at 4 and 7 dpi, and proteins (15 μg) were subjected to Western blotting and probed for expression of TAg and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (a loading control). The numbers above the lanes indicate the days after infection at which the cell lysates were harvested. M, mock-infected lysate. (B) Low-molecular-weight DNA was isolated at 0, 4, and 7 dpi and analyzed by real-time PCR. Data are presented as genome copy number per reaction, normalized to the control plasmid pRL-Null (control for purification efficiency). Samples were assayed in triplicate; results are representative of two independent experiments.
Importance of core origin and flanking sequences for species-specific BKV DNA replication.
To assess whether the lack of BKV DNA replication in murine cells is dependent upon core origin or origin flanking sequences, chimeric DNAs with the BKV core origin flanked by BKV (B-B-B) or mPyV sequences (P-B-B, B-B-P, and P-B-P) were constructed (Fig. 2) and their replication activity was analyzed following DNA transfection into cells. Exchanging the BKV early and late flanking region with mPyV sequences reduced the efficiency of chimeric DNA replication in human cells (Fig. 2, compare lane 1 with lanes 2 to 4). When transfected into mouse TCMK-1 cells that support mPyV DNA (P-P-P) replication in the presence of mPyV TAg (Fig. 2, lane 9), no replication of DNAs containing the BKV origin was detected (Fig. 2, lane 5), consistent with the results of the viral infection assays described in Fig. 1. Furthermore, none of the BKV-mPyV chimeric templates were replicated (Fig. 2, lanes 6 to 8). Although BKV and mPyV TAgs were expressed at similar levels in TCMK-1 cells (data not shown), BKV TAg did not support replication of any BKV origin tested in murine cells, whereas in these cells, mPyV TAg supported replication of its cognate origin (Fig. 2) and of chimeric DNAs with the mPyV core origin (data not shown). These data indicate that the BKV core origin and its cognate TAg are primary determinants of the lack of BKV replication in murine cells.
BKV TAg-dependent DNA replication in human cell extracts.
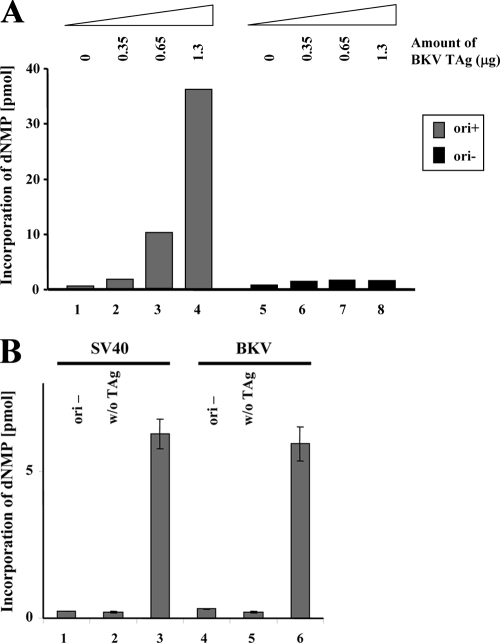
To define the cause of the lack of BKV replication in murine cells, an in vitro BKV TAg-dependent DNA replication system was established. Since insect cells efficiently express polyomavirus TAg proteins capable of supporting cell-free DNA replication (9, 68, 79, 89, 93, 94, 103), BKV TAg was expressed from baculovirus vectors in High Five insect cells and purified (data not shown). Archetype BKV, which is derived from human patients but does not replicate in cultured cells, was used as the source of DNA for these experiments. The insect cell extracts containing BKV TAg promoted BKV DNA replication in vitro in a dose-dependent manner when mixed with HeLa cell extracts (Fig. 3A, bars 1 to 4). Replication also depended on the presence of the BKV core origin (ori+) (Fig. 3A, compare bars 2 to 4 with bars 6 to 8). Similar results were obtained with purified BKV TAg (data not shown), whose activity was comparable to that of purified SV40 TAg with DNAs containing the SV40 origin (Fig. 3B, bars 3 and 6).
FIG. 3.
Cell-free BKV DNA replication by recombinant BKV TAg. (A) DNA synthesis in the presence of recombinant BKV TAg was measured by incorporation of dNMPs into DNA. Increasing amounts (0, 7, 13, and 26 μg) of High Five cell extracts containing recombinant BKV TAg (about 0, 0.35, 0.65, and 1.3 μg, respectively) were added to HeLa hypotonic extracts in the presence of DNA with a BKV origin of replication (250 ng of B-B-B, ori+, bars 1 to 4) or empty vector (ori−, bars 5 to 8). (B) Cell-free DNA replication in the presence of BKV and SV40 TAg. DNA replication in the presence of 200 ng of purified recombinant BKV TAg or SV40 TAg as indicated was measured using 40 μg of HeLa extract and 500 ng of DNA containing the SV40 or BKV origin of replication (ori+) or an empty vector (ori−). Incorporation of dNMPs into DNA was measured by scintillation counting. The DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
Restriction of replication of BKV origin-containing DNA.
Reflecting the lack of viral replication observed in cultured cells, murine cell extracts did not support BKV TAg-dependent replication of BKV DNAs, although these extracts supported the mPyV TAg-dependent replication of the mPyV origin (Fig. 4, compare bars 2 and 1) and resembled reactions carried out in parallel with SV40 TAg (Fig. 4, bar 3), and assays including mPyV DNA but lacking mPyV TAg (Fig. 4, bar 4). To ascertain the biochemical basis of the lack of BKV DNA replication, the murine extracts were supplemented with human Pol α-primase (hPol α-primase), RPA, or topoisomerase I. No incorporation of deoxynucleoside monophosphates (dNMPs) into BKV origin-containing DNA was observed when hPol α-primase and BKV TAg were added to murine extracts (Fig. 4, bar 6). In contrast, the addition of hPol α-primase resulted in SV40 TAg-dependent replication of SV40 origin-containing DNAs to about 60% of the level obtained with mPyV TAg and its cognate origin (Fig. 4, compare bars 7 and 1), consistent with published data (90). Similarly, purified human recombinant RPA (hRPA) added to murine extracts did not support replication of BKV DNA (Fig. 4, bar 9); however, addition of hRPA to the murine extracts stimulated mPyV DNA replication with mPyV TAg (Fig. 4, compare bars 1 and 8), suggesting that RPA levels are limiting in these extracts and that the purified hRPA does not inhibit DNA replication per se. This is consistent with previous data indicating that addition of RPA or Escherichia coli single-stranded DNA binding protein stimulated SV40 and mPyV DNA replication, respectively, in mouse cell extracts (26, 94). Addition of purified human recombinant topoisomerase I also did not support incorporation of dNMPs into BKV DNA (Fig. 4, bar 11), but it stimulated mPyV TAg-dependent DNA replication (Fig. 4, compare bars 1 and 10) nearly twofold, suggesting that topoisomerase I is also limiting in murine cell extracts, as has been previously observed in human cell extracts (87).
Murine proteins inhibit BKV TAg-dependent DNA replication.
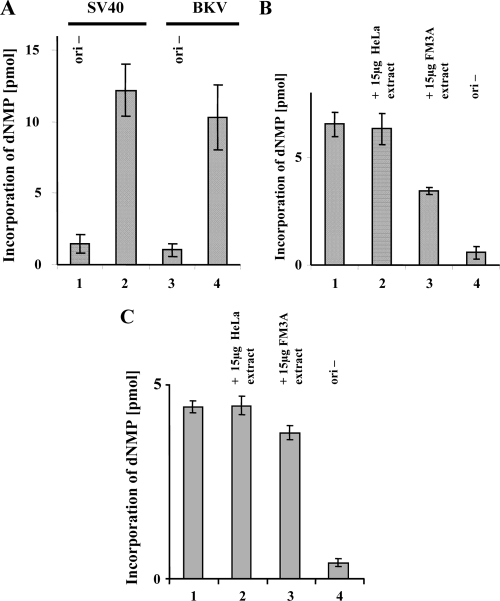
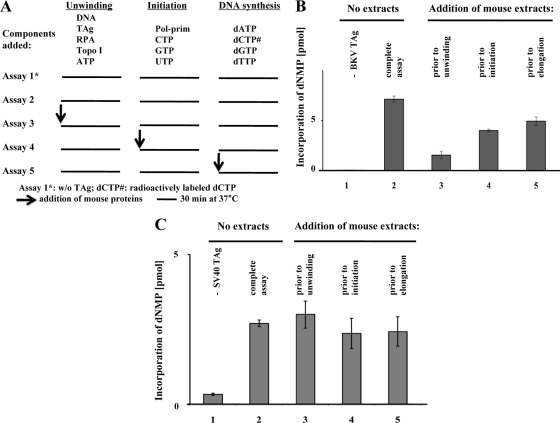
The lack of BKV TAg-dependent DNA replication in murine extracts was studied with a monopolymerase replication system comprised of purified hPol α-primase, RPA, and topoisomerase I (89). Addition of each of these purified human proteins individually did not suffice to support BKV DNA replication in mouse extracts (Fig. 4). However, the three human proteins mixed together, without murine cell extracts, were capable of BKV TAg-dependent DNA replication in vitro with efficiencies resembling those of the SV40 system (Fig. 5A). Replication in the BKV monopolymerase system was not affected by human cell extracts added immediately prior to BKV TAg (Fig. 5B, compare bars 1 and 2), whereas addition of murine extracts reduced the incorporation of dNMPs by almost 50% (compare bar 3 with bars 1 and 2). By comparison, the addition of murine extracts reduced the incorporation of dNMPs in the SV40 monopolymerase system by only 10% (Fig. 5C, compare bar 3 with bar 1), and the addition of human extracts to the SV40 system did not influence replication (compare bars 1 and 2). To determine at what step the inhibition occurs, murine extracts were introduced into the monopolymerase system at different stages of replication (Fig. 6A). When added prior to the addition of TAg and human replication factors, murine extracts inhibited BKV TAg-dependent replication by more than 75% (Fig. 6B, compare bars 2 and 3). In contrast, addition of murine extracts at a later stage (after DNA unwinding but prior to the initiation or the elongation reaction) had a lesser inhibitory effect (Fig. 6B, compare bars 4 and 5 with bars 2 and 3). These findings contrast with those for the SV40 system, where little inhibition by murine extracts is observed, regardless of the time of addition (Fig. 6C). However, these results resemble those recently reported with the SV40 monopolymerase system using polypeptides that interfere with the assembly of the initiation complex (98).
FIG. 5.
DNA synthesis with purified human proteins in the presence of BKV and SV40 TAg. (A) Incorporation of dNMPs into DNA containing an SV40 (bar 2) or BKV (bar 4) origin of replication in the presence of 200 ng of the respective viral TAg and 100 ng of purified hPol α-primase, 50 ng topoisomerase I, and 1000 ng RPA was measured (monopolymerase DNA replication system). Vectors without a functional viral origin (ori−) served as negative controls (bars 1 and 3). (B) The effect of human and mouse proteins on DNA synthesis by hPol α-primase was determined with a BKV origin of replication. The incorporation of radioactive dNMPs using the BKV origin of replication as a template was measured in the presence of buffer but no additional proteins or with 15 μg human or mouse cell extracts (bars 1, 2, and 3, respectively). DNA synthesis in the presence of DNA lacking an origin of replication served as a negative control (bar 4). (C) The effect of human and mouse proteins on the DNA synthesis by human DNA Pol α-primase was determined with an SV40 origin of replication. The incorporation of radioactive dNMPs was determined in the presence of buffer but no additional proteins or with 15 μg human or mouse cell extracts (bars 1, 2, and 3, respectively). DNA synthesis in the presence of DNA lacking an origin of replication served as a negative control (bar 4). Incorporation of dNMPs into DNA was measured by scintillation counting. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
FIG. 6.
DNA replication with purified human proteins in the presence of BKV TAg and mouse cell extracts. (A) The replication of polyomavirus DNA was biochemically separated into three consecutive reaction steps: the unwinding, initiation, and elongation reactions. In the presence of RPA, topoisomerase I, ATP, and an ATP-regenerating system, viral TAg unwinds viral DNA at 37°C for 30 min (unwinding reaction). To synthesize primers at the unwound origin of DNA replication (initiation reaction), hPol α-primase and the three remaining ribonucleotides were added, and oligoribonucleotide primers are synthesized during the incubation at 37°C for 30 min, whereas no DNA can be synthesized since deoxynucleoside triphosphates are lacking. Finally, deoxynucleoside triphosphates, which include radioactively labeled dCTP to monitor DNA synthesis via scintillation counting, are added and DNA is synthesized at 37°C for 30 min (elongation reaction). Mouse cell extracts capable of supporting mPyV DNA replication were added to the reactions prior to the specified step (as indicated by the arrows). The addition of buffer served as control for the influence of salt and dilutions. (B) Results of the monopolymerase assay using BKV TAg and template containing the BKV origin of replication. (C) Results of the monopolymerase assay using SV40 TAg and template containing the SV40 origin of replication. In panels B and C, bars 1 and 2 represent dNMP incorporation into DNA in the absence and presence of TAg, respectively, but without mouse proteins. For bar 3, mouse cell extracts were added to reaction components before the addition of hPol α-primase and ribonucleotides (prior to unwinding of DNA). For bar 4, mouse cell extracts were added after the unwinding reaction but before the addition of hPol α-primase (prior to initiation of DNA replication). For bar 5, mouse cell extracts were added after initiation of DNA replication but before addition of deoxynucleoside triphosphates (prior to elongation). Incorporation of dNMPs into DNA was measured by scintillation counting. DNA synthesis was determined in duplicate and repeated three times. The averages from these experiments and the standard deviations are presented.
Origin selectivity of BKV TAg-dependent DNA replication.
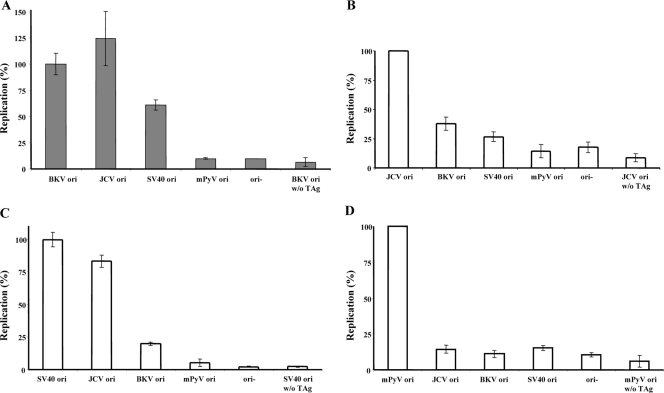
As the inhibition of BKV TAg-dependent DNA replication by murine extracts occurs predominantly before/during the origin binding and initiation, the origin selectivity of BKV TAg and other polyomavirus TAg proteins (JCV, SV40, and mPyV) was assessed. In human extracts, BKV TAg efficiently promoted replication of DNAs containing either a BKV origin or a JCV origin and, less well, that of DNAs containing an SV40 origin (Fig. 7A). JCV TAg promoted replication of DNA containing its cognate origin and, less well, replication of DNAs with BKV or SV40 origins (Fig. 7B). In turn, SV40 TAg supported replication of DNAs with its cognate origin or with a JCV origin (68) and, less well, DNAs with a BKV origin (Fig. 7C). As reported earlier, DNAs with the BKV, SV40, or JCV origin were not replicated by mPyV TAg and murine extracts (Fig. 7D). These observations point to subtle but perhaps significant differences between these TAgs and their interactions with cognate and noncognate origins that might explain the restriction of replication of these viruses in cells. Also, these findings extend reports of BKV TAg-dependent DNA replication of the JCV origin in human extracts and cells (50, 52).
FIG. 7.
Comparison of the replication of DNAs with different origins by polyomavirus TAg proteins in vitro. Replication assays were carried out as described in Materials and Methods. (A to C) Incorporation of dNMPs into DNA was measured in the presence of BKV (A), JCV (B), or SV40 (C) TAg protein and human (HeLa) cell extracts. (D) mPyV TAg-dependent DNA replication in mouse (FM3A) cell extracts. DNA synthesis in the presence of BKV, JCV, SV40, and mPyV origin-containing DNA but without the cognate TAg, as well as DNA synthesis of plasmid DNA without a viral origin in the presence of the indicated TAg, served as negative controls in all panels. All assays were carried out in triplicate, and the results presented are the averages and standard deviations from two independent experiments.
DISCUSSION
The regulation of BKV replication in human kidney tissues and the cause(s) of BKV reactivation in allografts leading to PVAN are not understood, and combined with the lack of suitable animal models, this makes the development of effective preventions or interventions difficult. A murine model of BKV infection would be of great benefit; however, BKV DNA is not replicated in murine cells. As shown in Fig. 1, BKV can infect murine cells and low levels of BKV TAg are expressed following infection by virions, but no BKV DNA replication occurs. However, these BKV TAg levels are most likely not the cause for the failure of BKV DNA replication in murine cells, since our previous findings indicate that expression of very low levels of BKV TAg in human RPTE cells suffices for viral replication and progeny production (1). The finding that the presence of BKV TAg is not sufficient for BKV DNA replication in murine cells is consistent with the results of BKV DNA replication in murine cells using overexpressed BKV TAg (Fig. 2 and data not shown). Although BKV TAg levels in murine cells were equivalent to those of mPyV TAg expressed in parallel, BKV TAg does not allow BKV DNA replication, whereas mPyV TAg efficiently supported mPyV DNA replication in these cells (Fig. 2). These data reveal that a factor other than the infection of murine cells and the expression level of BKV TAg is the regulatory step in the species specificity of BKV DNA replication.
Analyses of BKV TAg- and mPyV TAg-dependent replication of their cognate origin-containing DNAs in combination with heterologous flanking sequences revealed that the BKV core origin and TAg are primary determinants of the restriction of BKV replication in murine cells. To study the molecular basis of this restriction, a robust BKV TAg-dependent DNA replication in vitro was established with archetype BKV DNA sequences and analyzed with human and murine cell extracts (Fig. 3). As was observed with cellular assays of BKV DNA replication, murine extracts also did not support BKV TAg-dependent DNA replication. Furthermore, addition of replication-active hPol α-primase, RPA, and topoisomerase I to the murine extracts did not promote BKV DNA replication (Fig. 4), in contrast to similar studies of SV40 and mPyV DNA replication in heterologous systems (9, 94). Additional analyses of BKV TAg-dependent DNA replication with a monopolymerase system comprising these three human proteins revealed that murine cell extracts, but not human cell extracts, inhibit BKV DNA replication at an early stage (Fig. 5 and 6), perhaps during the unwinding of the core origin by BKV TAg. Such inhibition is not observed with SV40 TAg-dependent DNA synthesis in the monopolymerase system. The inhibitory activities may associate with and/or modify BKV TAg so as to interfere with its origin binding or unwinding activities and are consistent with the cell-based replication assays that indicate the BKV core origin sequences bound by TAg are of primary importance for the restriction of replication in murine cells.
The absence of BKV TAg-dependent DNA synthesis in murine extracts, even with the addition of human replication proteins, suggests that the restriction of BKV DNA replication in murine extracts differs from that observed for JCV and SV40 DNA replication. It is noteworthy that JCV TAg is reported to support replication of DNAs with its cognate origin in murine extracts (89). These differences also might be reflected by the origin selectivity of the virus TAg proteins for their cognate origins. All the experiments with murine extracts were carried out under conditions optimal for mPyV DNA replication, even though factors such as RPA and topoisomerase I are rate limiting (Fig. 4). Moreover, the stimulation of incorporation of dNMPs into mPyV DNA by RPA and topoisomerase I, and into SV40 DNA by hPol α-primase, indicates that these human proteins are functional in murine extracts.
Although introduction of the human replication proteins did not restore BKV DNA replication in murine extracts, one cannot rule out the possibility that the lack of BKV DNA replication in murine cells is mediated by an incompatibility of components of the host DNA replication complex. For instance, a murine initiation factor(s) might stably complex with BKV TAg, forming an inactive chimeric complex, analogous to what has been observed with SV40 DNA replication inhibition by polypeptides representing the protein-protein interaction regions of replication proteins (97, 98). Later steps of DNA replication involving other components, such as PCNA, replication factor C, and DNA polymerase δ/ɛ, required for elongation and polymerase switching might also contribute to the restriction of BKV DNA replication.
The replication assays using chimeric templates suggest that sequences flanking the core origin are unlikely to be primary determinants of the restriction of replication. However, chimeric templates might lack some important “cross talk” interactions between core origin flanking sequences and the core origin, which could be vital for DNA replication in vivo. For example, AP-1 stimulates both mPyV and SV40 DNA replication (29, 30, 39, 56, 100) but inhibits JCV replication (43, 75). NF-1 also has been shown to stimulate SV40 DNA replication (15, 63, 100); however, a closer look into different members of NF-1 family proteins revealed that NF-1D, expressed predominantly in permissive glial cells, stimulated JCV virus replication (61), while NF-1A, expressed predominantly in nonpermissive progenitor and HeLa cells, restricted JCV replication in these cells (74). NF-1 binding sites have been identified on BKV origin flanking sequences (11-13, 54), and AP-1 binding sites were reported in enhancers of BKV strains (54, 55). Their influence on BKV DNA replication needs to be elucidated.
Recently, DNA replication of SV40 and mPyV has been shown to activate and to utilize the ATM-mediated DNA damage response (20, 83), which can be detrimental for viral DNA replication due to a block to cell cycle progression at the G1/S checkpoint. To override the DNA damage response triggered by viral infection, SV40 TAg targets subunits of the MRN complex for degradation through its interaction with CUL7, an E3 ubiquitin ligase (41, 108, 111). It is possible that BKV TAg does not interact with murine CUL7 and therefore cannot mediate the degradation of the murine MRN complex, whereas this mechanism would be functional in human cells and extracts. In addition, components of the DNA replication machinery also participate in the DNA repair pathway (19, 70, 78). It is possible that the DNA damage response triggered by BKV infection of murine cells might differently modulate these components, causing restriction of BKV replication.
Although BKV is closely related to SV40 and JCV, our data indicate there to be distinct sites at which host-specific replication factors may conflict with viral DNA replication. Detailed analysis of the restriction of BKV DNA replication in murine cells may point to processes that maintain BKV DNA replication at low levels until it is stimulated in the transplant setting to activate DNA replication.
Acknowledgments
This work was supported by NIH grant R21 AI062848 to W.R.F.; by INTAS, Health Research Board, Ireland, and Science Foundation Ireland grants to H.P.N.; by NIH grant AI060584 to M.J.I.; by an NUIG fellowship to I.T.; and by the F. G. Novy Fellowship to J.R.A.
We thank John Lednicky and W. Deppert for kind gifts of reagents and Sarah Scanlon for advice.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Abend, J. R., J. A. Low, and M. J. Imperiale. 2007. Inhibitory effect of gamma interferon on BK virus gene expression and replication. J. Virol. 81272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atencio, I. A., F. F. Shadan, X. J. Zhou, N. D. Vaziri, and L. P. Villarreal. 1993. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J. Virol. 671424-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atencio, I. A., and L. P. Villarreal. 1994. Polyomavirus replicates in differentiating but not in proliferating tubules of adult mouse polycystic kidneys. Virology 20126-35. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, E. R., M. Naujokas, and J. A. Hassell. 1989. Requirements for species-specific papovavirus DNA replication. J. Virol. 635371-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya, S., H. E. Lorimer, and C. Prives. 1995. Murine polyomavirus and simian virus 40 large T antigens produce different structural alterations in viral origin DNA. J. Virol. 697579-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binggeli, S., A. Egli, S. Schaub, I. Binet, M. Mayr, J. Steiger, and H. H. Hirsch. 2007. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am. J. Transplant. 71131-1139. [DOI] [PubMed] [Google Scholar]
- 7.Borowiec, J. A., F. B. Dean, P. A. Bullock, and J. Hurwitz. 1990. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell 60181-184. [DOI] [PubMed] [Google Scholar]
- 8.Borowiec, J. A., and J. Hurwitz. 1988. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 73149-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brückner, A., F. Stadlbauer, L. A. Guarino, A. Brunahl, C. Schneider, C. Rehfuess, C. Previes, E. Fanning, and H. P. Nasheuer. 1995. The mouse DNA polymerase alpha-primase subunit p48 mediates species-specific replication of polyomavirus DNA in vitro. Mol. Cell. Biol. 151716-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo, A., A. Corallini, M. P. Grossi, L. Carra, P. G. Balboni, M. Negrini, G. Milanesi, G. Federspil, and G. Barbanti-Brodano. 1983. Episomal DNA of a BK virus variant in a human insulinoma. J. Med. Virol. 1237-49. [DOI] [PubMed] [Google Scholar]
- 11.Cassill, J. A., and S. Subramani. 1989. A naturally occurring deletion in the enhancer repeats of the human papovavirus BK optimizes early enhancer function at the expense of late promoter activity. Virology 170296-298. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty, T., and G. C. Das. 1989. Identification of HeLa cell nuclear factors that bind to and activate the early promoter of human polyomavirus BK in vitro. Mol. Cell. Biol. 93821-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty, T., and G. C. Das. 1991. Proteins of the nuclear factor-1 family act as an activator of the late promoter in human polyomavirus BK in vitro. J. Gen. Virol. 721935-1942. [DOI] [PubMed] [Google Scholar]
- 14.Chang, C. F., H. Tada, and K. Khalili. 1994. The role of a pentanucleotide repeat sequence, AGGGAAGGGA, in the regulation of JC virus DNA replication. Gene. 148309-314. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, L., and T. J. Kelly. 1989. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell 59541-551. [DOI] [PubMed] [Google Scholar]
- 16.Christensen, J. B., and M. J. Imperiale. 1995. Inactivation of the retinoblastoma susceptibility protein is not sufficient for the transforming function of the conserved region 2-like domain of simian virus 40 large T antigen. J. Virol. 693945-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corallini, A., G. Barbanti-Brodano, W. Bortoloni, I. Nenci, E. Cassai, M. Tampieri, M. Portolani, and M. Borgatti. 1977. High incidence of ependymomas induced by BK virus, a human papovavirus. J. Natl. Cancer Inst. 591561-1564. [DOI] [PubMed] [Google Scholar]
- 18.Corallini, A., M. Pagnani, P. Viadana, E. Silini, M. Mottes, G. Milanesi, G. Gerna, R. Vettor, G. Trapella, V. Silvani, et al. 1987. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int. J. Cancer 3960-67. [DOI] [PubMed] [Google Scholar]
- 19.Cortez, D. 2005. Unwind and slow down: checkpoint activation by helicase and polymerase uncoupling. Genes Dev. 191007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahl, J., J. You, and T. L. Benjamin. 2005. Induction and utilization of an ATM signaling pathway by polyomavirus. J. Virol. 7913007-13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalrymple, S. A., and K. L. Beemon. 1990. BK virus T antigens induce kidney carcinomas and thymoproliferative disorders in transgenic mice. J. Virol. 641182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLucia, A. L., B. A. Lewton, R. Tjian, and P. Tegtmeyer. 1983. Topography of sinmian virus 40 protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J. Virol. 46143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Vecchio, A. M., R. A. Steinman, and R. P. Ricciardi. 1989. An element of the BK virus enhancer required for DNA replication. J. Virol. 631514-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deyerle, K. L., F. G. Sajjadi, and S. Subramani. 1989. Analysis of origin of DNA replication of human papovavirus BK. J. Virol. 63356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deyerle, K. L., and S. Subramani. 1988. Linker scan analysis of the early regulatory region of human papovavirus BK. J. Virol. 623378-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eki, T., F. Hanaoka, T. Kohda, M. Seki, M. Ui, and T. Enomoto. 1995. Efficient replication of polyomavirus DNA in a cell-free system supplemented with Escherichia coli single-stranded DNA binding protein, which exhibits species-specificity in the requirement for DNA polymerase alpha-primase. J. Biochem. (Tokyo) 118435-441. [DOI] [PubMed] [Google Scholar]
- 27.Fishman, J. A. 2002. BK virus nephropathy—polyomavirus adding insult to injury. N. Engl. J. Med. 347527-530. [DOI] [PubMed] [Google Scholar]
- 28.Frick, D. N., and C. C. Richardson. 2001. DNA primases. Annu. Rev. Biochem. 7039-80. [DOI] [PubMed] [Google Scholar]
- 29.Guo, W., W. J. Tang, X. Bu, V. Bermudez, M. Martin, and W. R. Folk. 1996. AP1 enhances polyomavirus DNA replication by promoting T-antigen-mediated unwinding of DNA. J. Virol. 704914-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, Z. S., and M. L. DePamphilis. 1992. Specific transcription factors stimulate simian virus 40 and polyomavirus origins of DNA replication. Mol. Cell. Biol. 122514-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han Lee, E. D., C. C. Kemball, J. Wang, Y. Dong, D. C. Stapler, K. M. Hamby, S. Gangappa, K. A. Newell, T. C. Pearson, A. E. Lukacher, and C. P. Larsen. 2006. A mouse model for polyomavirus-associated nephropathy of kidney transplants. Am. J. Transplant. 6913-922. [DOI] [PubMed] [Google Scholar]
- 32.Harlow, E., P. Whyte, B. R. Franza, Jr., and C. Schley. 1986. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol. Cell. Biol. 61579-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris, K. F., J. B. Christensen, and M. J. Imperiale. 1996. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J. Virol. 702378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch, H. H. 2005. BK virus: opportunity makes a pathogen. Clin. Infect. Dis. 41354-360. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 791277-1286. [DOI] [PubMed] [Google Scholar]
- 36.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26365-369. [DOI] [PubMed] [Google Scholar]
- 37.Iftode, C., Y. Daniely, and J. A. Borowiec. 1999. Replication protein A (RPA): the eukaryotic SSB. Crit. Rev. Biochem. Mol. Biol. 24141-180. [DOI] [PubMed] [Google Scholar]
- 38.Imperiale, M. J. 2000. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology 2671-7. [DOI] [PubMed] [Google Scholar]
- 39.Ito, K., M. Asano, P. Hughes, H. Kohzaki, C. Masutani, F. Hanaoka, T. Kerppola, T. Curran, Y. Murakami, and Y. Ito. 1996. c-Jun stimulates origin-dependent DNA unwinding by polyomavirus large Tantigen. EMBO J. 155636-5646. [PMC free article] [PubMed] [Google Scholar]
- 40.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359295-300. [DOI] [PubMed] [Google Scholar]
- 41.Kasper, J. S., H. Kuwabara, T. Arai, S. H. Ali, and J. A. DeCaprio. 2005. Simian virus 40 large T antigen's association with the CUL7 SCF complex contributes to cellular transformation. J. Virol. 7911685-11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley, W. L., and C. Georgopoulos. 1997. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc. Natl. Acad. Sci. USA 943679-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim, J., S. Woolridge, R. Biffi, E. Borghi, A. Lassak, P. Ferrante, S. Amini, K. Khalili, and M. Safak. 2003. Members of the AP-1 family, c-Jun and c-Fos, functionally interact with JC virus early regulatory protein large T antigen. J. Virol. 775241-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowles, W. A. 2001. Propagation and assay of BK virus. Methods Mol. Biol. 16519-31. [DOI] [PubMed] [Google Scholar]
- 45.Lee, S. H., and J. Hurwitz. 1990. Mechanism of elongation of primed DNA by DNA polymerase delta, proliferating cell nuclear antigen, and activator 1. Proc. Natl. Acad. Sci. USA 875672-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, W., and E. Langhoff. 2006. Polyomavirus in human cancer development. Adv. Exp. Med. Biol. 577310-318. [DOI] [PubMed] [Google Scholar]
- 47.Li, L., B. L. Li, M. Hock, E. Wang, and W. R. Folk. 1995. Sequences flanking the pentanucleotide T-antigen binding sites in the polyomavirus core origin help determine selectivity of DNA replication. J. Virol. 697570-7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 49.Low, J. A., B. Magnuson, B. Tsai, and M. J. Imperiale. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 801361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch, K. J., and R. J. Frisque. 1991. Factors contributing to the restricted DNA replicating activity of JC virus. Virology 180306-317. [DOI] [PubMed] [Google Scholar]
- 51.Lynch, K. J., and R. J. Frisque. 1990. Identification of critical elements within the JC virus DNA replication origin. J. Virol. 645812-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch, K. J., S. Haggerty, and R. J. Frisque. 1994. DNA replication of chimeric JC virus-simian virus 40 genomes. Virology 204819-822. [DOI] [PubMed] [Google Scholar]
- 53.Major, E. O., and G. Di Mayorca. 1973. Malignant transformation of BHK21 clone 13 cells by BK virus—a human papovavirus. Proc. Natl. Acad. Sci. USA 703210-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markowitz, R. B., and W. S. Dynan. 1988. Binding of cellular proteins to the regulatory region of BK virus DNA. J. Virol. 623388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markowitz, R. B., S. Tolbert, and W. S. Dynan. 1990. Promoter evolution in BK virus: functional elements are created at sequence junctions. J. Virol. 642411-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin, M. E., J. Piette, M. Yaniv, W. J. Tang, and W. R. Folk. 1988. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc. Natl. Acad. Sci. USA 855839-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto, T., T. Eki, and J. Hurwitz. 1990. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc. Natl. Acad. Sci. USA 879712-9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mew, R. T., G. Lecatsas, O. W. Prozesky, and E. H. Harley. 1981. Characteristics of BK papovavirus DNA prepared directly from human urine. Intervirology 1614-19. [DOI] [PubMed] [Google Scholar]
- 59.Moens, U., T. Johansen, J. I. Johnsen, O. M. Seternes, and T. Traavik. 1995. Noncoding control region of naturally occurring BK virus variants: sequence comparison and functional analysis. Virus Genes 10261-275. [DOI] [PubMed] [Google Scholar]
- 60.Moens, U., and M. Van Ghelue. 2005. Polymorphism in the genome of non-passaged human polyomavirus BK: implications for cell tropism and the pathological role of the virus. Virology 331209-231. [DOI] [PubMed] [Google Scholar]
- 61.Monaco, M. C., B. F. Sabath, L. C. Durham, and E. O. Major. 2001. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J. Virol. 759687-9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monini, P., L. de Lellis, A. Rotola, D. Di Luca, T. Ravaioli, B. Bigoni, and E. Cassai. 1995. Chimeric BK virus DNA episomes in a papillary urothelial bladder carcinoma. Intervirology 38304-308. [DOI] [PubMed] [Google Scholar]
- 63.Muller, K., and N. Mermod. 2000. The histone-interacting domain of nuclear factor I activates simian virus 40 DNA replication in vivo. J. Biol. Chem. 2751645-1650. [DOI] [PubMed] [Google Scholar]
- 64.Muller, W. J., C. R. Mueller, A. M. Mes, and J. A. Hassell. 1983. Polyomavirus origin for DNA replication comprises multiple genetic elements. J. Virol. 47586-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murakami, Y., T. Eki, and J. Hurwitz. 1992. Studies on the initiation of simian virus 40 replication in vitro: RNA primer synthesis and its elongation. Proc. Natl. Acad. Sci. USA 89952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasheuer, H. P., and F. Grosse. 1988. DNA polymerase alpha-primase from calf thymus. Determination of the polypeptide responsible for primase activity. J. Biol. Chem. 2638981-8988. [PubMed] [Google Scholar]
- 67.Nasheuer, H. P., D. von Winkler, C. Schneider, I. Dornreiter, I. Gilbert, and E. Fanning. 1992. Purification and functional characterization of bovine RP-A in an in vitro SV40 DNA replication system. Chromosoma 102S52-S59. [DOI] [PubMed] [Google Scholar]
- 68.Nesper, J., R. W. Smith, A. R. Kautz, E. Sock, M. Wegner, F. Grummt, and H. P. Nasheuer. 1997. A cell-free replication system for human polyomavirus JC DNA. J. Virol. 717421-7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nethanel, T., and G. Kaufmann. 1990. Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J. Virol. 645912-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paulsen, R. D., and K. A. Cimprich. 2007. The ATR pathway: fine-tuning the fork. DNA Repair (Amsterdam) 6953-966. [DOI] [PubMed] [Google Scholar]
- 71.Pestryakov, P. E., K. Weisshart, B. Schlott, S. N. Khodyreva, E. Kremmer, F. Grosse, O. I. Lavrik, and H. P. Nasheuer. 2003. Human replication protein A. The C-terminal RPA70 and the central RPA32 domains are involved in the interactions with the 3′-end of a primer-template DNA. J. Biol. Chem. 27817515-17524. [DOI] [PubMed] [Google Scholar]
- 72.Portolani, M., G. Barbanti-Brodano, and M. L. Placa. 1975. Malignant transformation of hamster kidney cells by BK virus. J. Virol. 15420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poulin, D. L., and J. A. DeCaprio. 2006. The carboxyl-terminal domain of large T antigen rescues SV40 host range activity in trans independent of acetylation. Virology 349212-221. [DOI] [PubMed] [Google Scholar]
- 74.Ravichandran, V., and E. O. Major. 2008. DNA-binding transcription factor NF-1A negatively regulates JC virus multiplication. J. Gen. Virol. 891396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ravichandran, V., B. F. Sabath, P. N. Jensen, S. A. Houff, and E. O. Major. 2006. Interactions between c-Jun, nuclear factor 1, and JC virus promoter sequences: implications for viral tropism. J. Virol. 8010506-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reploeg, M. D., G. A. Storch, and D. B. Clifford. 2001. BK virus: a clinical review. Clin. Infect. Dis. 33191-202. [DOI] [PubMed] [Google Scholar]
- 77.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 78.Sancar, A., L. A. Lindsey-Boltz, K. Unsal-Kacmaz, and S. Linn. 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 7339-85. [DOI] [PubMed] [Google Scholar]
- 79.Schneider, C., K. Weisshart, L. A. Guarino, I. Dornreiter, and E. Fanning. 1994. Species-specific functional interactions of DNA polymerase alpha-primase with simian virus 40 (SV40) T antigen require SV40 origin DNA. Mol. Cell. Biol. 143176-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schub, O., G. Rohaly, R. W. Smith, A. Schneider, S. Dehde, I. Dornreiter, and H. P. Nasheuer. 2001. Multiple phosphorylation sites of DNA polymerase α-primase cooperate to regulate the initiation of DNA replication in vitro. J. Biol. Chem. 27638076-38083. [DOI] [PubMed] [Google Scholar]
- 81.Seki, M., T. Enomoto, T. Eki, A. Miyajima, Y. Murakami, F. Hanaoka, and M. Ui. 1990. DNA helicase and nucleoside-5′-triphosphatase activities of polyoma virus large tumor antigen. Biochemistry 291003-1009. [DOI] [PubMed] [Google Scholar]
- 82.Shah, K. V., R. W. Daniel, and J. D. Strandberg. 1975. Sarcoma in a hamster inoculated with BK virus, a human papovavirus. J. Natl. Cancer Inst. 54945-950. [PubMed] [Google Scholar]
- 83.Shi, Y., G. E. Dodson, S. Shaikh, K. Rundell, and R. S. Tibbetts. 2005. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J. Biol. Chem. 28040195-40200. [DOI] [PubMed] [Google Scholar]
- 84.Shimazu, T., Y. Komatsu, K. I. Nakayama, H. Fukazawa, S. Horinouchi, and M. Yoshida. 2006. Regulation of SV40 large T-antigen stability by reversible acetylation. Oncogene 257391-7400. [DOI] [PubMed] [Google Scholar]
- 85.Simmons, D. T., G. Loeber, and P. Tegtmeyer. 1990. Four major sequence elements of simian virus 40 large T antigen coordinate its specific and nonspecific DNA binding. J. Virol. 641973-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simmons, D. T., R. Roy, L. Chen, D. Gai, and P. W. Trowbridge. 1998. The activity of topoisomerase I is modulated by large T antigen during unwinding of the SV40 origin. J. Biol. Chem. 27320390-20396. [DOI] [PubMed] [Google Scholar]
- 87.Simmons, D. T., P. W. Trowbridge, and R. Roy. 1998. Topoisomerase I stimulates SV40 T antigen-mediated DNA replication and inhibits T antigen's ability to unwind DNA at nonorigin sites. Virology 242435-443. [DOI] [PubMed] [Google Scholar]
- 88.Small, J. A., G. Khoury, G. Jay, P. M. Howley, and G. A. Scangos. 1986. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc. Natl. Acad. Sci. USA 838288-8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith, R. W., and H. P. Nasheuer. 2003. Initiation of JC virus DNA replication in vitro by human and mouse DNA polymerase alpha-primase. Eur. J. Biochem. 2702030-2037. [DOI] [PubMed] [Google Scholar]
- 90.Smith, R. W., C. Steffen, F. Grosse, and H. P. Nasheuer. 2002. Species specificity of simian virus 40 DNA replication in vitro requires multiple functions of human DNA polymerase alpha. J. Biol. Chem. 27720541-20548. [DOI] [PubMed] [Google Scholar]
- 91.Sock, E., M. Wegner, and F. Grummt. 1991. DNA replication of human polyomavirus JC is stimulated by NF-I in vivo. Virology 182298-308. [DOI] [PubMed] [Google Scholar]
- 92.Soe, K., G. Dianov, H. P. Nasheuer, V. A. Bohr, F. Grosse, and T. Stevnsner. 2001. A human topoisomerase I cleavage complex is recognized by an additional human topisomerase I molecule in vitro. Nucleic Acids Res. 293195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stadlbauer, F., A. Brueckner, C. Rehfuess, C. Eckerskorn, F. Lottspeich, V. Förster, B. Y. Tseng, and H. P. Nasheuer. 1994. DNA replication in vitro by recombinant DNA-polymerase-α-primase. Eur. J. Biochem. 222781-793. [DOI] [PubMed] [Google Scholar]
- 94.Stadlbauer, F., C. Voitenleitner, A. Brückner, E. Fanning, and H. P. Nasheuer. 1996. Species-specific replication of simian virus 40 DNA in vitro requires the p180 subunit of human DNA polymerase alpha-primase. Mol. Cell. Biol. 1694-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stahl, H., P. Dröge, and R. Knippers. 1986. DNA helicase activity of SV40 large tumor antigen. EMBO J. 51939-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taguchi, F., K. Hara, J. Kajioka, and D. Nagaki. 1979. Isolation of BK virus from a patient with systemic lupus erythematosus (SLE). Microbiol. Immunol. 231131-1132. [DOI] [PubMed] [Google Scholar]
- 97.Taneja, P., I. Boche, H. Hartmann, H. P. Nasheuer, F. Grosse, E. Fanning, and K. Weisshart. 2007. Different activities of the largest subunit of replication protein A cooperate during SV40 DNA replication. FEBS Lett. 5813973-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taneja, P., H. P. Nasheuer, H. Hartmann, F. Grosse, E. Fanning, and K. Weisshart. 2007. Timed interactions between viral and cellular replication factors during the initiation of SV40 in vitro DNA replication. Biochem. J. 407313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turner, W. J., and M. E. Woodworth. 2001. DNA replication efficiency depends on transcription factor-binding sites. J. Virol. 755638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Noordaa, J. 1976. Infectivity, oncogenicity and transforming ability of BK virus and BK virus DNA. J. Gen. Virol. 30371-373. [DOI] [PubMed] [Google Scholar]
- 102.Waga, S., G. Bauer, and B. Stillman. 1994. Reconstitution of complete SV40 DNA replication with purified replication factors. J. Biol. Chem. 26910923-10934. [PubMed] [Google Scholar]
- 103.Wang, M., J. S. Park, M. Ishiai, J. Hurwitz, and S. H. Lee. 2000. Species specificity of human RPA in simian virus 40 DNA replication lies in T-antigen-dependent RNA primer synthesis. Nucleic Acids Res. 284742-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe, S., R. Zeng, Y. Aoki, T. Itoh, and K. i. Arai. 2001. Initiation of polyoma virus origin-dependent DNA replication through STAT5 activation by human granulocyte-macrophage colony-stimulating factor. Blood 971266-1273. [DOI] [PubMed] [Google Scholar]
- 105.Weisshart, K., H. Förster, E. Kremmer, B. Schlott, F. Grosse, and H. P. Nasheuer. 2000. Protein-protein interactions of the primase subunits p58 and p48 with simian virus 40 T antigen are required for efficient primer synthesis in a cell-free system. J. Biol. Chem. 27517328-17337. [DOI] [PubMed] [Google Scholar]
- 106.Wilderman, P. J., B. Hu, and M. E. Woodworth. 1999. Conformational changes in simian virus 40 rearranged regulatory regions: effects of the 21-base-pair promoters and their location. J. Virol. 7310254-10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wold, M. S., J. J. Li, and T. J. Kelly. 1987. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc. Natl. Acad. Sci. USA 843643-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu, X., D. Avni, T. Chiba, F. Yan, Q. Zhao, Y. Lin, H. Heng, and D. Livingston. 2004. SV40 T antigen interacts with Nbs1 to disrupt DNA replication control. Genes Dev. 181305-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie, A.-Y., V. P. Bermudez, and W. R. Folk. 2002. Stimulation of DNA replication from the polyomavirus origin by PCAF and GCN5 acetyltransferases: acetylation of large T antigen. Mol. Cell. Biol. 227907-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuzhakov, A., Z. Kelman, J. Hurwitz, and M. O'Donnell. 1999. Multiple competition reactions for RPA order the assembly of the DNA polymerase delta holoenzyme. EMBO J. 186189-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao, X., R. J. Madden-Fuentes, B. X. Lou, J. M. Pipas, J. Gerhardt, C. J. Rigell, and E. Fanning. 2008. Ataxia telangiectasia-mutated damage-signaling kinase- and proteasome-dependent destruction of Mre11-Rad50-Nbs1 subunits in simian virus 40-infected primate cells. J. Virol. 825316-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]